Integrated Approaches of Arsenic Remediation from Wastewater: A Comprehensive Review of Microbial, Bio-Based, and Advanced Technologies
Abstract
1. Introduction
2. Review Methods
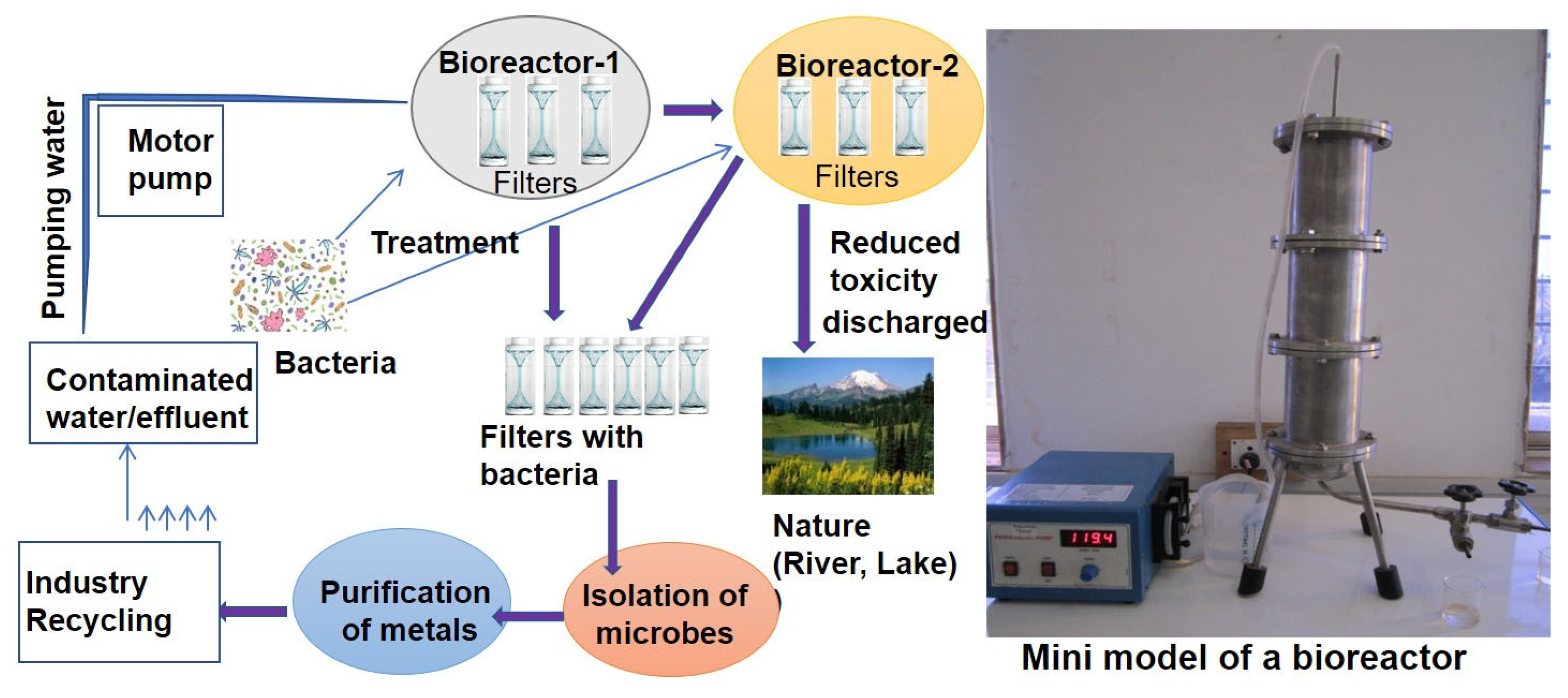
3. Microbial Remediation of Arsenic
4. Cellulose and Fruit-Peel-Based Adsorbents for Arsenic Remediation
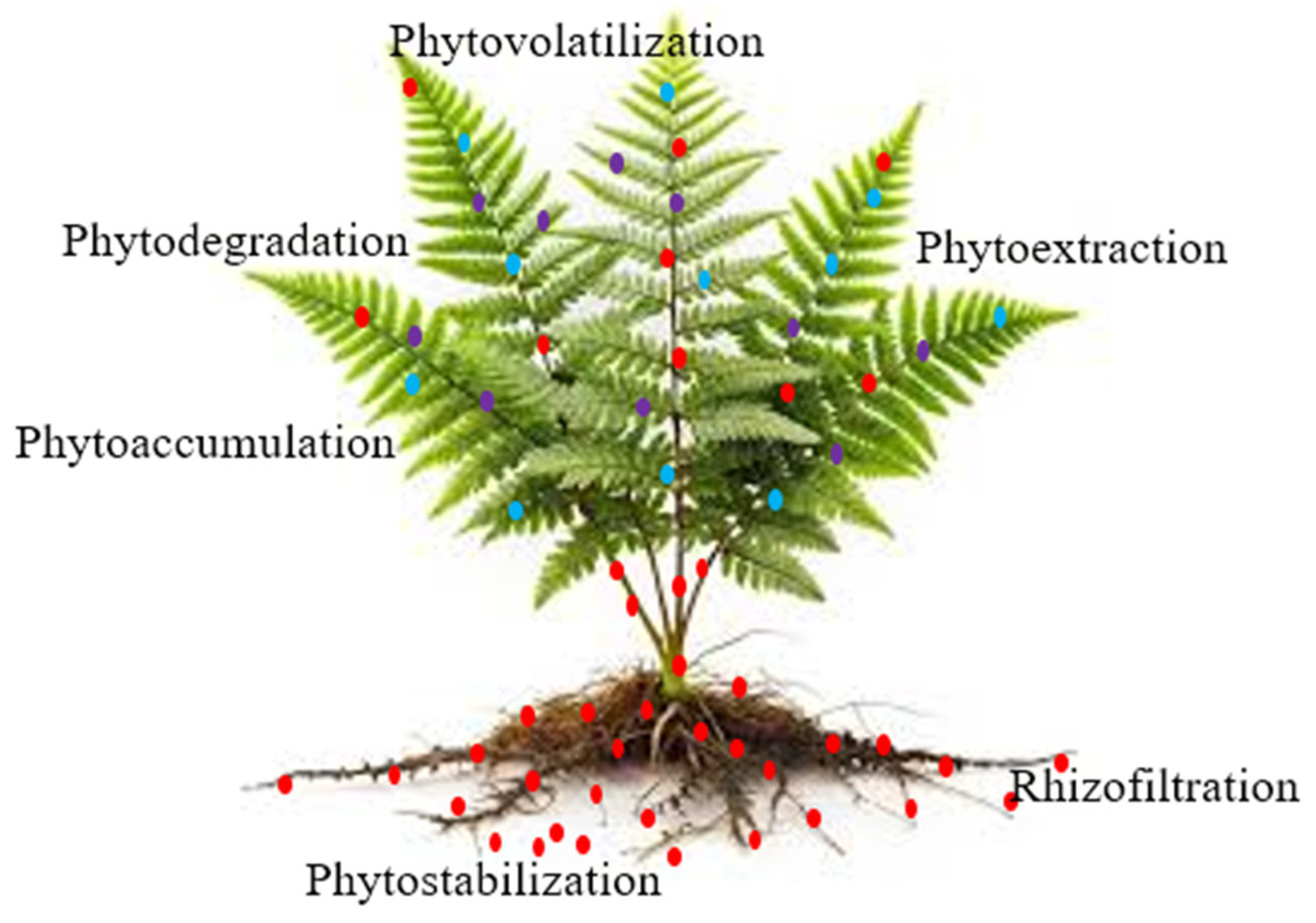
5. Plant-Based (Phytoremediation) Techniques of Arsenic Remediation
6. Biochar and Modified Biochar for Arsenic Remediation
7. Nanotechnology-Based Approaches for Arsenic Remediation
8. Integrated and Hybrid Technologies for Arsenic Remediation
9. Comparative Analysis and Performance Evaluation of Arsenic Remediation Technologies
10. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ATSDR Agency for Toxic Substances and Disease Registry Sciences. Substance Priority List ATSDR’s; Agency for Toxic Substances and Disease Registry Sciences: Atlanta, Georgia, 2019; pp. 1–9.
- Shankar, S.; Shanker, U.; Shikha. Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, W.; Lang, G.; Sun, Q.; Nan, T.; Li, X.; Ren, Y.; Li, Z. Worldwide Distribution, Health Risk, Treatment Technology, and Development Tendency of Geogenic High-Arsenic Groundwater. Water 2024, 16, 478. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Desale, P.; Kapadnis, B.P.; Hossain, K.; Saha, A.K.; Ghosh, S.; Olsson, B.; et al. Isolation and Characterization of a Lysinibacillus Strain B1-CDA Showing Potential for Bioremediation of Arsenics from Contaminated Water. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2014, 49, 1349–1360. [Google Scholar] [CrossRef]
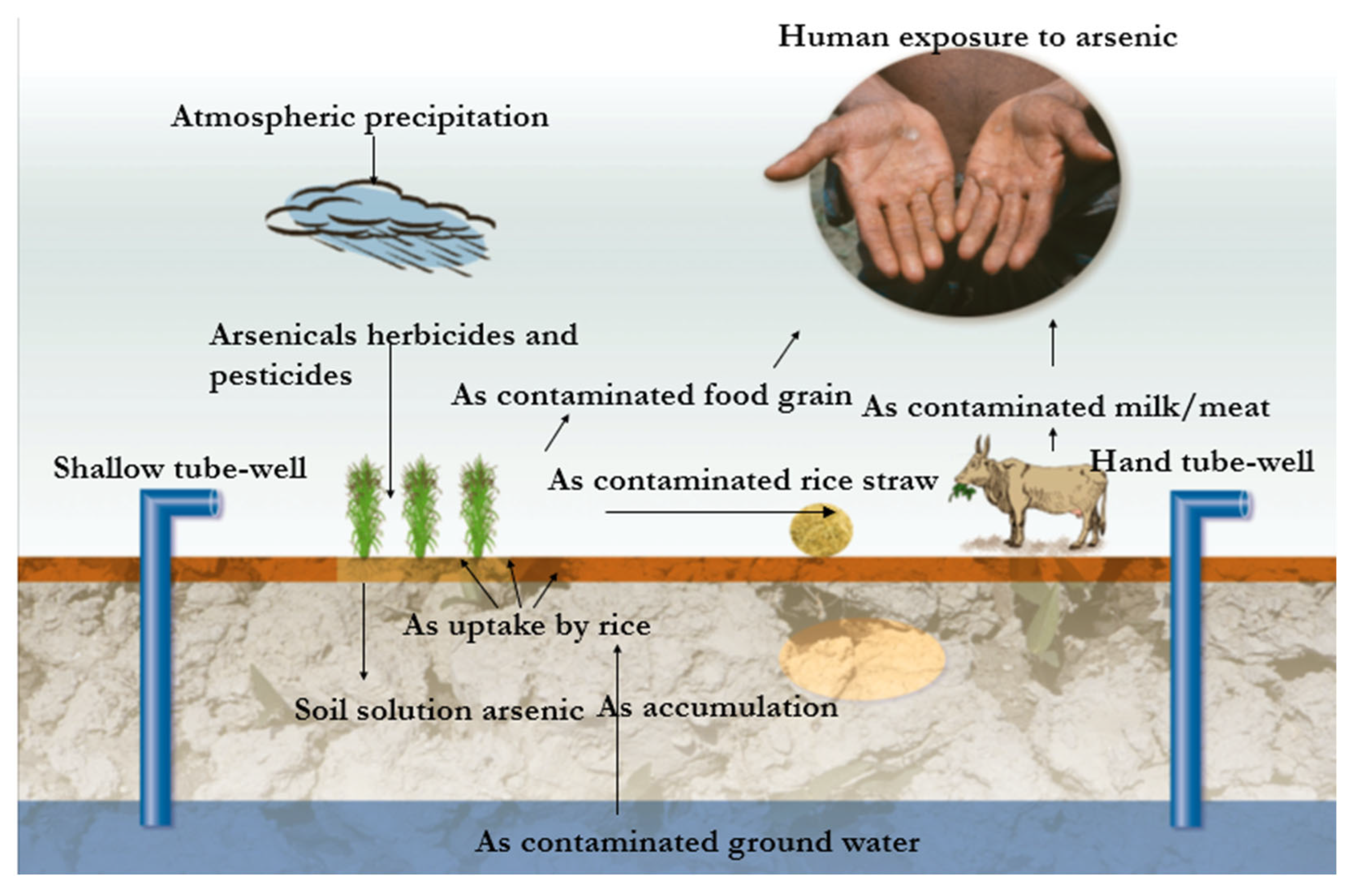
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental Source of Arsenic Exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.C.d.S.; Hott, R.d.C.; dos Santos, M.J.; Santos, M.S.; Andrade, T.G.; Bomfeti, C.A.; Rocha, B.A.; Barbosa, F.; Rodrigues, J.L. Arsenic in Mining Areas: Environmental Contamination Routes. Int. J. Environ. Res. Public Health 2023, 20, 4291. [Google Scholar] [CrossRef]
- Wang, N.; Ye, Z.; Huang, L.; Zhang, C.; Guo, Y.; Zhang, W. Arsenic Occurrence and Cycling in the Aquatic Environment: A Comparison between Freshwater and Seawater. Water 2023, 15, 147. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Bahar, M.M.; Naidu, R. Diffuse Soil Pollution from Agriculture: Impacts and Remediation. Sci. Total Environ. 2025, 962, 178398. [Google Scholar] [CrossRef]
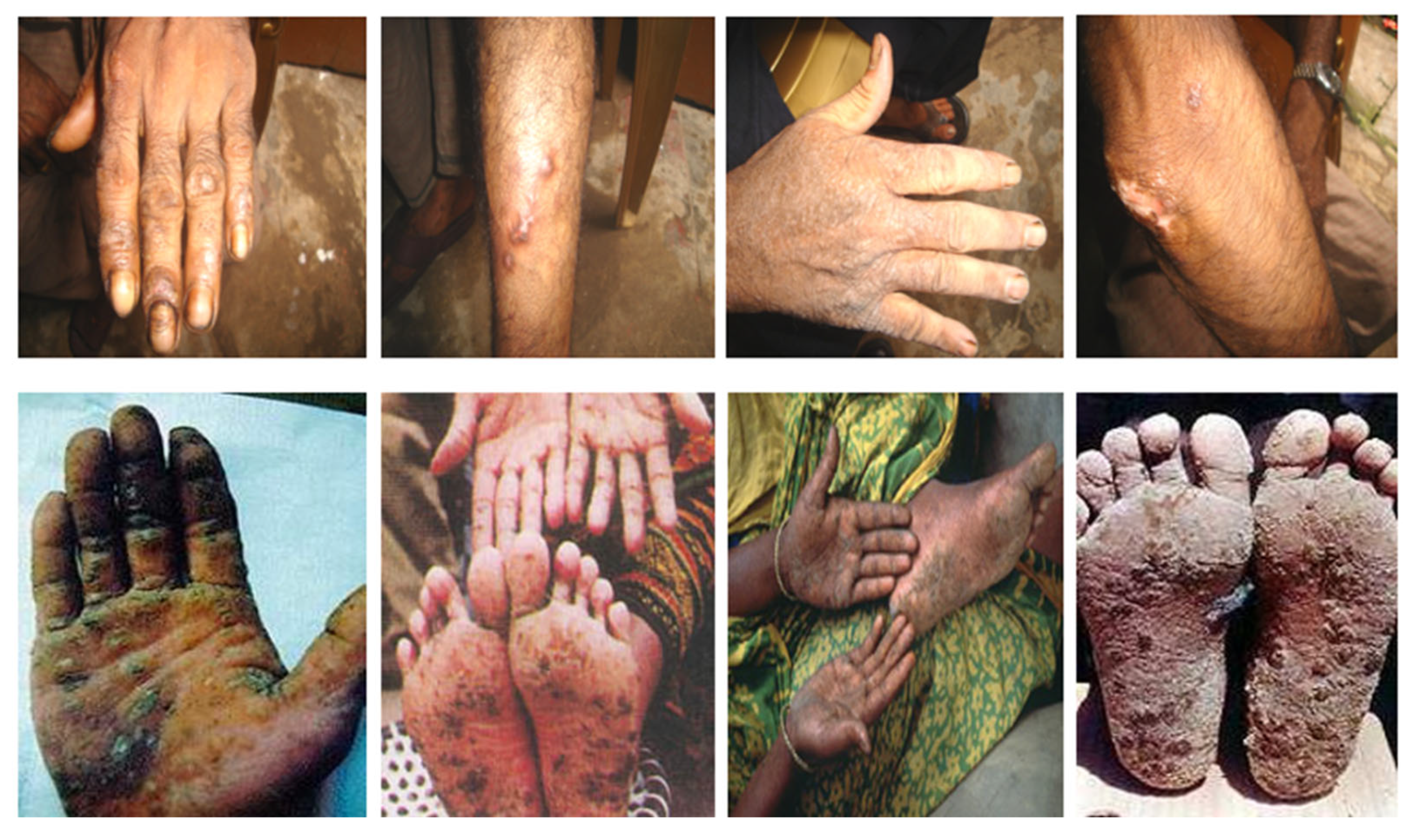
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Vasken Aposhian, H.; Graziano, J.H.; Thompson, C.; Suk, W.A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Ghosh, S.; Olsson, B.; Mandal, A. Comparative Genome Analysis of Lysinibacillus B1-CDA, a Bacterium That Accumulates Arsenics. Genomics 2015, 106, 384–392. [Google Scholar] [CrossRef]
- Karim, M.R.; Ali, N.; Hoque, M.A.; Haque, A.; Salam, K.A.; Rahman, A.; Islam, K.; Saud, Z.A.; Khalek, M.A.; Akhand, A.A.; et al. Association between Arsenic Exposure and Plasma Cholinesterase Activity: A Population Based Study in Bangladesh. Environ. Health A Glob. Access Sci. Source 2010, 9, 36. [Google Scholar] [CrossRef]
- Rahman, A. Bioremediation of Toxic Metals for Protecting Human Health and the Ecosystem. Doctoral Dissertation, Örebro University, Örebro, Sweden, 2016. [Google Scholar]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy Metal Ions Removal from Metal Plating Wastewater Using Electrocoagulation: Kinetic Study and Process Performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Nahar, N.; Rahman, A.; Nawani, N.N.; Ghosh, S.; Mandal, A. Phytoremediation of Arsenic from the Contaminated Soil Using Transgenic Tobacco Plants Expressing ACR2 Gene of Arabidopsis Thaliana. J. Plant Physiol. 2017, 218, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Elbana, T.A.; Magdi Selim, H.; Akrami, N.; Newman, A.; Shaheen, S.M.; Rinklebe, J. Freundlich Sorption Parameters for Cadmium, Copper, Nickel, Lead, and Zinc for Different Soils: Influence of Kinetics. Geoderma 2018, 324, 80–88. [Google Scholar] [CrossRef]
- Altıntıg, E.; Yenigun, M.; Sarı, A.; Altundag, H.; Tuzen, M.; Saleh, T.A. Facile Synthesis of Zinc Oxide Nanoparticles Loaded Activated Carbon as an Eco-Friendly Adsorbent for Ultra-Removal of Malachite Green from Water. Environ. Technol. Innov. 2021, 21, 101305. [Google Scholar] [CrossRef]
- Badmus, S.O.; Oyehan, T.A.; Saleh, T.A. Synthesis of a Novel Polymer-Assisted AlNiMn Nanomaterial for Efficient Removal of Sulfate Ions from Contaminated Water. J. Polym. Environ. 2021, 29, 2840–2854. [Google Scholar] [CrossRef]
- Saleh, T.A. Protocols for Synthesis of Nanomaterials, Polymers, and Green Materials as Adsorbents for Water Treatment Technologies. Environ. Technol. Innov. 2021, 24, 101821. [Google Scholar] [CrossRef]
- Abdel Salam, O.E.; Reiad, N.A.; ElShafei, M.M. A Study of the Removal Characteristics of Heavy Metals from Wastewater by Low-Cost Adsorbents. J. Adv. Res. 2011, 2, 297–303. [Google Scholar] [CrossRef]
- Rahman, A.; Haque, A.; Ghosh, S.; Shinu, P.; Attimarad, M. Modified Shrimp-Based Chitosan as an Emerging Adsorbent Removing Heavy Metals (Chromium, Nickel, Arsenic, and Cobalt) from Polluted Water. Sustainability 2023, 15, 2431. [Google Scholar] [CrossRef]
- Rahman, A. Promising and Environmentally Friendly Removal of Copper, Zinc, Cadmium, and Lead from Wastewater Using Modified Shrimp-Based Chitosan. Water 2024, 16, 184. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, N.; Nawani, N.N.; Jass, J.; Ghosh, S.; Olsson, B.; Mandal, A. Data in Support of the Comparative Genome Analysis of Lysinibacillus B1-CDA, a Bacterium That Accumulates Arsenics. Data Brief 2015, 5, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Yoshida, K.; Islam, M.M.; Kobayashi, G. Investigation of Efficient Adsorption of Toxic Heavy Metals (Chromium, Lead, Cadmium) from Aquatic Environment Using Orange Peel Cellulose as Adsorbent. Sustainability 2023, 15, 4470. [Google Scholar] [CrossRef]
- Nathan, R.J.; Barr, D.; Rosengren, R.J. Six Fruit and Vegetable Peel Beads for the Simultaneous Removal of Heavy Metals by Biosorption. Environ. Technol. 2020, 43, 1935–1952. [Google Scholar] [CrossRef]
- Nahar, N.; Rahman, A.; Mos, M.; Warzecha, T.; Algerin, M.; Ghosh, S.; Johnson-Brousseau, S.; Mandal, A. In Silico and in Vivo Studies of an Arabidopsis Thaliana Gene, ACR2, Putatively Involved in Arsenic Accumulation in Plants. J. Mol. Model. 2012, 18, 4249–4262. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Rahman, A.; Moś, M.; Warzecha, T.; Ghosh, S.; Hossain, K.; Nawani, N.N.; Mandal, A. In Silico and in Vivo Studies of Molecular Structures and Mechanisms of AtPCS1 Protein Involved in Binding Arsenite and/or Cadmium in Plant Cells. J. Mol. Model. 2014, 20, 2104. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a Low-Cost Adsorbent for Aqueous Heavy Metal Removal: A Review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A Critical Review on Arsenic Removal from Water Using Iron-Based Adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Lata, S.; Samadder, S.R. Removal of Arsenic from Water Using Nano Adsorbents and Challenges: A Review. J. Environ. Manage. 2016, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, H.; Chang, Z.; Liang, N.; Wu, M.; Pan, B. Biochar Modification to Enhance Arsenic Removal from Water: A Review. Environ. Geochem. Health 2023, 45, 2763–2778. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A Review of Biochar-Based Sorbents for Separation of Heavy Metals from Water. Int. J. Phytoremediation 2020, 22, 111–126. [Google Scholar] [CrossRef]
- Bhat, A.; Tian, F.; Singh, B. Advances in Nanomaterials and Colorimetric Detection of Arsenic in Water: Review and Future Perspectives. Sensors 2024, 24, 3889. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Taher, E.S.; Rashed, F.; Ghazanfar, S.; Shehata, A.M.; Mohammed, N.A.; Pascalau, R.; Smuleac, L.; Ibrahim, A.M.; Abdeen, A.; et al. The Arsenic Bioremediation Using Genetically Engineered Microbial Strains on Aquatic Environments: An Updated Overview. Heliyon 2024, 10, e36314. [Google Scholar] [CrossRef]
- William, V.U.; Magpantay, H.D. Arsenic and Microorganisms: Genes, Molecular Mechanisms, and Recent Advances in Microbial Arsenic Bioremediation. Microorganisms 2024, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Nawani, N.; Rahman, A.; Mandal, A. Chapter 12—Microbial Biomass for Sustainable Remediation of Wastewater. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Basu, S.; Dutta, A.; Mukherjee, S.K.; Hossain, S.T. Isolation and Characterization of an As(III) Oxidizing Bacterium, Acinetobacter sp. TMKU4 from Paddy Field for Possible Arsenic Decontamination. J. Hazard. Mater. Adv. 2023, 10, 100289. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Q.; Wang, G. Microbial Oxidation of Arsenite: Regulation, Chemotaxis, Phosphate Metabolism and Energy Generation. Front. Microbiol. 2020, 11, 569282. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, N.; Cai, X.; Wang, Z.; Cui, Y. Arsenic Redox Transformation by Pseudomonas sp. HN-2 Isolated from Arsenic-Contaminated Soil in Hunan, China. J. Environ. Sci. 2016, 47, 165–173. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Ye, L.; Jing, C. Arsenic Biotransformation in Industrial Wastewater Treatment Residue: Effect of Co-Existing Shewanella sp. ANA-3 and MR-1. J. Environ. Sci. 2022, 118, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Ehara, A.; Kasahara, Y.; Hamamura, N.; Amachi, S. Crossm Expression of Genes and Proteins Involved in Arsenic Respiration and Resistance in Dissimilatory Arsenate-Reducing. Appl. Environ. Microbiol. 2019, 85, e00763-19. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Beesley, L.; Zhang, Z.; Zhi, S.; Jia, Y.; Ding, Y. Microbial Arsenic Methylation in Soil and Uptake and Metabolism of Methylated Arsenic in Plants: A Review. Int. J. Environ. Res. Public Health 2019, 16, 5012. [Google Scholar] [CrossRef]
- Hemmat-Jou, M.H.; Liu, S.; Liang, Y.; Chen, G.; Fang, L.; Li, F. Microbial Arsenic Methylation in Soil-Water Systems and Its Environmental Significance. Sci. Total Environ. 2024, 944, 173873. [Google Scholar] [CrossRef]
- Wang, P.; Sun, G.; Jia, Y.; Meharg, A.A.; Zhu, Y. A Review on Completing Arsenic Biogeochemical Cycle: Microbial Volatilization of Arsines in Environment. J. Environ. Sci. 2014, 26, 371–381. [Google Scholar] [CrossRef]
- Tang, R.; Yuan, S.; Wang, Y.; Wang, W.; Wu, G.; Zhan, X.; Hu, Z. Arsenic Volatilization in Roxarsone-Loaded Digester: Insight into the Main Factors and ArsM Genes. Sci. Total Environ. 2020, 711, 135123. [Google Scholar] [CrossRef]
- Yang, P.; Ke, C.; Zhao, C.; Liu, B.; Xue, X.; Rensing, C.; Yang, S. ArsM-Mediated Arsenite Volatilization Is Limited by Efflux Catalyzed by As Efflux Transporters. Chemosphere 2020, 239, 124822. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Tang, X.; Xiao, Z.; Liu, H. Kinetic Research of Scorodite Formation via Oxidative Coprecipitation from Arsenic–Bearing Solution. Process Saf. Environ. Prot. 2024, 191, 658–675. [Google Scholar] [CrossRef]
- Kimura, K.; Okibe, N. Enhancing Biogenic Scorodite Formation Using Waste Iron Sludge: A Sustainable Approach for Arsenic Immobilization. Minerals 2025, 15, 56. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, M.; Liu, C.; Dong, L.; Zhou, J.; Yi, X.; Ji, D.; Qiao, J.; Tong, H. Arsenic Release from Microbial Reduction of Scorodite in the Presence of Electron Shuttle in Flooded Soil. J. Environ. Sci. 2023, 126, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Vega-Hernandez, S.; Weijma, J.; Buisman, C.J.N. Immobilization of Arsenic as Scorodite by a Thermoacidophilic Mixed Culture via As(III)-Catalyzed Oxidation with Activated Carbon. J. Hazard. Mater. 2019, 368, 221–227. [Google Scholar] [CrossRef]
- Tanaka, M.; Sasaki, K.; Okibe, N. Behavior of Sulfate Ions during Biogenic Scorodite Crystallization from Dilute As(III)-Bearing Acidic Waters. Hydrometallurgy 2018, 180, 144–152. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, T.; Pal, P.; Mitra, S.; Ghosh, S.K.; Soren, T.; Maiti, T.K. Bioprotective Mechanisms of Enterobacter sp. against Arsenic, Cadmium, and Lead Toxicity and Its Potential Role in Soil Bioremediation. J. Environ. Chem. Eng. 2025, 13, 115432. [Google Scholar] [CrossRef]
- Yang, H.C.; Rosen, B.P. New Mechanisms of Bacterial Arsenic Resistance. Biomed. J. 2016, 39, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kondakindi, V.R.; Pabbati, R.; Erukulla, P.; Maddela, N.R.; Prasad, R. Bioremediation of Heavy Metals-Contaminated Sites by Microbial Extracellular Polymeric Substances—A Critical View. Environ. Chem. Ecotoxicol. 2024, 6, 408–421. [Google Scholar] [CrossRef]
- Ullah, I.; Anwar, Y.; Siddiqui, M.F.; Alsulami, N.; Ullah, R. Phytoremediation of Arsenic (As) in Rice Plants, Mediated by Bacillus Subtilis Strain IU31 through Antioxidant Responses and Phytohormones Synthesis. Environ. Pollut. 2024, 355, 124207. [Google Scholar] [CrossRef]
- Nam, I.H.; Murugesan, K.; Ryu, J.; Kim, J.H. Arsenic (As) Removal Using Talaromyces sp. KM-31 Isolated from As-Contaminated Mine Soil. Minerals 2019, 9, 568. [Google Scholar] [CrossRef]
- Prithviraj, D.; Deboleena, K.; Neelu, N.; Noor, N.; Aminur, R.; Balasaheb, K.; Abul, M. Biosorption of Nickel by Lysinibacillus sp. BA2 Native to Bauxite Mine. Ecotoxicol. Environ. Saf. 2014, 107, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, H.; Afsheen, N.; Rafique, S.; Arshad, A.; Intisar, M.; Hussain, A.; Bilal, M.; Iqbal, H.M.N. Genetically Engineered Microorganisms for Environmental Remediation. Chemosphere 2023, 310, 136751. [Google Scholar] [CrossRef]
- Kumar Satyapal, G.; Kumar, R.; Kumar, S.; Shankar Singh, R.; Prashant; Kumar Ranjan, R.; Kumar, K.; Kumar Jha, A.; Pal Singh, N.; Haque, R.; et al. Cloning and Functional Characterization of Arsenite Oxidase (AoxB) Gene Associated with Arsenic Transformation in Pseudomonas sp. Strain AK9. Gene 2023, 850, 146926. [Google Scholar] [CrossRef]
- Maleki, F.; Shahpiri, A. Efficient and Specific Bioaccumulation of Arsenic in the Transgenic Escherichia Coli Expressing ArsR1 from Corynebacterium Glutamicum. BioMetals 2022, 35, 889–901. [Google Scholar] [CrossRef]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of Immobilized Bacteria for Environmental Bioremediation: A Review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Kuppan, N.; Padman, M.; Mahadeva, M.; Srinivasan, S.; Devarajan, R. A Comprehensive Review of Sustainable Bioremediation Techniques: Eco Friendly Solutions for Waste and Pollution Management. Waste Manag. Bull. 2024, 2, 154–171. [Google Scholar] [CrossRef]
- Banik, S.; Das, K.C.; Islam, M.S.; Salimullah, M. Recent Advancements and Challenges in Microbial Bioremediation of Heavy Metals Con-Tamination. JSM Biotechnol. Biomed. Eng. 2013, 2, 1035. [Google Scholar]
- Gonzalez, J.M.; Aranda, B. Microbial Growth under Limiting Conditions-Future Perspectives. Microorganisms 2023, 11, 1641. [Google Scholar] [CrossRef]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Saba; Andreasen, R.; Li, Y.; Rehman, Y.; Ahmed, M.; Meyer, R.L.; Sabri, A.N. Prospective Role of Indigenous Exiguobacterium Profundum PT2 in Arsenic Biotransformation and Biosorption by Planktonic Cultures and Biofilms. J. Appl. Microbiol. 2018, 124, 431–443. [Google Scholar] [CrossRef]
- Mallick, I.; Hossain, S.T.; Sinha, S.; Mukherjee, S.K. Brevibacillus sp. KUMAs2, a Bacterial Isolate for Possible Bioremediation of Arsenic in Rhizosphere. Ecotoxicol. Environ. Saf. 2014, 107, 236–244. [Google Scholar] [CrossRef]
- Khanam, R.; Moni, R.; Islam, M.Z.; Billah, M.M.; Zohora, U.S.; Sabrin, F.; Rahman, M.S. Study of an Arsenic Metabolizing Bacteria from Arsenic Contaminated Soil of Chandpur District, Bangladesh. Jahangirnagar Univ. J. Biol. Sci. 2019, 8, 57–65. [Google Scholar] [CrossRef]
- Bagade, A.V.; Bachate, S.P.; Dholakia, B.B.; Giri, A.P.; Kodam, K.M. Characterization of Roseomonas and Nocardioides spp. for arsenic transformation. J. Hazard. Mater. 2016, 318, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Bhakat, K.; Chakraborty, A.; Islam, E. Characterization of Arsenic Oxidation and Uranium Bioremediation Potential of Arsenic Resistant Bacteria Isolated from Uranium Ore. Environ. Sci. Pollut. Res. 2019, 26, 12907–12919. [Google Scholar] [CrossRef]
- Marwa, N.; Singh, N.; Srivastava, S.; Saxena, G.; Pandey, V.; Singh, N. Characterizing the Hypertolerance Potential of Two Indigenous Bacterial Strains (Bacillus Flexus and Acinetobacter Junii) and Their Efficacy in Arsenic Bioremediation. J. Appl. Microbiol. 2019, 126, 1117–1127. [Google Scholar] [CrossRef]
- Satyapal, G.K.; Mishra, S.K.; Srivastava, A.; Ranjan, R.K.; Prakash, K.; Haque, R.; Kumar, N. Possible Bioremediation of Arsenic Toxicity by Isolating Indigenous Bacteria from the Middle Gangetic Plain of Bihar, India. Biotechnol. Rep. 2018, 17, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Jamal, M.A.H.M.; Biswas, P.K.; Rahman, S.M.; Sharma, S.P.; Saha, S.K.; Hong, S.T.; Islam, M.R. Arsenic Remediation in Bangladeshi Rice Varieties with Enhance Plant Growth by Unique Arsenic-Resistant Bacterial Isolates. Geomicrobiol. J. 2020, 37, 130–142. [Google Scholar] [CrossRef]
- Zannier, F.; Portero, L.R.; Ordoñez, O.F.; Martinez, L.J.; Farías, M.E.; Albarracin, V.H. Polyextremophilic Bacteria from High Altitude Andean Lakes: Arsenic Resistance Profiles and Biofilm Production. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Bagade, A.; Nandre, V.; Paul, D.; Patil, Y.; Sharma, N.; Giri, A.; Kodam, K. Characterisation of Hyper Tolerant Bacillus Firmus L-148 for Arsenic Oxidation. Environ. Pollut. 2020, 261, 114124. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Sarkar, A. Characterization of Arsenite-Oxidizing Bacteria to Decipher Their Role in Arsenic Bioremediation. Prep. Biochem. Biotechnol. 2019, 49, 30–37. [Google Scholar] [CrossRef]
- Sher, S.; Tahir Ishaq, M.; Abbas Bukhari, D.; Rehman, A. Brevibacterium sp. Strain CS2: A Potential Candidate for Arsenic Bioremediation from Industrial Wastewater. Saudi J. Biol. Sci. 2023, 30, 103781. [Google Scholar] [CrossRef]
- Darshana Salaskar, S.P.K. Isolation and Identification of Arsenic Resistant Providencia Rettgeri (KDM3) from Industrial Effluent Contaminated Soil and Studies on Its Arsenic Resistance Mechanisma. J. Microb. Biochem. Technol. 2015, 7, 194–201. [Google Scholar] [CrossRef]
- Jebelli, M.A.; Maleki, A.; Amoozegar, M.A.; Kalantar, E.; Shahmoradi, B.; Gharibi, F. Isolation and Identification of Indigenous Prokaryotic Bacteria from Arsenic-Contaminated Water Resources and Their Impact on Arsenic Transformation. Ecotoxicol. Environ. Saf. 2017, 140, 170–176. [Google Scholar] [CrossRef]
- Banerjee, S.; Datta, S.; Chattyopadhyay, D.; Sarkar, P. Arsenic Accumulating and Transforming Bacteria Isolated from Contaminated Soil for Potential Use in Bioremediation. J. Environ. Sci. Health-Part A Toxic/Hazardous Subst. Environ. Eng. 2011, 46, 1736–1747. [Google Scholar] [CrossRef]
- Han, Y.H.; Yin, D.X.; Jia, M.R.; Wang, S.S.; Chen, Y.; Rathinasabapathi, B.; Chen, D.L.; Ma, L.Q. Arsenic-Resistance Mechanisms in Bacterium Leclercia adecarboxylata Strain As3-1: Biochemical and Genomic Analyses. Sci. Total Environ. 2019, 690, 1178–1189. [Google Scholar] [CrossRef]
- Tariq, A.; Ullah, U.; Asif, M.; Sadiq, I. Biosorption of Arsenic through Bacteria Isolated from Pakistan. Int. Microbiol. 2019, 22, 59–68. [Google Scholar] [CrossRef]
- Aguilar, N.C.; Faria, M.C.S.; Pedron, T.; Batista, B.L.; Mesquita, J.P.; Bomfeti, C.A.; Rodrigues, J.L. Isolation and Characterization of Bacteria from a Brazilian Gold Mining Area with a Capacity of Arsenic Bioaccumulation. Chemosphere 2020, 240, 124871. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Majhi, A.K.; Sarkar, A. The Role of Arsenate Reducing Bacteria for Their Prospective Application in Arsenic Contaminated Groundwater Aquifer System. Biocatal. Agric. Biotechnol. 2019, 20, 101218. [Google Scholar] [CrossRef]
- PAUL, T.; CHAKRABORTY, A.; ISLAM, E.; MUKHERJEE, S.K. Arsenic Bioremediation Potential of Arsenite-Oxidizing Micrococcus sp. KUMAs15 Isolated from Contaminated Soil. Pedosphere 2018, 28, 299–310. [Google Scholar] [CrossRef]
- Dey, U.; Chatterjee, S.; Mondal, N.K. Isolation and Characterization of Arsenic-Resistant Bacteria and Possible Application in Bioremediation. Biotechnol. Rep. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Shi, R.J.; Wang, T.; Lang, J.Q.; Zhou, N.; Ma, M.G. Multifunctional Cellulose and Cellulose-Based (Nano) Composite Adsorbents. Front. Bioeng. Biotechnol. 2022, 10, 891034. [Google Scholar] [CrossRef]
- El Mahdaoui, A.; Radi, S.; Elidrissi, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Moura, N.M.M. Progress in the Modification of Cellulose-Based Adsorbents for the Removal of Toxic Heavy Metal Ions. J. Environ. Chem. Eng. 2024, 12, 113870. [Google Scholar] [CrossRef]
- Amira, N.; Armir, Z.; Zulkifli, A.; Gunaseelan, S.; Palanivelu, S.D.; Salleh, K.M.; Hafiz, M.; Othman, C.; Zakaria, S. Polymers Regenerated Cellulose Products for Agricultural and Their Potential: A Review. Polymers 2021, 13, 13203586. [Google Scholar]
- Joshi, V.C.; Shukla, S.; Sharma, S. Designing of a Proficient Macro Porous Metallo-Polymer of Iron with Anion Functionality: A Sustainable Approach for Arsenic Remediation from Water. Desalin. Water Treat. 2024, 320, 100816. [Google Scholar] [CrossRef]
- Antony Jose, S.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A Comprehensive Review on Cellulose Nanofibers, Nanomaterials, and Composites: Manufacturing, Properties, and Applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, T.; Narayanan, R. Reduction of Heavy Metals from Textile Effluent with Activated Carbon from Wheat Husk. Pollut. Res. 2020, 39, S138–S142. [Google Scholar]
- Sanka, P.M.; Rwiza, M.J.; Mtei, K.M. Removal of Selected Heavy Metal Ions from Industrial Wastewater Using Rice and Corn Husk Biochar. Water Air Soil Pollut. 2020, 231, 244. [Google Scholar] [CrossRef]
- Reddy, A.K.; Jaisankar, V. Adsorption Treatment Of Heavy Metal Removal From Simulated Waste Water Using Rice Husk Activated Carbon (RHAC) And Its Polyvinylpyrrolidone (PVP) Composite As An Adsorbent. J. Water Environ. Sci. 2019, 3, 460–470. [Google Scholar]
- Meez, E.; Rahdar, A.; Kyzas, G.Z. Sawdust for the Removal of Heavy Metals from Water: A Review. Molecules 2021, 26, 4318. [Google Scholar] [CrossRef]
- Dias, M.; Pinto, J.; Henriques, B.; Figueira, P.; Fabre, E.; Tavares, D.; Vale, C.; Pereira, E. Nutshells as Efficient Biosorbents to Remove Cadmium, Lead, and Mercury from Contaminated Solutions. Int. J. Environ. Res. Public Health 2021, 18, 1580. [Google Scholar] [CrossRef]
- Singh, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Immobilised Apple Peel Bead Biosorbent for the Simultaneous Removal of Heavy Metals from Cocktail Solution. Cogent Environ. Sci. 2019, 5, 1673116. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Hashim, N.; Abdullah, S.; Abdullah, N.; Mohamed, A.; Asshaary Daud, M.A.; Aidil Muzakkar, K.F. Adsorption of Heavy Metals on Banana Peel Bioadsorbent. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; Volume 1532. [Google Scholar] [CrossRef]
- Ashfaq, A.; Nadeem, R.; Bibi, S.; Rashid, U.; Hanif, A.; Jahan, N.; Ashfaq, Z.; Ahmed, Z.; Adil, M.; Naz, M. Efficient Adsorption of Lead Ions from Synthetic Wastewater Using Agrowaste-Based Mixed Biomass (Potato Peels and Banana Peels). Water 2021, 13, 3344. [Google Scholar] [CrossRef]
- Akinhanmi, T.F.; Ofudje, E.A.; Adeogun, A.I.; Aina, P.; Joseph, I.M. Orange Peel as Low-Cost Adsorbent in the Elimination of Cd(II) Ion: Kinetics, Isotherm, Thermodynamic and Optimization Evaluations. Bioresour. Bioprocess. 2020, 7, 34. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Cerrillo-Gonzalez, M.M.; Paz-Garcia, J.M.; Rodriguez-Maroto, J.M.; Arhoun, B. Valorization of Lemon Peel Waste as Biosorbent for the Simultaneous Removal of Nickel and Cadmium from Industrial Effluents. Environ. Technol. Innov. 2021, 21, 101380. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Dutta, S.; Saxena, V.K. A Review on Biosorptive Removal of Dyes and Heavy Metals from Wastewater Using Watermelon Rind as Biosorbent. Environ. Adv. 2020, 2, 100007. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Ighalo, J.O.; Adaobi Igwegbe, C.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Pashalidis, I.; Kalderis, D. Sunflower-Biomass Derived Adsorbents for Toxic/Heavy Metals Removal from (Waste) Water. J. Mol. Liq. 2021, 342, 117540. [Google Scholar] [CrossRef]
- Ezeonuegbu, B.A.; Machido, D.A.; Whong, C.M.Z.; Japhet, W.S.; Alexiou, A.; Elazab, S.T.; Qusty, N.; Yaro, C.A.; Batiha, G.E.S. Agricultural Waste of Sugarcane Bagasse as Efficient Adsorbent for Lead and Nickel Removal from Untreated Wastewater: Biosorption, Equilibrium Isotherms, Kinetics and Desorption Studies. Biotechnol. Rep. 2021, 30, e00614. [Google Scholar] [CrossRef]
- Bernard, E.; Jimoh, A.; Odigure, J.O. Heavy Metals Removal from Industrial Wastewater by Activated Carbon Prepared from Coconut Shell. Res. J. Chem. Sci. 2013, 3, 3–9. [Google Scholar]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption Capability of Brewed Tea Waste in Waters Containing Toxic Lead(II), Cadmium (II), Nickel (II), and Zinc(II) Heavy Metal Ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef]
- Guo, X.Y.; Liang, S.; Tian, Q.H. Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption Using Modified Orange Peel as Adsorbent. Adv. Mater. Res. 2011, 236–238, 237–240. [Google Scholar] [CrossRef]
- Huynh, A.T.; Chen, Y.C.; Tran, B.N.T. A Small-Scale Study on Removal of Heavy Metals from Contaminated Water Using Water Hyacinth. Processes 2021, 9, 1802. [Google Scholar] [CrossRef]
- Hegazy, I.; Ali, M.E.A.; Zaghlool, E.H.; Elsheikh, R. Heavy Metals Adsorption from Contaminated Water Using Moringa Seeds/ Olive Pomace Byproducts. Appl. Water Sci. 2021, 11, 95. [Google Scholar] [CrossRef]
- Patel, H. Batch and Continuous Fixed Bed Adsorption of Heavy Metals Removal Using Activated Charcoal from Neem (Azadirachta indica) Leaf Powder. Sci. Rep. 2020, 10, 16895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, Y.; Gao, S.; Wu, S. Sustainable and Efficient Wastewater Treatment Using Cellulose-Based Hydrogels: A Review of Heavy Metal, Dye, and Micropollutant Removal Applications. Separations 2025, 12, 72. [Google Scholar] [CrossRef]
- Massimi, L.; Giuliano, A.; Astolfi, M.L.; Congedo, R.; Masotti, A.; Canepari, S. Efficiency Evaluation of Food Waste Materials for the Removal of Metals and Metalloids from Complex Multi-Element Solutions. Materials 2018, 9, 334. [Google Scholar] [CrossRef]
- Shil, R.K.; Rahman, I.M.; Sakai, Y.; Marumoto, M.; Rocky, M.M.H.; Endo, M.; Wong, K.H.; Mashio, A.S.; Hasegawa, H. Iron- and Zirconium-Modified Nanocellulose Adsorbent: Broad-Range Selectivity Test for Potentially Toxic Elements and Effective Arsenite Removal. Water Air Soil Pollut. 2025, 236, 482. [Google Scholar] [CrossRef]
- Guo, X.; Du, Y.; Chen, F.; Park, H.S.; Xie, Y. Mechanism of Removal of Arsenic by Bead Cellulose Loaded with Iron Oxyhydroxide (β-FeOOH): EXAFS Study. J. Colloid Interface Sci. 2007, 314, 427–433. [Google Scholar] [CrossRef]
- Tibolla, H.; Pelissari, F.M.; Menegalli, F.C. Cellulose Nanofibers Produced from Banana Peel by Chemical and Enzymatic Treatment. LWT 2014, 59, 1311–1318. [Google Scholar] [CrossRef]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of Fruit Waste Derived Bio-Adsorbents for Wastewater Treatment: A Review. J. Hazard. Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Darmenbayeva, A.; Rajasekharan, R.; Massalimova, B.; Bektenov, N.; Taubayeva, R.; Bazarbaeva, K.; Kurmanaliev, M.; Mukazhanova, Z.; Nurlybayeva, A.; Bulekbayeva, K.; et al. Cellulose-Based Sorbents: A Comprehensive Review of Current Advances in Water Remediation and Future Prospects. Molecules 2024, 29, 5969. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Verma, Y.; Lai, C.W.; Naushad, M.; Iqbal, J.; Kumar, A.; Dhiman, P. Biochar and Biosorbents Derived from Biomass for Arsenic Remediation. Heliyon 2024, 10, e36288. [Google Scholar] [CrossRef] [PubMed]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (Modified) Natural Adsorbents for Arsenic Remediation: A Review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. NPJ Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- McCarty, K.M.; Hanh, H.T.; Kim, K.W. Arsenic Geochemistry and Human Health in South East Asia. Rev. Environ. Health 2011, 26, 71–78. [Google Scholar] [CrossRef]
- Neisan, R.S.; Saady, N.M.C.; Bazan, C.; Zendehboudi, S. Optimization of Arsenic Removal from Water Using Novel Renewable Adsorbents Derived from Orange Peels. Waste Manag. Bull. 2025, 3, 21–35. [Google Scholar] [CrossRef]
- Vijayarani, A. Biosorption of Arsenic (III) by Using Lemon Peel Powder as Low Cost Effective Biosorbent. J. Nat. Sci. Res. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Y.; Aierken, A.; Zhang, M.; Fang, P.; Fan, Y.; Ming, Z. Mono/Competitive Adsorption of Arsenic(III) and Nickel(II) Using Modified Green Tea Waste. J. Taiwan Inst. Chem. Eng. 2016, 60, 213–221. [Google Scholar] [CrossRef]
- Nasehir Khan, M.N.; Mohd Arif Zainol, M.R.R.; Mohamad Yusop, M.F.; Ahmad, M.A. Turning Waste into Wonder: Arsenic Removal Using Rice Husk Based Activated Carbon. J. Eng. Res. 2024, in press. [CrossRef]
- Asif, Z.; Chen, Z. Removal of Arsenic from Drinking Water Using Rice Husk. Appl. Water Sci. 2017, 7, 1449–1458. [Google Scholar] [CrossRef]
- Gyawali, D.; Poudel, M.; Gautam, B.; Neupane, B.B.; Paudyal, H.; Ghimire, K.N. Zirconium-Modified Citrus Limetta Peel for Effective Removal of Arsenic from Ground Water. J. Water Process Eng. 2024, 68, 106283. [Google Scholar] [CrossRef]
- Poudel, B.R.; Aryal, R.L.; Bhattarai, S.; Koirala, A.R.; Gautam, S.K.; Ghimire, K.N.; Pant, B.; Park, M.; Paudyal, H.; Pokhrel, M.R. Agro-Waste Derived Biomass Impregnated with TiO2 as a Potential Adsorbent for Removal of as(III) from Water. Catalysts 2020, 10, 1125. [Google Scholar] [CrossRef]
- Peng, Y.; Xiao, H.Y.; Cheng, X.Z.; Chen, H.M. Removal of Arsenic from Wastewater by Using Pretreating Orange Peel. Adv. Mater. Res. 2013, 773, 889–892. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Sharif, F.; Bashir, S.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; et al. Arsenic Removal by Natural and Chemically Modified Water Melon Rind in Aqueous Solutions and Groundwater. Sci. Total Environ. 2018, 645, 1444–1455. [Google Scholar] [CrossRef]
- Johnson, V.E.; Liao, Q.; Jallawide, B.W.; Anaman, R.; Amanze, C.; Huang, P.; Cao, W.; Ding, C.; Shi, Y. Simultaneous Removal of As(V) and Pb(II) Using Highly-Efficient Modified Dehydrated Biochar Made from Banana Peel via Hydrothermal Synthesis. Colloids Surf. A Physicochem. Eng. Asp. 2023, 663, 6–11. [Google Scholar] [CrossRef]
- Gupta, A.; Vidyarthi, S.R.; Sankararamakrishnan, N. Concurrent Removal of As(III) and As(V) Using Green Low Cost Functionalized Biosorbent—Saccharum officinarum Bagasse. J. Environ. Chem. Eng. 2015, 3, 113–121. [Google Scholar] [CrossRef]
- Gyawali, D.; Poudel, S.; Poudel, M.; Ghimire, K.N.; Pokhrel, M.R.; Basnet, P.; Bahadur BK, K.; Paudyal, H. Synthesis, Characterization and As(III) Scavenging Behaviours of Mango Peel Waste Loaded with Zr(IV) Ion from Contaminated Water. Heliyon 2024, 10, e36496. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, M.; Kumari, A.; Shrestha, S.; Shrestha, B. Arsenic Removal Fromwater by Adsorption onto Iron Oxide/Nano-Porous Carbon Magnetic Composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef]
- Liu, X.; Ao, H.; Xiong, X.; Xiao, J.; Liu, J. Arsenic Removal from Water by Iron-Modified Bamboo Charcoal. Water Air Soil Pollut. 2012, 223, 1033–1044. [Google Scholar] [CrossRef]
- Thapa, S.; Pokhrel, M.R. Removal of As(III) from Aqueous Solution Using Fe(III) Loaded Pomegranate Waste. J. Nepal Chem. Soc. 2013, 30, 29–36. [Google Scholar] [CrossRef]
- Setyono, D.; Valiyaveettil, S. Chemically Modified Sawdust as Renewable Adsorbent for Arsenic Removal from Water. ACS Sustain. Chem. Eng. 2014, 2, 2722–2729. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Apple Peels—A Versatile Biomass for Water Purification? ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef]
- Letechipia, J.O.; González-Trinidad, J.; Júnez–Ferreira, H.E.; Bautista–Capetillo, C.; Robles Rovelo, C.O.; Contreras Rodríguez, A.R. Removal of Arsenic from Semiarid Area Groundwater Using a Biosorbent from Watermelon Peel Waste. Heliyon 2023, 9, e13251. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Bundschuh, J.; et al. Exploring the Arsenic Removal Potential of Various Biosorbents from Water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.H.; Ranjan, D.; Talat, M. Rice Polish for the Removal of Arsenic from Aqueous Solution: Optimization of Process Variables. Ind. Eng. Chem. Res. 2009, 48, 4194–4201. [Google Scholar] [CrossRef]
- Shafique, U.; Ijaz, A.; Salman, M.; uz Zaman, W.; Jamil, N.; Rehman, R.; Javaid, A. Removal of Arsenic from Water Using Pine Leaves. J. Taiwan Inst. Chem. Eng. 2012, 43, 256–263. [Google Scholar] [CrossRef]
- Saqib, A.N.S.; Waseem, A.; Khan, A.F.; Mahmood, Q.; Khan, A.; Habib, A.; Khan, A.R. Arsenic Bioremediation by Low Cost Materials Derived from Blue Pine (Pinus wallichiana) and Walnut (Juglans regia). Ecol. Eng. 2013, 51, 88–94. [Google Scholar] [CrossRef]
- Amin, N.; Kaneco, S.; Kitagawa, T.; Begum, A.; Katsumata, H.; Suzuki, T.; Ohta, K. Removal of Arsenic in Aqueous Solutions by Adsorption onto Waste Rice Husk. Ind. Eng. Chem. Res. 2006, 45, 8105–8110. [Google Scholar] [CrossRef]
- Lavanya, M.B.; Viswanath, D.S.; Sivapullaiah, P.V. Phytoremediation: An Eco-Friendly Approach for Remediation of Heavy Metal-Contaminated Soils-A Comprehensive Review. Environ. Nanotechnol. Monit. Manag. 2024, 22, 100975. [Google Scholar] [CrossRef]
- Yang, C.; Han, N.; Inoue, C.; Yang, Y.L.; Nojiri, H.; Ho, Y.N.; Chien, M.F. Rhizospheric Plant-Microbe Synergistic Interactions Achieve Efficient Arsenic Phytoextraction by Pteris vittata. J. Hazard. Mater. 2022, 434, 128870. [Google Scholar] [CrossRef]
- Kafle, A.; Timilsina, A.; Gautam, A.; Adhikari, K.; Bhattarai, A.; Aryal, N. Phytoremediation: Mechanisms, Plant Selection and Enhancement by Natural and Synthetic Agents. Environ. Adv. 2022, 8, 100203. [Google Scholar] [CrossRef]
- Favas, P.J.C.; Pratas, J.; Prasad, M.N.V. Accumulation of Arsenic by Aquatic Plants in Large-Scale Field Conditions: Opportunities for Phytoremediation and Bioindication. Sci. Total Environ. 2012, 433, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hammond, C.M.; Root, R.A.; Maier, R.M.; Chorover, J. Mechanisms of Arsenic Sequestration by Prosopis Juliflora during the Phytostabilization of Metalliferous Mine Tailings. Environ. Sci. Technol. 2018, 52, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Limmer, M.; Burken, J. Phytovolatilization of Organic Contaminants. Environ. Sci. Technol. 2016, 50, 6632–6643. [Google Scholar] [CrossRef]
- Guarino, F.; Miranda, A.; Castiglione, S.; Cicatelli, A. Arsenic Phytovolatilization and Epigenetic Modifications in Arundo donax L. Assisted by a PGPR Consortium. Chemosphere 2020, 251, 126310. [Google Scholar] [CrossRef]
- Zhao, F.; Han, Y.; Shi, H.; Wang, G.; Zhou, M.; Chen, Y. Arsenic in the Hyperaccumulator Pteris vittata: A Review of Benefits, Toxicity, and Metabolism. Sci. Total Environ. 2023, 896, 165232. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, S.H.; Kim, J.G. Evaluation of Factors Affecting Arsenic Uptake by Brassica juncea in Alkali Soil after Biochar Application Using Partial Least Squares Path Modeling (PLS-PM). Chemosphere 2021, 275, 130095. [Google Scholar] [CrossRef]
- Qadir, M.; Hussain, A.; Shah, M.; Hamayun, M.; Al-Huqail, A.A.; Iqbal, A.; Ali, S. Improving Sunflower Growth and Arsenic Bioremediation in Polluted Environments: Insights from Ecotoxicology and Sustainable Mitigation Approaches. Heliyon 2024, 10, e33078. [Google Scholar] [CrossRef]
- Martínez-Martínez, J.G.; Rosales-Loredo, S.; Hernández-Morales, A.; Arvizu-Gómez, J.L.; Carranza-Álvarez, C.; Macías-Pérez, J.R.; Rolón-Cárdenas, G.A.; Pacheco-Aguilar, J.R. Bacterial Communities Associated with the Roots of Typha spp. and Its Relationship in Phytoremediation Processes. Microorganisms 2023, 11, 1587. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, J.; Ding, G.; Cai, H.; Shi, L.; Qiu, G.; Wang, X.; Wang, S.; Wang, C. Arsenite Transporter OsNIP3;5 Modulates Phosphate Starvation Responses via Regulating Arsenite Translocation in Rice. Plant Physiol. Biochem. 2025, 225, 5–9. [Google Scholar] [CrossRef]
- Nabi, A.; Naeem, M.; Aftab, T.; Khan, M.M.A.; Ahmad, P. A Comprehensive Review of Adaptations in Plants under Arsenic Toxicity: Physiological, Metabolic and Molecular Interventions. Environ. Pollut. 2021, 290, 118029. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front. Plant Sci. 2020, 11, 513099. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ho, Y.N.; Inoue, C.; Chien, M.F. Long-Term Effectiveness of Microbe-Assisted Arsenic Phytoremediation by Pteris vittata in Field Trials. Sci. Total Environ. 2020, 740, 140137. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a Highly Arsenic Polluted Site, Using Pteris vittata L. And Arbuscular Mycorrhizal Fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef]
- Kohda, Y.H.T.; Endo, G.; Kitajima, N.; Sugawara, K.; Chien, M.F.; Inoue, C.; Miyauchi, K. Arsenic Uptake by Pteris vittata in a Subarctic Arsenic-Contaminated Agricultural Field in Japan: An 8-Year Study. Sci. Total Environ. 2022, 831, 154830. [Google Scholar] [CrossRef]
- Tan, H.W.; Pang, Y.L.; Lim, S.; Chong, W.C. A State-of-the-Art of Phytoremediation Approach for Sustainable Management of Heavy Metals Recovery. Environ. Technol. Innov. 2023, 30, 103043. [Google Scholar] [CrossRef]
- Lwanga, I.C.; Bhattacharya, S.; Dey, S.; Mandal, S.; Gupta, K.; Biswas, J.K.; Sengupta, S.; Watts, M. Phytoremediation of Arsenic: A State-of-the-Art Review with Special Emphasis on Modern Biotechnological Approaches. Total Environ. Eng. 2025, 2, 100014. [Google Scholar] [CrossRef]
- Srivastava, S.; Shukla, A.; Rajput, V.D.; Kumar, K.; Minkina, T.; Mandzhieva, S.; Shmaraeva, A.; Suprasanna, P. Arsenic Remediation through Sustainable Phytoremediation Approaches. Minerals 2021, 11, 936. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Tiwari, P.; Upadhyay, M.K.; Srivastava, S.; Dwivedi, S.; Dhankher, O.P.; Tripathi, R.D. Biotechnological Strategies for Remediation of Arsenic-Contaminated Soils to Improve Soil Health and Sustainable Agriculture. Soil Environ. Health 2024, 2, 100061. [Google Scholar] [CrossRef]
- Gomes, M.P. Nanophytoremediation: Advancing Phytoremediation Efficiency through Nanotechnology Integration. Discov. Plants 2025, 2, 8. [Google Scholar] [CrossRef]
- Ha, N.T.H.; Ha, N.T.; Nga, T.T.H.; Minh, N.N.; Anh, B.T.K.; Hang, N.T.A.; Duc, N.A.; Nhuan, M.T.; Kim, K.W. Uptake of Arsenic and Heavy Metals by Native Plants Growing near Nui Phao Multi-Metal Mine, Northern Vietnam. Appl. Geochem. 2019, 108, 104368. [Google Scholar] [CrossRef]
- Wei, X.; Zhou, Y.; Tsang, D.C.W.; Song, L.; Zhang, C.; Yin, M.; Liu, J.; Xiao, T.; Zhang, G.; Wang, J. Hyperaccumulation and Transport Mechanism of Thallium and Arsenic in Brake Ferns (Pteris vittata L.): A Case Study from Mining Area. J. Hazard. Mater. 2020, 388, 121756. [Google Scholar] [CrossRef]
- Li, R.; Dong, F.; Yang, G.; Zhang, W.; Zong, M.; Nie, X.; Zhou, L.; Babar, A.; Liu, J.; Ram, B.K.; et al. Characterization of Arsenic and Uranium Pollution Surrounding a Uranium Mine in Southwestern China and Phytoremediation Potential. Pol. J. Environ. Stud. 2020, 29, 173–185. [Google Scholar] [CrossRef]
- Pan, P.; Lei, M.; Qiao, P.; Zhou, G.; Wan, X.; Chen, T. Potential of Indigenous Plant Species for Phytoremediation of Metal(Loid)-Contaminated Soil in the Baoshan Mining Area, China. Environ. Sci. Pollut. Res. 2019, 26, 23583–23592. [Google Scholar] [CrossRef] [PubMed]
- Onyia, P.C.; Ozoko, D.C.; Ifediegwu, S.I. Phytoremediation of Arsenic-Contaminated Soils by Arsenic Hyperaccumulating Plants in Selected Areas of Enugu State, Southeastern, Nigeria. Geol. Ecol. Landsc. 2021, 5, 308–319. [Google Scholar] [CrossRef]
- Eze, V.C.; Harvey, A.P. Extractive Recovery and Valorisation of Arsenic from Contaminated Soil through Phytoremediation Using Pteris Cretica. Chemosphere 2018, 208, 484–492. [Google Scholar] [CrossRef]
- Lim, M.; McBride, M.B.; Kessler, A. Arsenic Bioaccumulation by Eruca Sativa Is Unaffected by Intercropping or Plant Density. Water Air Soil Pollut. 2017, 228, 364. [Google Scholar] [CrossRef]
- Leão, G.A.; de Oliveira, J.A.; Felipe, R.T.A.; Farnese, F.S. Phytoremediation of Arsenic-Contaminated Water: The Role of Antioxidant Metabolism of Azolla caroliniana Willd. (Salviniales). Acta Bot. Bras. 2017, 31, 161–168. [Google Scholar] [CrossRef]
- Mishra, S.; Wellenreuther, G.; Mattusch, J.; Stärk, H.J.; Küpper, H. Speciation and Distribution of Arsenic in the Nonhyperaccumulator Macrophyte Ceratophyllum demersum. Plant Physiol. 2013, 163, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Jasrotia, S.; Kansal, A.; Mehra, A. Performance of Aquatic Plant Species for Phytoremediation of Arsenic-Contaminated Water. Appl. Water Sci. 2017, 7, 889–896. [Google Scholar] [CrossRef]
- Mirza, N.; Mahmood, Q.; Pervez, A.; Ahmad, R.; Farooq, R.; Shah, M.M.; Azim, M.R. Phytoremediation Potential of Arundo Donax in Arsenic-Contaminated Synthetic Wastewater. Bioresour. Technol. 2010, 101, 5815–5819. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.L.; Yang, M.X.; Lv, S.M.; Tan, A.J. The Effect of Chelating Agents on Iron Plaques and Arsenic Accumulation in Duckweed (Lemna minor). J. Hazard. Mater. 2021, 419, 126410. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K. Heavy Metals and Arsenic Phytoremediation Potential of Invasive Alien Wetland Plants Phragmites karka and Arundo donax: Water-Energy-Food (W-E-F) Nexus Linked Sustainability Implications. Bioresour. Technol. Rep. 2021, 15, 100741. [Google Scholar] [CrossRef]
- Ye, W.L.; Khan, M.A.; McGrath, S.P.; Zhao, F.J. Phytoremediation of Arsenic Contaminated Paddy Soils with Pteris vittata Markedly Reduces Arsenic Uptake by Rice. Environ. Pollut. 2011, 159, 3739–3743. [Google Scholar] [CrossRef]
- Thathong, V.; Tantamsapya, N.; Yossapol, C.; Liao, C.H.; Wirojanagud, W.; Padungthon, S. Role of Colocasia esculenta L. Schott in Arsenic Removal by a Pilot-Scale Constructed Wetland Filled with Laterite Soil. Heliyon 2019, 5, e01233. [Google Scholar] [CrossRef]
- de Souza, T.D.; Borges, A.C.; Braga, A.F.; Veloso, R.W.; Teixeira de Matos, A. Phytoremediation of Arsenic-Contaminated Water by Lemna Valdiviana: An Optimization Study. Chemosphere 2019, 234, 402–408. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, F.J.; Huang, Q.; Williams, P.N.; Sun, G.X.; Zhu, Y.G. Arsenic Uptake and Speciation in the Rootless Duckweed Wolffia Globosa. New Phytol. 2009, 182, 421–428. [Google Scholar] [CrossRef]
- Li, B.; Gu, B.; Yang, Z.; Zhang, T. The Role of Submerged Macrophytes in Phytoremediation of Arsenic from Contaminated Water: A Case Study on Vallisneria natans (Lour.). Hara. Ecotoxicol. Environ. Saf. 2018, 165, 224–231. [Google Scholar] [CrossRef]
- de Souza, T.D.; Borges, A.C.; Teixeira de Matos, A.; Veloso, R.W.; Braga, A.F. Optimization of Arsenic Phytoremediation Using Eichhornia Crassipes. Int. J. Phytoremediation 2018, 20, 1129–1135. [Google Scholar] [CrossRef]
- de Campos, F.V.; de Oliveira, J.A.; da Silva, A.A.; Ribeiro, C.; dos Santos Farnese, F. Phytoremediation of Arsenite-Contaminated Environments: Is Pistia stratiotes L. a Useful Tool? Ecol. Indic. 2019, 104, 794–801. [Google Scholar] [CrossRef]
- Goswami, C.; Majumder, A.; Misra, A.K.; Bandyopadhyay, K. Arsenic Uptake by Lemna minor in Hydroponic System. Int. J. Phytoremediation 2014, 16, 1221–1227. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Vázquez, S.; Carpena-Ruiz, R.O.; Esteban, E.; Peñalosa, J.M. Using Mediterranean Shrubs for the Phytoremediation of a Soil Impacted by Pyritic Wastes in Southern Spain: A Field Experiment. J. Environ. Manage. 2011, 92, 1584–1590. [Google Scholar] [CrossRef]
- Petelka, J.; Abraham, J.; Bockreis, A.; Deikumah, J.P.; Zerbe, S. Soil Heavy Metal(Loid) Pollution and Phytoremediation Potential of Native Plants on a Former Gold Mine in Ghana. Water Air Soil Pollut. 2019, 230, 267. [Google Scholar] [CrossRef]
- Rosli, R.A.; Harumain, Z.A.S.; Zulkalam, M.F.; Hamid, A.A.A.; Sharif, M.F.; Mohamad, M.A.N.; Noh, A.L.; Shahari, R. Phytoremediation of Arsenic in Mine Wastes by Acacia mangium. Remediation 2021, 31, 49–59. [Google Scholar] [CrossRef]
- Trivedi, Y.; Sharma, M.; Mishra, R.K.; Sharma, A.; Joshi, J.; Gupta, A.B.; Achintya, B.; Shah, K.; Vuppaladadiyamd, A.K. Biochar Potential for Pollutant Removal during Wastewater Treatment: A Comprehensive Review of Separation Mechanisms, Technological Integration, and Process Analysis. Desalination 2025, 600, 118509. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of Adsorption and Functionalization of Biochar for Pesticides: A Review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Lataf, A.; Jozefczak, M.; Vandecasteele, B.; Viaene, J.; Schreurs, S.; Carleer, R.; Yperman, J.; Marchal, W.; Cuypers, A.; Vandamme, D. The Effect of Pyrolysis Temperature and Feedstock on Biochar Agronomic Properties. J. Anal. Appl. Pyrolysis 2022, 168, 105728. [Google Scholar] [CrossRef]
- Naseefa, P.K.; Sukanya, V.; Nimitha, K.; Sruthi, M.; Shanthi, T.R.; Harilal, C.C. A Comparative Account of the Sugarcane Bagasse and Rice Husk Biochar on the Physicochemical and Biological Properties of Soils from Heterogeneous Agroecosystems. Commun. Soil Sci. Plant Anal. 2025, 56, 1013–1027. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of Feedstock Type and Pyrolysis Temperature on Potential Applications of Biochar. J. Anal. Appl. Pyrolysis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of Temperature and Processing Conditions on Biochar Chemical Properties and Their Influence on Soil C and N Transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Gęca, M.; Khalil, A.M.; Tang, M.; Bhakta, A.K.; Snoussi, Y.; Nowicki, P.; Wiśniewska, M.; Chehimi, M.M. Surface Treatment of Biochar-Methods, Surface Analysis and Potential Applications: A Comprehensive Review. Surfaces 2023, 6, 179–213. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Omvesh; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Li, C.; Chen, Y.; Zheng, L.; Ding, D.; Shan, S. Synergistic Mechanism of Iron Manganese Supported Biochar for Arsenic Remediation and Enzyme Activity in Contaminated Soil. J. Environ. Manag. 2023, 347, 119127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Y.; Li, B.; Huang, W.; Qin, J.; Li, H.; Chen, G. Hydrous Zirconium Oxide Modified Biochar for in Situ Remediation of Arsenic Contaminated Agricultural Soil. J. Environ. Chem. Eng. 2022, 10, 108360. [Google Scholar] [CrossRef]
- Ullah, I.; Baig, S.A.; Zaheer, H.; Shams, D.F.; Bibi, H.; Khan, W.; Xu, X.; Danish, M. Application of Magnetically Recoverable Biochar Amended Zirconium Adsorbent Composite for Enhanced As(III, V) Removal from Aqueous Solutions. Water Air Soil Pollut. 2025, 236, 46. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, X.; Fu, Q.; Hu, H.; Miao, F.; Huang, C.; Zhu, J. Renewable and Efficient Removal of Arsenic from Contaminated Water by Modified Biochars Derived from As-Enriched Plant. Bioresour. Technol. 2023, 387, 3–8. [Google Scholar] [CrossRef]
- Deepshikaa, R.; Prasanthrajan, M.; Rahale, C.S.; Kanna, S.U.; Mahendiran, R.; Parthiban, K.T.; Geethalakshmi, V. Advancements in Nanobiochar for Environmental Remediation: A Comprehensive Review. Plant Sci. Today 2024, 11, 527–547. [Google Scholar] [CrossRef]
- Islam, M.S.; Magid, A.S.I.A.; Chen, Y.; Weng, L.; Arafat, M.Y.; Khan, Z.H.; Ma, J.; Li, Y. Arsenic and Cadmium Load in Rice Tissues Cultivated in Calcium Enriched Biochar Amended Paddy Soil. Chemosphere 2021, 283, 131102. [Google Scholar] [CrossRef] [PubMed]
- Cruz, G.J.F.; Mondal, D.; Rimaycuna, J.; Soukup, K.; Gómez, M.M.; Solis, J.L.; Lang, J. Agrowaste Derived Biochars Impregnated with ZnO for Removal of Arsenic and Lead in Water. J. Environ. Chem. Eng. 2020, 8, 103800. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic Removal by Perilla Leaf Biochar in Aqueous Solutions and Groundwater: An Integrated Spectroscopic and Microscopic Examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Shaheen, S.M.; Rinklebe, J.; Wang, H.; Murtaza, B.; Islam, E.; Farrakh Nawaz, M.; et al. Arsenic Removal by Japanese Oak Wood Biochar in Aqueous Solutions and Well Water: Investigating Arsenic Fate Using Integrated Spectroscopic and Microscopic Techniques. Sci. Total Environ. 2018, 621, 1642–1651. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, R.; Chen, S.; Zhu, J.; Wu, P.; Huang, J.; Qi, S. Arsenic(III) Removal from Aqueous Solution Using TiO2-Loaded Biochar Prepared by Waste Chinese Traditional Medicine Dregs. RSC Adv. 2022, 12, 7720–7734. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Miranda Avilés, R.; Serafin Muñoz, A.H.; Rocha Amador, D.O.; Perez Rodriguez, R.Y.; Hernández Anguiano, J.H.; Julia Navarro, C.; Zha, X.; Moncada, D.; de Jesús Puy Alquiza, M.; et al. Efficient Arsenic Removal from Water Using Iron-Impregnated Low-Temperature Biochar Derived from Henequen Fibers: Performance, Mechanism, and LCA Analysis. Sci. Rep. 2024, 14, 20769. [Google Scholar] [CrossRef]
- Devrajani, S.K.; Ahmed, Z.; Qambrani, N.A.; Kanwal, S.; Sundaram, U.M.; Mubarak, N.M. Mechanism of Arsenic Removal Using Brown Seaweed Derived Impregnated with Iron Oxide Biochar for Batch and Column Studies. Sci. Rep. 2024, 14, 18102. [Google Scholar] [CrossRef]
- Nham, N.T.; Al Tahtamouni, T.M.; Nguyen, T.D.; Huong, P.T.; Jitae, K.; Viet, N.M.; Van Noi, N.; Phuong, N.M.; Anh, N.T.H. Synthesis of Iron Modified Rice Straw Biochar toward Arsenic from Groundwater. Mater. Res. Express 2019, 6, 115528. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Hassan, E.B.; Pittman, C.U.; Vega, A.S.; Mlsna, T.E. Assessing South American Guadua Chacoensis Bamboo Biochar and Fe3O4 Nanoparticle Dispersed Analogues for Aqueous Arsenic(V) Remediation; Elsevier: Amsterdam, The Netherlands, 2020; Volume 706, ISBN 6623251618. [Google Scholar]
- Chen, C.K.; Chen, J.J.; Nguyen, N.T.; Le, T.T.; Nguyen, N.C.; Chang, C.T. Specifically Designed Magnetic Biochar from Waste Wood for Arsenic Removal. Sustain. Environ. Res. 2021, 31, 29. [Google Scholar] [CrossRef]
- He, R.; Peng, Z.; Lyu, H.; Huang, H.; Nan, Q.; Tang, J. Synthesis and Characterization of an Iron-Impregnated Biochar for Aqueous Arsenic Removal. Sci. Total Environ. 2018, 612, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Pham, T.H.; Nguyen Thi, H.T.; Nguyen, T.N.; Nguyen, M.V.; Tran Dinh, T.; Nguyen, M.P.; Do, T.Q.; Phuong, T.; Hoang, T.T.; et al. Synthesis of Iron-Modified Biochar Derived from Rice Straw and Its Application to Arsenic Removal. J. Chem. 2019, 2019, 5295610. [Google Scholar] [CrossRef]
- Ahmad, I.; Ghaffar, A.; Zakir, A.; Khan, Z.U.H.; Saeed, M.F.; Rasool, A.; Jamal, A.; Mihoub, A.; Marzeddu, S.; Boni, M.R. Activated Biochar Is an Effective Technique for Arsenic Removal from Contaminated Drinking Water in Pakistan. Sustainability 2022, 14, 14523. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Ye, Z.; Zhao, Q.; Li, Y.; Wu, Y.; Zhang, W.; Zhang, H. Removal of Arsenite and Arsenate from Contaminated Water Using Fe-ZrO-Modified Biochar. J. Environ. Chem. Eng. 2022, 10, 108765. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of Arsenic by Magnetic Biochar Prepared from Pinewood and Natural Hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef]
- Lou, S.; Liu, B.; Qin, Y.; Zeng, Y.; Zhang, W.; Zhang, L. Enhanced Removal of As(III) and As(V) from Water by a Novel Zirconium-Chitosan Modified Spherical Sodium Alginate Composite. Int. J. Biol. Macromol. 2021, 176, 304–314. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lamb, D.; Rahman, M.M.; Bahar, M.M.; Sanderson, P. Adsorption-Desorption Behavior of Arsenate Using Single and Binary Iron-Modified Biochars: Thermodynamics and Redox Transformation. ACS Omega 2022, 7, 101–117. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharya, T.; Shaikh, W.A.; Chakraborty, S.; Owens, G.; Naushad, M. Valorization of Fruit Waste-Based Biochar for Arsenic Removal in Soils. Environ. Res. 2022, 213, 113710. [Google Scholar] [CrossRef]
- Hoareau, C.E.; Kabeya, C. Soil Remediation by Nanotechnology: Valuating Materials, Mechanisms, and Environmental Impacts. Ind. Domest. Waste Manag. 2024, 4, 132–142. [Google Scholar] [CrossRef]
- Anjum, M.; Miandad, R.; Waqas, M.; Gehany, F.; Barakat, M.A. Remediation of Wastewater Using Various Nano-Materials. Arab. J. Chem. 2019, 12, 4897–4919. [Google Scholar] [CrossRef]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered Nanomaterials for Water Treatment and Remediation: Costs, Benefits, and Applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef]
- Chai, F.; Zhang, R.; Min, X.; Yang, Z.; Chai, L.; Zhao, F. Highly Efficient Removal of Arsenic (III/V) from Groundwater Using NZVI Functionalized Cellulose Nanocrystals Fabricated via a Bioinspired Strategy. Sci. Total Environ. 2022, 842, 6–11. [Google Scholar] [CrossRef]
- Pham, P.; Rashid, M.; Cai, Y.; Yoshinaga, M.; Dionysiou, D.D.; O’Shea, K. Removal of as(III) from Water Using the Adsorptive and Photocatalytic Properties of Humic Acid-Coated Magnetite Nanoparticles. Nanomaterials 2020, 10, 1604. [Google Scholar] [CrossRef]
- Kuroda, K.; Lu, B.; Hama, Y.; Yang, Y. Recent Progress in Photocatalysts for Oxidation of As(III) and Photocatalyst-Impregnated Adsorbents for Removing Aqueous Arsenic. Curr. Opin. Environ. Sci. Health 2023, 35, 4–9. [Google Scholar] [CrossRef]
- Zhang, F.S.; Itoh, H. Photocatalytic Oxidation and Removal of Arsenite from Water Using Slag-Iron Oxide-TiO2 Adsorbent. Chemosphere 2006, 65, 125–131. [Google Scholar] [CrossRef]
- Spanu, D.; Dal Santo, V.; Malara, F.; Naldoni, A.; Turolla, A.; Antonelli, M.; Dossi, C.; Marelli, M.; Altomare, M.; Schmuki, P.; et al. Photoelectrocatalytic Oxidation of As(III) over Hematite Photoanodes: A Sensible Indicator of the Presence of Highly Reactive Surface Sites. Electrochim. Acta 2018, 292, 828–837. [Google Scholar] [CrossRef]
- Tan, P.T.; Thu Hien, L.T.; Anh, N.N.; Minh, P.N.; Van Trinh, P.; Van Hao, N. Graphene Oxide-Carbon Nanotube-Magnetite Nanocomposites for Efficient Arsenic Removal from Aqueous Solutions. RSC Adv. 2025, 15, 20792–20809. [Google Scholar] [CrossRef]
- Su, H.; Ye, Z.; Hmidi, N. High-Performance Iron Oxide–Graphene Oxide Nanocomposite Adsorbents for Arsenic Removal. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 161–172. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Saeidirad, M.; Qazi, F.S.; Kashtiaray, A.; Ganjali, F.; Tian, Y.; Maleki, A. Regulation of Porosity in MOFs: A Review on Tunable Scaffolds and Related Effects and Advances in Different Applications. J. Environ. Chem. Eng. 2022, 10, 108836. [Google Scholar] [CrossRef]
- Sadiq, S.; Khan, S.; Khan, I.; Khan, A.; Humayun, M.; Wu, P.; Usman, M.; Khan, A.; Alanazi, A.F.; Bououdina, M. A Critical Review on Metal-Organic Frameworks (MOFs) Based Nanomaterials for Biomedical Applications: Designing, Recent Trends, Challenges, and Prospects. Heliyon 2024, 10, e25521. [Google Scholar] [CrossRef]
- Ulaş, F.; Yüksel, E.; Dinçer, D.; Dababat, A.; İmren, M. Recent Advances in Plant-Based Green Synthesis of Nanoparticles: A Sustainable Approach for Combating Plant-Parasitic Nematodes. Sustainability 2025, 17, 4152. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Meena, R.K. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Chen, T.L.; Kim, H.; Pan, S.Y.; Tseng, P.C.; Lin, Y.P.; Chiang, P.C. Implementation of Green Chemistry Principles in Circular Economy System towards Sustainable Development Goals: Challenges and Perspectives. Sci. Total Environ. 2020, 716, 136998. [Google Scholar] [CrossRef]
- Babaei, M.; Tayemeh, M.B.; Jo, M.S.; Yu, I.J.; Johari, S.A. Trophic Transfer and Toxicity of Silver Nanoparticles along a Phytoplankton-Zooplankton-Fish Food Chain. Sci. Total Environ. 2022, 842, 156807. [Google Scholar] [CrossRef]
- Li, F.; Li, R.; Lu, F.; Xu, L.; Gan, L.; Chu, W.; Yan, M.; Gong, H. Adverse Effects of Silver Nanoparticles on Aquatic Plants and Zooplankton: A Review. Chemosphere 2023, 338, 139459. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-Supported Nanoscale Zero-Valent Iron: New Findings on Simultaneous Adsorption of Cd(II), Pb(II), and As(III) in Aqueous Solution and Soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Khodakarami, M.; Honaker, R. Photothermal Self-Floating Aerogels Based on Chitosan Functionalized with Polydopamine and Carbon Nanotubes for Removal of Arsenic from Wastewater. Sci. Total Environ. 2024, 912, 169519. [Google Scholar] [CrossRef]
- Sheikhmohammadi, A.; Dahaghin, Z.; Mohseni, S.M.; Sarkhosh, M.; Azarpira, H.; Atafar, Z.; Abtahi, M.; Rezaei, S.; Sardar, M.; Masoudi, H.; et al. The Synthesis and Application of the SiO2@Fe3O4@MBT Nanocomposite as a New Magnetic Sorbent for the Adsorption of Arsenate from Aqueous Solutions: Modeling, Optimization, and Adsorption Studies. J. Mol. Liq. 2018, 255, 313–323. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hafez, I.; Tajvidi, M.; Amirbahman, A. Highly Efficient Iron Oxide Nanoparticles Immobilized on Cellulose Nanofibril Aerogels for Arsenic Removal from Water. Nanomaterials 2021, 11, 2818. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, H.; Zhang, H.B.; Cao, H.Z.; Hou, G.Y.; Tang, Y.P.; Zheng, G.Q. Graphene Oxide/CuFe2O4 Foam as an Efficient Absorbent for Arsenic Removal from Water. Chem. Eng. J. 2018, 334, 1808–1819. [Google Scholar] [CrossRef]
- Wu, K.; Jing, C.; Zhang, J.; Liu, T.; Yang, S.; Wang, W. Magnetic Fe3O4 @CuO Nanocomposite Assembled on Graphene Oxide Sheets for the Enhanced Removal of Arsenic(III/V) from Water. Appl. Surf. Sci. 2019, 466, 746–756. [Google Scholar] [CrossRef]
- Bui, T.H.; Kim, C.; Hong, S.P.; Yoon, J. Effective Adsorbent for Arsenic Removal: Core/Shell Structural Nano Zero-Valent Iron/Manganese Oxide. Environ. Sci. Pollut. Res. 2017, 24, 24235–24242. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeong, H.M.; Lee, G.H.; Jung, Y.W.; Jo, S.G.; Kang, J.K. Agglomeration-Free Fe3O4anchored via Nitrogen Mediation of Carbon Nanotubes for High-Performance Arsenic Adsorption. J. Environ. Chem. Eng. 2021, 9, 104772. [Google Scholar] [CrossRef]
- Torasso, N.; Vergara-Rubio, A.; Pereira, R.; Martinez-Sabando, J.; Baudrit, J.R.V.; Cerveny, S.; Goyanes, S. An in Situ Approach to Entrap Ultra-Small Iron Oxide Nanoparticles inside Hydrophilic Electrospun Nanofibers with High Arsenic Adsorption. Chem. Eng. J. 2023, 454, 140168. [Google Scholar] [CrossRef]
- Fang, Z.; Li, Y.; Huang, C.; Liu, Q. Amine Functionalization of Iron-Based Metal-Organic Frameworks MIL-101 for Removal of Arsenic Species: Enhanced Adsorption and Mechanisms. J. Environ. Chem. Eng. 2023, 11, 110155. [Google Scholar] [CrossRef]
- Akoto, J.D.; Chai, F.; Repo, E.; Yang, Z.; Wang, D.; Zhao, F.; Liao, Q.; Chai, L. Polyethyleneimine Stabilized Nanoscale Zero-Valent Iron-Magnetite (Fe3O4@nZVI-PEI) for the Enhanced Removal of Arsenic from Acidic Aqueous Solution: Performance and Mechanisms. J. Environ. Chem. Eng. 2022, 10, 108589. [Google Scholar] [CrossRef]
- Shao, P.; Ding, L.; Luo, J.; Luo, Y.; You, D.; Zhang, Q.; Luo, X. Lattice-Defect-Enhanced Adsorption of Arsenic on Zirconia Nanospheres: A Combined Experimental and Theoretical Study. ACS Appl. Mater. Interfaces 2019, 11, 29736–29745. [Google Scholar] [CrossRef]
- Karki, B.; Pandey, P.; Rajbhandari, R.; Joshi, S.; Koirala, A.R.; Sharma, R.K.; Pant, H.R. Facile Synthesis of Magnetic Activated Carbon Composite for Arsenic Adsorption. J. Inst. Eng. 2019, 15, 71–78. [Google Scholar] [CrossRef]
- Bi, X.; Zeng, C.; Westerhoff, P. Adsorption of Arsenic Ions Transforms Surface Reactivity of Engineered Cerium Oxide Nanoparticles. Environ. Sci. Technol. 2020, 54, 9437–9444. [Google Scholar] [CrossRef]
- Raj, S.K.; Sharma, V.; Yadav, A.; Indurkar, P.D.; Kulshrestha, V. Nano-Alumina Wrapped Carbon Microspheres for Ultrahigh Elimination of Pentavalent Arsenic and Fluoride from Potable Water. J. Ind. Eng. Chem. 2023, 117, 402–413. [Google Scholar] [CrossRef]
- Asadi Haris, S.; Dabagh, S.; Mollasalehi, H.; Ertas, Y.N. Alginate Coated Superparamagnetic Iron Oxide Nanoparticles as Nanocomposite Adsorbents for Arsenic Removal from Aqueous Solutions. Sep. Purif. Technol. 2023, 310, 123193. [Google Scholar] [CrossRef]
- Wang, H.; Qi, X.; Yan, G.; Shi, J. Copper-Doped ZIF-8 Nanomaterials as an Adsorbent for the Efficient Removal of As(V) from Wastewater. J. Phys. Chem. Solids 2023, 179, 111408. [Google Scholar] [CrossRef]
- Uppal, H.; Chawla, S.; Joshi, A.G.; Haranath, D.; Vijayan, N.; Singh, N. Facile Chemical Synthesis and Novel Application of Zinc Oxysulfide Nanomaterial for Instant and Superior Adsorption of Arsenic from Water. J. Clean. Prod. 2019, 208, 458–469. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Singh, P.N.; Tara, N.; Pal, S.; Chaudhry, S.A.; Sinha, I. Arsenic Removal from Water by Starch Functionalized Maghemite Nano-Adsorbents: Thermodynamics and Kinetics Investigations. Colloids Interface Sci. Commun. 2020, 36, 100263. [Google Scholar] [CrossRef]
- Qu, G.; Jia, P.; Zhang, T.; Li, Z.; Chen, C.; Zhao, Y. UiO-66(Zr)-Derived t-Zirconia with Abundant Lattice Defect for Remarkably Enhanced Arsenic Removal. Chemosphere 2022, 288, 132594. [Google Scholar] [CrossRef]
- Asheghmoalla, M.; Mehrvar, M. Integrated and Hybrid Processes for the Treatment of Actual Wastewaters Containing Micropollutants: A Review on Recent Advances. Processes 2024, 12, 339. [Google Scholar] [CrossRef]
- Unimke, A.A.; Okezie, O.; Mohammed, S.E.; Mmuoegbulam, A.O.; Abdullahi, S.; Ofon, U.A.; Olim, D.M.; Badamasi, H.; Galadima, A.I.; Fatunla, O.K.; et al. Microbe-Plant-Nanoparticle Interactions: Role in Bioremediation of Petroleum Hydrocarbons. Water Sci. Technol. 2024, 90, 2870–2893. [Google Scholar] [CrossRef]
- Wang, C.; Hong, M.; Wang, J.; Liao, R.; Liu, S.; Yu, S.; Liu, Y.; Yang, B.; Qiu, G. Significant Enhancement of Acidithiobacillus Ferrooxidans on the Synergistic Removal of As(III) by Pyrite and Red Mud: Migration and Transformation of As and Fe. Chem. Eng. J. 2025, 519, 165167. [Google Scholar] [CrossRef]
- Babechuk, M.G.; Weisener, C.G.; Fryer, B.J.; Paktunc, D.; Maunders, C. Microbial Reduction of Ferrous Arsenate: Biogeochemical Implications for Arsenic Mobilization. Appl. Geochem. 2009, 24, 2332–2341. [Google Scholar] [CrossRef]
- Aljuboury, D.A.D.A.; Palaniandy, P.; Abdul Aziz, H.B.; Feroz, S. Treatment of Petroleum Wastewater by Conventional and New Technologies—A Review. Glob. Nest J. 2017, 19, 439–452. [Google Scholar] [CrossRef]
- Park, H.; Choi, H. As(III) Removal by Hybrid Reactive Membrane Process Combined with Ozonation. Water Res. 2011, 45, 1933–1940. [Google Scholar] [CrossRef]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for Arsenic Removal: Challenges, Recent Developments, and Perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef]
- Rahman, M.; Uddin, N.; Parvez, M.H. Bio-Based Nanomaterials for Groundwater Arsenic Remediation: Mechanisms, Challenges, and Future Perspectives. Nanomaterials 2025, 15, 933. [Google Scholar] [CrossRef]
- Li, M.; Wang, Z.; Feng, Z.; Lu, J.; Chen, D.; Chen, C.; He, H.; Zhang, Q.; Chen, X.; Morel, J.L.; et al. New Insights into Efficient Iron Sulfide Oxidation for Arsenic Immobilization by Microaerophilic and Acidophilic Fe(II)-Oxidizing Bacteria under Micro-Oxygen and Acidic Conditions. J. Hazard. Mater. 2025, 489, 4–9. [Google Scholar] [CrossRef]
- Haukelidsaeter, S.; Boersma, A.S.; Piso, L.; Lenstra, W.K.; van Helmond, N.A.G.M.; Schoonenberg, F.; van der Pol, E.; Hurtarte, L.C.C.; van der Wielen, P.W.J.J.; Behrends, T.; et al. Efficient Chemical and Microbial Removal of Iron and Manganese in a Rapid Sand Filter and Impact of Regular Backwash. Appl. Geochem. 2024, 162, 105904. [Google Scholar] [CrossRef]
- Maceiras, R.; Perez-Rial, L.; Alfonsin, V.; Feijoo, J.; Lopez, I. Biochar Amendments and Phytoremediation: A Combined Approach for Effective Lead Removal in Shooting Range Soils. Toxics 2024, 12, 520. [Google Scholar] [CrossRef]
- Sarma, H.; Shyam, S.; Zhang, M.; Guerriero, G. Nano-Biochar Interactions with Contaminants in the Rhizosphere and Their Implications for Plant-Soil Dynamics. Soil Environ. Health 2024, 2, 100095. [Google Scholar] [CrossRef]
- Banerji, T.; Chaudhari, S. Arsenic Removal from Drinking Water by Electrocoagulation Using Iron Electrodes- an Understanding of the Process Parameters. J. Environ. Chem. Eng. 2016, 4, 3990–4000. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Gourich, B.; Chafi, M.; Stiriba, Y.; Vial, C.; Drogui, P.; Naja, J. Electrocoagulation Process in Water Treatment: A Review of Electrocoagulation Modeling Approaches. Desalination 2017, 404, 1–21. [Google Scholar] [CrossRef]
- Mandeep; Shukla, P. Microbial Nanotechnology for Bioremediation of Industrial Wastewater. Front. Microbiol. 2020, 11, 590631. [Google Scholar] [CrossRef]
- Ahmad, A.; Heijnen, L.; de Waal, L.; Battaglia-Brunet, F.; Oorthuizen, W.; Pieterse, B.; Bhattacharya, P.; van der Wal, A. Mobility and Redox Transformation of Arsenic during Treatment of Artificially Recharged Groundwater for Drinking Water Production. Water Res. 2020, 178, 115826. [Google Scholar] [CrossRef]
- Shamshad, J.; Ur Rehman, R. Innovative Approaches to Sustainable Wastewater Treatment: A Comprehensive Exploration of Conventional and Emerging Technologies. Environ. Sci. Adv. 2024, 4, 189–222. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khan, K.H.; Parvez, M.M.; Irizarry, N.; Uddin, M.N. Nanocomposites with Optimized Nanoparticle Dispersion and Enhanced Functionalities for Industrial Applications. Processes 2025, 13, 994. [Google Scholar] [CrossRef]

| Microorganism | Arsenic Resistance | Mechanism/Reaction | Arsenic Species Targeted | Adsorption/Removal% Efficiency | Optimal Conditions | Reference |
|---|---|---|---|---|---|---|
| Exiguobacterium Profundum PT2 | Resists 25·2 mg/g As | Biosorption | As(V) and As(III) | Reduced 3·73 mmol in 48 h | pH 7; 37 °C | [65] |
| Brevibacillus sp. | Resists 265 mM of As(V) and 17 mM of As(III) | Reduction and oxidation | As(V) and As(III) | Removed ~40% of As | pH 7.1; 37 °C | [66] |
| Bacillus aryabhatti | Able to grow up to 500 mM As(V) | Reduction | As(V) and As(III) | pH 7; 60 °C | [67] | |
| Roseomonas sp. | Resists 50 mM of As(V) and 2 mM of As(III) | Oxidation | As(V) and As(III) | Oxidized 2 mM As(III) in 60 h | pH 7.1; 37 °C | [68] |
| Microbacterium, Micrococcus, Shinella, and Bacillus sp. | Resists more than 400 mM As(V) and 8 mM As(III) | Reduction and oxidation | As(V) and As(III) | NA | pH 9; 30 °C | [69] |
| Nocardioides sp. | Resists 100 mM of As(V) and 5 mM of As(III) | Reduction | As(V) and As(III) | Reduced 2 mM As(V) in 36 h | pH 7; 37 °C | [68] |
| Bacillus flexus and Acinetobacter junii | Capable of growing at 150 mmol L−1 As (V) and 70 mmol L−1 As (III). | Biosorption | As(V) and As(III) | 8 mg/g | pH 8; 30 °C | [70] |
| Pseudomonas sp. AK1 | Able to grow at 13 mmol As (III). | Oxidation | As(III) | 25% reduction in 72 h | pH 7; 30 °C | [71] |
| Bacillus sp., Acinetobacter sp. | Able to grow up to 300 and 350 mM As(V) | Bioaccumulation | As(V) or As(III) | 848.33 mg/g dry cell weight | pH 7; 30 °C | [72] |
| Pseudomonas sp. AK9 | Able to grow at 15 mmol As (III). | Oxidation | As(III) | 25% reduction in 72 h | pH 7; 30 °C | [71] |
| Acinetobacter sp. and Exiguobacterium sp. | viable even at concentrations of 350 mM As(V) and up to As(III) 15 mM | Bioaccumulation | As(V) or As(III) | 60% | pH 7; 35 °C | [73] |
| Bacillus firmus | tolerated 3 M As(V) and 75 mM As(III) | Oxidation | As(V) or As(III) | 77% in 15 days | pH 9; 30 °C | [74] |
| Bacillus sp. | Viable at concentrations of 1000 mM As(V) and up to 70 mM of As(III) | Oxidation | As(III) | 88% | pH 7; 33.5 °C | [75] |
| Brevibacterium sp. CS2 | MIC of 280 mM As(V) and 40 mM of As(III) | Oxidation | As(III) | 32 and 46% in wastewater and distilled water, respectively, in 8 days | pH 7; 37 °C | [76] |
| Providencia rettgeri | Viable at concentrations of 133.3 mM As(V) | Bioaccumulation | As(V) | NA | pH 7; room temp | [77] |
| Lysinibacillus sp. | MIC of 500 mM As(V) | Reduction | As(V) | Reduced 50% | pH 7; 37 °C | [4] |
| Pseudomonas sp. | Tolerable concentration of As(III) up to 3250 mg/L and As(V) up to 20,280 mg/L | Oxidation and reduction | As(V) or As(III) | Bacterium exhibited 48% of As(III) and 78% of As(V) transformation | pH 7.5; 25 °C | [78] |
| Pseudomonas sp. HN-2 | Oxidation | As(III) | Oxidized 92.0% of As(III) to As(V) in 3 h | pH 7; 37 °C | [38] | |
| Pseudomonas sp. and Acinetobacter sp. | MIC of 125 mM As(V) and 50 mM of As(III) | Oxidation and reduction | As(V) or As(III) | NA | pH 7; 37 °C | [79] |
| Leclercia adecarboxylata | Tolerated up to 100 mM As(V) and 10 mM As(III) | Reduction | As(V) | Harbored a typical As(V) reductase gene (arsC) | pH 7; 30 °C | [80] |
| Pseudomonas aeruginosa | The MIC was 7 g/L for As(V) and 1.4 g/L for As(III) | Oxidation and reduction | As(III) | 98 mg/g | pH 7; 37 °C | [81] |
| Bacillus cereus | Resistant to 3000 mg/L of As | Oxidation reduction | As(V) or As(III) | Reduced 71.88% of As(III) and 85.72% of As(V) | pH 6.8; 30 °C | [82] |
| Lysinibacillus boronitolerans | Resistant to 3000 mg/L of As | Oxidation reduction | As(V) or As(III) | Reduced 71.88% of As(III) and 85.72% of As(V) | pH 6.8; 30 °C | [82] |
| Bacillus sp. | MIC of 500 mM of As(V) | Reduction | As(V) | As(V) reduction efficiency was optimized to 72% | pH 6.8; 30 °C | [83] |
| Micrococcus sp. | Capable of growing at 150 mmol L−1 As (V) and 70 mmol L−1 As (III). | Oxidation | As(V) or As(III) | Reduced 91.04% | pH 7; 30 °C | [84] |
| Bacillus sp. | MIC As(V) up to 4500 ppm and 600 ppm of As(III) | Oxidation and reduction | As(V) or As(III) | 51.45% As(III) and 53.29% As(V) | 30 ± 1 °C | [85] |
| Aneurinibacillus aneurinilyticus | MIC As(V) up to 4500 ppm and 600 ppm of As(III) | Oxidation and reduction | As(V) or As(III) | 51.99% As(III) and 50.37% As(V) | 30 ± 1 °C | [85] |
| Adsorbent/Material | Modification/Type | As Species Targeted | Adsorption Capacity (mg/g)/Removal Efficiency (%) | Optimal pH | Mechanism/Key Advantages | Reference |
|---|---|---|---|---|---|---|
| Orange peel | Modified titanium dioxide (TiO2) | 10.91 mg/g | 4.2 | High surface area, eco-friendly | [121] | |
| Shrimp-based chitosan | Modified by 1.5% HCl and 5% NaOH | As(V) | 98.5%; 15.92 mg/g | 7 | Abundant supply at low costs | [20] |
| Lemon peel | NB | As(III) | 72.34% | 6 | Low-cost and sustainable biosorbent | [122] |
| Green tea Waste | Modified by Ca(OH)2 | As(III) | 0.4212 mg/g | 3; 33 °C | High surface area, eco-friendly | [123] |
| Rice husk | Alkaline activation | As(V) | 15–30 mg/L | 3 | Agro-waste utilization | [124] |
| Rice husk | NB | As(V) | 90.7% | 8 | Cost-effective and biodegradable | [125] |
| Citrus limetta peel | Zirconium-modified | As(V) | 75.86 mg/g | 5.8 | Low-cost and sustainable biosorbent | [126] |
| Pomegranate peels | Modified by TiO2 | As(III) | 76.92 mg/g | pH = 7, T = 25 °C | Cheap, easy-going | [127] |
| Orange peel | Ca(OH)2-modified | As(V) | 43.69 mg/g | 5.5 | Low-cost and eco-friendly adsorbents | [128] |
| Watermelon rind | Modified by citric acid | As(III), As(V) | As(III) (99%) and As(V) (98%) | 8.2 | Cheap, easy-going | [129] |
| Banana peel | Calcium nitrate, diammonium Hydrogen phosphate, sulfuric acid, ferric nitrate -modified | As(V) | 98.7% | 4–6 | Ligand exchange, electrostatic | [130] |
| Sugarcane bagasse | Thiol-functionalized | As(III), As(V) | 28.57 mg/g | 7 | Low cost, green | [131] |
| Mango peel | Zr(IV) | As(III) | 87.32%; 45.52 mg/g | 10.18 | Adsorption via carbon matrix | [132] |
| Sugarcane bagasse | Activation using H3PO4 | As(III) | 6.69 mg/g | 8 | Abundant supply at low costs | [133] |
| Bamboo charcoal | Iron-modified | As(III), As(V) | 7.23 mg/g | 4–5 | High surface area, large pore volume, and low cost | [134] |
| Pomegranate waste | Fe(III)-loaded | As(III) | 50 mg/g | 9 | Low-cost bioadsorbent | [135] |
| Sawdust | Treated using ZrO2 | As(III), As(V) | 29 mg/g (AsIII) and 12 mg/g (AsV) | 7 | Environmentally friendly and cost-effective | [136] |
| Apple peel | Zirconium-loaded | As(III), As(V) | 5.68 mg/g | 2–6 | Low cost, green | [137] |
| Watermelon peel | NM | As(III), As(V) | 99.99% | 5.5–7.5 | Low cost, high efficiency | [138] |
| Java plum seeds | NM | As(III), As(V) | 78% As(III) and 67% As(V) | 7 for As(III) and 5.3 for As(V) | Inexpensive, effective, and sustainable | [139] |
| Egg shell | NM | As(III), As(V) | 87% As(III) and 71% As(V) | 7 for As(III) and 4 for As(V) | Inexpensive, effective, and sustainable | [139] |
| Water chestnut shell | NM | As(III), As(V) | 75% | 7 | Inexpensive, effective, and sustainable | [139] |
| Corn cob | NM | As(III), As(V) | 67% | 7 | Inexpensive, effective, and sustainable | [139] |
| Tea waste | NM | As(III), As(V) | 74% | 7 | Inexpensive, effective, and sustainable | [139] |
| Pomegranate peel | NM | As(III), As(V) | 65% | 9 | Inexpensive, effective, and sustainable | [139] |
| Rice polish | NM | As(III), As(V) | As(III) (41.18 μg/g) and As(V) (49 μg/g) | 6.84 for As(III) and 4.29 for As(V) | Cheap, easy-going | [140] |
| Chir pine leaves | NM | As(III), As(V) | 3.27 mg/g | 4 | Exothermic, spontaneous, and favorable | [141] |
| Blue pine wood shavings | NM | As(III), As(V) | 97% | 10 | Simplicity and easy operation, and handling | [142] |
| Walnut shell | NM | As(III), As(V) | 88% | 10–11 | Simplicity and easy operation, and handling | [142] |
| Chick pea testa | NM | As(III), As(V) | 35% | 8 | Simplicity and easy operation, and handling | [142] |
| Rice husk | NM | As(III), As(V) | 96% | 6.5 | Environmentally friendly, cost-effective, and biodegradable | [143] |
| Plant Species | Type | Max As Uptake (mg/kg) | Mechanism | Advantages | Reference |
|---|---|---|---|---|---|
| Pteris vittata | Fern | >2000 | Phytoextraction | Fast-growing, high As accumulation | [151] |
| Pteris vittata | Fern | 1860 | Phytoextraction and phytostabilization | Potential candidate for As removal in soils and sediments | [166] |
| Pteris vittata L. | Fern | 7215–11,110 | Phytoaccumulation | Capable of co-hyperaccumulating high As levels | [167] |
| Artemisia divarica | Dicotyledons | 47.26 | Phytoextraction | Low-cost, prevents pollution, enables fast recycling | [168] |
| Pteris ensiformis | Fern | 1091 | phytoextraction | high biomass, wide occurrence, and rapid growth | [169] |
| Pteridium aquilinum | Fern | 622 | Rhizofiltration | Eco-friendly, solar-powered | [170] |
| Pteris cretica | Fern | 4875 | Phytoaccumulation | Phosphomolybdic acid from Pteris cretica is converted to Mg3(PO4)2, a potential fertilizer | [171] |
| Eruca sativa | Herb | 0.1560–0.1630 | Phytoaccumulation | Fast growth, high biomass | [172] |
| Azolla caroliniana | Fern | 386.1 | Phytoaccumulation | Rapid growth and reproduction, high surface area | [173] |
| Ceratophyllum demersum | Hornwort | 60% | Phytoaccumulation | Fast-growing, high As uptake, low maintenance. | [174] |
| Cladophora sp. | Algae | 6 mg/ L | Phytoaccumulation | Rapid growth, high surface area | [175] |
| Chlorodesmis | Algae | 4 mg/L (40–50%) | Phytoaccumulation | Fast growth rate, low-cost, and eco-friendly | [175] |
| Arundo donax | Reed | 600 μg/ L | Phytoaccumulation | Plant growth was observed within an As concentration range of 50–600 μg/L | [176] |
| Lemna minor | Duckweed | 8.70–15.02% | Rhizofiltration | Low maintenance and cost-effective | [177] |
| Eichornia crassipes | Water hyacinth | 39.2% | Rhizofiltration | Extensive root system and cost-effective | [107] |
| Phragmites karka | Tall reed | 46% | Phytoaccumulation | Large biomass and surface area | [178] |
| Pteris vitatta | Fern | 3.5–11.4% | Phytoaccumulation | Fast-growing, large biomass | [179] |
| Genetically Modified Arabidopsis thaliana | Herb | As uptake 28 µg/g in the shoot and 2400 µg/g in the root | Phytoaccumulation | Small size, low space requirement, and easy cultivation | [14] |
| Colocasia esculenta | Angiosperm | 89% | Phytoaccumulation | High biomass, efficient uptake, and accumulation | [180] |
| Lemna valdiviana | Duckweed | 1190 mg/kg (82% removal) | Phytoextraction | Low maintenance and cost-effective | [181] |
| Wolfia globosa | Watermeal | >1000 mg/kg | Phytoaccumulation/ Phytofiltration | High surface area, fast growth rate | [182] |
| Vallisneria natans | Grass | 58.11–66.21% | Phytoextraction | Low maintenance and fast growth rate | [183] |
| Eichhornia crassipes | Water hyacinth | 83% | Phytoaccumulation/ Phytoextraction | Extensive root system and cost-effective | [184] |
| Pistia stratiotes | Water lettuce | Root and leaf content 1120.40 µg/g and 31.60 µg/g, respectively | Phytoaccumulation | Free-floating, rapid growth, and high biomass | [185] |
| Lemna minor | Duckweed | >70% | Phytoaccumulation | Low maintenance and cost-effective | [186] |
| Viola macedonica | Herb | 783 mg/kg | Phytostabilization | Extensive root system, adaptability to various soils | [187] |
| Viola arsenica | Herb | 2124 mg/kg | Phytostabilization | Extensive root system, adaptability to various soils | [187] |
| Leucaena leucocephala | Dicotyledons | 6.83 | Bioaccumulation and phytoextraction | Potential for both fertility improvement and heavy metal(loid) hazard prevention | [188] |
| Acacia mangium | Dicotyledons | 1549 | Phytostabilization | Able to survive on arsenic- conc. up to 500 mg/kg | [189] |
| Retama sphaerocarpa | Shrub | >88% | Phytostabilization | Deep root system, biomass production, and drought tolerance | [187] |
| Biochar Type | Pyrolysis Temp (°C) | Modification | Arsenic Species Targeted | Removal%/Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|---|---|
| Corncob biochar | 450 | Amendment materials with Fe, Mn | As(III), As(V) | Reduced 51.2–54.1% | [203] |
| Coffee husk and corncob biochar | 600 | Impregnated with ZnO | As(V) | 25.9 mg/g | [204] |
| Pomegranate peels | 400 | Impregnated with TiO2 | As (III) | 76.92 mg/g | [127] |
| Perilla leaf biochar | 700 | Not modified | As(III), As(V) | (97–100%) | [205] |
| Oak wood biochar | 500 | Not modified | As(III), As(V) | 4 mg/g (92 to 100%) | [206] |
| Chinese traditional medicine dregs waste biochar | 450 | Iron-doped TiO2 Modification | As(III) | 58.45 mg/g | [207] |
| Henequen fibers biochar | 260 | FeCl3·6H2O modification | As(V) | 8.98 mg/g | [208] |
| Brown seaweed biochar | 400 | FeCl3·6H2O modification | As(V) | 0.83 mg/g 96.7% | [209] |
| Rice straw biochar | 500 | FeSO4·7H2O and FeCl3·6H2O | As(V) | 26.9 mg/g | [210] |
| Bamboo biochar | 700 | Fe3O4 modified | As(V) | 13.9 mg/g (100%) | [211] |
| Wood waste biochar | 600 | Promoted by FeCl3 and KMnO4 | As (III) | 81% and 0.72 mg/g | [212] |
| Corn straw biochar | 600 | Treated with FeCl3 | As(V) | 6.80 mg/g | [213] |
| Rice straw biochar | 500 | Using FeCl3 modification | As(V) | 28.49 mg/g | [214] |
| Cotton stalks biochar | 400 | Treated with nitric acid (HNO3) and Base (NaOH) | As(III) | 157 µg/g | [215] |
| Durian shells biochar | 500 | Fe-ZrO-modified | As(III), As(V) | As(III) 46.7 and As(V) 47.8 mg/g | [216] |
| Pine wood biochar | 600 | Birnessite | As(V) | 910 µg/g | [217] |
| Pine wood biochar | 600 | Mn oxide | As(V) | 590 µg/g | [217] |
| Corncob biochar | 350 | Modified with zirconium (CCB@Fe3O4-Zr with Zr to Fe3O4) | As(III), As(V) | 81% (As(III)), 99% (As(V)) removal | [200] |
| Chitosan biochar | 531.96 | Modified sodium alginate (Zr-CTS/SA) | As(III), As(V) | 43.19 mg/g (As(III)), 76.78 mg/g (As(V)) | [218] |
| Pristine biochar | 300 | Iron (Fe) and binary zirconium–iron (Zr–Fe)-modified | As(V) | 67.28 | [219] |
| Fruit waste biochar | 500 | Not modified | As(V) | 88.8 ± 0.04% | [220] |
| Nanomaterial | Functionalization/Support | Target Arsenic Species | Adsorption Capacity (mg/g) | Advantages | Reference |
|---|---|---|---|---|---|
| Zero-valent iron (Z-NZVI) | Zeolite | As(III) | 11.52 (mg/g) | Improved kinetics, high surface area, and dispersion | [239] |
| CNT/Ch, and PDA@CNT/Ch | Chitosan aerogel | As(III) | 94% | Highly functional at low pH | [240] |
| SiO2@Fe3O4@MBT | Silica (SiO2), Fe3O4 is embedded/coated for magnetism | As(V) | 95.77% | Fast adsorption and high surface area | [241] |
| Cellulose nanofibril aerogels/Fe-IONPs | Cellulose nanofibril aerogels | As(III), As(V) | As(III) 48 mg/g, As(V)91 mg/g | High porosity | [242] |
| Graphene oxide/CuFe2O4 foam | Graphene oxide (GO) and copper ferrite composite | As(III), As(V) | 44 (mg/g) | High adsorption capacity, eco-friendly, and reusable | [243] |
| Magnetic Fe3O4@CuO nanocomposite | Graphene oxide (GO) | As(III), As(V) | As(III) 70.36 mg/g; As(V) 62.60 mg/g | High adsorption capacity, eco-friendly, and durable | [244] |
| Core/shell structural nZVI/Mn oxide | Manganese oxide shell | As(III), As(V) | 29.4 | Improved selectivity, high adsorption capacity | [245] |
| Fe3O4 NP-NCNT hybrid | Agglomeration-free Fe3O4 | As(III), As(V) | 69% As(III), 35% As(V) | High affinity for arsenic, regeneration | [246] |
| Hydrophilic poly(vinyl alcohol) (PVA) nanofibers | Polymeric matrix | As(V) | 3.5 mg/g | Excellent dispersion and surface availability of ions | [247] |
| Iron-based metal–organic frameworks MIL-101 [NH2-MIL-101(Fe)] | Iron-based metal | As(III), As(V) | As(V) and As (III) were 148 and 153 mg/g | Fast kinetics, high surface area and porosity | [248] |
| Fe3O4@Nzvi-PEI | Polyethyleneimine | As(III), As(V) | 95.8% As(III), 90.5 As(V) | Fast kinetics, high reduction capacity | [249] |
| Zr-UiO-66-SH-A | Zirconium oxide (ZrO2) | As(III), As(V) | As(III) and As(V) 90.7 and 98.8 mg/g | High affinity for arsenic, regeneration, and reusability | [250] |
| AC/Fe3O4 | Magnetic iron oxide | As(III), As(V) | As(III) 70% and As(V) 29% | High adsorption capacity, high specific surface area. | [251] |
| Cerium oxide (CeO2) nanoparticles (NPs) | Cerium oxide | As(III), As(V) | 451 mg/g As(III), 119 mg/g As(V) | Eco-friendly, large surface area, and porosity | [252] |
| Nano-alumina-coated carbon microspheres (Al-CMs) | Carbon microsphere (CM) core | As(V) | 68 mg/g | Reusability of adsorbent | [253] |
| Alginate-coated superparamagnetic Iron Oxide nanoparticles (SPIONs) | Alginate beads (SPIONs-Alg) | As(V) | 99% (240.08 mg/g) | High adsorption capacity, eco-friendly, and biocompatible | [254] |
| Copper-doped ZIF-8 nanomaterials | Zeolitic imidazolate | As(III), As(V) | 238.11 mg/g As (III) and 10–350 mg/g As(V) | Fast kinetics, reusability, and high surface area | [255] |
| Zinc oxysulfide nanomaterials (ZnOxS1-x) | Silica (SiO2) | As(III), As(V) | 299.4 (99.9%) | High affinity for arsenic, low cost, and abundance | [256] |
| Starch-functionalized maghemite nanoparticles (g-Fe2O3@starch) | Starch polymer | As(III) | 8.88 | Cost-effective synthesis, eco-friendly and biocompatible | [257] |
| Zr-metal–organic framework (UiO-66)-derived t-ZrO2 | Zirconium oxide (ZrO2) | As(III), As(V) | 352.1 mg/g | Faster adsorption rate and ultrahigh uptake | [258] |
| Technology | Mechanism | Efficiency | Cost | Advantages | Limitations | Suitable Application |
|---|---|---|---|---|---|---|
| Microbial Remediation | Oxidation/reduction | Moderate–High | Low–Medium | Eco-friendly, selective transformation | Slower process, sensitive to environmental conditions | Groundwater, wastewater, wetlands |
| Phytoremediation | Uptake and accumulation by plants | Moderate | Low | Low-cost, green, improves soil health | Time-consuming and biomass disposal issues | Contaminated soil and wetlands |
| Biochar and Modified Biochar | Adsorption and immobilization | Moderate–High | Low–Medium | Abundant materials, easy application | Risk of desorption depends on biochar quality | Soil remediation, filtration units |
| Nanotechnology-Based Methods | Adsorption, redox transformation | High (>95%) | Medium–High | High capacity, fast kinetics | Potential nanotoxicity, high cost | Point-of-use water treatment |
| Membrane Filtration | Size exclusion/adsorptive filtration | Very High (>99%) | High | Precise removal, effective even at low concentrations | Expensive, fouling, energy-demanding | Urban and industrial wastewater |
| Adsorption | Adsorption and immobilization | High | Low-medium | Ease of operation, low cost, fast kinetics | Requires solid waste disposal, Non-destructive for contaminants | Groundwater, urban and industrial wastewater |
| Electrocoagulation | In situ coagulant generation | High | Medium | Sludge-free, chemical-less | Power needs, electrode passivation | Decentralized treatment plants |
| Hybrid/Integrated Systems | Combined bio-physico-chemical | Very High | Variable | Synergistic effects, site-specific design | Requires monitoring and integration expertise | Groundwater, urban and industrial wastewater |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, A. Integrated Approaches of Arsenic Remediation from Wastewater: A Comprehensive Review of Microbial, Bio-Based, and Advanced Technologies. Toxics 2025, 13, 768. https://doi.org/10.3390/toxics13090768
Rahman A. Integrated Approaches of Arsenic Remediation from Wastewater: A Comprehensive Review of Microbial, Bio-Based, and Advanced Technologies. Toxics. 2025; 13(9):768. https://doi.org/10.3390/toxics13090768
Chicago/Turabian StyleRahman, Aminur. 2025. "Integrated Approaches of Arsenic Remediation from Wastewater: A Comprehensive Review of Microbial, Bio-Based, and Advanced Technologies" Toxics 13, no. 9: 768. https://doi.org/10.3390/toxics13090768
APA StyleRahman, A. (2025). Integrated Approaches of Arsenic Remediation from Wastewater: A Comprehensive Review of Microbial, Bio-Based, and Advanced Technologies. Toxics, 13(9), 768. https://doi.org/10.3390/toxics13090768







