Effectiveness and Remediation Mechanisms of Geo-Electrochemical Technology for Arsenic Removal in Paddy Soil from Northern Guangxi
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Source
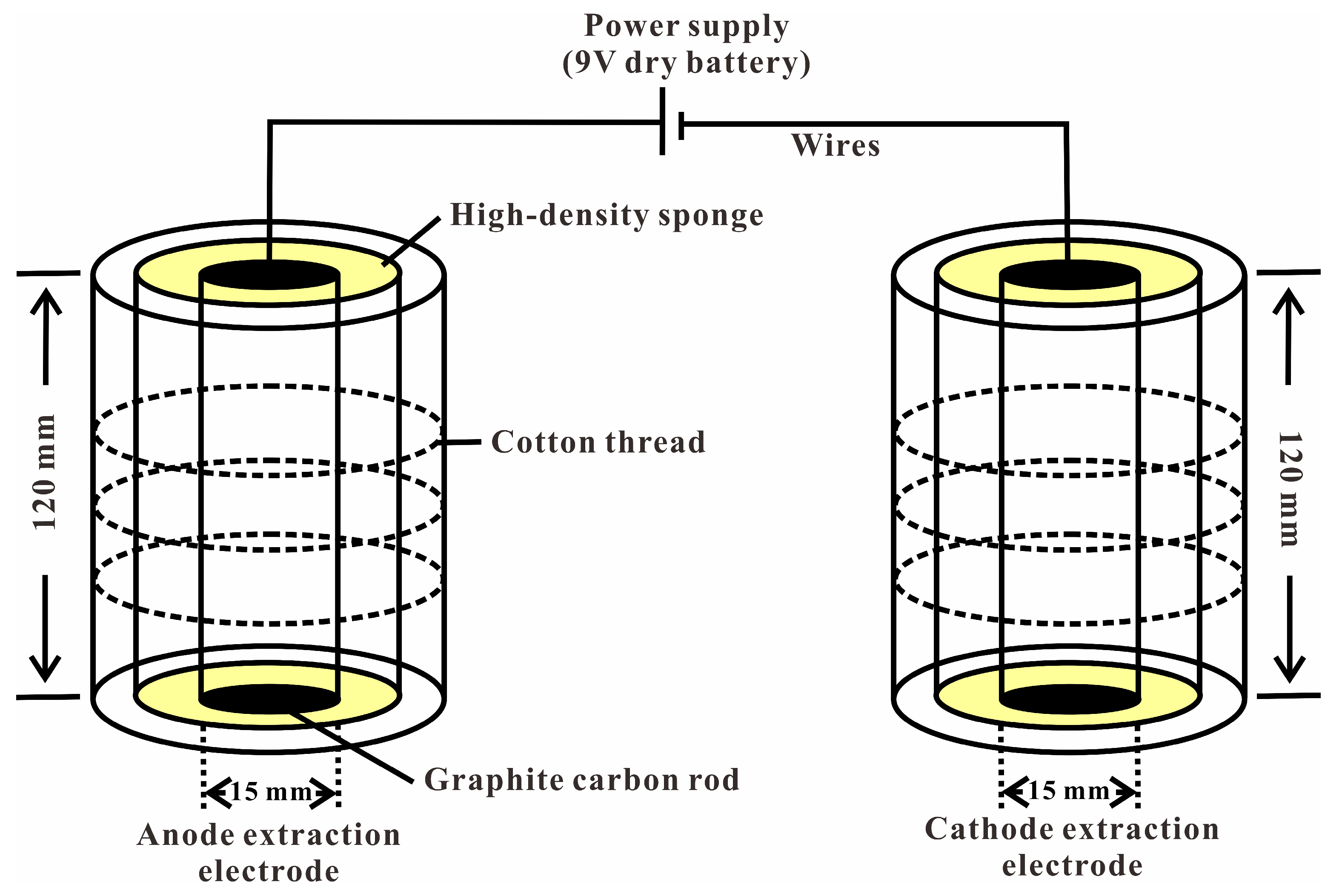
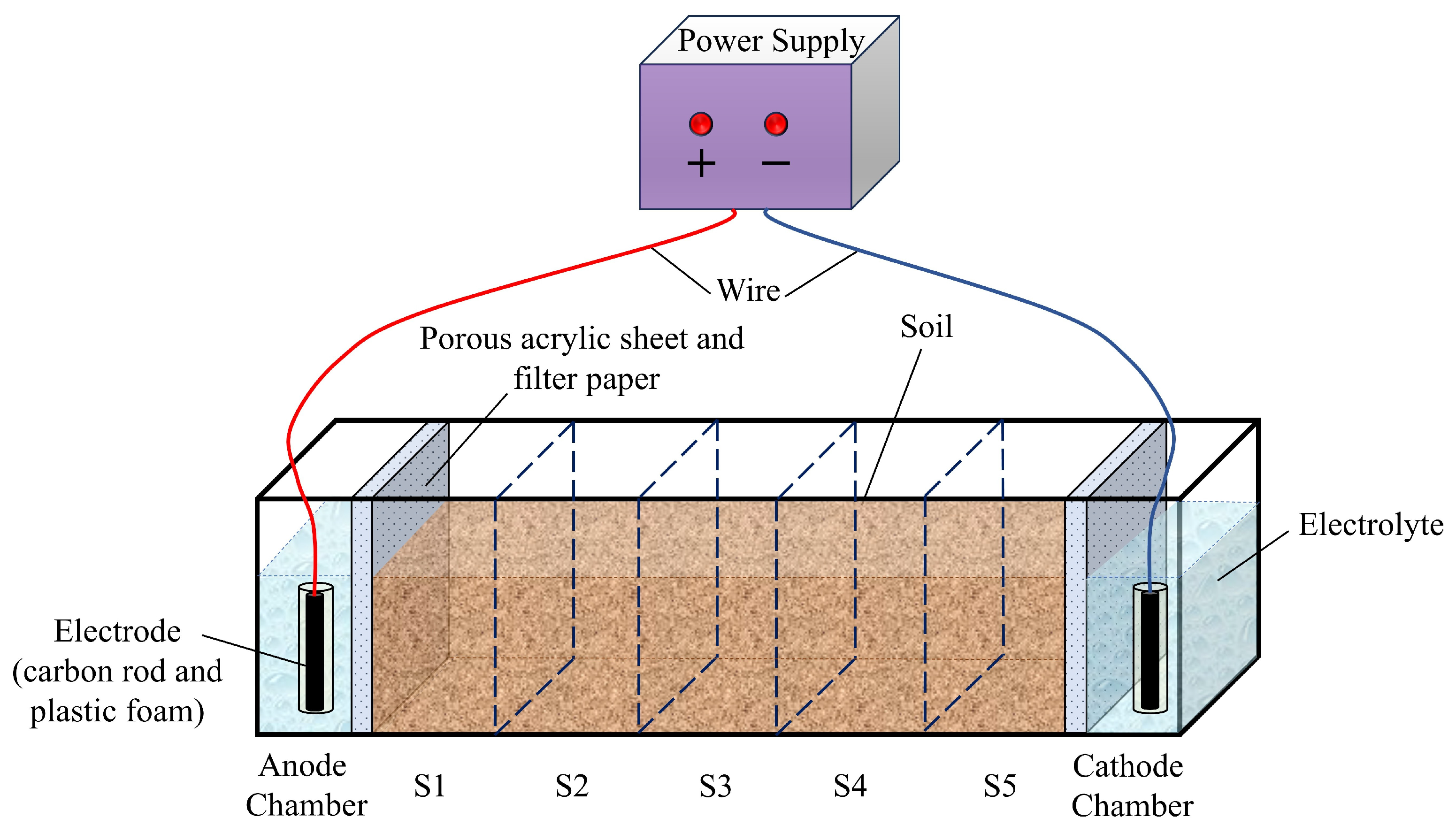
2.2. Experimental Setup
2.3. Multi-Factor Combinatorial Optimization
2.4. Analytical Methods
2.5. Data Processing
3. Results and Discussion
3.1. Physical, Chemical, and Contaminant Characteristics of Soil
3.2. Changes in pH
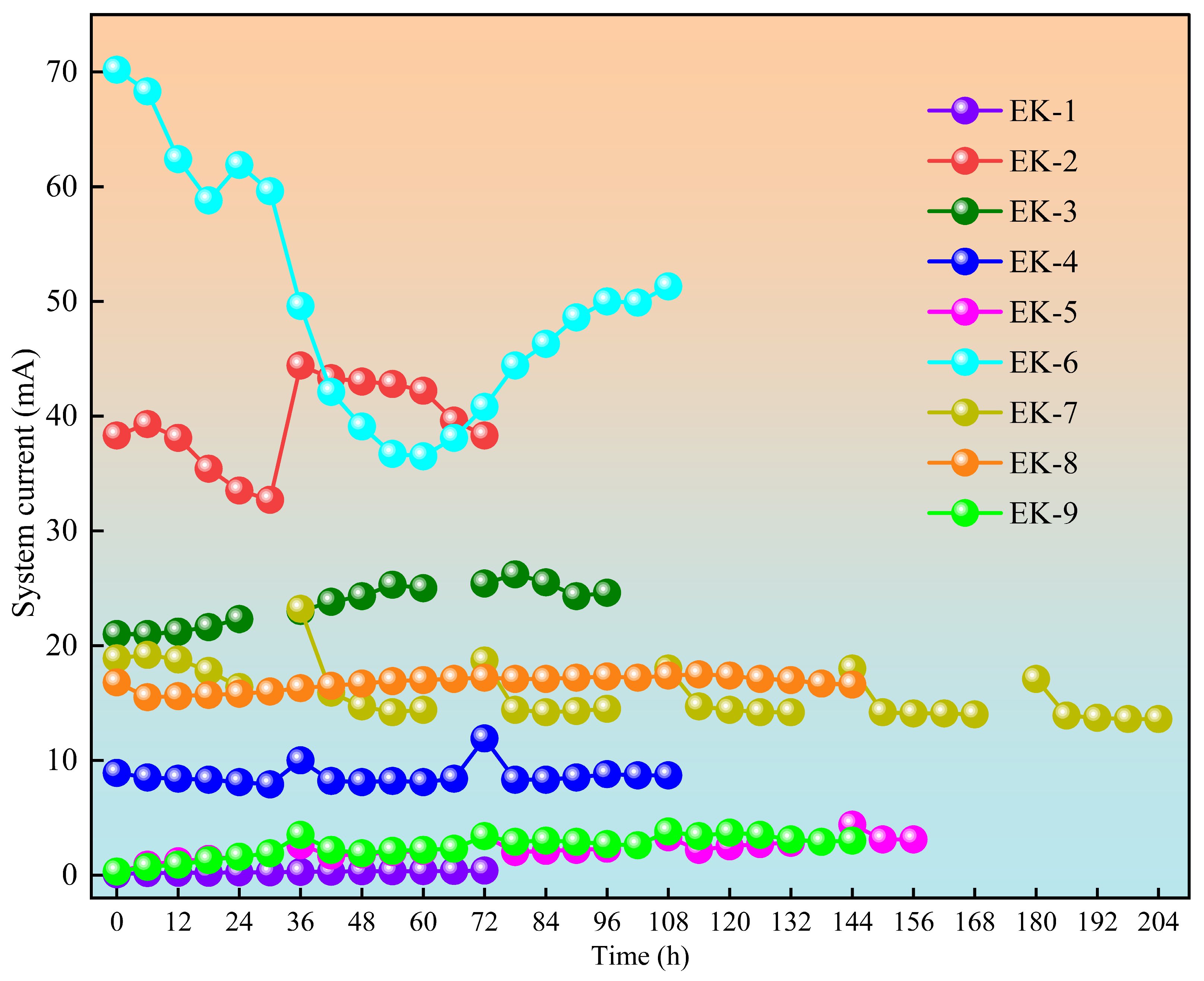
3.3. Current Variation
3.4. Analysis of Arsenic Speciation in Post-Remediation Soil
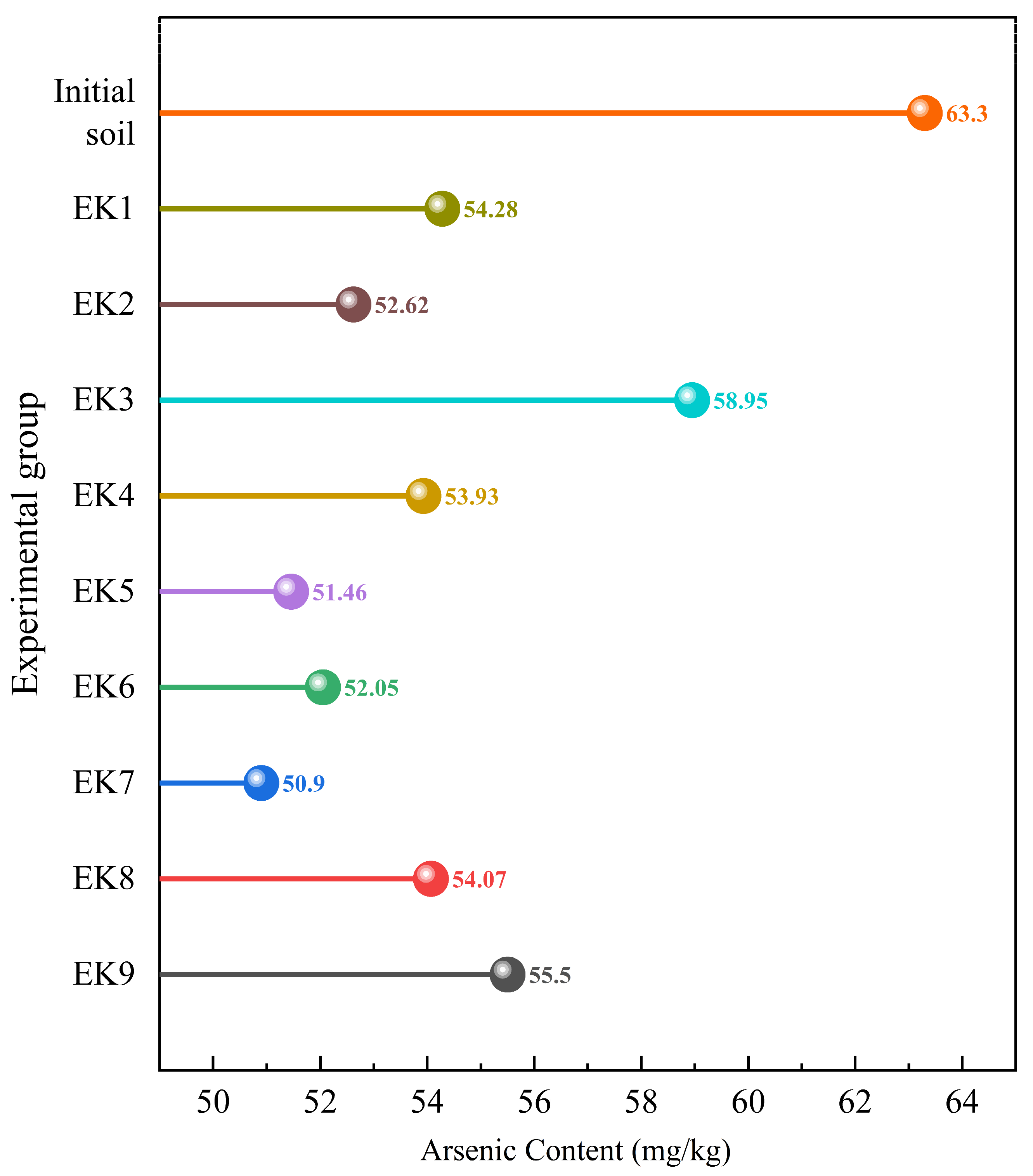
3.5. Analysis of Arsenic Removal Results
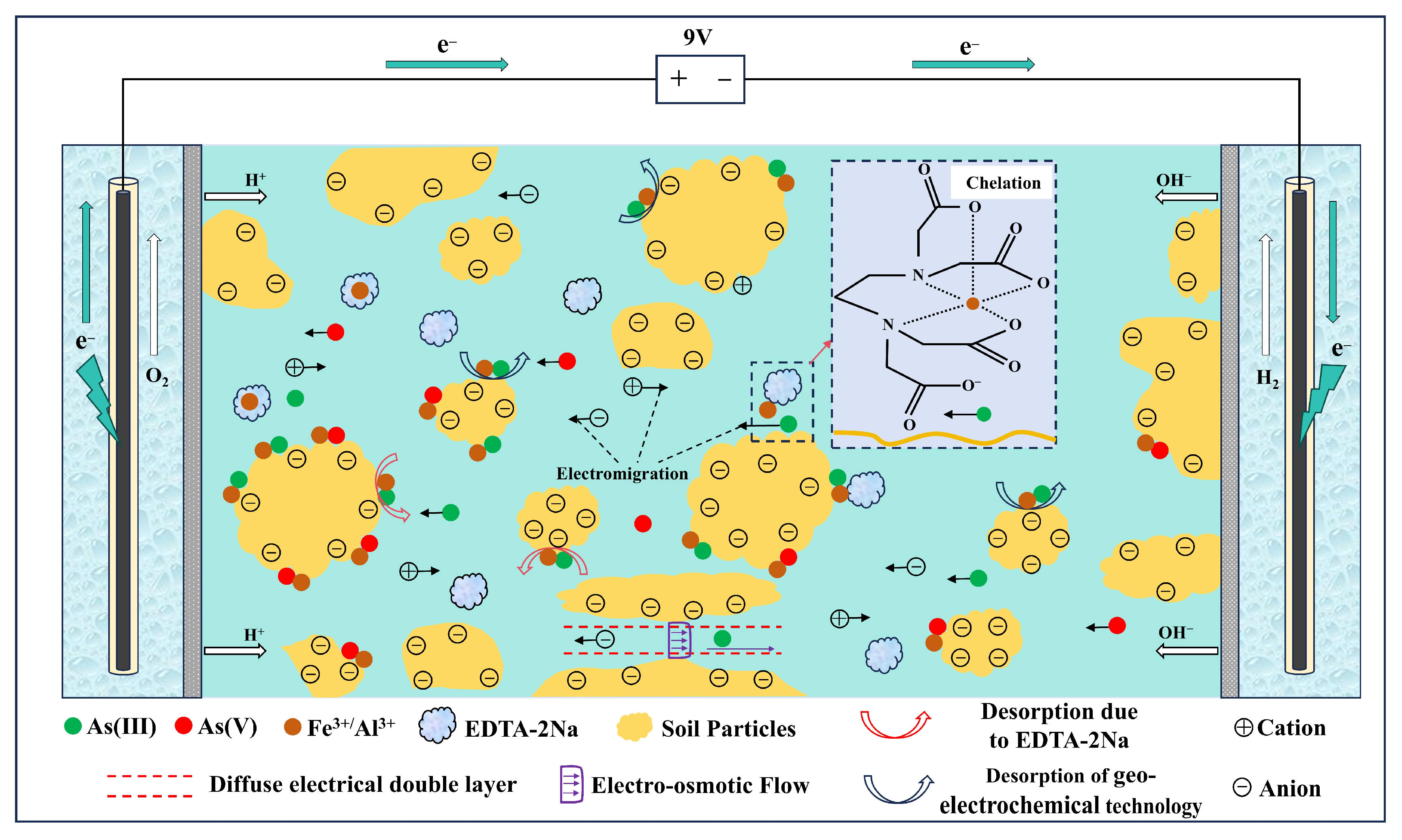
3.6. Mechanistic Analysis of Geo-Electrochemical Technology Coupled with EDTA-2Na for Remediation of Arsenic-Contaminated Soil
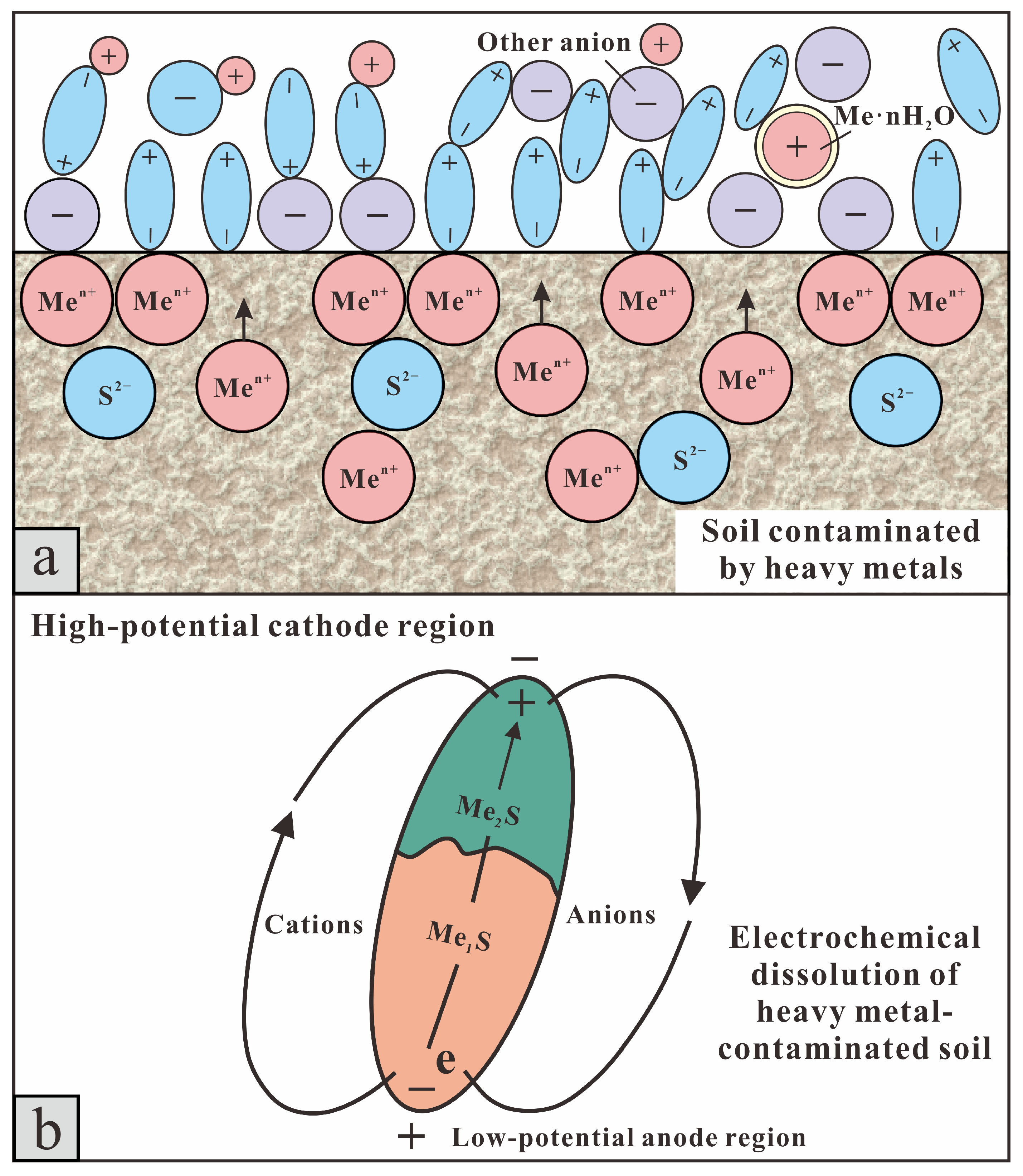
- (i)
- (ii)
- EDTA-2Na-assisted detachment and chelation—the displacement of arsenic species from soil surfaces through ligand exchange, followed by the formation of mobile As–EDTA complexes [48].
4. Conclusions
- (1)
- Following geo-electrochemical remediation, the residual fraction of arsenic in the soil is transformed into more mobile forms, resulting in an increased proportion of mobile arsenic species.
- (2)
- Group EK7 achieved the highest arsenic removal rate of approximately 19.59%. The results of the orthogonal experiment revealed that the type of electrolyte had the most significant impact on the arsenic removal efficiency, followed by the power supply duration and the voltage gradient, while the power supply mode had a minimal effect. The optimal conditions for removal of arsenic from soil were identified as a power supply duration of 108 h, a voltage gradient of 0.6 V/cm, continuous power supply mode, and EDTA-2Na as the electrolyte.
- (3)
- The remediation effect of geo-electrochemical treatment combined with EDTA-2Na in arsenic-contaminated soil is due to a multiphase electrochemical reaction mechanism, integrating the “effective collision-type” reactions driven by micro-electric field effects derived from the electrokinetic process (involving galvanic and electrolytic cells) with the “activation transition-type” chelation reactions facilitated by EDTA-2Na.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Telo da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; He, X. Remediation of Cadmium Contaminated Soil by Modified Gangue Material: Characterization, Performance and Mechanisms. Chemosphere 2022, 290, 133347. [Google Scholar] [CrossRef]
- Peng, J.; Song, Y.; Yuan, P.; Cui, X.; Qiu, G. The Remediation of Heavy Metals Contaminated Sediment. J. Hazard. Mater. 2009, 161, 633–640. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Luo, Y.; Guo, C.; Ma, X.; Fan, J.; Chen, J.; Geng, N. Occurrence, Accumulation, and Health Risks of Heavy Metals in Chinese Market Baskets. Sci. Total Environ. 2022, 829, 154597. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Rozbu, M.R.; Chowdhury, A.T.; Nuzhat, S.; Rafa, N.; Mahlia, T.M.I.; Ong, H.C.; Mofijur, M. Heavy Metal Toxicity, Sources, and Remediation Techniques for Contaminated Water and Soil. Environ. Technol. Innov. 2022, 25, 102114. [Google Scholar] [CrossRef]
- Dan, S.F.; Udoh, E.C.; Wang, Q. Contamination and Ecological Risk Assessment of Heavy Metals, and Relationship with Organic Matter Sources in Surface Sediments of the Cross River Estuary and Nearshore Areas. J. Hazard. Mater. 2022, 438, 129531. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rahman, I.M.M.; Datta, R.R.; Kosugi, C.; Mashio, A.S.; Maki, T.; Hasegawa, H. Arsenic Speciation and Biotransformation by the Marine Macroalga Undaria Pinnatifida in Seawater: A Culture Medium Study. Chemosphere 2019, 222, 705–713. [Google Scholar] [CrossRef]
- Wu, C.; Zou, Q.; Xue, S.-G.; Pan, W.-S.; Yue, X.; Hartley, W.; Huang, L.; Mo, J.-Y. Effect of Silicate on Arsenic Fractionation in Soils and Its Accumulation in Rice Plants. Chemosphere 2016, 165, 478–486. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic Removal Technologies and Future Trends: A Mini Review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Chirakkara, R.A.; Reddy, K.R. Electrokinetic-Enhanced Phytoremediation of Soils: Status and Opportunities. Chemosphere 2013, 93, 626–636. [Google Scholar] [CrossRef]
- Luo, J.; Cai, L.; Qi, S.; Wu, J.; Sophie Gu, X. A Multi-Technique Phytoremediation Approach to Purify Metals Contaminated Soil from e-Waste Recycling Site. J. Environ. Manag. 2017, 204, 17–22. [Google Scholar] [CrossRef]
- Fu, R.; Wen, D.; Xia, X.; Zhang, W.; Gu, Y. Electrokinetic Remediation of Chromium (Cr)-Contaminated Soil with Citric Acid (CA) and Polyaspartic Acid (PASP) as Electrolytes. Chem. Eng. J. 2017, 316, 601–608. [Google Scholar] [CrossRef]
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F.A. Electrical Field: A Historical Review of Its Application and Contributions in Wastewater Sludge Dewatering. Water Res. 2010, 44, 2381–2407. [Google Scholar] [CrossRef]
- Kang, M.; Guo, H.; Zhu, W.; Luo, X.; Yang, J. The Improvement and Application of the Geoelectrochemical Exploration Method. Appl. Sci. 2023, 13, 2735. [Google Scholar] [CrossRef]
- Liu, P.; Luo, X.; Wen, M.; Anand, R.; Thorne, R.; Zheng, C.; Zhang, J. Application of the Geoelectrochemical Extraction Method to the Xiyi Pb-Zn Deposit in Southwestern China. J. Geochem. Explor. 2019, 203, 1–26. [Google Scholar] [CrossRef]
- Liu, P.; Luo, X.; Wen, M.; Zhang, J.; Zheng, C.; Gao, W.; Ouyang, F. Geoelectrochemical Anomaly Prospecting for Uranium Deposits in Southeastern China. Appl. Geochem. 2018, 97, 226–237. [Google Scholar] [CrossRef]
- Liu, P.; Luo, X.; Wen, M.; Zhang, J.; Gao, W.; Ouyang, F.; Duan, X. Using Electrogeochemical Approach to Explore Buried Gold Deposits in an Alpine Meadow-Covered Area. Acta Geochim. 2018, 37, 402–413. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, M.; Liu, P.; Jiang, Y. Removal of Heavy Metal Cd Element from Paddy Soil by Geo-Electrochemical Technology. Appl. Sci. 2023, 13, 11685. [Google Scholar] [CrossRef]
- Luo, X.; Ouyang, F.; Hu, Y.; Wen, M.; Wang, B. Low Voltage Bipolar Geo-Electric Extraction Device; Patent CN201096644. 2008. Available online: https://d.wanfangdata.com.cn/patent/ChdQYXRlbnROZXdTb2xyOVMyMDI1MDgxORIpWkxfQ04yMDA3MjAxMjU3MDguNV9DTjIwMTA5NjY0NFlfMjAwODA4MDYaCHdxNDJiN25z (accessed on 23 August 2025).
- Ji, L.; Si, Y.; Liu, H.; Song, X.; Zhu, W.; Zhu, A. Application of Orthogonal Experimental Design in Synthesis of Mesoporous Bioactive Glass. Microporous Mesoporous Mater. 2014, 184, 122–126. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Accta Pe Dologica Sinica: Beijing, China, 2000.
- GB/T 25282-2010; Soil and Sediment-Sequential Extraction Procedure of Speciation of 13 Trace Elements. National Library of Standards: Beijing, China, 2010. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=3CD527C889212919CFEC80932D514455&refer=outter (accessed on 23 August 2025).
- Wei, F.; Chen, J.; Wu, Y. Chinese Soil Element Background Values; China Environment Science Press: Beijing, China, 1990. [Google Scholar]
- Ma, Q.; Wei, Z.; Wu, Q. Effectiveness and Mechanisms of Chemical Leaching Combined with Electrokinetic Technology on the Remediation of Heavy Metal Contaminated Soil. Environ. Sci. 2023, 44, 1668–1677. [Google Scholar]
- Yao, W.; Cai, Z.; Sun, S.; Romantschuk, M.; Sinkkonen, A.; Sun, Y.; Wang, Q. Electrokinetic-Enhanced Remediation of Actual Arsenic-Contaminated Soils with Approaching Cathode and Fe0 Permeable Reactive Barrier. J. Soils Sediments 2020, 20, 1526–1533. [Google Scholar] [CrossRef]
- Borodin, O. Challenges with Prediction of Battery Electrolyte Electrochemical Stability Window and Guiding the Electrode—Electrolyte Stabilization. Curr. Opin. Electrochem. 2019, 13, 86–93. [Google Scholar] [CrossRef]
- Baek, K.; Kim, D.-H.; Park, S.-W.; Ryu, B.-G.; Bajargal, T.; Yang, J.-S. Electrolyte Conditioning-Enhanced Electrokinetic Remediation of Arsenic-Contaminated Mine Tailing. J. Hazard. Mater. 2009, 161, 457–462. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, W.; Hu, W.; Kang, N. Experiment Study and Mechanism Analysis of Chelating Agent Enhanced on Electrokinetic Removal of Copper and Lead from Loess. Chin. J. Geotech. Eng. 2024, 46, 2183–2191. Available online: https://www.cgejournal.com/cn/article/doi/10.11779/CJGE20220737 (accessed on 23 August 2025).
- Xu, H.; Cang, L.; Song, Y.; Yang, J. Influence of Electrode Configuration on Electrokinetic-Enhanced Persulfate Oxidation Remediation of PAH-Contaminated Soil. Environ. Sci. Pollut. Res. 2020, 27, 44355–44367. [Google Scholar] [CrossRef]
- Hu, W.; Cheng, W.-C.; Wen, S. Investigating the Effect of Degree of Compaction, Initial Water Content, and Electric Field Intensity on Electrokinetic Remediation of an Artificially Cu- and Pb-Contaminated Loess. Acta Geotech. 2023, 18, 937–949. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Liu, Y. Effect of Electrode-Orientated Electrokinetic Enhancement on Phytoremediation on Arsenic Contaminated Soil. Environ. Eng. 2020, 38, 228–233. Available online: http://hjgc.ic-mag.com/cn/article/doi/10.13205/j.hjgc.202010036 (accessed on 23 August 2025).
- Shinta, Y.; Zaman, B.; Sumiyati, S. Citric Acid and EDTA as Chelating Agents in Phytoremediation of Heavy Metal in Polluted Soil: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 896, 012023. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Lin, J.; Cui, X.; Wang, G. Study on Electrokinetic Remediation Efficiencies of Different Fraction of Heavy Metals in Soils. Chin. J. Environ. Eng. 2010, 4, 2585–2589. Available online: https://www.sciengine.com/CJEE/doi/10.0000/j.cjee.20101137 (accessed on 23 August 2025).
- Zeng, H.; Cheng, F.; Zhang, X.; Wu, B.; Guo, S. Effect of the Approaching Electrode on the Soil Arsenic Migration and Speciation Transformation during Electrokinetic Remediation. Chin. J. Environ. Eng. 2023, 17, 1294–1302. Available online: https://kns.cnki.net/kcms2/article/abstract?v=bugI047JRxit3KuuVWvOeARarlEW2mKwmgFBO-jW3VIGrXW4pkklyK_0fUL_ugIJZo2EYzD4n7Ebn0BJzledvtz8arqXYnxx-S1dX19gFr41RvajeLr4S2uq78WyvSUS6bWTpQgNfvNbEXxMOAOysS94T-78hOG-NqUDfGTIAzHhoFDKLWXXyQ==&uniplatform=NZKPT&langu (accessed on 23 August 2025).
- Cancès, B.; Juillot, F.; Morin, G.; Laperche, V.; Alvarez, L.; Proux, O.; Hazemann, J.-L.; Brown, G.E.; Calas, G. XAS Evidence of As(V) Association with Iron Oxyhydroxides in a Contaminated Soil at a Former Arsenical Pesticide Processing Plant. Environ. Sci. Technol. 2005, 39, 9398–9405. [Google Scholar] [CrossRef]
- Krause, E.; Ettel, V.A. Solubilities and Stabilities of Ferric Arsenates. In Crystallization and Precipitation; Strathdee, G., Klein, M., Melis, L., Eds.; Pergamon: Elmsford, NY, USA, 1987; pp. 195–210. ISBN 978-0-08-035751-5. Available online: https://www.sciencedirect.com/science/article/pii/B9780080357515500325 (accessed on 23 August 2025).
- Huang, J.; Zhang, H. Redox Reactions of Iron and Manganese Oxides in Complex Systems. Front. Environ. Sci. Eng. 2020, 14, 76. [Google Scholar] [CrossRef]
- Deng, Z.; Ren, D.; Kang, C.; Huang, C.; Guo, H. Simulation on Phenanthrene Migration in Electrokinetic Remediation of Soil. Chin. J. Environ. Eng. 2020, 14, 789–806. Available online: https://kns.cnki.net/kcms2/article/abstract?v=bugI047JRxgNcS2ubMGQEY9OoUKiFQQqZqfVgUOj8j8R1mflUtm6KnpXVu2jI1qQpGmfh9sEPhgoi0_5X69mgMZQ2UyPWsGPIvx1ZSMLWBq83o2L468_e8oZ5c3oiVGgelWT0kr6CRC2ShUdmzyN5UEJRGs8kwqJmHUGeyjGXq6sVa3IiagQKA==&uniplatform=NZKPT&language=CHS (accessed on 23 August 2025).
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic Remediation for the Removal of Heavy Metals in Soil: Limitations, Solutions and Prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef]
- Wang, R.; Chu, Z.; Song, N.; Wang, K.; Liu, J.; Wang, F. Removal of Heavy Metals from Residual Sludge by Electrokinetic Remediation Technology. J. Agric. Resour. Environ. 2019, 36, 798–805. Available online: https://kns.cnki.net/kcms2/article/abstract?v=bugI047JRxhLpodOWWdGY3BKrYBIDoLov4oSpBMAVKNfBDPT2OezRiahr-bhchUlx_W9VT2VmX7TvzOqBN05Hg2BGVvcbzPGvAtDNXYMj85wETJAT1pYb (accessed on 23 August 2025).
- Volkov, A.G.; Deamer, D.W.; Tanelian, D.L.; Markin, V.S. Electrical Double Layers at the Oil/Water Interface. Prog. Surf. Sci. 1996, 53, 1–134. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation Techniques for Heavy Metal-Contaminated Soils: Principles and Applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Xie, Y.; Zhu, F.; Wang, J.; Shi, H. Analysis of Resistivity Variation under Direct Current Field Force. Chin. J. Soil Sci. 2014, 45, 1364–1369. Available online: https://kns.cnki.net/kcms2/article/abstract?v=bugI047JRxhF2gxmK2erHWAkR-Gh4ilgTP69j1u3TojO6cdE9U42xdIq60VtKNu6zn0zd2jckiiTNZyDhsCFArSwUxWS3ajNgDZpq_9RTpPtcgpkGq_r94RdZj8QTmm7wqBQG-419KLJvnp6uRsXJp1fPR_n3T67DDyKErSg7tMGgakwa-aT3Q==&uniplatform=NZKPT&language=CHS (accessed on 23 August 2025).
- Bai, H.; Lin, X.H.; Zhang, C.; Ee, L.Y.; Low, K.M.; Phua, T.W.; He, L.; Li, S.F.Y. Accelerated De-Chelation of EDTA-Metal Complexes: A Novel and Versatile Approach for Wastewater and Solid Waste Remediation. Chem. Eng. J. Adv. 2024, 19, 100633. [Google Scholar] [CrossRef]
- Xu, L.; Dai, H.; Wei, S. Advances of Washing Agents in Remediation of Heavy Metal Contaminated Soil. China Environ. Sci. 2021, 41, 5237–5244. Available online: https://kns.cnki.net/kcms2/article/abstract?v=bugI047JRxjXcQcdCBg4jO8yLvyUyl65fTgV2ysFBNEUF5EZMGhPeFf5_KLdcSCGlu6XUh0H8jVhl4-xvw54smPdIza_ObzCbxyCg7Twc6Qncg_BWlWaifCvacoYz58fzULA3PCdcHSii5mxggJ6twDJ1kFXis9D6ncuBI0HTRGm5zGt4TIi5A==&uniplatform=NZKPT&language=CHS (accessed on 23 August 2025).
- Kovács, A.; Nemcsok, D.S.; Kocsis, T. Bonding Interactions in EDTA Complexes. J. Mol. Struct. THEOCHEM 2010, 950, 93–97. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, Y.; Peng, P. Effect of Chelating Agent on Pteris Vittata for Remediation of Arsenic-Contaminated Soil. Environ. Eng. 2021, 39, 204–209+119. Available online: https://kns.cnki.net/kcms2/article/abstract?v=bugI047JRxiNfgdx3Lp_MsCpiM8bF3WRtEH4a53VU9CRE3cxV8sAoL-6aedaToK80xOSdr4Ng0sLEerq12yTqlRIo5NudyazB0NSNsqrnrkYp6A8NoYp6II7-QSzugy3XI4xkSnxMrKoXtPa7Tt42a9sM1dkRbYqkjAMwfN9Jv4=&uniplatform=NZKPT&language=CHS (accessed on 23 August 2025).
- Palma, L.D.; Ferrantelli, P. Copper Leaching from a Sandy Soil: Mechanism and Parameters Affecting EDTA Extraction. J. Hazard. Mater. 2005, 122, 85–90. [Google Scholar] [CrossRef]
- Gluhar, S.; Kaurin, A.; Lestan, D. Soil Washing with Biodegradable Chelating Agents and EDTA: Technological Feasibility, Remediation Efficiency and Environmental Sustainability. Chemosphere 2020, 257, 127226. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Review on Remediation Technologies for Arsenic-Contaminated Soil. Front. Environ. Sci. Eng. 2019, 14, 24. [Google Scholar] [CrossRef]
- Zhang, P.; Li, P.; Ping, Y.; Xu, H.; Zhang, Z.; Zhao, F.; Zeng, G.; Huang, P.; Yang, Z. Anionic Surfactant-Activated Remediation of Pb, Cd, As Contaminated Soil by Electrochemical Technology. Sci. Total Environ. 2024, 952, 175889. [Google Scholar] [CrossRef]



| Factor | Power Supply Duration | Voltage Gradient | Power Supply Mode | Electrolyte Type |
|---|---|---|---|---|
| Level 1 | 72 h | 0.3 V/cm | Continuous | Double deionized water |
| Level 2 | 108 h | 0.6 V/cm | Electrode reversal (every 36 h) | 0.1 mol/L EDTA-2Na |
| Level 3 | 144 h | 0.9 V/cm | Intermittent (24 h on/12 h off) | 0.1 mol/L citric acid |
| Experimental Group | Power Supply Duration (h) | Voltage Gradient (V/cm) | Power Supply Mode | Electrolyte Type |
|---|---|---|---|---|
| EK1 | 72 | 0.3 | Continuous | Double deionized water |
| EK2 | 72 | 0.6 | Electrode reversal | EDTA-2Na |
| EK3 | 72 | 0.9 | Intermittent | Citric acid |
| EK4 | 108 | 0.3 | Electrode reversal | Citric acid |
| EK5 | 108 | 0.6 | Intermittent | Double deionized water |
| EK6 | 108 | 0.9 | Continuous | EDTA-2Na |
| EK7 | 144 | 0.3 | Intermittent | EDTA-2Na |
| EK8 | 144 | 0.6 | Continuous | Citric acid |
| EK9 | 144 | 0.9 | Electrode reversal | Double deionized water |
| Parameter | Result | Risk Screening Value | Risk Intervention Value | Background Value in Guangxi |
|---|---|---|---|---|
| pH | 6.11 | 4.9 | ||
| Electrical conductivity (μs/cm) | 10.60 | |||
| Water content (%) | 45 | |||
| The content of As (mg/kg) | 63.30 | 30 | 150 | 24.80 |
| Run | Power Supply Duration (h) | Voltage Gradient (V/cm) | Power Supply Mode | Electrolyte Type | Arsenic Removal Rate (%) |
|---|---|---|---|---|---|
| EK1 | 72 | 0.3 | Continuous | Double deionized water | 14.24 |
| EK2 | 72 | 0.6 | Electrode reversal | EDTA-2Na | 16.87 |
| EK3 | 72 | 0.9 | Intermittent | Citric acid | 6.87 |
| EK4 | 108 | 0.3 | Electrode reversal | Citric acid | 14.81 |
| EK5 | 108 | 0.6 | Intermittent | Double deionized water | 18.70 |
| EK6 | 108 | 0.9 | Continuous | EDTA-2Na | 17.77 |
| EK7 | 144 | 0.3 | Intermittent | EDTA-2Na | 19.59 |
| EK8 | 144 | 0.6 | Continuous | Citric acid | 14.57 |
| EK9 | 144 | 0.9 | Electrode reversal | Double deionized water | 12.32 |
| Kj1 | 37.97 | 48.64 | 46.58 | 45.26 | |
| Kj2 | 51.27 | 50.14 | 43.99 | 54.22 | |
| Kj3 | 46.48 | 36.95 | 45.16 | 36.24 | |
| kj1 | 12.66 | 16.21 | 15.53 | 15.09 | |
| kj2 | 17.09 | 16.71 | 14.66 | 18.07 | |
| kj3 | 15.49 | 12.32 | 15.05 | 12.08 | |
| R | 4.43 | 4.39 | 0.86 | 5.99 |
| Variance Source | Sum of Squares (SS) | Degrees of Freedom (df) | Mean Square (MS) | F-Value | Significance (p = 0.05) |
|---|---|---|---|---|---|
| Power supply duration | 30.19 | 2 | 15.09 | 26.55 | * |
| Voltage gradient | 34.66 | 2 | 17.33 | 30.48 | * |
| Power supply mode | 1.14 | 2 | 0.57 | 1.00 | ns |
| Electrolyte type | 53.82 | 2 | 26.91 | 47.33 | * |
| Total | 119.94 | 8 |
| Experimental Group | EK1 | EK2 | EK3 | EK4 | EK5 | EK6 | EK7 | EK8 | EK9 |
|---|---|---|---|---|---|---|---|---|---|
| Ec (kW·h/mg) | 0.02 | 2.39 | 3.52 | 0.9 | 0.16 | 4.34 | 1.41 | 2.35 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Wen, M.; Sun, Y.; Liu, P.; Ma, Y.; Zhang, C.; Zhang, X. Effectiveness and Remediation Mechanisms of Geo-Electrochemical Technology for Arsenic Removal in Paddy Soil from Northern Guangxi. Toxics 2025, 13, 728. https://doi.org/10.3390/toxics13090728
Jiang Y, Wen M, Sun Y, Liu P, Ma Y, Zhang C, Zhang X. Effectiveness and Remediation Mechanisms of Geo-Electrochemical Technology for Arsenic Removal in Paddy Soil from Northern Guangxi. Toxics. 2025; 13(9):728. https://doi.org/10.3390/toxics13090728
Chicago/Turabian StyleJiang, Yuxiong, Meilan Wen, Yao Sun, Panfeng Liu, Yunxue Ma, Caiyun Zhang, and Xiaohan Zhang. 2025. "Effectiveness and Remediation Mechanisms of Geo-Electrochemical Technology for Arsenic Removal in Paddy Soil from Northern Guangxi" Toxics 13, no. 9: 728. https://doi.org/10.3390/toxics13090728
APA StyleJiang, Y., Wen, M., Sun, Y., Liu, P., Ma, Y., Zhang, C., & Zhang, X. (2025). Effectiveness and Remediation Mechanisms of Geo-Electrochemical Technology for Arsenic Removal in Paddy Soil from Northern Guangxi. Toxics, 13(9), 728. https://doi.org/10.3390/toxics13090728






