Distribution of Legacy and Emerging PFASs in a Terrestrial Ecosystem Located near a Fluorochemical Manufacturing Facility
Abstract
1. Introduction
2. Materials and Methods
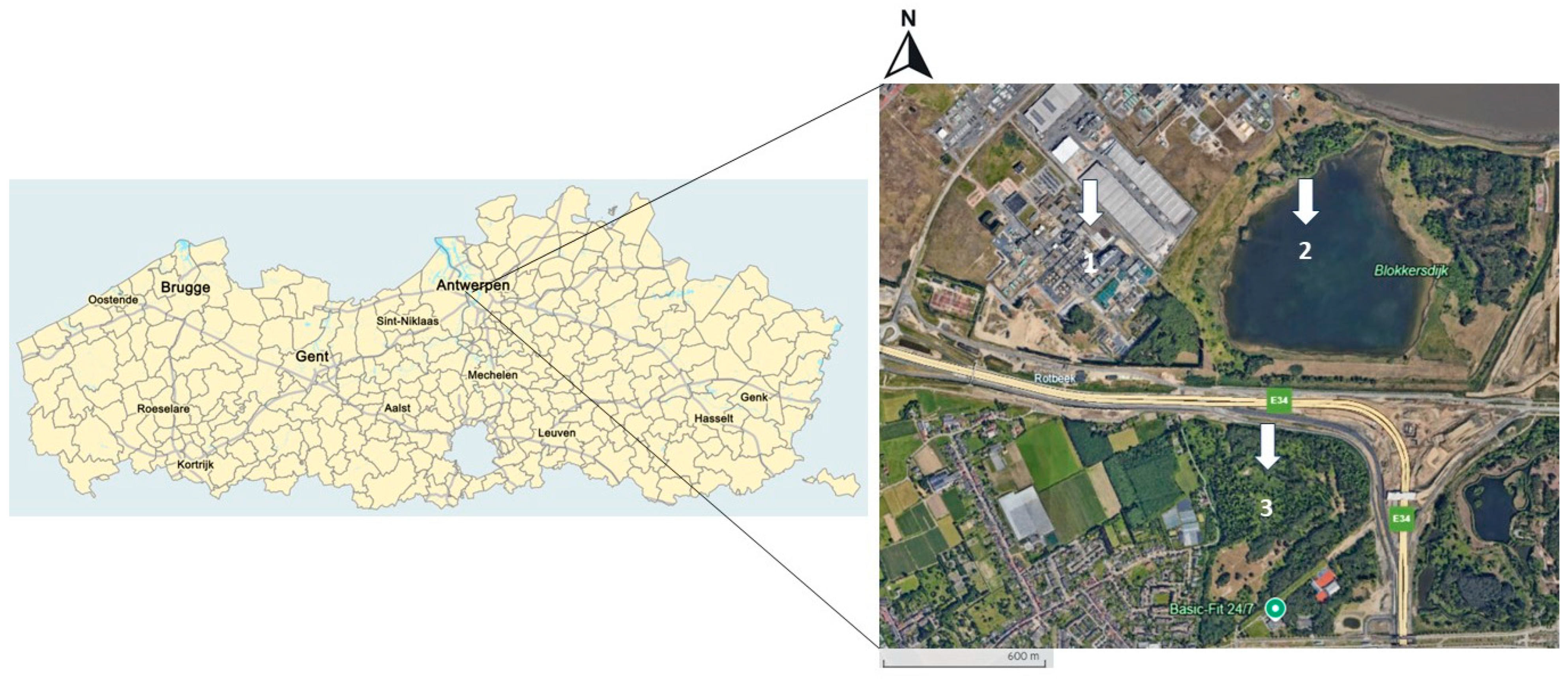
2.1. Study Area and Sample Collection
2.2. PFAS Analysis
Quality Control and Assurance
2.3. Statistical Analysis
3. Results
3.1. Soil PFAS Profiles and Concentrations
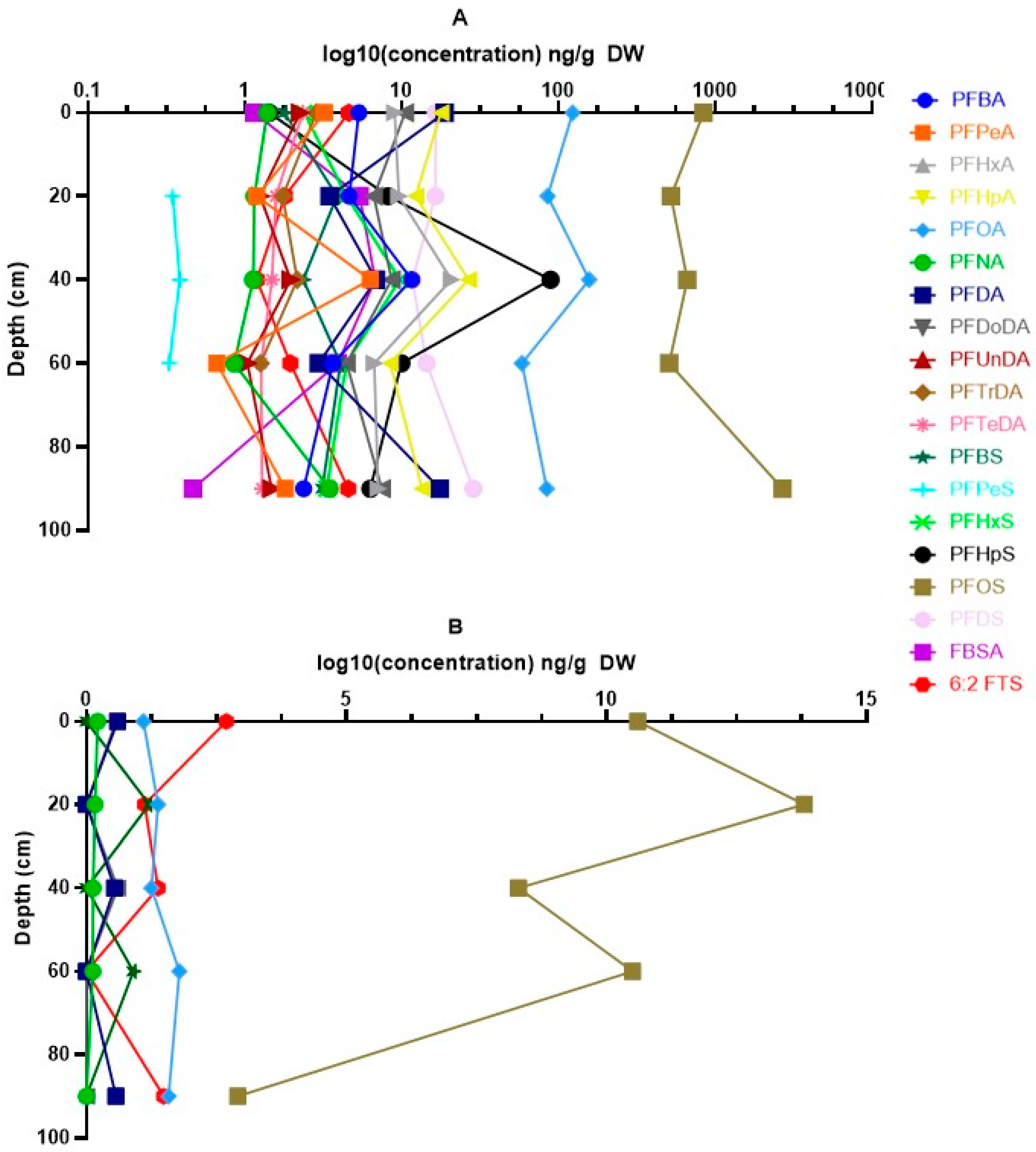
3.2. Vertical Distribution of PFASs in Soil
3.3. PFAS Profiles and Concentrations in Nettles
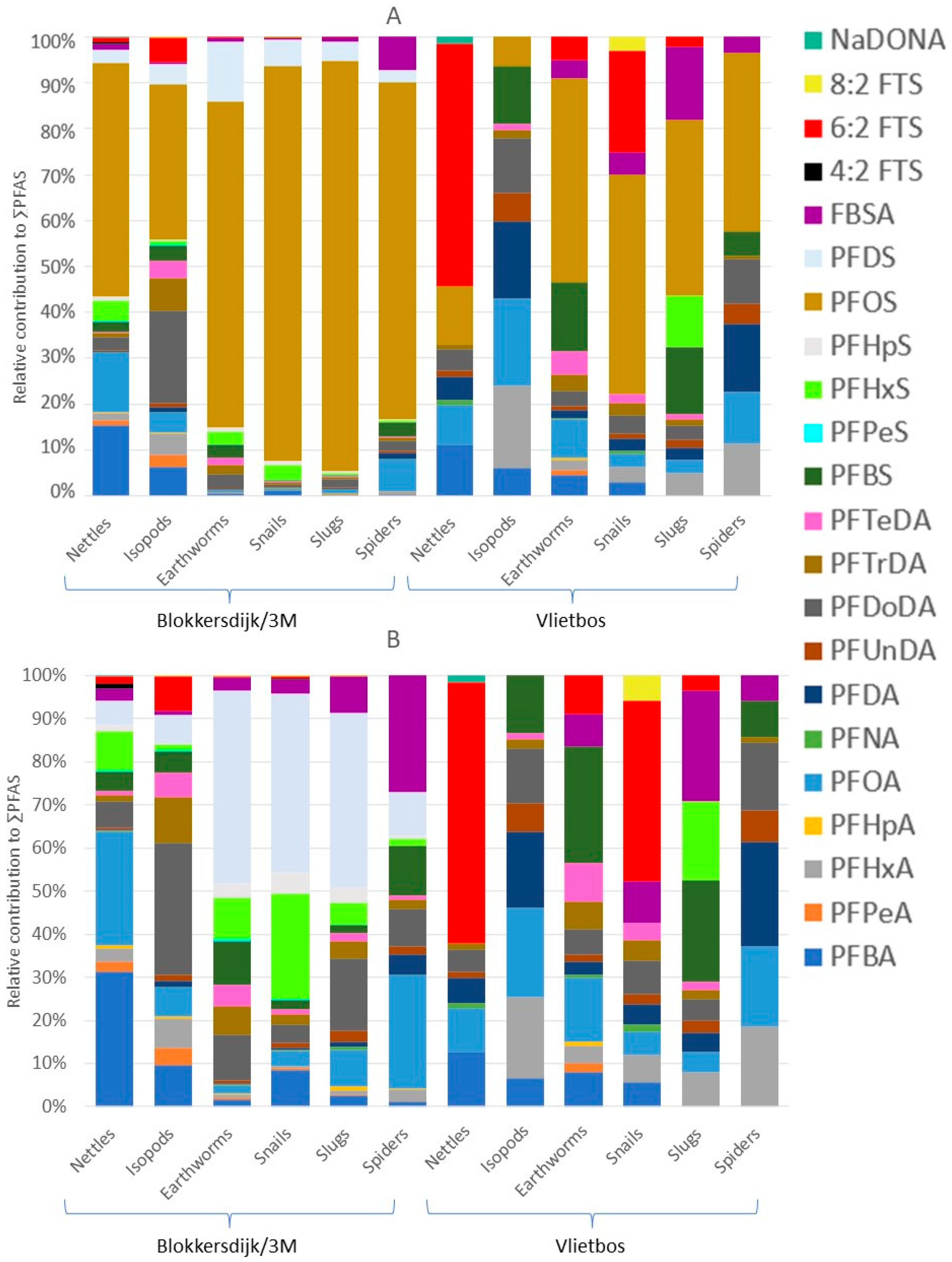
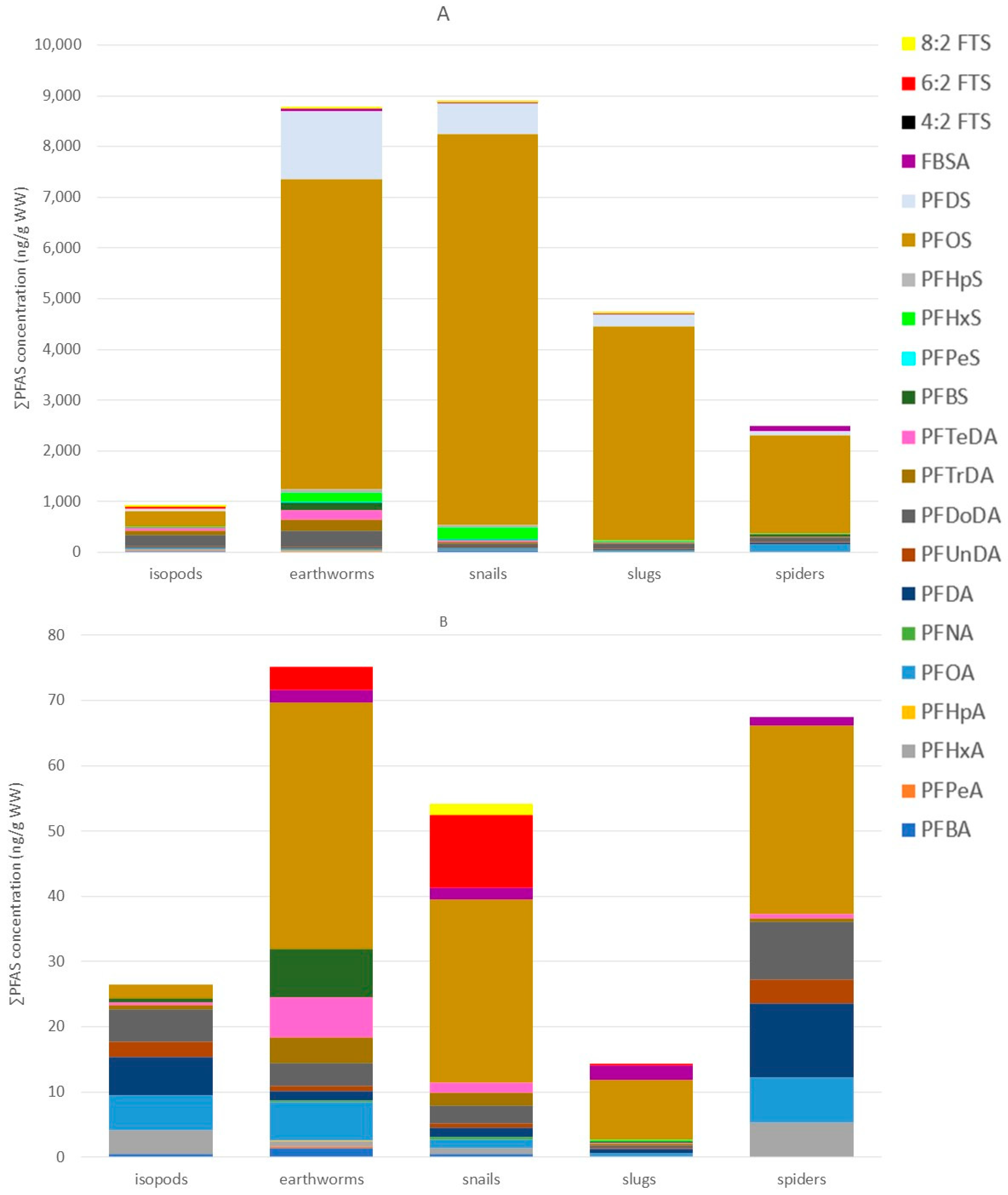
3.4. PFAS Profiles, Concentrations, and Taxon-Specific Differences in Terrestrial Invertebrates
3.5. PFAS Profiles, Concentrations, and Matrix-Dependent Differences and Similarities in Great Tit Plasma and Feathers
3.6. Associations Among Terrestrial Matrices
4. Discussion
4.1. Soil PFAS Profiles and Concentrations
4.2. Vertical Distribution of PFASs in Soil
4.3. PFAS Profiles and Concentrations in Nettles
4.4. PFAS Profiles, Concentrations, and Taxon-Specific PFAS Accumulation in Terrestrial Invertebrates
4.5. PFAS Profiles, Concentrations, and Matrix-Dependent Differences and Similarities in Great Tit Plasma and Feathers
4.6. Associations Among Terrestrial Matrices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFASs | Per- and poly-fluoroalkyl substances |
| TOC | Total organic carbon |
| PP | Polypropylene |
| UPLC-MS/MS | Ultra-performance liquid chromatography coupled tandem mass spectrometry |
| ACN | Acetonitrile |
| LOQ | Limit of quantification |
| MLE | Maximum likelihood estimation |
| PFOS | Perfluorooctanesulfonic acid |
| PFOA | Perfluorooctanoic acid |
| PFHpA | Perfluoroheptanoic acid |
| PFHxA | Perfluorohexanoic acid |
| PFBA | Perfluorobutanoic acid |
| PFBS | Perfluorobutane sulfonic acid |
| FTS | Fluorotelomer sulfonic acid |
| PFNA | Perfluorononanoic acid |
| PFDA | Perfluorodecanoic acid |
| PFUnDA | Perfluoroundecanoic acid |
| PFDoDA | Perfluorododecanoic acid |
| PFHxS | Perfluorohexane sulfonic acid |
| PFPeA | Perfluoropentanoic acid |
| PFHpS | Perfluoroheptane sulfonic acid |
| PFDS | Perfluorodecane sulfonic acid |
| FBSA | Perfluorobutane sulfonamide |
| PFTrDA | Perfluorotridecanoic acid |
| PFTeDA | Perfluorotetradecanoic acid |
| PFCA | Perfluorocarboxylic acid |
| PFSA | Perfluorosulfonic acid |
References
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef] [PubMed]
- Groffen, T.; Lopez-Antia, A.; D’Hollander, W.; Prinsen, E.; Eens, M.; Bervoets, L. Perfluoroalkylated acids in the eggs of great tits (Parus major) near a fluorochemical plant in Flanders, Belgium. Environ. Pollut. 2017, 228, 140–148. [Google Scholar] [CrossRef]
- Conder, J.M.; Hoke, R.A.; Wolf, W.d.; Russell, M.H.; Buck, R.C. Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Robinson, B.H. The Phytomanagement of PFAS-Contaminated Land. Int. J. Environ. Res. Public Health 2022, 19, 6817. [Google Scholar] [CrossRef]
- Li, Y.; Oliver, D.P.; Kookana, R.S. A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 628–629, 110–120. [Google Scholar] [CrossRef]
- Rijnders, J.; Bervoets, L.; Prinsen, E.; Eens, M.; Beemster, G.T.S.; AbdElgawad, H.; Groffen, T. Perfluoroalkylated acids (PFAAs) accumulate in field-exposed snails (Cepaea sp.) and affect their oxidative status. Sci. Total Environ. 2021, 790, 148059. [Google Scholar] [CrossRef]
- Washington, J.W.; Yoo, H.; Ellington, J.J.; Jenkins, T.M.; Libelo, E.L. Concentrations, distribution, and persistence of perfluoroalkylates in sludge-applied soils near Decatur, Alabama, USA. Environ. Sci. Technol. 2010, 44, 8390–8396. [Google Scholar] [CrossRef] [PubMed]
- Röhler, K.; Haluska, A.A.; Susset, B.; Liu, B.; Grathwohl, P. Long-term behavior of PFAS in contaminated agricultural soils in Germany. J. Contam. Hydrol. 2021, 241, 103812. [Google Scholar] [CrossRef] [PubMed]
- Rupp, J.; Guckert, M.; Berger, U.; Drost, W.; Mader, A.; Nödler, K.; Nürenberg, G.; Schulze, J.; Söhlmann, R.; Reemtsma, T. Comprehensive target analysis and TOP assay of per- and polyfluoroalkyl substances (PFAS) in wild boar livers indicate contamination hot-spots in the environment. Sci. Total Environ. 2023, 871, 162028. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.F.; Aristizabal-Henao, J.J.; Aufmuth, J.; Awkerman, J.; Bowden, J.A. Survey of per- and polyfluoroalkyl substances (PFAS) in surface water collected in Pensacola, FL. Heliyon 2022, 8, e10239. [Google Scholar] [CrossRef] [PubMed]
- Jouanneau, W.; Bårdsen, B.J.; Herzke, D.; Johnsen, T.V.; Eulaers, I.; Bustnes, J.O. Spatiotemporal Analysis of Perfluoroalkyl Substances in White-Tailed Eagle (Haliaeetus albicilla) Nestlings from Northern Norway-A Ten-Year Study. Environ. Sci. Technol. 2020, 54, 5011–5020. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Dauwe, T.; Meyer, J.; Maes, K.; Bervoets, L.; Eens, M. High levels of PFOS in eggs of three bird species in the neighbourhood of a fluoro-chemical plant. Ecotoxicol. Environ. Saf. 2017, 139, 165–171. [Google Scholar] [CrossRef]
- Groffen, T.; Rijnders, J.; Verbrigghe, N.; Verbruggen, E.; Prinsen, E.; Eens, M.; Bervoets, L. Influence of soil physicochemical properties on the depth profiles of perfluoroalkylated acids (PFAAs) in soil along a distance gradient from a fluorochemical plant and associations with soil microbial parameters. Chemosphere 2019, 236, 124407. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Y.; Shi, Y.; Wang, P.; Jones, K.; Sweetman, A.J.; Johnson, A.C.; Zhang, M.; Zhou, Y.; Lu, X.; et al. Crop bioaccumulation and human exposure of perfluoroalkyl acids through multi-media transport from a mega fluorochemical industrial park, China. Environ. Int. 2017, 106, 37–47. [Google Scholar] [CrossRef]
- Groffen, T.; Prinsen, E.; Devos Stoffels, O.A.; Maas, L.; Vincke, P.; Lasters, R.; Eens, M.; Bervoets, L. PFAS accumulation in several terrestrial plant and invertebrate species reveals species-specific differences. Environ. Sci. Pollut. Res. Int. 2022, 30, 23820–23835. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, G.; Dong, C.; Yang, R.; Pei, Z.; Li, Y.; Li, A.; Zhang, Q.; Jiang, G. Occurrence, bioaccumulation and trophodynamics of per- and polyfluoroalkyl substances (PFAS) in terrestrial and marine ecosystems of Svalbard, Arctic. Water Res. 2025, 271, 122979. [Google Scholar] [CrossRef]
- Herzke, D.; Nikiforov, V.; Yeung, L.W.Y.; Moe, B.; Routti, H.; Nygård, T.; Gabrielsen, G.W.; Hanssen, L. Targeted PFAS analyses and extractable organofluorine—Enhancing our understanding of the presence of unknown PFAS in Norwegian wildlife. Environ. Int. 2023, 171, 107640. [Google Scholar] [CrossRef]
- Hopkins, K.E.; McKinney, M.A.; Saini, A.; Letcher, R.J.; Karouna-Renier, N.K.; Fernie, K.J. Characterizing the Movement of Per- and Polyfluoroalkyl Substances in an Avian Aquatic–Terrestrial Food Web. Environ. Sci. Technol. 2023, 57, 20249–20260. [Google Scholar] [CrossRef] [PubMed]
- Heimstad, E.S.; Nygård, T.; Moe, B.; Herzke, D. New insights from an eight-year study on per- and polyfluoroalkyl substances in an urban terrestrial ecosystem. Environ. Pollut. 2024, 347, 123735. [Google Scholar] [CrossRef] [PubMed]
- Gkika, I.S.; Xie, G.; van Gestel, C.A.M.; Ter Laak, T.L.; Vonk, J.A.; van Wezel, A.P.; Kraak, M.H.S. Research Priorities for the Environmental Risk Assessment of Per- and Polyfluorinated Substances. Environ. Toxicol. Chem. 2023, 42, 2302–2316. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.D.; Peng, M.Y.; Liu, H.B.; Yang, J.Y. Concentration and distribution of metals, total fluorine, per- and poly-fluoroalkyl substances (PFAS) in vertical soil profiles in industrialized areas. Chemosphere 2022, 302, 134855. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, G.; Li, M.; Li, J.; Liang, X.; Yang, X.; Guo, J.; Wang, T.; Zhu, L. Three-dimensional spatial distribution of legacy and novel poly/perfluoroalkyl substances in the Tibetan Plateau soil: Implications for transport and sources. Environ. Int. 2022, 158, 107007. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Eens, M.; Bervoets, L. Per- and polyfluoroalkyl substances (PFAS) in homegrown crops: Accumulation and human risk assessment. Chemosphere 2024, 364, 143208. [Google Scholar] [CrossRef]
- Jha, G.; Kankarla, V.; McLennon, E.; Pal, S.; Sihi, D.; Dari, B.; Diaz, D.; Nocco, M. Per- and Polyfluoroalkyl Substances (PFAS) in Integrated Crop-Livestock Systems: Environmental Exposure and Human Health Risks. Int. J. Environ. Res. Public Health 2021, 18, 12550. [Google Scholar] [CrossRef]
- Wang, W.; Rhodes, G.; Ge, J.; Yu, X.; Li, H. Uptake and accumulation of per- and polyfluoroalkyl substances in plants. Chemosphere 2020, 261, 127584. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Cureton, P.; Hoke, R.A.; Houde, M.; Kumar, A.; Kurias, J.; Lanno, R.; McCarthy, C.; Newsted, J.; Salice, C.J.; et al. Assessing the Ecological Risks of Per- and Polyfluoroalkyl Substances: Current State-of-the Science and a Proposed Path Forward. Environ. Toxicol. Chem. 2021, 40, 564–605. [Google Scholar] [CrossRef]
- Groffen, T.; Eens, M.; Bervoets, L. Do concentrations of perfluoroalkylated acids (PFAAs) in isopods reflect concentrations in soil and songbirds? A study using a distance gradient from a fluorochemical plant. Sci. Total Environ. 2019, 657, 111–123. [Google Scholar] [CrossRef]
- D’Hollander, W.; De Bruyn, L.; Hagenaars, A.; de Voogt, P.; Bervoets, L. Characterisation of perfluorooctane sulfonate (PFOS) in a terrestrial ecosystem near a fluorochemical plant in Flanders, Belgium. Environ. Sci. Pollut. Res. Int. 2014, 21, 11856–11866. [Google Scholar] [CrossRef]
- Koch, A.; Jonsson, M.; Yeung, L.W.Y.; Kärrman, A.; Ahrens, L.; Ekblad, A.; Wang, T. Per- and Polyfluoroalkyl-Contaminated Freshwater Impacts Adjacent Riparian Food Webs. Environ. Sci. Technol. 2020, 54, 11951–11960. [Google Scholar] [CrossRef] [PubMed]
- Fremlin, K.M.; Elliott, J.E.; Letcher, R.J.; Harner, T.; Gobas, F.A.P.C. Developing Methods for Assessing Trophic Magnification of Perfluoroalkyl Substances within an Urban Terrestrial Avian Food Web. Environ. Sci. Technol. 2023, 57, 12806–12818. [Google Scholar] [CrossRef]
- Grønnestad, R.; Vázquez, B.P.; Arukwe, A.; Jaspers, V.L.B.; Jenssen, B.M.; Karimi, M.; Lyche, J.L.; Krøkje, Å. Levels, Patterns, and Biomagnification Potential of Perfluoroalkyl Substances in a Terrestrial Food Chain in a Nordic Skiing Area. Environ. Sci. Technol. 2019, 53, 13390–13397. [Google Scholar] [CrossRef]
- Gkika, I.S.; Arie Vonk, J.; ter Laak, T.L.; van Gestel, C.A.M.; Dijkstra, J.; Groffen, T.; Bervoets, L.; Kraak, M.H.S. Strong bioaccumulation of a wide variety of PFAS in a contaminated terrestrial and aquatic ecosystem. Environ. Int. 2025, 202, 109629. [Google Scholar] [CrossRef]
- Dauwe, T.; Bervoets, L.; Blust, R.; Pinxten, R.; Eens, M. Are eggshells and egg contents of Great and Blue Tits suitable as indicators of heavy metal pollution? Belg. J. Zool. 1999, 129, 439–447. [Google Scholar]
- Eens, M.; Pinxten, R.; Verheyen, R.F.; Blust, R.; Bervoets, L. Great and Blue Tits as Indicators of Heavy Metal Contamination in Terrestrial Ecosystems. Ecotoxicol. Environ. Saf. 1999, 44, 81–85. [Google Scholar] [CrossRef]
- Janssens, E.; Dauwe, T.; Bervoets, L.; Eens, M. Heavy metals and selenium in feathers of great tits (Parus major) along a pollution gradient. Environ. Toxicol. Chem. 2001, 20, 2815–2820. [Google Scholar] [CrossRef]
- Jaspers, V.L.B.; Covaci, A.; Herzke, D.; Eulaers, I.; Eens, M. Bird feathers as a biomonitor for environmental pollutants: Prospects and pitfalls. TrAC Trends Anal. Chem. 2019, 118, 223–226. [Google Scholar] [CrossRef]
- Lasters, R.; Groffen, T.; Bervoets, L.; Eens, M. Perfluoroalkyl acid (PFAA) profile and concentrations in two co-occurring tit species: Distinct differences indicate non-generalizable results across passerines. Sci. Total Environ. 2021, 761, 143301. [Google Scholar] [CrossRef]
- Lopez-Antia, A.; Groffen, T.; Lasters, R.; AbdElgawad, H.; Sun, J.; Asard, H.; Bervoets, L.; Eens, M. Perfluoroalkyl Acids (PFAAs) Concentrations and Oxidative Status in Two Generations of Great Tits Inhabiting a Contamination Hotspot. Environ. Sci. Technol. 2019, 53, 1617–1626. [Google Scholar] [CrossRef]
- Groffen, T.; Buytaert, J.; Prinsen, E.; Bervoets, L.; Eens, M. Per- and Polyfluoroalkyl Substances (PFAS) Accumulation, Reproductive Impairment, and Associations with Nestling Body Condition in Great (Parus major)- and Blue Tits (Cyanistes caeruleus) Living near a Hotspot in Belgium. Toxics 2024, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Dauwe, T.; Van de Vijver, K.; De Coen, W.; Eens, M. PFOS levels in the blood and liver of a small insectivorous songbird near a fluorochemical plant. Environ. Int. 2007, 33, 357–361. [Google Scholar] [CrossRef]
- Groffen, T.; Lasters, R.; Bervoets, L.; Prinsen, E.; Eens, M. Are Feathers of a Songbird Model Species (The Great Tit, Parus major) Suitable for Monitoring Perfluoroalkyl Acids (PFAAs) in Blood Plasma? Environ. Sci. Technol. 2020, 54, 9334–9344. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Groffen, T.; Lasters, R.; Lemière, F.; Willems, T.; Eens, M.; Bervoets, L.; Prinsen, E. Development and validation of an extraction method for the analysis of perfluoroalkyl substances (PFASs) in environmental and biotic matrices. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1116, 30–37. [Google Scholar] [CrossRef]
- Groffen, T.; Bervoets, L.; Jeong, Y.; Willems, T.; Eens, M.; Prinsen, E. A rapid method for the detection and quantification of legacy and emerging per- and polyfluoroalkyl substances (PFAS) in bird feathers using UPLC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1172, 122653. [Google Scholar] [CrossRef]
- Powley, C.R.; George, S.W.; Ryan, T.W.; Buck, R.C. Matrix Effect-Free Analytical Methods for Determination of Perfluorinated Carboxylic Acids in Environmental Matrixes. Anal. Chem. 2005, 77, 6353–6358. [Google Scholar] [CrossRef]
- Villanueva, P. MLE-Based Procedure for Left-Censored Data Excel Spreadsheet; Office of Pesticide Programs: Washington, DC, USA; U.S. Environmental Protection Agency: Washington, DC, USA, 2005.
- ERM. 1st Gefaseerd Beschrijvend Bodemonderzoek; ERM: Antwerpen, Belgium, 2022. [Google Scholar]
- Kim, H.H.; Gilak Hakimabadi, S.; Pham, A.L. Treatment of electrochemical plating wastewater by heterogeneous photocatalysis: The simultaneous removal of 6:2 fluorotelomer sulfonate and hexavalent chromium. RSC Adv. 2021, 11, 37472–37481. [Google Scholar] [CrossRef]
- KMI. Characteristics of Some Climatological Parameters. 2018. Available online: https://www.meteo.be/nl/unpublish/algemeen-klimaat-belgie/parameters#:~:text=De%20absolute%20jaarlijkse%20minima%20schommelen,lager%20dan%20%E2%80%9310%C2%B0C (accessed on 13 August 2025).
- Campos Pereira, H.; Ullberg, M.; Kleja, D.B.; Gustafsson, J.P.; Ahrens, L. Sorption of perfluoroalkyl substances (PFASs) to an organic soil horizon—Effect of cation composition and pH. Chemosphere 2018, 207, 183–191. [Google Scholar] [CrossRef]
- Fabregat-Palau, J.; Vidal, M.; Rigol, A. Modelling the sorption behaviour of perfluoroalkyl carboxylates and perfluoroalkane sulfonates in soils. Sci. Total Environ. 2021, 801, 149343. [Google Scholar] [CrossRef]
- Fabregat-Palau, J.; Vidal, M.; Rigol, A. Examining sorption of perfluoroalkyl substances (PFAS) in biochars and other carbon-rich materials. Chemosphere 2022, 302, 134733. [Google Scholar] [CrossRef]
- Krippner, J.; Falk, S.; Brunn, H.; Georgii, S.; Schubert, S.; Stahl, T. Accumulation Potentials of Perfluoroalkyl Carboxylic Acids (PFCAs) and Perfluoroalkyl Sulfonic Acids (PFSAs) in Maize (Zea mays). J. Agric. Food Chem. 2015, 63, 3646–3653. [Google Scholar] [CrossRef]
- Gredelj, A.; Polesel, F.; Trapp, S. Model-based analysis of the uptake of perfluoroalkyl acids (PFAAs) from soil into plants. Chemosphere 2020, 244, 125534. [Google Scholar] [CrossRef]
- Che, J.; Xu, C.; Song, X.; Ding, X.; Ali, M.; Chen, H. Bioaccumulation of PFASs in cabbage collected near a landfill site in China: Laboratory and field investigations. Sci. Total Environ. 2024, 906, 167578. [Google Scholar] [CrossRef]
- Brendel, S.; Fetter, É.; Staude, C.; Vierke, L.; Biegel-Engler, A. Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under REACH. Environ. Sci. Eur. 2018, 30, 9. [Google Scholar] [CrossRef]
- Speiser, B. Food and Feeding Behaviour; CABI: Wallingford, UK, 2001; pp. 259–288. [Google Scholar] [CrossRef]
- Price, J.B.; Holdich, D.M. An ultrastructural study of the integument during the moult cycle of the woodlouse, Oniscus asellus (Crustacea, Isopoda). Zoomorphologie 1980, 95, 250–263. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Distribution and partitioning of perfluoroalkyl carboxylic acids in surface soil, plants, and earthworms at a contaminated site. Sci. Total Environ. 2019, 647, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Wang, M.; Park, J.-S.; Petreas, M.; Bernstein, L.; Anton-Culver, H.; Nelson, D.O.; Reynolds, P. Time Trends in Per- and Polyfluoroalkyl Substances (PFASs) in California Women: Declining Serum Levels, 2011–2015. Environ. Sci. Technol. 2018, 52, 277–287. [Google Scholar] [CrossRef]
- Roos, A.M.; Gamberg, M.; Muir, D.; Kärrman, A.; Carlsson, P.; Cuyler, C.; Lind, Y.; Bossi, R.; Rigét, F. Perfluoroalkyl substances in circum-ArcticRangifer: Caribou and reindeer. Environ. Sci. Pollut. Res. 2022, 29, 23721–23735. [Google Scholar] [CrossRef]
- Dauwe, T.; Janssens, E.; Bervoets, L.; Blust, R.; Eens, M. Heavy-Metal Concentrations in Female Laying Great Tits (Parus major) and Their Clutches. Arch. Environ. Contam. Toxicol. 2005, 49, 249–256. [Google Scholar] [CrossRef]
- Kannan, K.; Franson, J.C.; Bowerman, W.W.; Hansen, K.J.; Jones, P.D.; Giesy, J.P. Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses. Environ. Sci. Technol. 2001, 35, 3065–3070. [Google Scholar] [CrossRef]
- Jaspers, V.L.; Voorspoels, S.; Covaci, A.; Eens, M. Can predatory bird feathers be used as a non-destructive biomonitoring tool of organic pollutants? Biol. Lett. 2006, 2, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; Letcher, R.J. Comparative tissue and body compartment accumulation and maternal transfer to eggs of perfluoroalkyl sulfonates and carboxylates in Great Lakes herring gulls. Environ. Pollut. 2012, 162, 40–47. [Google Scholar] [CrossRef]
- Hopkins, K.E.; McKinney, M.A.; Letcher, R.J.; Fernie, K.J. The influence of environmental and ecological factors on the accumulation and distribution of short- and long-chain perfluoroalkyl acids in a mid-trophic avian insectivore. Environ. Pollut. 2023, 321, 121133. [Google Scholar] [CrossRef] [PubMed]
- Løseth, M.E.; Briels, N.; Flo, J.; Malarvannan, G.; Poma, G.; Covaci, A.; Herzke, D.; Nygård, T.; Bustnes, J.O.; Jenssen, B.M.; et al. White-tailed eagle (Haliaeetus albicilla) feathers from Norway are suitable for monitoring of legacy, but not emerging contaminants. Sci. Total Environ. 2019, 647, 525–533. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buytaert, J.; Eens, M.; Bervoets, L.; Groffen, T. Distribution of Legacy and Emerging PFASs in a Terrestrial Ecosystem Located near a Fluorochemical Manufacturing Facility. Toxics 2025, 13, 689. https://doi.org/10.3390/toxics13080689
Buytaert J, Eens M, Bervoets L, Groffen T. Distribution of Legacy and Emerging PFASs in a Terrestrial Ecosystem Located near a Fluorochemical Manufacturing Facility. Toxics. 2025; 13(8):689. https://doi.org/10.3390/toxics13080689
Chicago/Turabian StyleBuytaert, Jodie, Marcel Eens, Lieven Bervoets, and Thimo Groffen. 2025. "Distribution of Legacy and Emerging PFASs in a Terrestrial Ecosystem Located near a Fluorochemical Manufacturing Facility" Toxics 13, no. 8: 689. https://doi.org/10.3390/toxics13080689
APA StyleBuytaert, J., Eens, M., Bervoets, L., & Groffen, T. (2025). Distribution of Legacy and Emerging PFASs in a Terrestrial Ecosystem Located near a Fluorochemical Manufacturing Facility. Toxics, 13(8), 689. https://doi.org/10.3390/toxics13080689





