Amniotic Fluid and Ocean Water: Evolutionary Echoes, Chemical Parallels, and the Infiltration of Micro- and Nanoplastics
Abstract
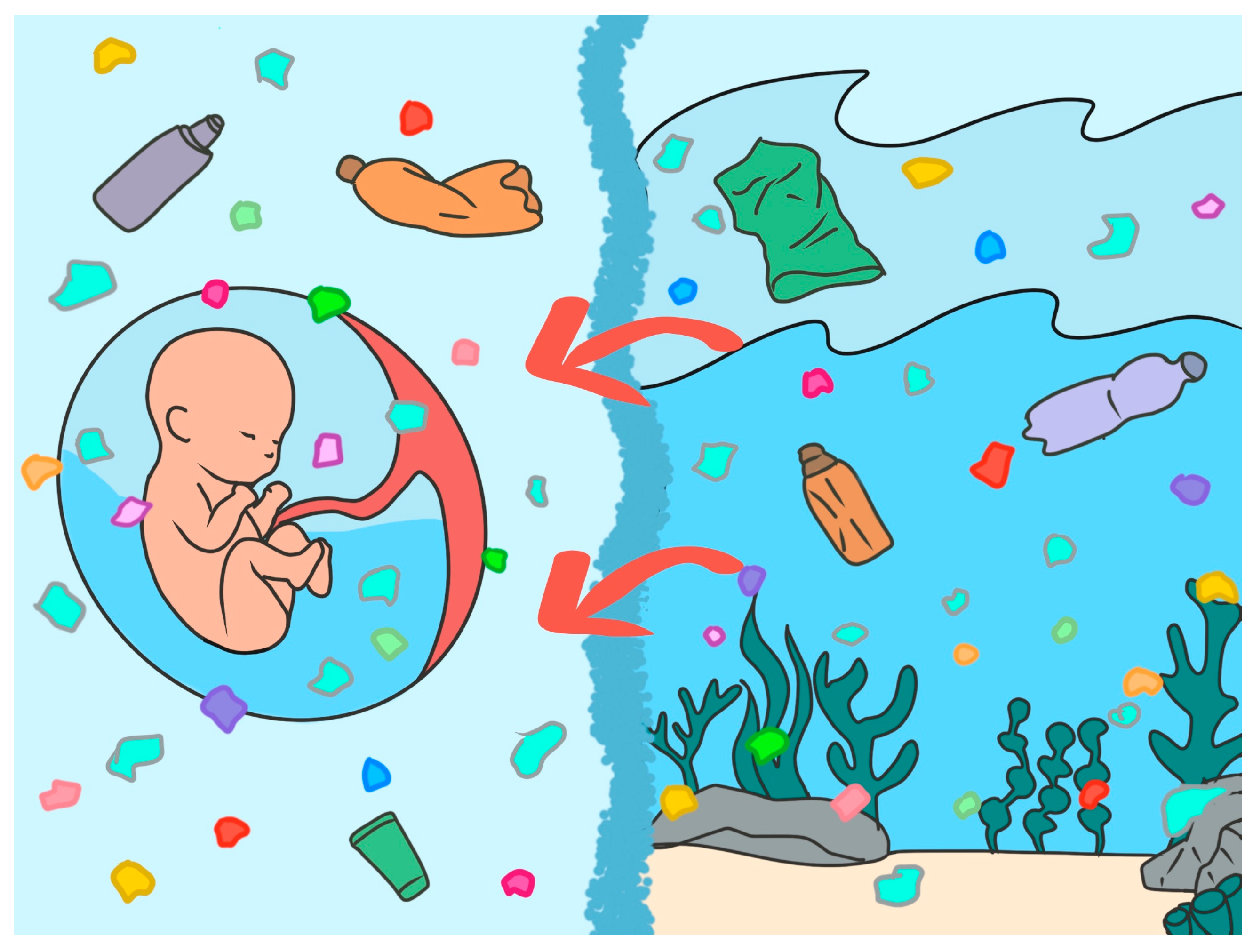
1. Introduction
1.1. The Aquatic Origins of Life: The Geochemical and Physiological Context
1.2. Evolutionary Continuity: Ionic Parallels in Amniotic Fluid
2. Claude Bernard’s Legacy and the Evolution of Homeostasis
3. Physiological Implications: Water Compartments and Dynamic Equilibrium
| Substance | Seawater (mmol/L) | Amniotic Fluid (mmol/L, 2nd–3rd Trimester) | Reference |
|---|---|---|---|
| Na+ | ≈470 | 130–140 | [46,47,48,49,50,51,52] |
| Cl− | ≈545 | 100–125 | |
| K+ | ≈10 | 3–6 | |
| Ca2+ | ≈10 | 1.5–2.4 | |
| Mg2+ | ≈53 | 1.0–2.0 | |
| SO42− | ≈28 | 0.3–0.5 | |
| HCO3− | ≈2.3 | 18–23 | |
| δ Minor and trace ions: Br−, Sr2+, F−, H4SiO4. | Bromide (Br−): 0.84 Strontium (Sr2+): 0.091 Fluoride (F−): 0.068 Dissolved silica (H4SiO4): 0.02–0.10 | Bromide (Br−): 0.02–0.08 Strontium (Sr2+): <0.01. Fluoride (F−): 0.002–0.01 Dissolved silica (H4SiO4): 0.01–0.03 | |
| pH | 7.5–8.5 | 7.0–7.4 | |
| Salinity (total) | ~35‰ | ~0.5–1.5‰ | [10,47,51,52] |
| Dissolved gases (O2, CO2, N2) | Present (O2 often 150–300 μmol/kg) | Present at physiological partial pressures | [47,52,53] |
| Urea | Present (μM) range; variable by region) | (~4–7 mmol/L) variable in gestation. | [39,47] |
| Glucose | Trace (nM–μM; rapidly consumed) | ≈15–40 mg/dL; <10 mg/dL suggests inflammation | [54,55,56] |
| Lipids (e.g., lecithin and sphingomyelin) | Present | Traces | [55,56,57,58,59] |
| Cell | Phytoplankton, prokaryotes, microeukaryotes | Fetal epithelial cells; leukocytes | [10,60,61] |
| RNA | RNA virus | cfRNA | [59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] |
| DNA | eDNA 0.1–88 µg/L up to tens of µg/L; exceptional cases (coastal hotspots) ~5000 µg/L. | Total cfDNA in the range of 50–300 µg/L, with ~10–20% of fetal origin. | |
| Proteins | 0.1% | 1–3% total volume | |
| ϕ Bacteria | 105–106 cells/mL (e.g., Prochlorococcus, Pelagibacter) | Traditionally sterile; recent studies report trace DNA, likely contamination? * | |
| ϕ Viruses | ≈107 particles/mL (mostly bacteriophages) | Absent unless there is a maternal–fetal infection (CMV, parvovirus, Zika) | |
| ϕ Fungi/Other eukaryotes | Marine yeasts, saprophytic fungi, protists | Rare; usually pathological (e.g., Candida in chorioamnionitis) | |
| Microplastics/Nanoplastics | From ~10−7 to 10 particles/L globally (wide range), with well-documented cases ≈2.2 particles/L for 32–651 µm in the Atlantic. | Presence is documented, but available works do not yet report comparable volumetric values; one report indicates ~1.5 particles per sample (volume not reported), and data remain preliminary | [79,80,81,82,83,84] |
3.1. In Summary
3.2. Environmental Toxicants and MNPs: A New Threat to Life’s Aquatic Niche
4. The One Health Paradigm: Linking Ocean and Amniotic Fluid
5. Conclusions: Protecting the Fluids of Life
6. Future Directions and Implications for Policy and Research
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Branscomb, E.; Russell, M.J. Frankenstein or a Submarine Alkaline Vent: Who is Responsible for Abiogenesis? BioEssays 2018, 40, 1700182. [Google Scholar] [CrossRef] [PubMed]
- Brack, A. Life in the solar system. Adv. Space Res. 1999, 24, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J. Fougerite: Free Energy Converter for Life’s Conception. In “Metal Ions and the Route to Life” Wolfgang Nitschke; Simon Duval, S., Ed.; Metal-Ions-in-Life-Sciences (MiLS) Series; Taylor and Francis: Abingdon, UK, 2025; pp. 341–374. [Google Scholar]
- Russell, M. A Self-sustaining Serpentinization Mega-engine Feeds the Fougerite Nanoengines Implicated in the Emergence of Guided Metabolism. Front. Microbiol. 2023, 14, 1083. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y. Hypothesis for Molecular Evolution in the Pre-Cellular Stage of the Origin of Life. Wiley Interdiscip. Rev. RNA 2025, 16, e70001. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Barge, L.M.; Bhartia, R.; Bocanegra, D.; Bracher, P.J.; Branscomb, E.; Kidd, R.; McGlynn, S.; Meier, D.H.; Nitschke, W.; et al. The drive to life on wet and icy worlds. Astrobiology 2014, 14, 308–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ueda, H.; Shibuya, T. Composition of the Primordial Ocean Just after Its Formation: Constraints from the Reactions between the Primitive Crust and a Strongly Acidic, CO2-Rich Fluid at Elevated Temperatures and Pressures. Minerals 2021, 11, 389. [Google Scholar] [CrossRef]
- Smith, V.J.; Deshmukh, M.; Wallach, T. The Molecular Ecology of Amniotic Fluid. J. Reprod. Immunol. 2024, 158, 104093. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D.; Gulbis, B.; Collins, W.P.; Zaidi, J.; Campbell, S. Investigation of the acid–base balance of coelomic and amniotic fluids in early human pregnancy. Am. J. Obstet. Gynecol. 1994, 170 Pt 1, 1365–1369. [Google Scholar] [CrossRef]
- Underwood, M.A.; Gilbert, W.M.; Sherman, M.P. Amniotic fluid: Not just fetal urine anymore. J. Perinatol. 2005, 25, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Suliburska, J.; Kocyłowski, R.; Komorowicz, I.; Grzesiak, M.; Bogdański, P.; Barałkiewicz, D. Concentrations of Mineral in Amniotic Fluid and Their Relations to Selected Maternal and Fetal Parameters. Biol. Trace Elem. Res. 2016, 172, 37–45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, S.J. From Claude Bernard to Walter Cannon. Emergence of the concept of homeostasis. Appetite 2008, 51, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. Homeostasis: The Underappreciated and Far Too Often Ignored Central Organizing Principle of Physiology. Front. Physiol. 2020, 11, 200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasmussen, J.M.; Thompson, P.M.; Entringer, S.; Buss, C.; Wadhwa, P.D. Fetal programming of human energy homeostasis brain networks: Issues and considerations. Obes. Rev. 2022, 23, e13392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reynolds, R.M.; Labad, J.; Buss, C.; Ghaemmaghami, P.; Raikkonen, K. Transmitting biological effects of stress in utero: Implications for mother and offspring. Psychoneuroendocrinology 2013, 38, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Salmaso, N.; Tomasi, S.; Vaccarino, F.M. Neurodevelopmental origins of health and disease. Curr. Opin. Neurol. 2014, 27, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hammerling, U. Membrane potential, the Na⁺/K⁺-ATPase and ATP consumption. Physiol. Rev. 2019, 99, 789–831. [Google Scholar] [CrossRef]
- Alberts, B. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2015; Chapter 14. Ion gradients and transport. [Google Scholar]
- Tobias, A.; Ballard, B.D.; Mohiuddin, S.S. Physiology, Water Balance. [Updated 2022 Oct 3]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541059/ (accessed on 1 September 2025).
- Guyton, A.C.; Hall, J.E. (Eds.) Body Fluid Compartments in Adults. In Textbook of Medical Physiology, 13th ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 75–87. [Google Scholar]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R. The renin–angiotensin–aldosterone system. Am. J. Hypertens 2002, 15 Pt 2, 147S–157S. [Google Scholar]
- Verkman, A.S. Roles of aquaporin-mediated water transport in fluid homeostasis. J. Physiol. 2011, 589 Pt 9, 1795–1805. [Google Scholar]
- Crowley, S.D.; Gurley, S.B.; Oliverio, M.I.; Pazmino, A.K.; Griffiths, R.; Flannery, P.J.; Spurney, R.F.; Kim, H.S.; Smithies, O.; Le, T.H.; et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J. Clin. Investig. 2005, 115, 1092–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damkier, H.H.; Brown, P.D.; Praetorius, J. Epithelial pathways in choroid plexus fluid secretion. News Physiol. Sci. 2010, 25, 18–24. [Google Scholar]
- Ducas, R.; Saini, B.S.; Yamamura, K.; Bhagra, C.; Marini, D.; Silversides, C.K.; Roche, S.L.; Colman, J.M.; Kingdom, J.C.; Sermer, M.; et al. Maternal and Fetal Hemodynamic Adaptations to Pregnancy and Clinical Outcomes in Maternal Cardiac Disease. Can. J. Cardiol. 2021, 37, 1942–1950. [Google Scholar] [CrossRef]
- De Bold, A.J.; Borenstein, H.B.; Veress, A.T.; Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981, 28, 89–94. [Google Scholar] [CrossRef]
- Millero, F.J.; Feistel, R.; Wright, D.W.; McDougall, T.J. The composition of Standard Seawater and the definition of the Reference-Composition Salinity Scale. Deep-Sea Res. I 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Viero, C.; Pitard, B.; Rent, G. Electrolyte composition and volume changes of amniotic fluid throughout gestation. Prenat. Diagn. 2006, 26, 361–368. [Google Scholar] [CrossRef]
- Brace, R.A. Volume, composition, and acidity of fluid recovered from fetal lungs in utero. Am. J. Obstet. Gynecol. 1997, 177, 1542–1547. [Google Scholar]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubí, E.; Lane, N. The Origin of Life in Alkaline Hydrothermal Vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Waypa, G.B.; Marks, J.D. Osmotic disruption of cell volume and membrane potential. Clin. Exp. Pharmacol. Physiol. 2012, 39, 620–626. [Google Scholar] [CrossRef]
- Hohmann, S.; Nielsen, S. (Eds.) Molecular Biology and Physiology of Water and Solute Transport; Springer Science+Business Media: New York, NY, USA, 2000; ISBN 978-1-4613-5439-0/978-1-4615-1203-5. [Google Scholar] [CrossRef]
- Sterling, P. Allostasis: A model of predictive regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gao, B.; Lei, L.; Liu, S.; Li, H.; Guo, M. Intercellular flow dominates the poroelasticity of multicellular tissues. Nat. Phys. 2025, 21, 1311–1318. [Google Scholar] [CrossRef]
- Fitzsimmons, E.D.; Bajaj, T. Embryology, Amniotic Fluid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Pullano, J.G.; Cohen-Addad, N.; Apuzzio, J.J.; Ganesh, V.L.; Josimovich, J.B. Water and salt conservation in the human fetus and newborn. I. Evidence for a role of fetal prolactin. J. Clin. Endocrinol. Metab. 1989, 69, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- NOAA Sea Water. Available online: https://www.noaa.gov/jetstream/ocean/sea-water (accessed on 1 September 2025).
- LibreTexts. Chemistry and Geochemistry of the Oceans. Available online: https://chem.libretexts.org/Bookshelves/Environmental_Chemistry/Geochemistry_(Lower)/02%3A_The_Hydrosphere/2.03%3A_Chemistry_and_geochemistry_of_the_oceans (accessed on 1 September 2025).
- EBSCOhost. Seawater Composition. Available online: https://www.ebsco.com/research-starters/oceanography/seawater-composition (accessed on 1 September 2025).
- Tsukimori, K.; Fukushima, K.; Tsukimori, M.; Nakano, H. Determination of reference values for electrolytes in amniotic fluid during normal pregnancy. J. Ultrasound Med. 2007, 26, 1635–1639. [Google Scholar]
- Gizzo, S.; Noventa, M.; Fagherazzi, S.; Lamparelli, L.; Ancona, E.; Di Gangi, S.; Nardelli, G.B. Update on amniotic fluid: From physiological role to clinical utility. J. Matern. Fetal Neonatal Med. 2012, 25, 1040–1046. [Google Scholar]
- Brace, R.A. Physiology of amniotic fluid volume regulation. Am. J. Physiol. 1983, 244, F541–F548. [Google Scholar] [CrossRef]
- Cole, D.E.; Baldwin, L.S.; Stirk, L.J. Concentrations of sulfate in human amniotic fluid. Clin. Chim. Acta 1992, 206, 287–293. [Google Scholar]
- Pressman, E.K.; Cavanaugh, J.L.; Mingione, M.J.; Woods, J.R. Amniotic fluid acid–base balance in normal pregnancy. Am. J. Obstet. Gynecol. 1996, 174 Pt 1, 225–230. [Google Scholar]
- Pilson, M.E.Q. An Introduction to the Chemistry of the Sea, 2nd ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Millero, F.J. Chemical Oceanography; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Santolaya, J.; Faro, R. Amniotic fluid physiology. Clin. Obstet. Gynecol. 1994, 37, 199–202. [Google Scholar]
- Painter, S.C.; Sanders, R.; Waldron, H.N.; Lucas, M.I.; Torres-Valdes, S. Urea distribution and uptake in the Atlantic Ocean between 50° N and 50° S. Mar. Ecol. Prog. Ser. 2008, 368, 53–63. [Google Scholar] [CrossRef]
- Khadjeh, G.H.; Ranjbar, R.; Salehi, M.; Banankhojasteh, S.M. Biochemical evaluation of amniotic fluid during different stages of gestation in the goat. Iranian J. Vet. Res. Univ. Shiraz 2007, 8, 3. [Google Scholar]
- Tong, X.L.; Wang, L.; Gao, T.B.; Qin, Y.G.; Qi, Y.Q.; Xu, Y.P. Potential function of amniotic fluid in fetal development, novel insights by comparing the composition of human amniotic fluid with umbilical cord and maternal serum at mid and late gestation. J. Chin. Med. Assoc. 2009, 72, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Xie, F. Spatiotemporal dynamics of dissolved organic matter in Asia’s longest river: Linking isotopes, land use, and anthropogenic impacts. Environ. Res. 2025, 285 Pt 3, 122491. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Jimenez, C.; Lohda, A.K.; Nores, J.; Hanaoka, S.; Avila, C.; Callahan, R.; Mazor, M.; Hobbins, J.C.; Diamond, M.P. Amniotic fluid glucose concentration: A rapid and simple method for the detection of intraamniotic infection in preterm labor. Am. J. Obstet. Gynecol. 1990, 163, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Gluck, L.; Kulovich, M.V. Lecithin/sphingomyelin ratios in amniotic fluid in prediction of fetal lung maturity. Am. J. Obstet. Gynecol. 1971, 109, 440–445. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar]
- John, K. Volkman, A review of sterol markers for marine and terrigenous organic matter. Org. Geochem. 1986, 9, 83–99. [Google Scholar] [CrossRef]
- Holzgreve, W.; Hahn, S. Fetal cells in maternal circulation and cell-free fetal DNA. Hum. Reprod. Update 2002, 8, 139–147. [Google Scholar]
- Koh, W.; Pan, W.; Gawad, C.; Fan, H.C.; Kerchner, G.A.; Wyss-Coray, T.; Blumenfeld, Y.J.; El-Sayed, Y.Y.; Quake, S.R. Noninvasive in vivo monitoring of tissue-specific global gene expression in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 7361–7366, Erratum in: Proc. Natl. Acad. Sci. USA 2014, 111, 11223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Benner, R. Chemical composition and reactivity. In Biogeochemistry of Marine Dissolved Organic Matter; Hansell, D.A., Carlson, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Cho, C.-K.J.; Shan, S.J.; Winsor, E.J.; Diamandis, E.P. Diamandis, Proteomics Analysis of Human Amniotic Fluid *. Mol. Cell. Proteom. 2007, 6, 1406–1415. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suttle, C.A. Marine viruses--major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Amend, A. From dandruff to deep-sea vents: Malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog. 2014, 10, e1004277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez-Muñoz, M.E.; Arrieta, M.-C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky, M.; Pliskova, L.; Menon, R.; Kutova, R.; Musilova, I.; Maly, J.; Andrys, C. Microbial load of umbilical cord blood Ureaplasma species and Mycoplasma hominis in preterm prelabor rupture of membranes. J. Matern. Neonatal Med. 2014, 27, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Kwon, J.Y. Trophoblast-microbiome interaction: A new paradigm on immune regulation. Am. J. Obstet. Gynecol. 2015, 213, S131–S137. [Google Scholar] [CrossRef]
- Pietramellara, G.; Ascher, J.; Borgogni, F.; Ceccherini, M.T.; Guerri, G.; Nannipieri, P. Extracellular DNA in soil and sediment: Fate and ecological relevance. Biol. Fertil. Soils. 2009, 45, 219–235. [Google Scholar] [CrossRef]
- Dell’Anno, A.; Danovaro, R. Extracellular DNA plays a key role in deep-sea ecosystem functioning. Science 2005, 309, 2179. [Google Scholar] [CrossRef]
- Torti, A.; Lever, M.A.; Jørgensen, B.B. Origin, dynamics, and implications of extracellular DNA pools in marine sediments. Mar Genom. 2015, 24 Pt 3, 185–196. [Google Scholar] [CrossRef]
- Wang, S.; Tian, R.; Bi, Y.; Meng, F.; Zhang, R.; Wang, C.; Wang, D.; Liu, L.; Zhang, B. A review of distribution and functions of extracellular DNA in the environment and wastewater treatment systems. Chemosphere 2024, 359, 142264. [Google Scholar] [CrossRef] [PubMed]
- Paul, J.H.; Jeffrey, W.H.; DeFlaun, M.F. Dynamics of extracellular DNA in the marine environment. Appl. Environ. Microbiol. 1987, 53, 170–179. [Google Scholar] [CrossRef]
- Bianchi, D.W.; Chiu, R.W. Sequencing of Circulating Cell-free DNA during Pregnancy. N. Engl. J. Med. 2018, 379, 464–473. [Google Scholar] [CrossRef]
- Lo, Y.M.D.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Finning, K.; Martin, P.; Summers, J.; Massey, E.; Poole, G.; Daniels, G. Fetal genotyping for the KEL1 and RHD alleles by analysis of maternal plasma DNA. Transfusion 2002, 42, 1079–1085. [Google Scholar] [CrossRef]
- Tsui, N.B.Y.; Chiu, R.W.K.; Lo, Y.M.D. Epigenetic approaches for the analysis of fetal nucleic acids in maternal plasma. Trends Mol. Med. 2002, 8, 454–458. [Google Scholar]
- Halfar, J.; Čabanová, K.; Vávra, K.; Delongová, P.; Motyka, O.; Špaček, R.; Kukutschová, J.; Šimetka, O.; Heviánková, S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere 2023, 343, 140301. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liang, L.; Li, Q.; Li, N.; Zhu, X.; Zhang, L. Association between microplastics in human amniotic fluid and pregnancy outcomes: Detection and characterization using Raman spectroscopy and pyrolysis GC/MS. J. Hazard. Mater. 2025, 482, 136637. [Google Scholar] [CrossRef] [PubMed]
- Pabortsava, E.; Lampitt, R.S. High concentrations of plastic hidden beneath the surface of the Atlantic Ocean. Nat. Commun. 2020, 11, 4073. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Ubeda, B.; Hernández-León, S.; Palma, A.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A.; et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; A van Franeker, J.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. Available online: https://research.wur.nl/en/publications/a-global-inventory-of-small-floating-plastic-debris/fingerprints/ (accessed on 1 September 2025). [CrossRef]
- Banchi, P.; Colitti, B.; Opsomer, G.; Rota, A.; Van Soom, A. The dogma of the sterile uterus revisited: Does microbial seeding occur during fetal life in humans and animals? Reproduction 2023, 167, e230078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banchi, P.; Bertero, A.; Corrò, M.; Colitti, B.; Maniscalco, L.; Van Soom, A.; Rota, A. Approaching the sterile womb theory in dogs and cats: A multi-technique investigation. Theriogenology 2025, 233, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.J.; Romero, R.; Greenberg, J.M.; Winters, A.D.; Galaz, J.; Gomez-Lopez, N.; Theis, K.R. Is there a placental microbiota? A critical review and re-analysis of published placental microbiota datasets. BMC Microbiol. 2023, 23, 76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Płotka-Wasylka, J.; Mulkiewicz, E.; Lis, H.; Godlewska, K.; Kurowska-Susdorf, A.; Sajid, M.; Lambropoulou, D.; Jatkowska, N. Endocrine disrupting compounds in the baby’s world—A harmful environment to the health of babies. Sci. Total Environ. 2023, 881, 163350. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Ma, Y.; Ruan, J.; You, S.; Ma, J.; Yu, H.; Zhao, J.; Zhang, K.; Yang, Q.; Jin, L.; et al. The invisible Threat: Assessing the reproductive and transgenerational impacts of micro- and nanoplastics on fish. Environ. Int. 2024, 183, 108432. [Google Scholar] [CrossRef]
- Dai, Y.; Han, R.; Yao, Z.; Yan, H.; Liu, Z.; Liu, X.; Yue, T.; Zhao, J.; Wang, Z.; Xing, B. Intergenerational transfer of micro(nano)plastics in different organisms. J. Hazard. Mater. 2025, 488, 137404. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. Available online: https://jambeck.engr.uga.edu/wp-content/uploads/2022/02/science.1260352-Jambeck-et-al-2015.pdf (accessed on 1 September 2025). [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in commercial bivalves from China. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic Pollution in Table Salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef] [PubMed]
- Qian, N.; Gao, X.; Lang, X.; Deng, H.; Bratu, T.M.; Chen, Q.; Stapleton, P.; Yan, B.; Min, W. Rapid single-particle chemical imaging of nanoplastics by SRS microscopy. Proc. Natl. Acad. Sci. USA 2024, 121, e2300582121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Inaudi, P.; Sicurella, G.M.; Rivoira, L.; Favilli, L.; Bracco, P.; Bertinetti, S.; Abollino, O.; Bruzzoniti, M.C.; Isaja, V.; Giacomino, A. Pollution profiling in Italian honeys: Elements and microplastics as comprehensive indicators of environmental contamination and food safety. Sci. Total Environ. 2025, 993, 179981. [Google Scholar] [CrossRef] [PubMed]
- Nabawy, N.M.; Ibrahim, S.A.; Abd El-Hameid, N.A.; Ghonemy, O.I.; Shaalan, W.M. Effects of Microplastics on Gene Expression, Muscular Performance, and Immunological Responses in Nile Tilapia (Oreochromis niloticus): Seasonal and Habitat Variations. Mar. Biotechnol. 2025, 27, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Fan, L.; Wang, J.; Xie, S.; Zhang, C.; Zhou, J.; Zhang, L.; Xu, G.; Zou, J. Insight into the immune and microbial response of the white-leg shrimp Litopenaeus vannamei to microplastics. Mar. Environ. Res. 2021, 169, 105377. [Google Scholar] [CrossRef] [PubMed]
- Liebezeit, G.; Liebezeit, E. Synthetic particles as contaminants in German beers. Food Addit. Contam. Part A 2014, 31, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Liu, X.; Mei, T.; Zhang, Y.; Pi, F.; Dai, H.; Zhou, Y.; Wang, J. Reducing microplastics in tea infusions released from filter bags by pre-washing method: Quantitative evidences based on Raman imaging and Py-GC/MS. Food Chem. 2024, 445, 138740. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of microplastics in white wines capped with polyethylene stoppers using micro-Raman spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, P.; Pirsaheb, M.; Amin, A.A.; Kianpour, S.; Hossini, H. Microplastic pollution in table salt and sugar: Occurrence, qualification and quantification and risk assessment. J. Food Compos. Anal. 2025, 119, 105261. [Google Scholar] [CrossRef]
- Visentin, E.; Niero, G.; Benetti, F.; O’Donnell, C.; De Marchi, M. Assessing microplastic contamination in milk and dairy products. NPJ Sci. Food 2025, 9, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.P.; Huang, X.H.; Chen, J.N.; Dong, M.; Zhang, Y.Y.; Qin, L. Pouring hot water through drip bags releases thousands of microplastics into coffee. Food Chem. 2023, 415, 135717. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.T.; Hoang, H.M.; Duong, D.D. Micro-and nanoplastic contamination in beverages in Vietnam. Environ. Monit. Assess. 2025, 197, 823. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Dewika, M.; Markandan, K.; Nagaratnam, S.; Irfan, N.A.; Abdah, M.A.A.M.; Ruwaida, J.N.; Sara, Y.Y.; Khalid, M. Assessing the concentration, distribution and characteristics of suspended microplastics in the Malaysian indoor environment. Sci. Total Environ. 2025, 959, 178049. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Guo, H.; Fu, H.; Yao, K. Microplastics in indoor and outdoor environments in China: Characteristic and human exposure risk assessment. Ecotoxicol. Environ. Saf. 2024, 287, 117328. [Google Scholar] [CrossRef] [PubMed]
- Torres-Agullo, A.; Karanasiou, A.; Moreno, T.; Lacorte, S. Airborne microplastic particle concentrations and characterization in indoor urban microenvironments. Environ. Pollut. 2022, 308, 119707. [Google Scholar] [CrossRef] [PubMed]
- Coşgun, M.S.; Gündoğdu, S.; Şahin, Ü.A.; Ayvaz, B.U.; Onat, B.; Ayvaz, C. Microplastics in the indoor air of subway station in Istanbul. Air Qual. Atmos. Health 2025, 18, 2213–2227. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Amouei Torkmahalleh, M.; Saeedi, R.; Aibaghi, R.; Faraji Ghasemi, F. Suspended fine particulate matter (PM2.5), microplastics (MPs), and polycyclic aromatic hydrocarbons (PAHs) in air: Their possible relationships and health implications. Environ. Res. 2021, 192, 110339. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.S.; Taylor, M.P.; Wilson, S.P. Quantification and exposure assessment of microplastics in Australian indoor house dust. Environ. Pollut. 2021, 283, 117064. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; MacDonald, A.; Allen, S.; Allen, D. The potential for a plastic recycling facility to release microplastic pollution and possible filtration remediation effectiveness. J. Hazard. Mater. Adv. 2023, 10, 100309. [Google Scholar] [CrossRef]
- Mokammel, A.; Naddafi, K.; Has-sanvand, M.S.; Nabizadeh, R.; Faridi, S.; Noruzzade, E.; Yaghmaeian, K. Airborne microplastics pollution in municipal solid waste processing and disposal complex: Concentration, characterization, and composition. Emerg. Contam. 2025, 11, 100459. [Google Scholar] [CrossRef]
- Novotna, J.; Tunak, M.; Militky, J.; Kremenakova, D.; Wiener, J.; Novakova, J.; Sevcu, A. Release of Microplastic Fibers from Polyester Knit Fleece during Abrasion, Washing, and Drying. ACS Omega 2025, 10, 14241–14249. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.; Lü, F.; Zhang, H.; Wang, W.; Shao, L.; Ye, J.; He, P. Is incineration the terminator of plastics and microplastics? J. Hazard. Mater. 2021, 401, 123429. [Google Scholar] [CrossRef] [PubMed]
- Mutshekwa, T.; Mulaudzi, F.; Maiyana, V.P.; Mofu, L.; Munyai, L.F.; Murungweni, F.M. Atmospheric deposition of microplastics in urban, rural, forest environments: A case study of Thulamela Local Municipality. PLoS ONE 2025, 20, e0313840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Wang, L.; Kannan, K. Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 2020, 134, 105314. [Google Scholar] [CrossRef]
- Not, C.; Chan, K.; So, M.W.K.; Lau, W.; Tang, L.T.; Cheung, C.K.H. State of microbeads in facial scrubs: Persistence and the need for broader regulation. Environ. Sci. Pollut. Res. Int. 2025, 32, 11063–11071. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bikiaris, N.; Nikolaidis, N.F.; Barmpalexis, P. Microplastics (MPs) in Cosmetics: A Review on Their Presence in Personal-Care, Cosmetic, and Cleaning Products (PCCPs) and Sustainable Alternatives from Biobased and Biodegradable Polymers. Cosmetics 2024, 11, 145. [Google Scholar] [CrossRef]
- Esmeralda, V.G.; Patterson, J.; Shelciya, S. Preliminary study on the ejection of microplastics from different types of face masks. J. Occup. Environ. Hyg. 2025, 22, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wontor, K.; Cizdziel, J.V.; Lu, H. Distribution and characteristics of microplastics in beach sand near the outlet of a major reservoir in north Mississippi, USA. Micropl. Nanopl. 2022, 2, 10. [Google Scholar] [CrossRef]
- Kwon, S.; Zambrano, M.C.; Venditti, R.A.; Frazier, R.; Zambrano, F.; Gonzalez, R.W.; Pawlak, J.J. Microfiber shedding from nonwoven materials including wipes and meltblown nonwovens in air and water environments. Environ. Sci. Pollut. Res. 2022, 29, 60584–60599. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Masciarelli, E.; Casorri, L.; Di Luigi, M.; Beni, C.; Valentini, M.; Costantini, E.; Aielli, L.; Reale, M. Microplastics in Agricultural Crops and Their Possible Impact on Farmers’ Health: A Review. Int. J. Environ. Res. Public Health 2025, 22, 45. [Google Scholar] [CrossRef]
- Savva, K.; Llorca, M.; Borrell, X.; Bertran-Solà, O.; Farré, M.; Moreno, T. Granulated rubber in playgrounds and sports fields: A potential source of atmospheric plastic-related contaminants and plastic additives after runoff events. J. Hazard. Mater. 2024, 479, 135697. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhou, W.; Hu, J.; Wu, C.; Niu, J.; Naidu, R. Paint has the potential to release microplastics, nanoplastics, inorganic nanoparticles, and hybrid materials. Environ. Sci. Eur. 2024, 36, 17. [Google Scholar] [CrossRef]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Healt. Ann. Glob. Health 2023, 89, 23. Available online: https://annalsofglobalhealth.org/articles/10.5334/aogh.4056 (accessed on 1 September 2025). [CrossRef] [PubMed]
- Chauhan, R.; Archibong, A.E.; Ramesh, A. Imprinting and Reproductive Health: A Toxicological Perspective. Int. J. Mol. Sci. 2023, 24, 16559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubin, A.E.; Zucker, I. Interactions of microplastics and organic compounds in aquatic environments: A case study of augmented joint toxicity. Chemosphere 2022, 289, 133212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Guo, J.L.; Xue, J.C.; Bai, C.L.; Guo, Y. Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment. Environ. Pollut. 2021, 291, 118106. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman Microspectroscopy Detection and Characterisation of Microplastics in Human Breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragusa, A.; Cristiano, L.; Di Vinci, P.; Familiari, G.; Nottola, S.A.; Macchiarelli, G.; Svelato, A.; De Luca, C.; Rinaldo, D.; Neri, I.; et al. Artificial plasticenta: How polystyrene nanoplastics affect in-vitro cultured human trophoblast cells. Front. Cell Dev. Biol. 2025, 13, 1539600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lopez, G.L.; Lamarre, A. The impact of micro- and nanoplastics on immune system development and functions: Current knowledge and future directions. Reprod. Toxicol. 2025, 135, 108951. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Guo, J.; Yang, R.; Wang, H.; Sun, Y.; Chen, B.; Dong, R. The Association Between Microplastics and Microbiota in Placentas and Meconium: The First Evidence in Humans. Environ. Sci. Technol. 2023, 57, 17774–17785. [Google Scholar] [CrossRef] [PubMed]
- Amereh, F.; Amjadi, N.; Mohseni-Bandpei, A.; Isazadeh, S.; Mehrabi, Y.; Eslami, A.; Naeiji, Z.; Rafiee, M. Placental plastics in young women from general population correlate with reduced foetal growth in IUGR pregnancies. Environ. Pollut. 2022, 314, 120174. [Google Scholar] [CrossRef] [PubMed]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef]
- So, Y.H.; Shin, H.S.; Lee, S.H.; Moon, H.J.; Jang, H.J.; Lee, E.-H.; Jung, E.-M. Maternal exposure to polystyrene microplastics impairs social behavior in mouse offspring with a potential neurotoxicity. NeuroToxicology 2023, 99, 206–216. [Google Scholar] [CrossRef]
- Chen, J.; Yan, L.; Zhang, Y.; Liu, X.; Wei, Y.; Zhao, Y.; Li, K.; Shi, Y.; Liu, H.; Lai, W.; et al. Maternal exposure to nanopolystyrene induces neurotoxicity in offspring through P53-mediated ferritinophagy and ferroptosis in the rat hippocampus. J. Nanobiotechnol. 2024, 22, 651. [Google Scholar] [CrossRef]
- Ma, Q.; Lei, J.; Pang, Y.; Shen, Y.; Zhang, T. Nanoplastics: AComprehensive Review of Central Nervous System Impacts. Env. Health 2025. [Google Scholar] [CrossRef]
- Wu, X.; Leung, T.; Jima, D.D.; Iyangbe, M.; Bang, J. Developing a feasible fast-track testing method for developmental neurotoxicity studies: Alternative model for risk assessment of micro- and nanoplastics. Front. Toxicol. 2025, 7, 1567225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, M.; Sharma, K.; Bunkar, S.K.; John, P.; Bhatnagar, P. Nano polystyrene induced changes in anxiety and learning behaviour are mediated through oxidative stress and gene disturbance in mouse brain regions. Neurotoxicology 2023, 99, 139–151. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Y.; Zhang, W.; Shen, T.; Li, H.; Wu, J.; Zhang, L.; Qin, L.; Chen, R.; Gu, W.; et al. Lipidomics and transcriptomics insight into impacts of microplastics exposure on hepatic lipid metabolism in mice. Chemosphere 2022, 308 Pt 3, 136591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Lee, Y.H.; Hsu, Y.H.; Chiu, I.J.; Huang, C.C.; Huang, C.C.; Chia, Z.C.; Lee, C.P.; Lin, Y.F.; Chiu, H.W. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 2021, 129, 57003. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Body-Malapel, M. Immunotoxicity and intestinal effects of nano- and microplastics: A review of the literature. Part. Fibre Toxicol. 2020, 17, 57. [Google Scholar] [CrossRef]
- Wei, W.; Li, Y.; Lee, M.; Andrikopoulos, N.; Lin, S.; Chen, C.; Leong, D.T.; Ding, F.; Song, Y.; Ke, P.C. Anionic nanoplastic exposure induces endothelial leakiness. Nat. Commun. 2022, 13, 4757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.; Yuan, H.; Wang, W.; Xu, Y.; Zhang, J.; Xu, H.; Fu, F. Co-exposure to polystyrene microplastics and lead aggravated ovarian toxicity in female mice via the PERK/eIF2α signaling pathway. Ecotoxicol. Environ. Saf. 2022, 243, 113966. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hao, F.; Liang, H.; Liu, W.; Guo, Y. Exposure to polystyrene nanoplastics impairs sperm metabolism and pre-implantation embryo development in mice. Front. Cell Dev. Biol. 2025, 13, 1562331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, L.; Wan, Z.; Luo, T.; Fu, Z.; Jin, Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 2018, 631–632, 449–458. [Google Scholar] [CrossRef]
- Malinowska, K.; Tarhonska, K.; Foksiński, M.; Sicińska, P.; Jabłońska, E.; Reszka, E.; Zarakowska, E.; Gackowski, D.; Górecka, K.; Balcerczyk, A.; et al. Impact of Short-Term Exposure to Non-Functionalized Polystyrene Nanoparticles on DNA Methylation and Gene Expression in Human Peripheral Blood Mononuclear Cells. Int. J. Mol. Sci. 2024, 25, 12786. [Google Scholar] [CrossRef]
- Ullah, F.; Wang, P.Y.; Saqib, S.; Zhao, L.; Ashraf, M.; Khan, A.; Khan, W.; Khan, A.; Chen, Y.; Xiong, Y.C. Toxicological complexity of microplastics in terrestrial ecosystems. iScience 2025, 28, 111879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habumugisha, T.; Zhang, Z.; Uwizewe, C.; Yan, C.; Ndayishimiye, J.C.; Rehman, A.; Zhang, X. Toxicological review of micro- and nano-plastics in aquatic environments: Risks to ecosystems, food web dynamics and human health. Ecotoxicol. Environ. Saf. 2024, 278, 116426. [Google Scholar] [CrossRef] [PubMed]
- WHO. One Health. Available online: https://www.who.int/health-topics/one-health#tab=tab_1 (accessed on 1 September 2025).
- Pitt, S.J.; Gunn, A. The One Health Concept. Br. J. Biomed. Sci. 2024, 81, 12366. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacKendrick, N. Plastic childhood: An environmental sociology of toys. Sociol. Perspect. 2014, 57, 507–529. [Google Scholar]
- Sunyach, C.; Antonelli, B.; Tardieu, S.; Marcot, M.; Perrin, J.; Bretelle, F. Environmental Health in Perinatal and Early Childhood: Awareness, Representation, Knowledge and Practice of Southern France Perinatal Health Professionals. Int. J. Environ. Res. Public Health 2018, 15, 2259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trasande, L.; Massey, R.I.; DiGangi, J.; Geiser, K.; Olanipekun, A.I.; Gallagher, L. How Developing Nations Can Protect Children From Hazardous Chemical Exposures While Sustaining Economic Growth. Health Aff. 2011, 30, 2400–2409. [Google Scholar] [CrossRef]
- Rummel, C.D.; Löder, M.G.; Fricke, N.F.; Lang, T.; Griebeler, E.M.; Janke, M.; Gerdts, G. Plastic ingestion by pelagic and demersal fish from the North Sea and Baltic Sea. Mar. Pollut. Bull. 2016, 102, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S., 3rd; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- 171 Rist, S.; Almroth, B.C.; Hartmann, N.B.; Karlsson, T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018, 626, 720–726. [Google Scholar] [CrossRef]
- Sharma, S.; Chatterjee, S. Microplastic pollution, a threat to marine ecosystem and human health: A short review. Environ. Sci. Pollut. Res. 2017, 24, 21530–21547. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hollman, P.; Mendoza-Hill, J.J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; FAO Fisheries and Aquaculture Technical Paper No. 615; FAO: Rome, Italy, 2017. [Google Scholar]
- Santillo, D.; Miller, K.; Johnston, P. Microplastic contamination in aquatic environments: Source, fate and potential impacts on marine organisms. Mar. Pollut. Bull. 2022, 176, 113446. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The One Health Concept: 10 Years Old and a Long Road Ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef]
- Rock, M.; Buntain, B.J.; Hatfield, J.; Hallgrimsson, B. The Evolution of One Health: A Decade of Progress and Challenges for the Future. Front. Vet. Sci. 2019, 6, 306. [Google Scholar] [CrossRef]
- Cox, K.D.; Covernton, G.A.; Davies, H.L.; Dower, J.F.; Juanes, F.; Dudas, S.E. Human Consumption of Microplastics. Environ Sci. Technol. 2019, 53, 7068–7074, Erratum in: Environ. Sci. Technol. 2020, 54, 10974. https://doi.org/10.1021/acs.est.0c04032. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J. Pollution and children’s health. Sci. Total Environ. 2018, 650 Pt 2, 2389–2394. [Google Scholar] [CrossRef]
- Cordiner, M.A.; Gibb, E.L.; Kisiel, Z.; Roth, N.X.; Biver, N.; Bockelée-Morvan, D.; Boissier, J.; Bonev, B.P.; Charnley, S.B.; Coulson, I.M.; et al. A D/H ratio consistent with Earth’s water in Halley-type comet 12P from ALMA HDO mapping. Nat. Astron. 2025. [Google Scholar] [CrossRef]
- Ragusa, A.; Principi, G.; Matta, M. Pregnancy in the Era of the Environmental Crisis: Plastic and Pollution. Clin. Exp. Obstet. Gynecol. 2022, 49, 216. [Google Scholar] [CrossRef]
- Ragusa, A.; De Luca, C.; Zucchelli, E.; Rinaldo, D.; Svelato, A. Plastic, microplastic, and the inconsistency of human thought. Front. Public Health 2023, 11, 1145240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Serrano-Aguirre, L.; Prieto, M.A. Can bioplastics always offer a truly sustainable alternative to fossil-based plastics? Microb. Biotechnol. 2024, 17, e14458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McVeigh, K.; Bryce, E. Plastic Pollution Talks Fail as Negotiators in Geneva Reject Draft Treaties. First Published on Friday 15 August 2025. Available online: https://www.theguardian.com/environment/2025/aug/15/plastic-pollution-talks-geneva-treaty (accessed on 1 September 2025).
- United Nations Development Programme. Combatting Plastic Pollution for Sustainable Development: A Snapshot of UNDP’s Work in 12 Countries; UNDP: New York, NY, USA, 2024; Available online: https://www.undp.org/sites/g/files/zskgke326/files/2024-11/undp_combatting_plastic_pollution_for_sustainable_development.pdf (accessed on 1 September 2025).

| Sample Matrix | Location | Contaminant | Concentration (Unit) | Analytical Method | Reference |
|---|---|---|---|---|---|
| Mytilus edulis (blue mussel) and Bivalves, Mytilus edulis and Crassostrea gigas | North Sea (cultured) Supermarkets in Brittany, France. | Microplastics | 0.36 ± 0.07 particles/g ww. And M. edulis contains on average 0.36 ± 0.07 particles g(−1) (wet weight), while a plastic load of 0.47 ± 0.16 particles g(−1) ww was detected in C. gigas. | Micro-FTIR imaging and Raman spectrometer was operated at a laser wavelength of 785 nm (diode), and high-resolution spectra were recorded in three spectral windows. The microscope has 5, 20, and 50 objectives, with spot sizes of approximately 50, 10, and 4 mm, respectively. | [92,93] |
| Scapharca subcrenata (ark clam) | China (fish market) | Microplastics | 10.5 particles/g ww | Micro-FTIR spectroscopy | [93] |
| Table salt (sea salt) | China (supermarkets) | Microplastics | 550–681 particles/kg | FTIR spectroscopy | [94] |
| Bottled water | Global | Microplastics | 325 particles/L | µ-FTIR imaging | [95] |
| Bottled water | Global | Nanoplastics | 240,000 particles/L | SRS microscopy | [95] |
| Honey | Italy (various origins) | Microplastics | 62 particles/kg | Micro-FTIR imaging | [96] |
| Oreochromis niloticus (Nile tilapia, fillet) | Laboratory RAS | Microplastics | 0.14 ± 0.32 µg/g ww | Pyrolysis-GC–MS | [97] |
| Litopenaeus vannamei (white-leg shrimp) | Thailand (pond) | Microplastics | 1.69 ± 0.13 particles/g ww | Micro-FTIR imaging | [98] |
| Beers (24 brands) | Germany (supermarkets) | Microplastics | 2–79 fibers/L | Optical microscopy | [99] |
| Tea bags in the tea infusions (plastic teabag) | Laboratory simulation | Microplastics | microplastics released from tea bags in the tea infusions ranged from 80 to 1288 pieces (micron-sized) and 0 to 63.755 μg (submicron-sized) per filter bag. | Laser confocal micro-Raman and direct classical least squares and pyrolysis-gas chromatography/mass spectrometry | [100] |
| White wine (PE stopper) | Europe (retail) | Microplastics | up to 5857 particles/L | Micro-Raman spectroscopy | [101] |
| Sugar | Various origins | Microplastics | 217 ± 123 fibers/kg; 32 ± 7 fragments/kg | Optical microscopy | [102] |
| Cow’s milk | Italy (supermarket) | Microplastics | 204–1004 particles/100 mL | Raman spectroscopy | [103] |
| Brewed coffee (plastic drip bag) | Laboratory simulation | Microplastics | >10,000 particles/cup | Stereomicroscopy and FTIR imaging | [104] |
| Soft drinks | Various markets | Microplastics | 9 particles/L | FTIR stereoscopy and stereomicroscopy | [105] |
| Fresh cheese | Italy (retail) | Microplastics | 1280 particles/kg | FTIR-ATR imaging | [103] |
| Ripened cheese | Italy (retail) | Microplastics | 1857 particles/kg | FTIR-ATR imaging |
| Sample Matrix | Location | Contaminant | Concentration | Analytical Method | Reference |
|---|---|---|---|---|---|
| Indoor residential air | Apartments | Microplastics | Variable | FPA-µFTIR imaging | [106] |
| Indoor apartments and office air | Apartments | Microplastics | Variable | Gravimetric analysis, stereomicroscopy, and Raman spectroscopy | [107] |
| Outdoor urban air | Residential streets (China) | Microplastics | 2.66 ± 1.76 particles m−3 | µFTIR imaging | [108] |
| Public bus cabin air | City buses (multiple cities) | Microplastics | 17.3 ± 2.4 particles m−3 | FPA-µFTIR imaging | [109] |
| Subway platform air | Metro stations (multiple cities) | Microplastics | 5.8 ± 1.9 particles m−3 | FPA-µFTIR imaging | [110] |
| PM2.5 fraction of urban air | Outdoor urban monitoring sites | Microplastics (fraction) | 3–7% of PM2.5 mass | Review of ambient-PM studies | [111] |
| Indoor household dust | Households (Australia) | Microplastics | 2046 ± 830 particles/g dust | µFTIR imaging | [112] |
| Plastic recycling plant air | Recycling facility | Microplastics | 5.97 106–1.12 × 108 MP m−3 | fluorescence microscopy analysis | [113] |
| Waste sorting facility air | Municipal solid waste | Microplastics | From 1.7 to 24.7 N/m3 with an average (±standard deviation) of 6.54 ± 5.08 N/m3. | Raman spectroscopy | [114] |
| Textile manufacturing | Czech Republic. | Microfibers | Amount of microplastic fibers released from the fleece fabric increased continuously until the third to fifth washing cycle, after which the released amount was nearly constant. | washing process | [115] |
| Incineration | 12 mass burn incinerators. China | Microplastics | 1.9–565 n/kg | Micro-Fourier transform infrared spectroscopy. | [116] |
| Rural ambient air and forest | Thulamela Local Municipality | Microplastics | Ranging from 90.51 ± 15.19–355.64 ± 47.65 particles/m2/day, with an overall average of 211.87 ± 31.44 particles/m2/day. | FTIR | [117] |
| Sample Matrix | Location/Context | Contaminant | Concentration | Analytical Method | Reference |
|---|---|---|---|---|---|
| Household dust | Private homes | Microplastics | 38–120,000 µg/g (median: 5900 µg/g) | Various | [118] |
| Facial exfoliating scrub (commercial) | UAE market (2019–2020) | Microplastic beads | Up to 6298 ± 1543 beads per g product | FTIR imaging | [119] |
| Rinse-off cosmetics (face wash, body scrub) | Global survey of PCCPs | Microplastics | Geometric mean 2 162 particles/g; 0.04 g plastic per g product | µFTIR imaging | [120] |
| Surgical masks (used) | Consumer use | Microplastics/fibers | 18.27 ± 4.1 items released per mask | ATR-FTIR spectroscopy | [121] |
| Beach sand | Coastal sand | Microplastics | Average of 590 ± 360, with 950 ± 100 in the lower wrack zone, 540 ± 40 in the upper wrack zone, and 270 ± 30 in areas between | µ-FTIR. | [122] |
| Nonwoven wet wipes (mechanical abrasion) | Lab simulation | Microfibers | 60–4000 microfibers released per cm2 | SEM + µFTIR imaging | [123] |
| Synthetic polyester T-shirt | Machine washes | Microfibers | 700,000 fibres could be released from an average 6 kg wash load of acrylic fabric | Abrasion chamber + microscopy | [124] |
| Agricultural plastic (farmers’ health) | Field (vegetable farm, Italy) | Microplastics | Various | [125] | |
| Playground rubber crumb (sports fields) | Outdoor playgrounds | Micro-/nanoplastics | Up to 30,426 ng/m3 | SEC-HRMS | [126] |
| Interior wall paint surfaces | Residential walls | Microplastics, nanoplastics, inorganic nanoparticles | SEM + Raman spectroscopy | [127] |
| System/Endpoint | Plastic Type and Size | Exposure Regimen | Key Outcomes | Analytical Methods | Reference |
|---|---|---|---|---|---|
| Neurological | Polystyrene NPs (~50–100 nm) | Oral gavage in mice, 28 days | Impaired learning and memory; ↑ ROS; lipid peroxidation in hippocampus | Morris water maze, ROS assay, histology | [144] |
| Endocrine | Polystyrene MPs (5 μm) + Lead | Oral exposure, mice, 35 days | Thyroid hormone disruption (↓ T4, ↑ TSH), altered ovarian steroidogenesis | ELISA, histopathology | [145] |
| Hepatic | Polystyrene NPs (50 nm) | Oral gavage in mice, 28 days | Hepatic steatosis, ↑ ALT/AST, oxidative stress | Biochemical assays, liver histology | [145] |
| Renal | Polystyrene MPs (5 μm) | Oral exposure in mice, 8 weeks | Tubular damage, oxidative stress, mitochondrial dysfunction | Histology, oxidative stress biomarkers | [146] |
| Immunological | Polystyrene MPs (0.5–5 μm) | Oral gavage, mice, 6 weeks | Splenic inflammation, cytokine imbalance (↑ TNF-α, IL-6) | ELISA, flow cytometry | [147] |
| Cardiovascular | Polystyrene NPs (80 nm) | Intravenous injection, mice, acute | Endothelial dysfunction, ↑ inflammatory markers | Echocardiography, histology | [148] |
| Reproductive | Polystyrene NPs (50 nm) | Oral exposure, male mice, 35 days | ↓ Sperm motility, abnormal morphology, testosterone reduction | Sperm analysis, ELISA, histology | [149,150] |
| Developmental (Fetal/placental) | Polystyrene NPs (20–200 nm) | Maternal exposure, mice, gestation | Placental transfer, fetal growth restriction, neurobehavioral abnormalities | Placental histology, neurobehavioral assays | [141] |
| Gut Microbiome | Polystyrene MPs (5 μm) | Oral gavage, mice, 6 weeks | Dysbiosis (↓ Firmicutes, ↑ Bacteroidetes), increased gut permeability | 16S rRNA sequencing, histology | [151] |
| Hematopoietic | Polystyrene NPs (50 nm) | In vitro human hematopoietic stem cells | DNA hypomethylation, impaired differentiation | Epigenetic assays, flow cytometry | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragusa, A. Amniotic Fluid and Ocean Water: Evolutionary Echoes, Chemical Parallels, and the Infiltration of Micro- and Nanoplastics. Toxics 2025, 13, 776. https://doi.org/10.3390/toxics13090776
Ragusa A. Amniotic Fluid and Ocean Water: Evolutionary Echoes, Chemical Parallels, and the Infiltration of Micro- and Nanoplastics. Toxics. 2025; 13(9):776. https://doi.org/10.3390/toxics13090776
Chicago/Turabian StyleRagusa, Antonio. 2025. "Amniotic Fluid and Ocean Water: Evolutionary Echoes, Chemical Parallels, and the Infiltration of Micro- and Nanoplastics" Toxics 13, no. 9: 776. https://doi.org/10.3390/toxics13090776
APA StyleRagusa, A. (2025). Amniotic Fluid and Ocean Water: Evolutionary Echoes, Chemical Parallels, and the Infiltration of Micro- and Nanoplastics. Toxics, 13(9), 776. https://doi.org/10.3390/toxics13090776







