Association of Prenatal Polycyclic Aromatic Hydrocarbons Exposure, DNA Hydroxymethylation, and Neurodevelopment at 0 and 2 Years of Age
Highlights
- Prenatal PAH exposure was significantly associated with impaired neurobehavioral development in 2-year-old children.
- The 5-hydroxymethylcytosine levels showed a positive correlation with neurobehavioral development scores.
- 5-Hydroxymethylcytosine levels in BDNF and MeCP2 genes mediated the association between prenatal PAH exposure and impaired neurobehavioral development.
- The 5-hydroxymethylcytosine may serve as a novel epigenetic biomarker for predicting PAH-induced neurodevelopmental deficits in offspring.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Personal Interview
2.3. Urine Collection and PAH Metabolites Analysis
2.4. Umbilical Cord Collection and BPDE Analysis
2.5. Global DNA 5-hmC in Cord Blood Analysis
2.6. BDNF and MeCP2 Gene Promoter Hydroxymethylated DNA Immunoprecipitation Analysis
2.7. Outcomes
2.8. Covariates
2.9. Statistical Analysis
3. Results
3.1. Characteristics of the Participants
3.2. PAH Metabolites Levels
3.3. PAH Metabolites and Neurobehavioral Developmental Scores
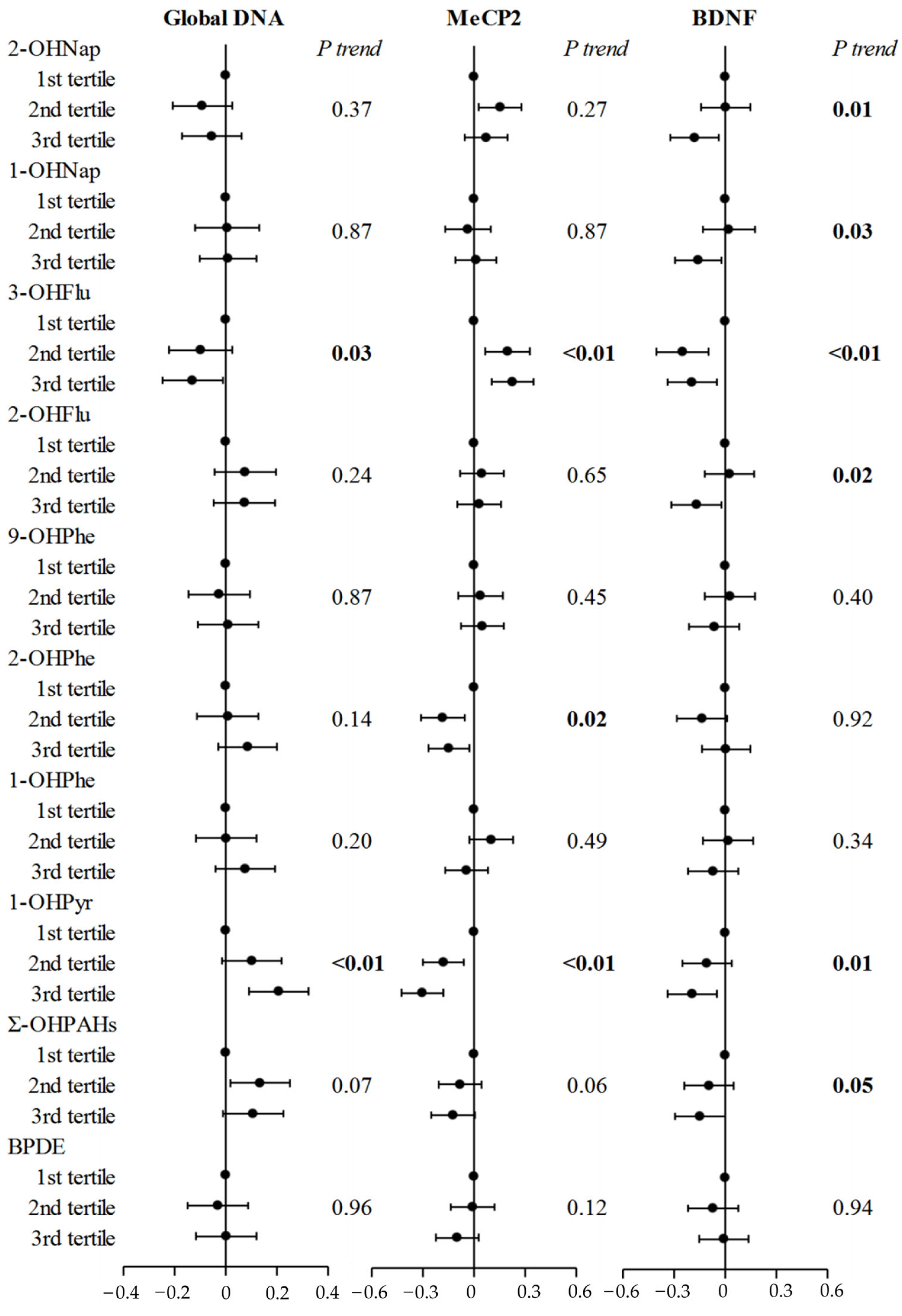
3.4. PAH Metabolites and Cord Blood 5-hmC Levels
3.5. The Associations Between 5-hmC and Neurodevelopmental Indexes
3.6. Mediation Analyses
3.7. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAHs | Polycyclic aromatic hydrocarbons |
| 5-hmC | 5-Chydroxymethylcytosine |
| ELISA | Enzyme linked immunosorbent assay |
| ChIP | Chromatin immunoprecipitation |
| HPLC-MS/MS | High-performance liquid chromatography with tandem mass spectrometry |
| NBNA | Neonatal Behavioral Neurological Assessment |
| GDSs | Gesell Developmental Scales |
| 1-OHPyr | 1-Hydroxypyrene |
| BDNF | Brain-derived neurotrophic factor |
| MeCP2 | Methyl CpG binding protein 2 |
| 5-mC | 5-Methyl-cytosine |
| TET | Ten-eleven translocation |
| BPDE | (+)Benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide |
| 3-OHChr | 3-Hydroxychrysene |
| 6-OHChr | 6-Hydroxychrysene |
| 9-OHBap | 9-Hydroxybenzo[a]pyrene |
| 2-OHPhe | 2-Hydroxyphenanthrene |
| BMI | Body mass index |
| Ʃ-OHPAH | Sum of 11 individual PAH metabolites |
| 2-OHNap | 2-Hydroxynaphthalene |
| 1-OHNap | 1-Hydroxynaphthalene |
| 3-OHFlu | 3-Hydroxyfluorene |
| 2-OHFlu | 2-Hydroxyfluorene |
| 9-OHPhe | 9-Hydroxyphenanthrene |
| 1-OHPhe | 1-Hydroxyphenanthrene |
| LOD | Limit of detection |
| DQ | Development quotient |
References
- Weinstein, J.R.; Asteria-Peñaloza, R.; Diaz-Artiga, A.; Davila, G.; Hammond, S.K.; Ryde, I.T.; Meyer, J.N.; Benowitz, N.; Thompson, L.M. Exposure to polycyclic aromatic hydrocarbons and volatile organic compounds among recently pregnant rural Guatemalan women cooking and heating with solid fuels. Int. J. Hyg. Environ. Health 2017, 220, 726–735. [Google Scholar] [CrossRef]
- White, A.J.; Bradshaw, P.T.; Herring, A.H.; Teitelbaum, S.L.; Beyea, J.; Stellman, S.D.; Steck, S.E.; Mordukhovich, I.; Eng, S.M.; Engel, L.S.; et al. Exposure to multiple sources of polycyclic aromatic hydrocarbons and breast cancer incidence. Environ. Int. 2016, 89–90, 185–192. [Google Scholar] [CrossRef]
- Coppieters, N.; Dieriks, B.V.; Lill, C.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol. Aging 2014, 35, 1334–1344. [Google Scholar] [CrossRef]
- Julie, B.; Herbstman, D.T.; Zhu, D.; Qu, L.; Sjödin, A.; Li, Z.; Camann, D.; Perera, F.P. Prenatal Exposure to Polycyclic Aromatic Hydrocarbons, Benzo[a]pyrene–DNA Adduct, and Genomic DNA Methylation in Cord Blood. Environ. Health Perspect. 2012, 120, 733–738. [Google Scholar]
- Santiago, M.; Antunes, C.; Guedes, M.; Sousa, N.; Marques, C.J. TET enzymes and DNA hydroxymethylation in neural development and function—How critical are they? Genomics 2014, 104, 334–340. [Google Scholar] [CrossRef]
- Weber, A.R.; Krawczyk, C.; Robertson, A.B.; Kusnierczyk, A.; Vagbo, C.B.; Schuermann, D.; Klungland, A.; Schar, P. Biochemical reconstitution of TET1-TDG-BER-dependent active DNA demethylation reveals a highly coordinated mechanism. Nat. Commun. 2016, 7, 10806. [Google Scholar] [CrossRef]
- Etchegaray, J.P.; Chavez, L.; Huang, Y.; Ross, K.N.; Choi, J.; Martinez-Pastor, B.; Walsh, R.M.; Sommer, C.A.; Lienhard, M.; Gladden, A.; et al. The histone deacetylase SIRT6 controls embryonic stem cell fate via TET-mediated production of 5-hydroxymethylcytosine. Nat. Cell Biol. 2015, 17, 545–557. [Google Scholar] [CrossRef]
- Hahn, M.A.; Qiu, R.; Wu, X.; Li, A.X.; Zhang, H.; Wang, J.; Jui, J.; Jin, S.G.; Jiang, Y.; Pfeifer, G.P.; et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell Rep. 2013, 3, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Kafer, G.R.; Li, X.; Horii, T.; Suetake, I.; Tajima, S.; Hatada, I.; Carlton, P.M. 5-Hydroxymethylcytosine Marks Sites of DNA Damage and Promotes Genome Stability. Cell Rep. 2016, 14, 1283–1292. [Google Scholar] [CrossRef]
- Li, S.; Papale, L.A.; Zhang, Q.; Madrid, A.; Chen, L.; Chopra, P.; Keles, S.; Jin, P.; Alisch, R.S. Genome-wide alterations in hippocampal 5-hydroxymethylcytosine links plasticity genes to acute stress. Neurobiol. Dis. 2016, 86, 99–108. [Google Scholar] [CrossRef][Green Version]
- Spiers, H.; Hannon, E.; Schalkwyk, L.C.; Bray, N.J.; Mill, J. 5-hydroxymethylcytosine is highly dynamic across human fetal brain development. BMC Genom. 2017, 18, 738. [Google Scholar] [CrossRef]
- Alaghband, Y.; Bredy, T.W.; Wood, M.A. The role of active DNA demethylation and Tet enzyme function in memory formation and cocaine action. Neurosci. Lett. 2016, 625, 40–46. [Google Scholar] [CrossRef]
- Fasolino, M.; Zhou, Z. The Crucial Role of DNA Methylation and MeCP2 in Neuronal Function. Genes 2017, 8, 141. [Google Scholar] [CrossRef]
- Lubin, F.D.; Roth, T.L.; Sweatt, J.D. Epigenetic Regulation of bdnf Gene Transcription in the Consolidation of Fear Memory. J. Neurosci. 2008, 28, 10576–10586. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Goffin, D.; Johnson, B.S.; Zhou, Z. Loss of MeCP2 function is associated with distinct gene expression changes in the striatum. Neurobiol. Dis. 2013, 59, 257–266. [Google Scholar] [CrossRef]
- Gulmez Karaca, K.; Brito, D.V.C. MeCP2: A Critical Regulator of Chromatin in Neurodevelopment and Adult Brain Function. Int. J. Mol. Sci. 2019, 20, 4577. [Google Scholar] [CrossRef]
- Suri, D.; Vaidya, V.A. Glucocorticoid regulation of brain-derived neurotrophic factor: Relevance to hippocampal structural and functional plasticity. Neuroscience 2013, 239, 196–213. [Google Scholar] [CrossRef]
- Perera, F.; Phillips, D.H.; Wang, Y.; Roen, E.; Herbstman, J.; Rauh, V.; Wang, S.; Tang, D. Prenatal exposure to polycyclic aromatic hydrocarbons/aromatics, BDNF and child development. Environ. Res. 2015, 142, 602–608. [Google Scholar] [CrossRef]
- Hodjat, M.; Rahmani, S.; Khan, F.; Niaz, K.; Navaei-Nigjeh, M.; Mohammadi Nejad, S.; Abdollahi, M. Environmental toxicants, incidence of degenerative diseases, and therapies from the epigenetic point of view. Arch. Toxicol. 2017, 91, 2577–2597. [Google Scholar] [CrossRef]
- Sanchez-Guerra, M.; Zheng, Y.; Osorio-Yanez, C.; Zhong, J.; Chervona, Y.; Wang, S.; Chang, D.; McCracken, J.P.; Díaz, A.; Bertazzi, P.A.; et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: Results from the Beijing truck driver air pollution study. Epigenetics 2015, 10, 633–642. [Google Scholar] [CrossRef]
- Nie, J.; Li, J.; Cheng, L.; Deng, Y.; Li, Y.; Yan, Z.; Duan, L.; Niu, Q.; Tang, D. Prenatal polycyclic aromatic hydrocarbons metabolites, cord blood telomere length, and neonatal neurobehavioral development. Environ. Res. 2019, 174, 105–113. [Google Scholar] [CrossRef]
- Tang, D.; Li, T.Y.; Liu, J.J.; Zhou, Z.J.; Yuan, T.; Chen, Y.H.; Rauh, V.A.; Xie, J.; Perera, F. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ. Health Perspect. 2008, 116, 674–679. [Google Scholar] [CrossRef]
- Wei, H.; Feng, Y.; Liang, F.; Cheng, W.; Wu, X.; Zhou, R.; Wang, Y. Role of oxidative stress and DNA hydroxymethylation in the neurotoxicity of fine particulate matter. Toxicology 2017, 380, 94–103. [Google Scholar] [CrossRef]
- Cui, H.; Hou, J.; Ma, G. [Influences of rearing style on the intellectual development of infants]. Wei Sheng Yan Jiu 2001, 30, 362–364. [Google Scholar]
- Lee, J.; Kalia, V.; Perera, F.; Herbstman, J.; Li, T.; Nie, J.; Qu, L.R.; Yu, J.; Tang, D. Prenatal airborne polycyclic aromatic hydrocarbon exposure, LINE1 methylation and child development in a Chinese cohort. Envirnon. Int. 2017, 99, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gong, Y.J.; Cao, W.C.; Wang, R.X.; Wang, Y.X.; Liu, C.; Chen, Y.J.; Huang, L.L.; Ai, S.H.; Lu, W.Q.; et al. Prenatal urinary polycyclic aromatic hydrocarbon metabolites, global DNA methylation in cord blood, and birth outcomes: A cohort study in China. Environ. Pollut. 2018, 234, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Valeri, L.; Vanderweele, T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods 2013, 18, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Personal. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Llop, S.; Ballester, F.; Estarlich, M.; Ibarluzea, J.; Manrique, A.; Rebagliato, M.; Esplugues, A.; Iñiguez, C. Urinary 1-hydroxypyrene, air pollution exposure and associated life style factors in pregnant women. Sci. Total Environ. 2008, 407, 97–104. [Google Scholar] [CrossRef]
- Wu, M.T.; Simpson, C.D.; Christiani, D.C.; Hecht, S.S. Relationship of exposure to coke-oven emissions and urinary metabolites of benzo(a)pyrene and pyrene in coke-oven workers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 311–314. [Google Scholar]
- Hisamuddin, N.H.; Jalaludin, J. Children’s exposure to polycyclic aromatic hydrocarbon (PAHs): A review on urinary 1-hydroxypyrene and associated health effects. Rev. Environ. Health 2023, 38, 151–168. [Google Scholar] [CrossRef]
- Edwards, S.C.; Jedrychowski, W.; Butscher, M.; Camann, D.; Kieltyka, A.; Mroz, E.; Flak, E.; Li, Z.; Wang, S.; Rauh, V.; et al. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ. Health Perspect. 2010, 118, 1326–1331. [Google Scholar] [CrossRef]
- McCallister, M.M.; Maguire, M.; Ramesh, A.; Aimin, Q.; Liu, S.; Khoshbouei, H.; Aschner, M.; Ebner, F.F.; Hood, D.B. Prenatal exposure to benzo(a)pyrene impairs later-life cortical neuronal function. Neurotoxicology 2008, 29, 846–854. [Google Scholar] [CrossRef]
- Cathey, A.L.; Watkins, D.J.; Rosario, Z.Y.; Vélez Vega, C.M.; Loch-Caruso, R.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Polycyclic aromatic hydrocarbon exposure results in altered CRH, reproductive, and thyroid hormone concentrations during human pregnancy. Sci. Total Environ. 2020, 749, 141581. [Google Scholar] [CrossRef]
- Tellez-Plaza, M.; Tang, W.Y.; Shang, Y.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Ledesma, M.; Leon, M.; Laclaustra, M.; Pollak, J.; et al. Association of global DNA methylation and global DNA hydroxymethylation with metals and other exposures in human blood DNA samples. Environ. Health Perspect. 2014, 122, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Romero, C.; Hur, J.; Bender, D.E.; Delaney, C.E.; Cataldo, M.D.; Smith, A.L.; Yung, R.; Ruden, D.M.; Callaghan, B.C.; Feldman, E.L. Identification of epigenetically altered genes in sporadic amyotrophic lateral sclerosis. PLoS ONE 2012, 7, e52672. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Xia, Q.; Sun, X.; Yu, H. Phototoxicity and environmental transformation of polycyclic aromatic hydrocarbons (PAHs)-light-induced reactive oxygen species, lipid peroxidation, and DNA damage. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2012, 30, 1–41. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, H.; Zhang, H.; Wang, W.; Liu, Y.; Fan, Y. Smoking modify the effects of polycyclic aromatic hydrocarbons exposure on oxidative damage to DNA in coke oven workers. Int. Arch. Occup. Environ. Health 2017, 90, 423–431. [Google Scholar] [CrossRef]
- Chia, N.; Wang, L.; Lu, X.; Senut, M.C.; Brenner, C.; Ruden, D.M. Hypothesis: Environmental regulation of 5-hydroxymethylcytosine by oxidative stress. Epigenetics 2011, 6, 853–856. [Google Scholar] [CrossRef]
- Coulter, J.B.; O’Driscoll, C.M.; Bressler, J.P. Hydroquinone Increases 5-Hydroxymethylcytosine Formation through Ten Eleven Translocation 1 (TET1) 5-Methylcytosine Dioxygenase. J. Biol. Chem. 2013, 288, 28792–28800. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Rifas-Shiman, S.L.; Godderis, L.; Duca, R.C.; Navas-Acien, A.; Litonjua, A.A.; DeMeo, D.L.; Brennan, K.J.; Amarasiriwardena, C.J.; Hivert, M.F.; et al. Prenatal Exposure to Mercury: Associations with Global DNA Methylation and Hydroxymethylation in Cord Blood and in Childhood. Environ. Health Perspect. 2017, 125, 087022. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, S.; Jin, L.; Wang, L.; Ren, A. Decreased global DNA hydroxymethylation in neural tube defects: Association with polycyclic aromatic hydrocarbons. Epigenetics 2019, 14, 1019–1029. [Google Scholar] [CrossRef]
- Gontier, G.; Iyer, M.; Shea, J.M.; Bieri, G.; Wheatley, E.G.; Ramalho-Santos, M.; Villeda, S.A. Tet2 Rescues Age-Related Regenerative Decline and Enhances Cognitive Function in the Adult Mouse Brain. Cell Rep. 2018, 22, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Villar-Menéndez, I.; Blanch, M.; Tyebji, S.; Pereira-Veiga, T.; Albasanz, J.L.; Martín, M.; Ferrer, I.; Pérez-Navarro, E.; Barrachina, M. Increased 5-Methylcytosine and Decreased 5-Hydroxymethylcytosine Levels are Associated with Reduced Striatal A2AR Levels in Huntington’s Disease. NeuroMol. Med. 2013, 15, 295–309. [Google Scholar] [CrossRef]
- Duan, R.; Fu, Q.; Sun, Y.; Li, Q. Epigenetic clock: A promising biomarker and practical tool in aging. Ageing Res. Rev. 2022, 81, 101743. [Google Scholar] [CrossRef] [PubMed]
- Ladd-Acosta, C. Epigenetic Signatures as Biomarkers of Exposure. Curr. Environ. Health Rep. 2015, 2, 117–125. [Google Scholar] [CrossRef]
- Eriksson, J.G. Developmental Origins of Health and Disease—From a small body size at birth to epigenetics. Ann. Med. 2016, 48, 456–467. [Google Scholar] [CrossRef]
- Laura, G.; Stefano, P.; Saal, F.S.; Vom Paola, P. The effects of bisphenol A on emotional behavior depend upon the timing of exposure, age and gender in mice. Horm. Behav. 2013, 63, 598–605. [Google Scholar] [CrossRef]
- Meredith, R.M. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: A framework for neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2015, 50, 180–188. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Zhang, H.; Zhang, H.; Wang, W.; Fan, Y. Urinary 1-hydroxypyrene and smoking are determinants of LINE-1 and AhRR promoter methylation in coke oven workers. Mutat. Res. 2018, 826, 33–40. [Google Scholar] [CrossRef]
- Yin, W.; Hou, J.; Xu, T.; Cheng, J.; Li, P.; Wang, L.; Zhang, Y.; Wang, X.; Hu, C.; Huang, C.; et al. Obesity mediated the association of exposure to polycyclic aromatic hydrocarbon with risk of cardiovascular events. Sci. Total Environ. 2018, 616–617, 841–854. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Participants a (n = 221) | Non-Participants (n = 179) | p-Value |
|---|---|---|---|
| Mother | |||
| Age (years) | 28.0 ± 3.8 | 27.3 ± 4.9 | 0.114 |
| Pre-pregnancy BMI (kg/m2) | 21.6 ± 3.2 | 21.3 ± 2.9 | 0.311 |
| Educational status | <0.001 | ||
| Middle school and below | 51 (23.1%) | 69 (38.5%) | |
| High school | 45 (20.4%) | 50 (27.9%) | |
| College and above | 125 (56.6%) | 60 (33.6%) | |
| Household income (CNY/year) | 0.048 | ||
| <30,000 | 88 (39.8%) | 89 (49.7%) | |
| 30,000–50,000 | 85 (38.5%) | 66 (36.9%) | |
| ≥50,000 | 48 (21.7%) | 24 (13.4%) | |
| Parity (n = 1) | 155 (70.1%) | 123 (68.7%) | 0.759 |
| Passive smoking (yes) | 102 (46.2%) | 96 (53.6%) | 0.102 |
| Child | |||
| Gender (male) | 116 (52.5%) | 97 (54.2%) | 0.735 |
| Gestational age (days) | 280.9 ± 7.8 | 279.5 ± 8.6 | 0.082 |
| Birth weight (g) | 3469.5 ± 438.7 | 3363.9 ± 433.0 | 0.018 |
| Birth length (cm) | 50.7 ± 2.1 | 50.0 ± 2.2 | <0.001 |
| Birth head circumference (cm) | 33.9 ± 1.8 | 33.8 ± 1.8 | 0.684 |
| NBNA scores | 38.7 ± 1.1 | 38.6 ± 1.1 | 0.909 |
| Motor | 111.6 ± 15.0 | - | - |
| Adaptive | 110.5 ± 14.6 | - | - |
| Language | 108.8 ± 17.2 | - | - |
| Social | 111.7 ± 14.5 | - | - |
| PAHs | Percent Detection (%) | GM | P25 | P50 | P75 |
|---|---|---|---|---|---|
| 2-OHNap | 98.6% | 0.063 | 0.015 | 0.072 | 0.230 |
| 1-OHNap | 55.2% | 0.009 | <LOD | 0.010 | 0.053 |
| 3-OHFlu | 52.5% | 0.009 | <LOD | 0.006 | 0.070 |
| 2-OHFlu | 76.0% | 0.036 | 0.003 | 0.077 | 0.212 |
| 9-OHPhe | 93.2% | 0.045 | 0.024 | 0.057 | 0.120 |
| 2-OHPhe | 91.9% | 0.078 | 0.046 | 0.133 | 0.194 |
| 1-OHPhe | 67.4% | 0.017 | <LOD | 0.024 | 0.131 |
| 1-OHPyr | 96.4% | 0.080 | 0.038 | 0.090 | 0.199 |
| 3-OHChr | 14.9% | 0.007 | <LOD | <LOD | <LOD |
| 6-OHChr | 18.6% | 0.006 | <LOD | <LOD | <LOD |
| 9-OHBap | 33.5% | 0.006 | <LOD | <LOD | 0.137 |
| ∑-OHPAHs | 100.0% | 0.754 | 0.343 | 0.687 | 1.724 |
| BPDE | 95.0% | 4.3 | 3.4 | 5.2 | 8.0 |
| 5-hmC | β (95% CI) | ||||
|---|---|---|---|---|---|
| NBNA | Motor | Adaptive | Language | Social | |
| Global DNA | −0.48 | −10.66 | −14.18 | −14.42 | −10.26 |
| (−0.89, −0.08) | (−15.98, −5.34) | (−19.11, −9.25) | (−20.36, −8.47) | (−15.23, −5.28) | |
| MeCP2 | 0.41 | 8.89 | 10.03 | 11.29 | 8.93 |
| (0.03, 0.80) | (3.26, 13.33) | (5.27, 14.80) | (5.64, 16.95) | (4.25, 13.61) | |
| BDNF | 0.10 | 7.77 | 0.14 | −0.15 | 1.51 |
| (−0.23, 0.44) | (3.45, 12.09) | (−4.13, 4.41) | (−5.19, 4.90) | (−2.65, 5.67) | |
| Exposure to Mediator (β1-OHPyr) | Mediator to Outcome (λM) | Mediated Effect (Indirect Effect, β1-OHPyr × λM) | Direct effect (λ1-OHPyr) | Mediated Proportion (%) | |
|---|---|---|---|---|---|
| Global DNA | |||||
| Motor | 0.15 | −9.10 | −1.34 | −3.36 | 28.51 |
| (0.07, 0.22) | (−14.57, −3.63) | (−2.63, −0.48) | (−6.43, −0.28) | ||
| Adaptive | 0.15 | −13.13 | −1.93 | −2.26 | 46.06 |
| (0.07, 0.22) | (−18.22, −8.04) | (−3.16, −1.01) | (−5.12, 0.60) | ||
| Language | 0.15 | −13.49 | −1.98 | −1.99 | 49.87 |
| (0.07, 0.22) | (−19.65, −7.33) | (−3.48, −0.90) | (−5.45, 1.47) | ||
| Social | 0.15 | −9.62 | −1.41 | −1.37 | 50.72 |
| (0.07, 0.22) | (−14.78, −4.47) | (−2.66, −0.61) | (−4.26, 1.53) | ||
| MeCP2 | |||||
| Motor | −0.20 | 6.41 | −1.28 | −3.41 | 27.29 |
| (−0.28, −0.12) | (1.11, 11.70) | (−2.36, −0.34) | (−6.60, −0.23) | ||
| Adaptive | −0.20 | 8.68 | −1.74 | −2.46 | 41.43 |
| (−0.28, −0.12) | (3.65, 13.71) | (−2.86, −0.77) | (−5.48, 0.56) | ||
| Language | −0.20 | 10.23 | −2.04 | −1.93 | 51.39 |
| (−0.28, −0.12) | (4.24, 16.22) | (−3.71, −0.75) | (−5.53, 1.67) | ||
| Social | −0.20 | 8.31 | −1.66 | −1.12 | 40.29 |
| (−0.28, −0.12) | (3.35, 13.27) | (−2.81, −0.72) | (−4.10, 1.86) | ||
| BDNF | |||||
| Motor | −0.13 | 6.74 | −0.89 | −3.80 | 18.98 |
| (−0.23, −0.04) | (2.40, 11.08) | (−2.20, −0.20) | (−6.84, −0.77) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cao, X.; Liu, C.; Cheng, L.; Niu, Q.; Nie, J. Association of Prenatal Polycyclic Aromatic Hydrocarbons Exposure, DNA Hydroxymethylation, and Neurodevelopment at 0 and 2 Years of Age. Toxics 2025, 13, 726. https://doi.org/10.3390/toxics13090726
Li J, Cao X, Liu C, Cheng L, Niu Q, Nie J. Association of Prenatal Polycyclic Aromatic Hydrocarbons Exposure, DNA Hydroxymethylation, and Neurodevelopment at 0 and 2 Years of Age. Toxics. 2025; 13(9):726. https://doi.org/10.3390/toxics13090726
Chicago/Turabian StyleLi, Jinyu, Xiaomin Cao, Chengjuan Liu, Lin Cheng, Qiao Niu, and Jisheng Nie. 2025. "Association of Prenatal Polycyclic Aromatic Hydrocarbons Exposure, DNA Hydroxymethylation, and Neurodevelopment at 0 and 2 Years of Age" Toxics 13, no. 9: 726. https://doi.org/10.3390/toxics13090726
APA StyleLi, J., Cao, X., Liu, C., Cheng, L., Niu, Q., & Nie, J. (2025). Association of Prenatal Polycyclic Aromatic Hydrocarbons Exposure, DNA Hydroxymethylation, and Neurodevelopment at 0 and 2 Years of Age. Toxics, 13(9), 726. https://doi.org/10.3390/toxics13090726







