Proteomics Reveals Mechanisms of Metabolic Dysregulation in Soman Neurotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Drug Administration
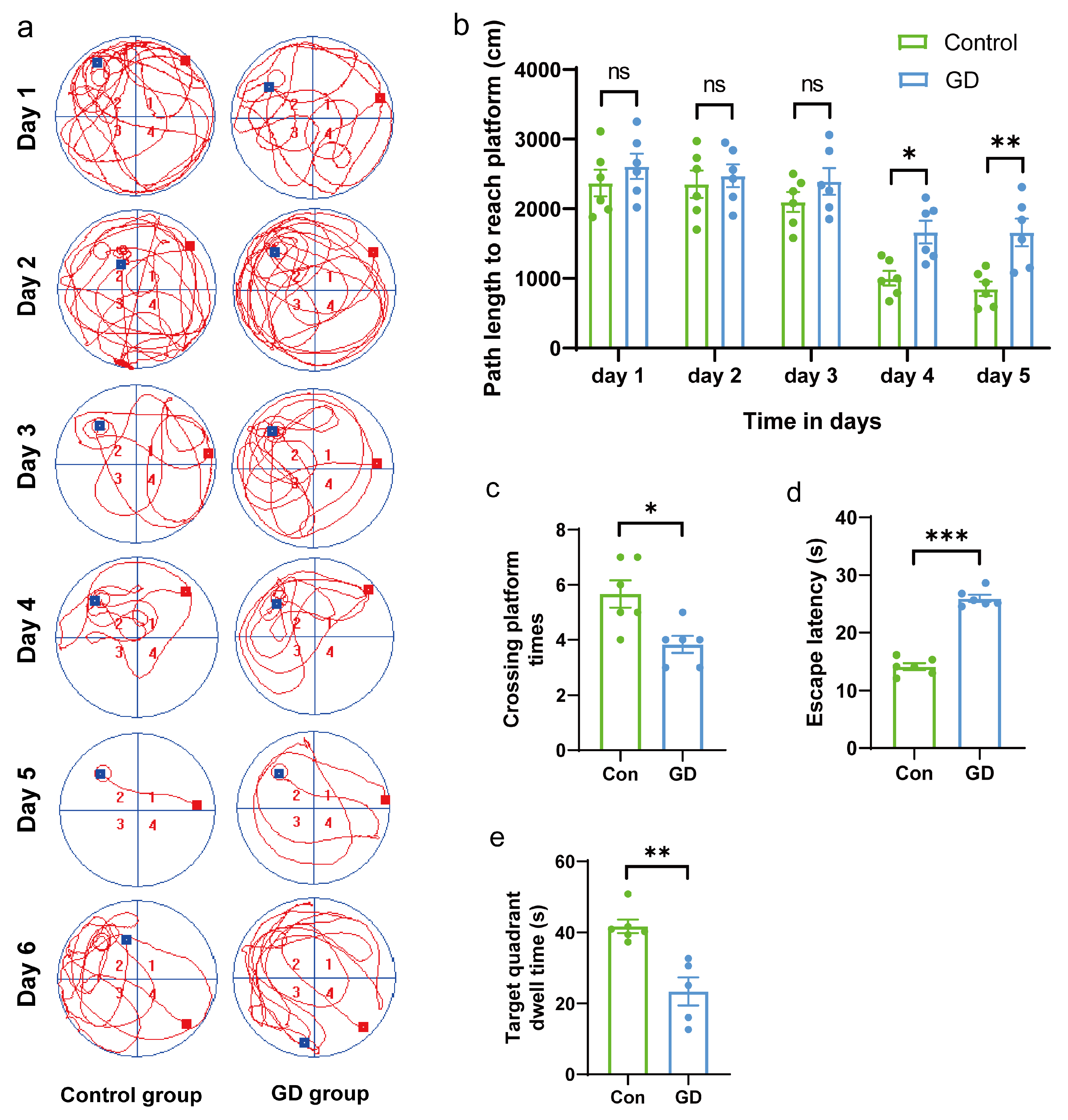
2.3. Morris Water Maze Experiment
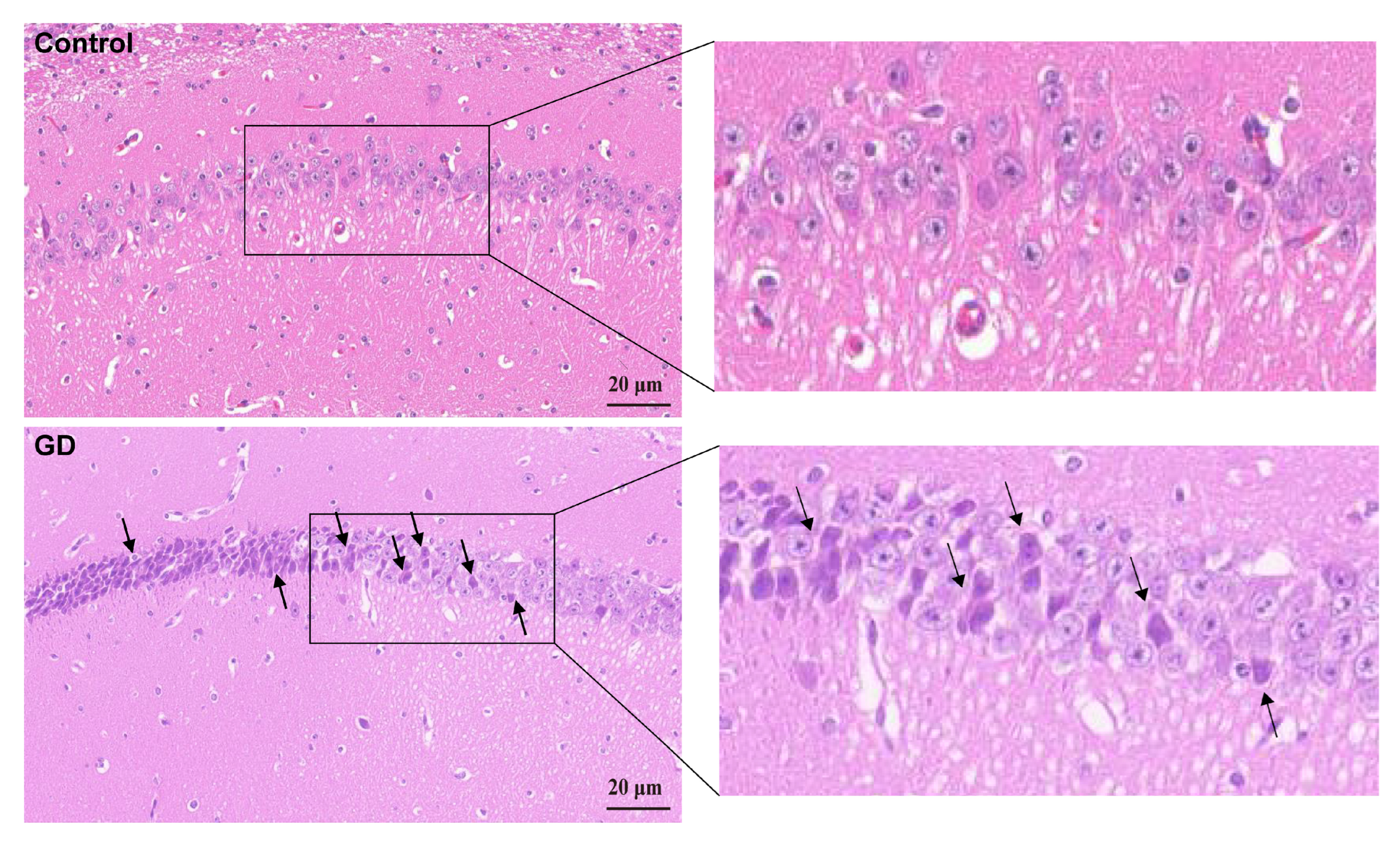
2.4. Histopathologic Study
2.5. Hippocampal Tissue Sample Preparation
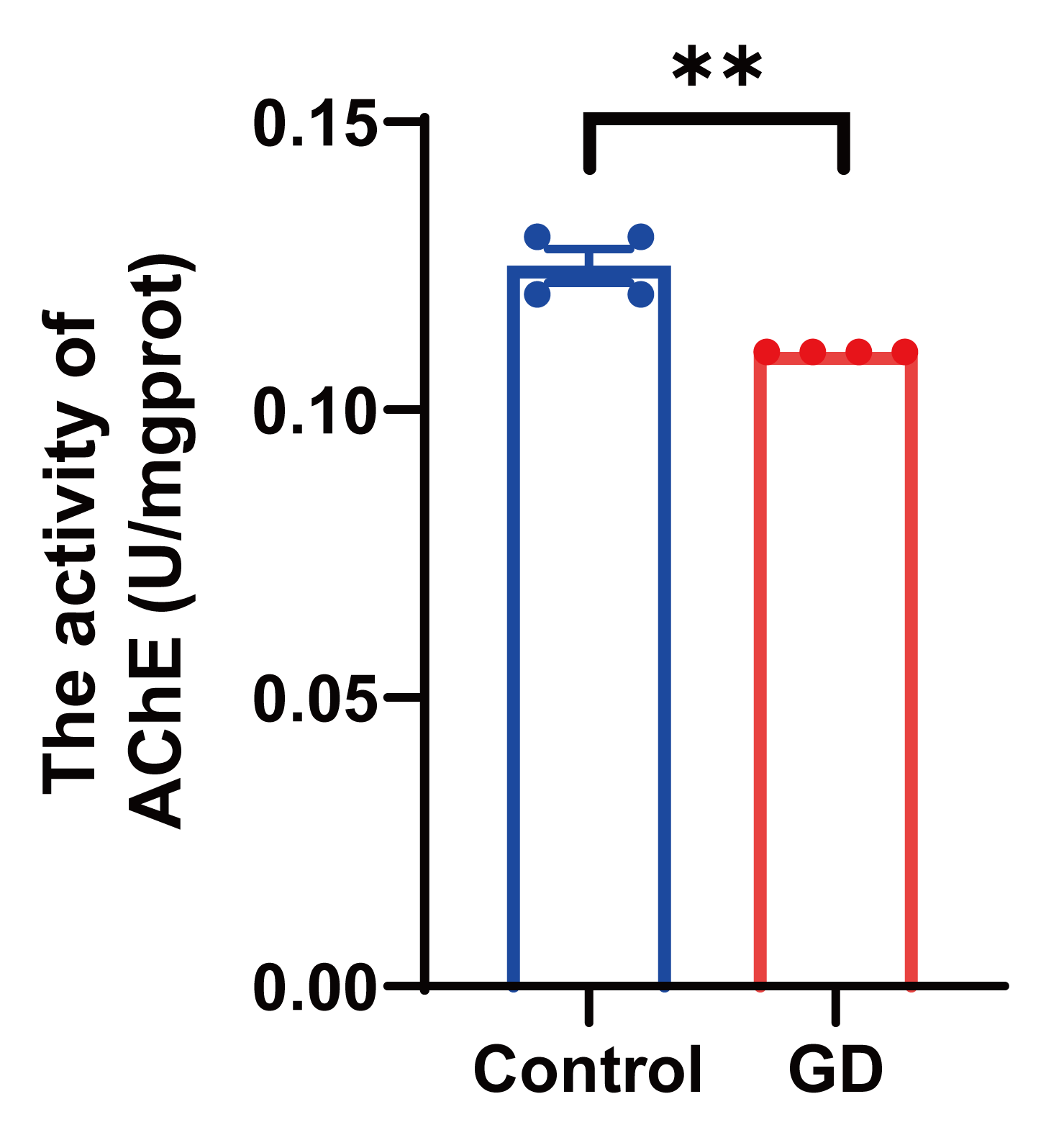
2.6. AChE Activity Assay
2.7. Enzyme-Linked Immunosorbent Assay
2.8. Determination of Metabolites in Specific Amino Acid Metabolic Pathways
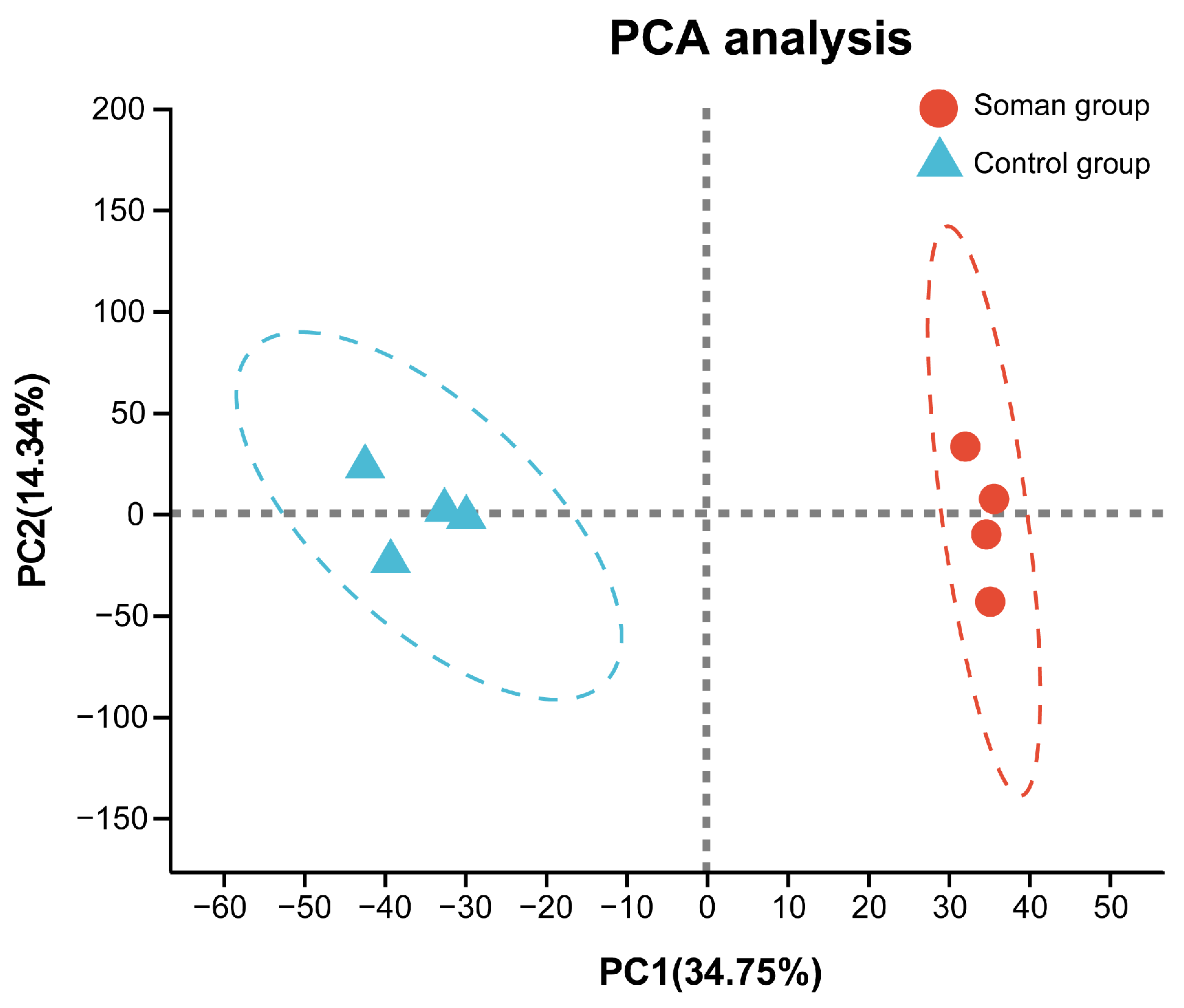
2.9. Proteomic Profile Analysis
2.10. Statistical Analysis and Bioinformatics Analysis
3. Results
3.1. Effects of GD on Spatial Learning and Memory in Guinea Pigs
3.2. Neuronal Changes in the Hippocampal Tissues of Guinea Pigs
3.3. AChE Activity in Guinea Pig Hippocampal Tissues After Repeated Exposure to Soman
3.4. Hippocampal Proteomic Profiles
3.5. Differential-Protein-Set Annotation Analysis
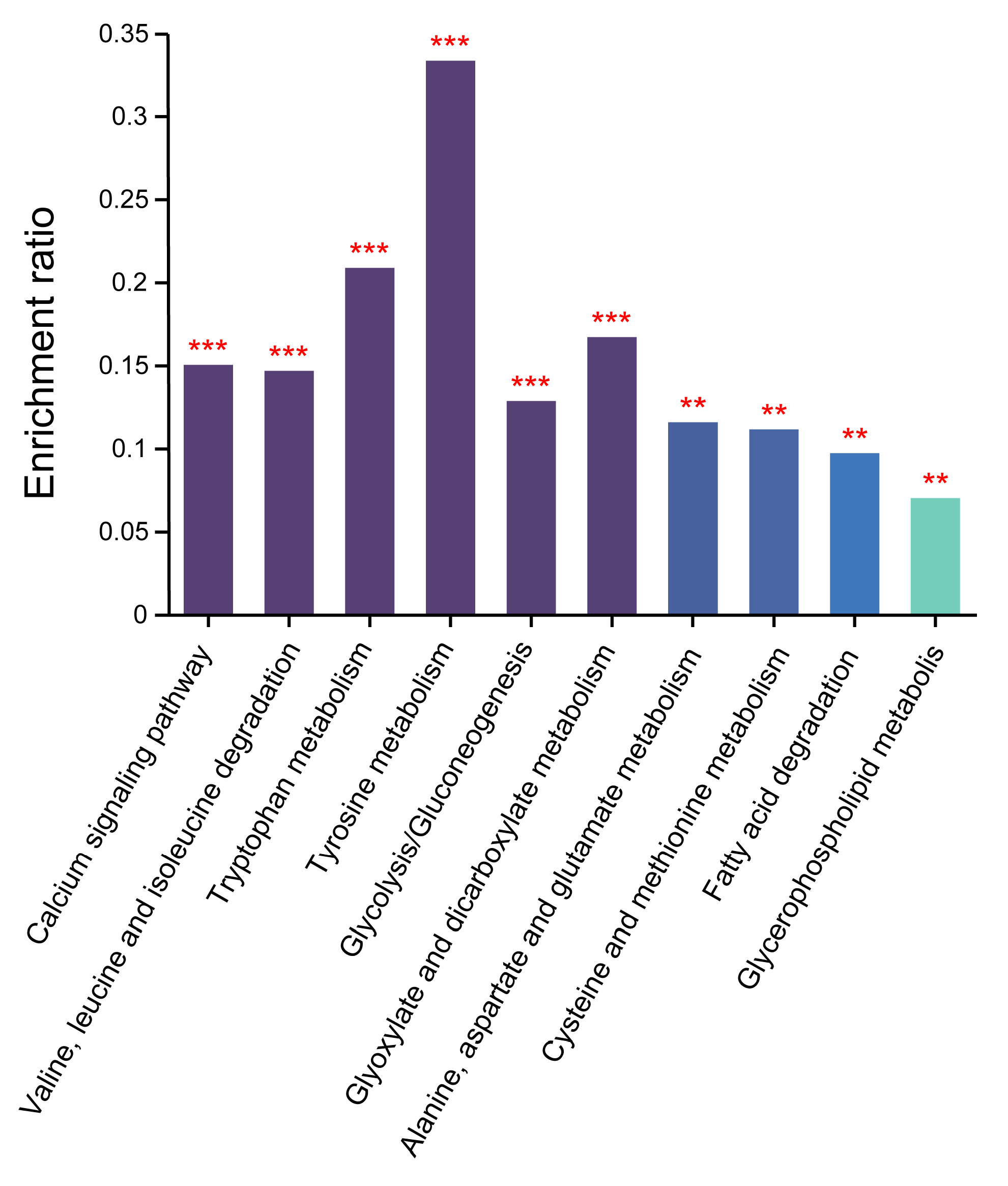
3.6. KEGG Functional Enrichment
3.6.1. Calcium Signal Transduction and Transport
3.6.2. Amino Acid Metabolism
3.6.3. Lipid Metabolism
3.6.4. Carbohydrate Metabolism
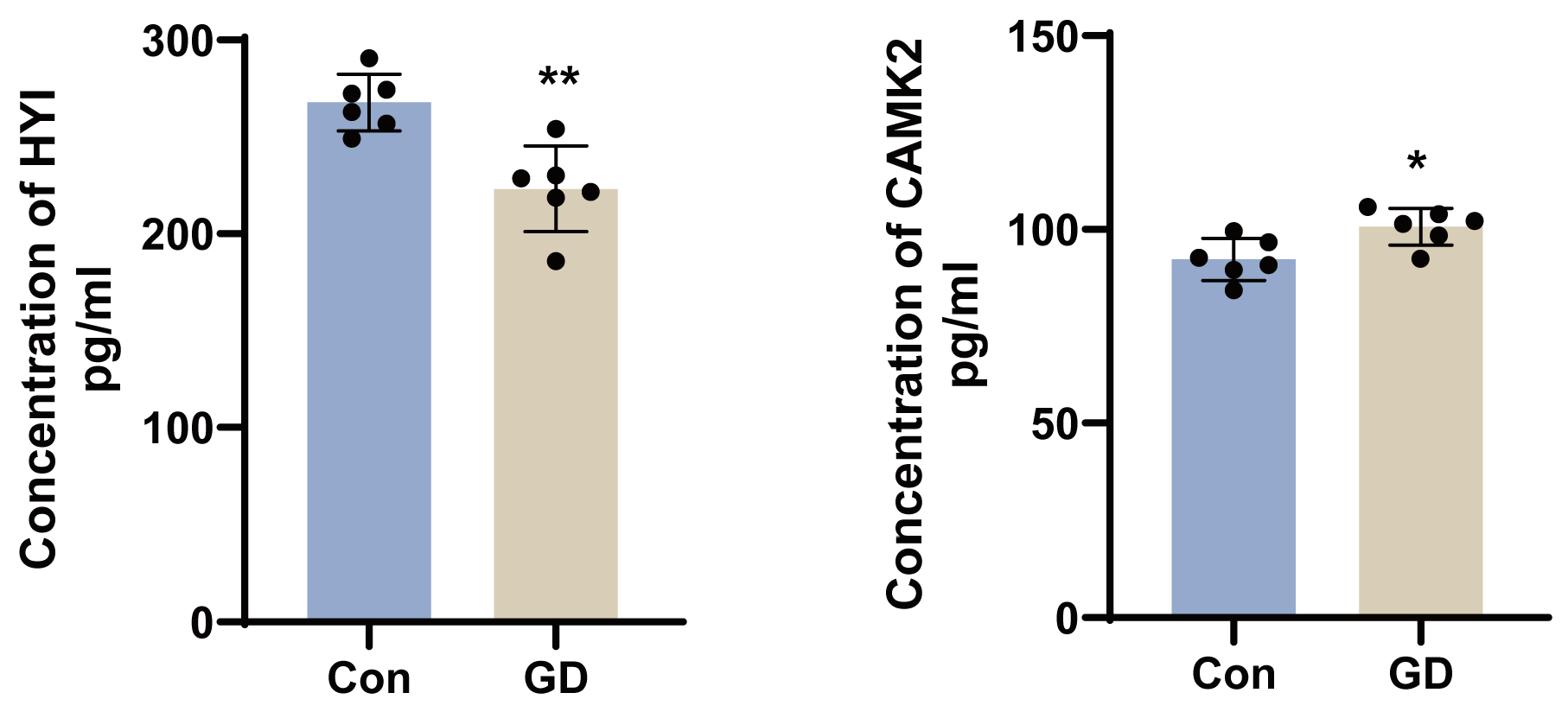
3.7. Validation of Key Proteins by ELISA
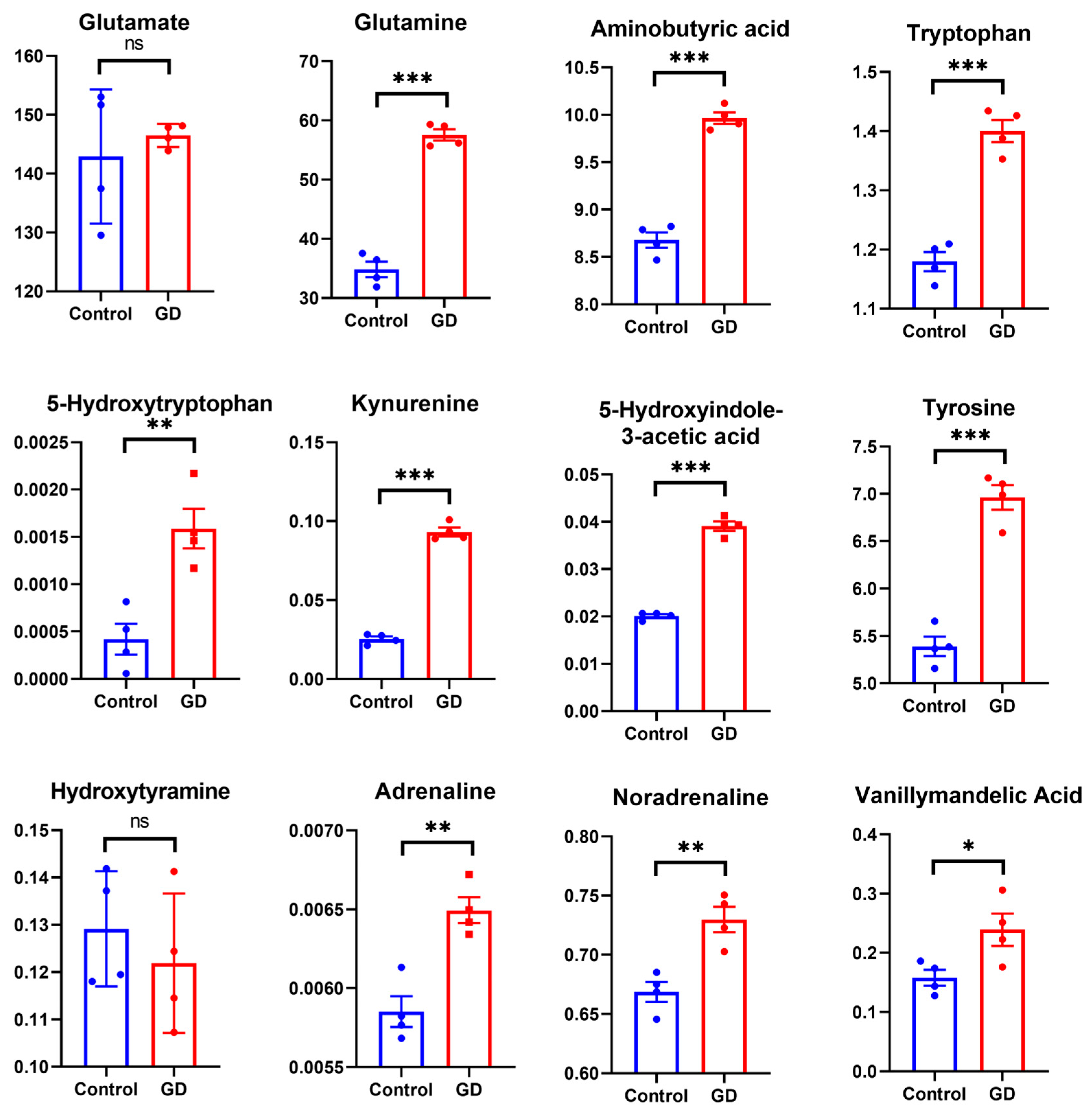
3.8. Effect of Soman on Metabolite Levels in Specific Amino Acid Metabolic Pathways
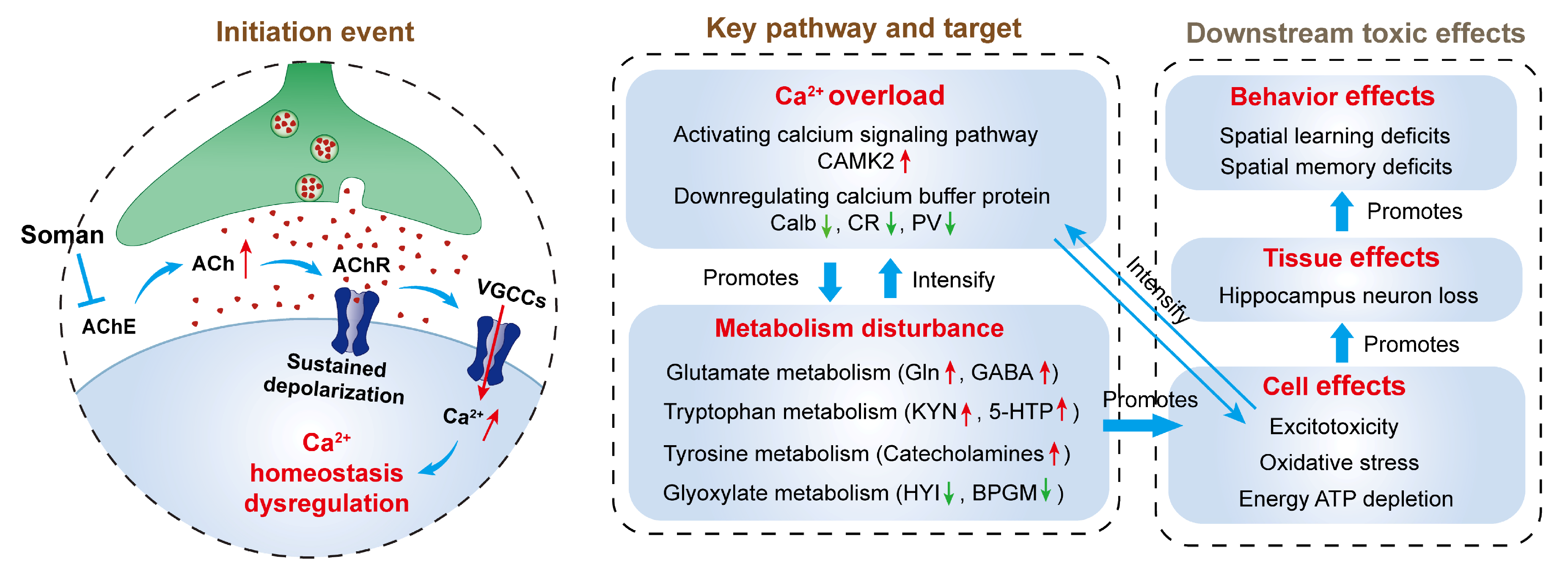
4. Discussion
4.1. Calcium Dysregulation
4.2. Amino Acid Catabolic Disruption
4.3. Glyoxylate and Dicarboxylate Metabolism Disruption
4.4. Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Toxicology of organophosphorus compounds in view of an increasing terrorist threat. Arch. Toxicol. 2016, 90, 2131–2145. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Jennifer, L.C.; Courtney, D.; Romolo, J.G. Central respiratory failure during acute organophosphate poisoning. Respir. Physiol. Neurobiol. 2013, 189, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Nixdorf-Bergweiler, B.E.; Edelmann, E.; van Brederode, J.F.M.; Alzheimer, C. Muscarinic Modulation of Morphologically Identified Glycinergic Neurons in the Mouse PreBötzinger Complex. Front. Cell. Neurosci. 2020, 13, 562. [Google Scholar] [CrossRef]
- Shao, X.M.; Feldman, J.L. Acetylcholine modulates respiratory pattern: Effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J. Neurophysiol. 2000, 83, 1243–1252. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Apland, J.P.; Figueiredo, T.H.; De Araujo Furtado, M.; Braga, M.F. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanisms of action, and medical countermeasures. Neuropharmacology 2020, 181, 108298. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.H., Jr.; Shih, T.M. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci. Biobehav. Rev. 1997, 21, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef]
- McPherson, C.A.; Harry, G.J. Assessing Neurotoxicant-Induced Inflammation in the Central Nervous System: Cytokine mRNA with Immunostaining of MicrogliaMicroglia Morphology. In Experimental Neurotoxicology Methods; Llorens, J., Barenys, M., Eds.; Springer: New York, NY, USA, 2021; pp. 277–304. [Google Scholar]
- Choi, D. Calcium-mediated neurotoxicity: Relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988, 11, 465–469. [Google Scholar] [CrossRef]
- Marambaud, P.; Dreses-Werringloer, U.; Vingtdeux, V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009, 4, 20–35. [Google Scholar] [CrossRef]
- Shimomura, Y.; Kitaura, Y. Physiological and pathological roles of branched-chain amino acids in the regulation of protein and energy metabolism and neurological functions. Pharmacol. Res. 2018, 133, 215–217. [Google Scholar] [CrossRef]
- Luo, C.; Tong, M.; Maxwell, D.; Saxena, A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chem.-Biol. Interact. 2008, 175, 261–266. [Google Scholar] [CrossRef]
- Garcia, S.J.; Aschner, M.; Syversen, T. Interspecies Variation in Toxicity of Cholinesterase Inhibitors. In Toxicology of Organophosphate & Carbamate Compounds; Gupta, R.C., Ed.; Academic Press: Burlington, NJ, USA, 2006; pp. 145–158. [Google Scholar]
- Fonnum, F.; Sterri, S.H. Factors modifying the toxicity of organophosphorous compounds including soman and sarin. Fundam. Appl. Toxicol. 1981, 1, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Keeble, E. Rodents: Biology and husbandry. In BSAVA Manual of Rodents and Ferrets; Keeble, E., Meredith, A., Eds.; BSAVA: Cheltenham, UK, 2009; pp. 1–17. [Google Scholar]
- Gordon, J.J.; Leadbeater, L. The prophylactic use of 1-methyl, 2-hydroxyiminomethylpyridinium methanesulfonate (P2S) in the treatment of organophosphate poisoning. Toxicol. Appl. Pharmacol. 1977, 40, 109–114. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, R.; Jin, Q.; Cui, Y.; Shi, J.; Chen, X.; Shi, T.; Zhang, Y.; Zhu, S.; Zong, X.; et al. Subacute sarin exposure disrupted the homeostasis of purine and pyrimidine metabolism in guinea pig striatum studied by integrated metabolomic, lipidomic and proteomic analysis. Toxicol. Lett. 2022, 367, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, W.; Yuan, P.; Zhu, P.; Fan, K.; Xia, Z.; Xu, S. The mechanism of CaMK2α-MCU-mitochondrial oxidative stress in bupivacaine-induced neurotoxicity. Free. Radic. Biol. Med. 2020, 152, 363–374. [Google Scholar]
- Otmakhov, N.; Gorbacheva, E.V.; Regmi, S.; Yasuda, R.; Hudmon, A.; Lisman, J. Excitotoxic Insult Results in a Long-Lasting Activation of CaMKIIα and Mitochondrial Damage in Living Hippocampal Neurons. PLoS ONE 2015, 10, e0120881. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; de Araujo Furtado, M.; Pidoplichko, V.I.; Braga, M.F.M. Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics 2023, 11, 866. [Google Scholar] [CrossRef]
- Deshpande, L.S.; Blair, R.E.; Phillips, K.F.; DeLorenzo, R.J. Role of the calcium plateau in neuronal injury and behavioral morbidities following organophosphate intoxication. Ann. N. Y. Acad. Sci. 2016, 1374, 176–183. [Google Scholar] [CrossRef]
- Song, M.; Kang, K.; Wang, S.; Zhang, C.; Zhao, X.; Song, F. Elevated intracellular Ca2+ functions downstream of mitodysfunction to induce Wallerian-like degeneration and necroptosis in organophosphorus-induced delayed neuropathy. Toxicology 2024, 504, 153812. [Google Scholar] [CrossRef]
- Griffioen, G. Calcium Dyshomeostasis Drives Pathophysiology and Neuronal Demise in Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 13243. [Google Scholar] [CrossRef]
- Alhowail, A.; Aldubayan, M. Mechanisms underlying cognitive impairment induced by prenatal caffeine exposure. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11909–11913. [Google Scholar]
- Yudkoff, M. Disorders of Amino Acid Metabolism. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 737–754. [Google Scholar]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A.; Waagepetersen, H.S. The glutamate/GABA-glutamine cycle: Aspects of transport, neurotransmitter homeostasis and ammonia transfer. J. Neurochem. 2006, 98, 641–653. [Google Scholar]
- Wang, W.; Wu, X.; Yang, C.S.; Zhang, J. An Unrecognized Fundamental Relationship between Neurotransmitters: Glutamate Protects against Catecholamine Oxidation. Antioxidants 2021, 10, 1564. [Google Scholar] [CrossRef]
- Jenner, P. Neurobiology of Amino Acids, Peptides and Trophic Factors. J. Neurol. Neurosurg. Psychiatry 1989, 52, 931–932. [Google Scholar] [CrossRef][Green Version]
- Almeida, A.; Moncada, S.; Bolaños, J.P. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat. Cell Biol. 2004, 6, 45–51. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mehrpour, O.; Forouzanfar, F.; Roshanravan, B.; Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: A review. Environ. Sci. Pollut. Res. 2020, 27, 24799–24814. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Neurotoxicity of organophosphate nerve agents. In Advances in Neurotoxicology; Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 79–112. [Google Scholar]
- Zimmer, L.A.; Ennis, M.; Shipley, M.T. Soman-induced seizures rapidly activate astrocytes and microglia in discrete brain regions. J. Comp. Neurol. 1997, 378, 482–492. [Google Scholar] [CrossRef]
- Willems, J.L.; Nicaise, M.; De Bisschop, H.C. Delayed neuropathy by the organophosphorus nerve agents soman and tabun. Arch. Toxicol. 1984, 55, 76–77. [Google Scholar] [CrossRef]
| Protein Name | Accession | Abbreviations | Pathway | Fold Change | p Value |
|---|---|---|---|---|---|
| Calcium/calmodulin-dependent protein kinase | A0A286XFT7 | CAMK2 | Calcium signal pathway | 1.963 | 0.000001 |
| Tyrosine 3-hydroxylase | H0VLS4 | TH | Tyrosine metabolism | 0.357 | 0.001205 |
| Sphingomyelin phosphodiesterase 3 | H0VT86 | SMPD3 | Glycerophospholipid metabolis | 56.657 | 0.001873 |
| Phosphoglycerate mutase | H0UTW8 | BPGM | Glycolysis/Gluconeogenesis | 0.021 | 0.000579 |
| Putative hydroxypyruvate isomerase | H0V732 | HYI | Glyoxylate and dicarboxylate metabolism | 0.4 | 0.000159 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zong, X.-X.; Jin, Q.; Shi, T.; Zhang, R.; Shi, J.; Wang, C.; Li, L. Proteomics Reveals Mechanisms of Metabolic Dysregulation in Soman Neurotoxicity. Toxics 2025, 13, 766. https://doi.org/10.3390/toxics13090766
Zong X-X, Jin Q, Shi T, Zhang R, Shi J, Wang C, Li L. Proteomics Reveals Mechanisms of Metabolic Dysregulation in Soman Neurotoxicity. Toxics. 2025; 13(9):766. https://doi.org/10.3390/toxics13090766
Chicago/Turabian StyleZong, Xing-Xing, Qian Jin, Tong Shi, Ruihua Zhang, Jingjing Shi, Chen Wang, and Liqin Li. 2025. "Proteomics Reveals Mechanisms of Metabolic Dysregulation in Soman Neurotoxicity" Toxics 13, no. 9: 766. https://doi.org/10.3390/toxics13090766
APA StyleZong, X.-X., Jin, Q., Shi, T., Zhang, R., Shi, J., Wang, C., & Li, L. (2025). Proteomics Reveals Mechanisms of Metabolic Dysregulation in Soman Neurotoxicity. Toxics, 13(9), 766. https://doi.org/10.3390/toxics13090766







