Assessment of Organic Pollutants Desorbed from Plastic Litter Items Stranded on Cadiz Beaches (SW Spain)
Highlights
- Most of the collected items in this study were associated with smoking, food consumption, and personal hygiene.
- FTIR analysis revealed a high prevalence of common polymers, particularly PET, PE, PP, and PVC.
- The SBSE (stir bar sorptive extraction) method in saline water was used to estimate the maximum desorbable fraction of organic contaminants from plastic debris.
- The most frequently detected fragrance was OTNE, followed by the insect repellent DEET.
- The results highlight how plastic beach litter can serve as a vector for environmental pollutants.
- They emphasize the urgent need for effective strategies to mitigate plastic pollution
Abstract
1. Introduction
2. Materials and Methods
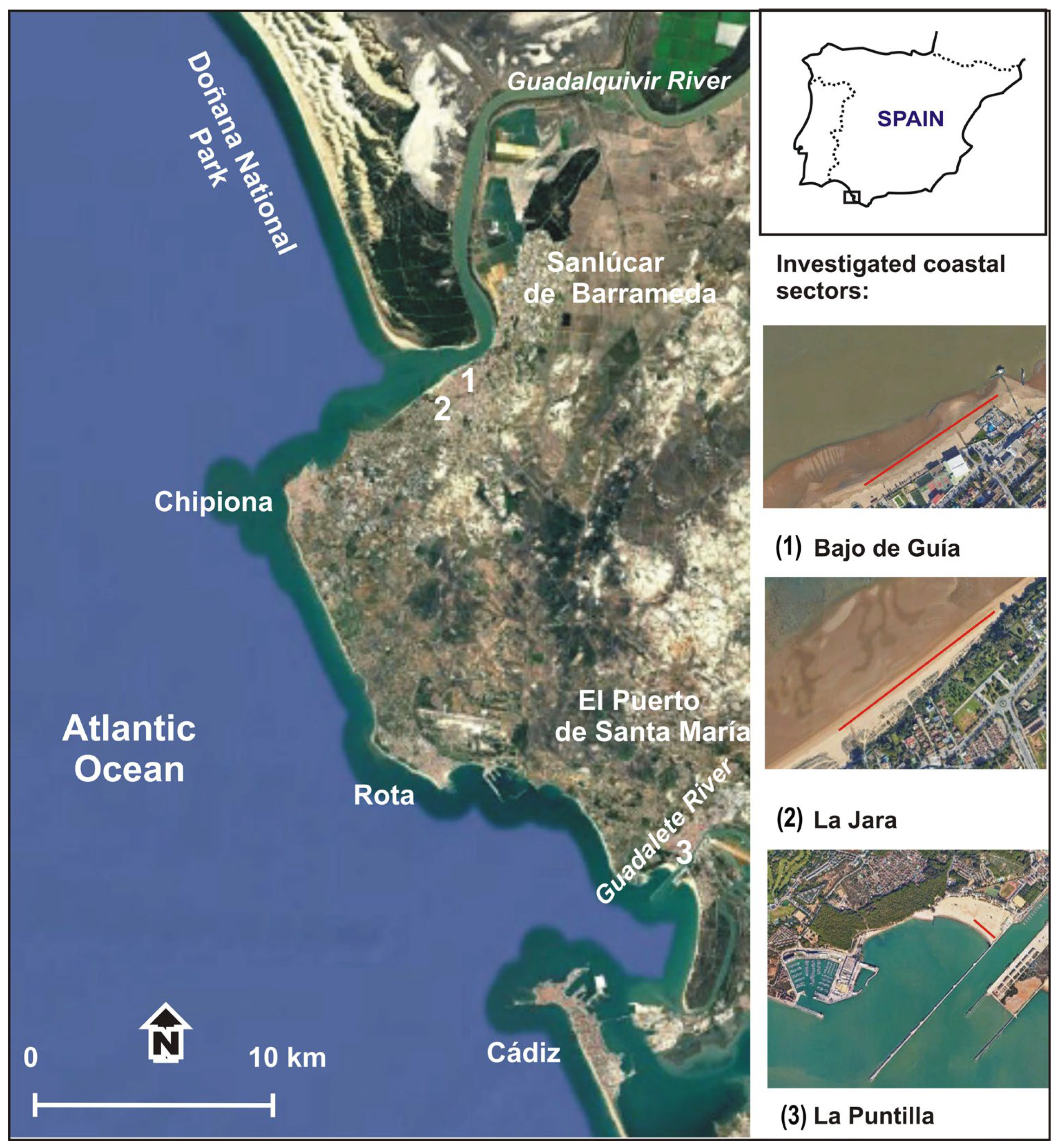
2.1. Study Area
2.2. Sampling Methodology
2.3. Sample Preparation for FTIRAnalysis on La Puntilla Beach Litter
2.4. Materials, Reagents, and Chemicals
2.5. Litter Leaching Through SBSE Extraction
2.6. Triple Quadrupole Mass Spectrometry Detection
3. Results and Discussion
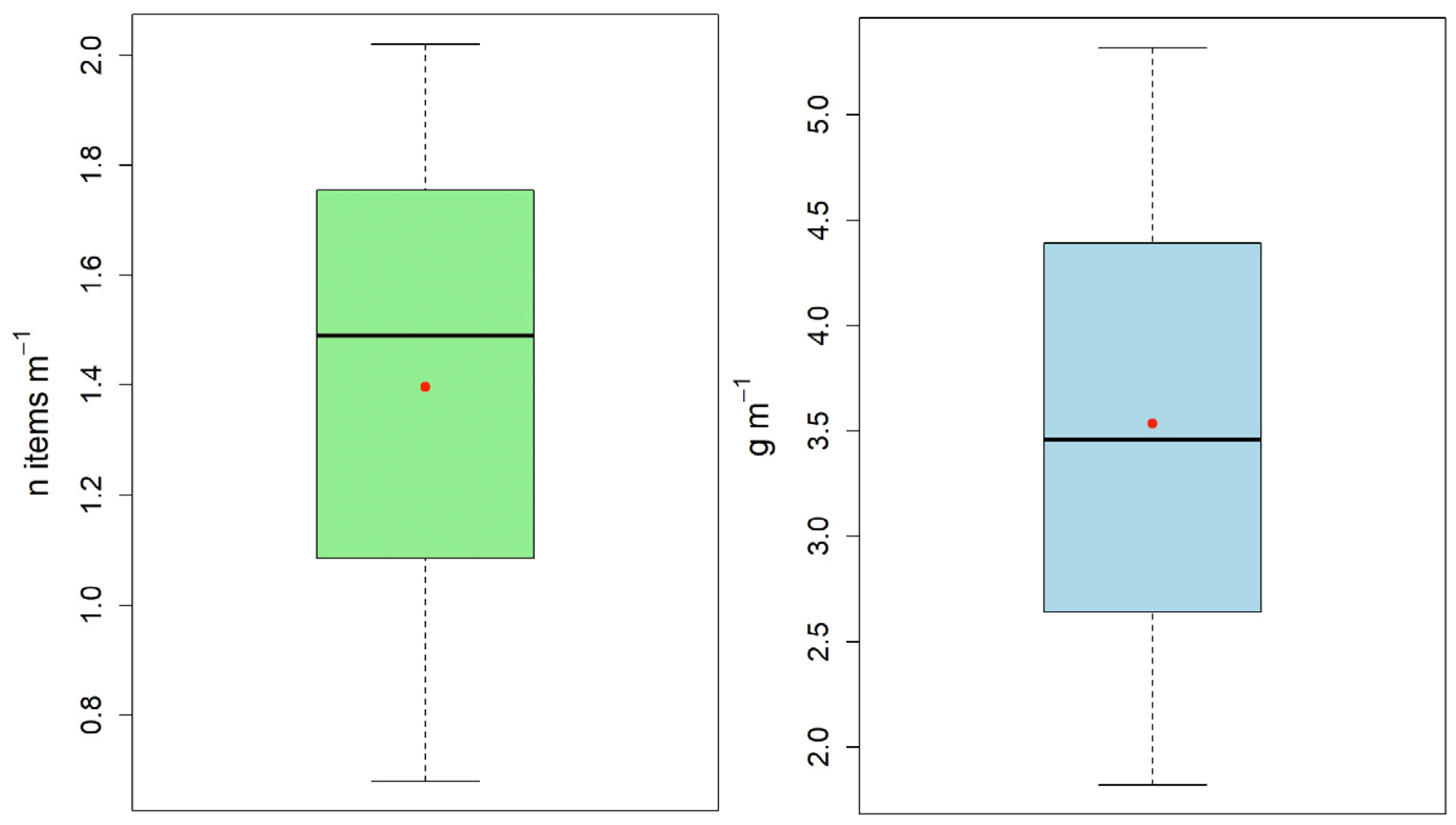
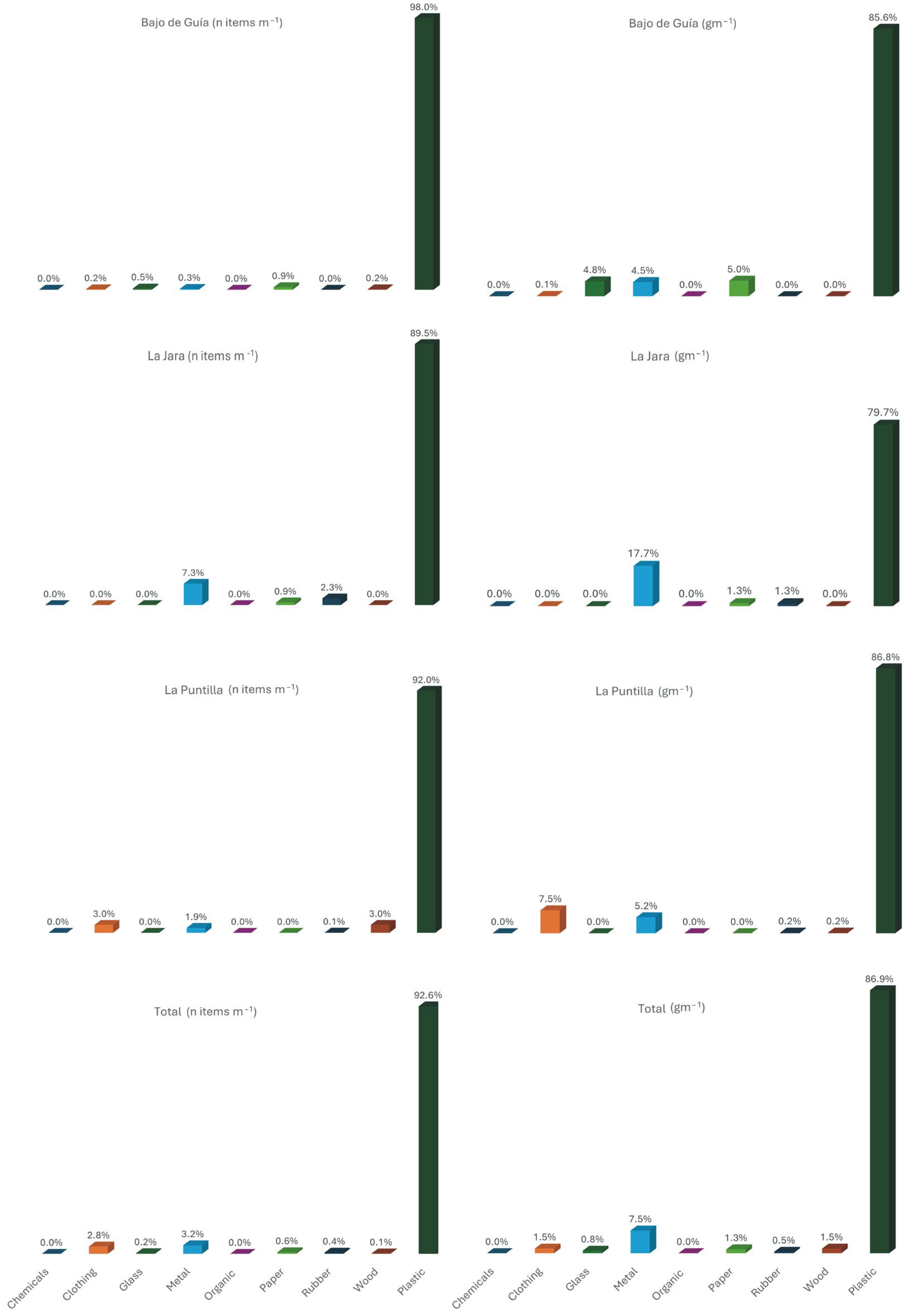
3.1. Litter Abundance and Characteristics
3.2. Identification of Composition from the Beach Litter from La Puntilla Beach by FTIR Analysis
3.3. Identification and Quantification of Organic Compounds
4. Conclusions
- Smoking-related materials contain high amounts of PAHs, especially at La Puntilla beach.
- Personal care products (PCPs), such as the wet wipes collected and used for desorption experiments, are a significant source of fragrances in the coastal environment. Concentrations of these compounds were one order of magnitude higher than in the rest of the analysed plastic litter categories. Additional co-leaching or sorption experiments must be done to prove the possible migration of fragrances from wipes to the rest of the materials.
- Plastic debris found at Bajo de Guia beach contained the highest concentrations of fragrances, pesticides, and insect repellent.
- The sources of contamination on the studied beaches are probably different.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDE | Dichlorodiphenyldichloroethylene |
| DDT | Dichlorodiphenyltrichloroethane |
| DEET | N,N-Diethyl-Meta-Toluamide |
| DOC | Dissolved organic carbon |
| EHMC | 2-Ethylhexyl-4-Methoxycinnamate |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GC—MS | Gas Chromatography—Mass Spectrometry |
| LOD | Limits of detection |
| LOQ | Limits of quantification |
| LS | Liquid–solid |
| MBC | Methylbenzylidene Camphor |
| MRM | Multiple reaction monitoring |
| MSFD | Marine Strategy Framework Directive |
| MTCS | Methyl-Triclosan |
| OTNE | Tetramethyl Acetyloctahydronaphthalenes |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PCBs | Polychlorinated Biphenyls |
| PDMS | Polydimethylsiloxane |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| POPs | Persistent organic pollutants |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl Chloride |
| S/N | Signal–noise |
| SBSE | Stir bar sorptive extraction |
| SW | Southwest |
| TCS | Triclosan |
| UV | Ultraviolet |
References
- Cheshire, A.; Adler, E.; Barbieère, J. UNEP/IOC Guidelines on Survey and Monitoring of Marine Litter; United Nations Environment Programme, Regional Seas Programme, Intergovernmental Oceanographic Commission, Integrated Coastal Area Management and Regional Programme: Nairobi, Kenya, 2009; ISBN 9789280730272. [Google Scholar]
- Coe, J.M.; Rogers, D.B. (Eds.) Marine Debris: Sources, Impacts and Solutions; Springer: New York, NY, USA, 1997; ISBN 978-1-4613-8488-5. [Google Scholar]
- Cocozza, P.; Scarrica, V.M.; Rizzo, A.; Serranti, S.; Staiano, A.; Bonifazi, G.; Anfuso, G. Microplastic Pollution from Pellet Spillage: Analysis of the Toconao Ship Accident along the Spanish and Portuguese Coasts. Mar. Pollut. Bull. 2025, 211, 117430. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ribes, L.; Basterretxea, G.; Palmer, M.; Tintoré, J. Origin and Abundance of Beach Debris in the Balearic Islands. Sci. Mar. 2007, 71, 305–314. [Google Scholar] [CrossRef]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Hong, S.; Lee, J.; Jang, Y.C.; Kim, Y.J.; Kim, H.J.; Han, D.; Hong, S.H.; Kang, D.; Shim, W.J. Impacts of Marine Debris on Wild Animals in the Coastal Area of Korea. Mar. Pollut. Bull. 2013, 66, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, G.W.; Koldewey, H.J.; Duncan, E.; Napper, I.E.; Niloy, M.N.H.; Nelms, S.E.; Sarker, S.; Bhola, S.; Nishat, B. Plastic Pollution in Aquatic Systems in Bangladesh: A Review of Current Knowledge. Sci. Total Environ. 2021, 761, 143285. [Google Scholar] [CrossRef]
- Nelms, S.E.; Duncan, E.M.; Patel, S.; Badola, R.; Bhola, S.; Chakma, S.; Chowdhury, G.W.; Godley, B.J.; Haque, A.B.; Johnson, J.A.; et al. Riverine Plastic Pollution from Fisheries: Insights from the Ganges River System. Sci. Total Environ. 2021, 756, 143305. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of Plastic Pollution in the Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef]
- Galgani, F.; Leauteà, J.P.; Moguedetà, P.; Souplet, A.; Verin, Y.; Carpentier, A.; Goragueràà, H.; Latrouiteàà, D.; Andral, B.; Cadiou, Y.; et al. Litter on the Sea Floor Along European Coasts. Mar. Pollut. Bull. 2000, 40, 516–527. [Google Scholar] [CrossRef]
- Moore, C.J. Synthetic Polymers in the Marine Environment: A Rapidly Increasing, Long-Term Threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef]
- Andrady, A.L. The Plastic in Microplastics: A Review. Mar. Pollut. Bull. 2017, 119, 12–22. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The Fate of Plastic in the Ocean Environment—A Minireview. Environ. Sci. Process Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef]
- Delre, A.; Goudriaan, M.; Morales, V.H.; Vaksmaa, A.; Ndhlovu, R.T.; Baas, M.; Keijzer, E.; de Groot, T.; Zeghal, E.; Egger, M.; et al. Plastic Photodegradation under Simulated Marine Conditions. Mar. Pollut. Bull. 2023, 187, 114544. [Google Scholar] [CrossRef] [PubMed]
- Barrick, A.; Champeau, O.; Chatel, A.; Manier, N.; Northcott, G.; Tremblay, L.A. Plastic Additives: Challenges in Ecotox Hazard Assessment. PeerJ 2021, 9, e11300. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic Products Leach Chemicals That InduceIn VitroToxicity under Realistic Use Conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef] [PubMed]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and Effects of Plastic Additives on Marine Environments and Organisms: A Review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of Known Plastic Packaging-Associated Chemicals and Their Hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef]
- Moore, R.E.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR) and Marine Plastics: Can Food Packaging Litter Act as a Dispersal Mechanism for AMR in Oceanic Environments? Mar. Pollut. Bull. 2020, 150, 110702. [Google Scholar] [CrossRef]
- Nakashima, E.; Isobe, A.; Kako, S.; Itai, T.; Takahashi, S. Quantification of Toxic Metals Derived from Macroplastic Litter on Ookushi Beach, Japan. Environ. Sci. Technol. 2012, 46, 10099–10105. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Bonifazi, G.; Fiore, L.; Pelosi, C.; Serranti, S. Evaluation of Plastic Packaging Waste Degradation in Seawater and Simulated Solar Radiation by Spectroscopic Techniques. Polym. Degrad. Stab. 2023, 207, 110215. [Google Scholar] [CrossRef]
- Qiu, S.-Q.; Huang, G.-Y.; Fang, G.-Z.; Li, X.-P.; Lei, D.-Q.; Shi, W.-J.; Xie, L.; Ying, G.-G. Chemical Characteristics and Toxicological Effects of Leachates from Plastics under Simulated Seawater and Fish Digest. Water Res. 2022, 209, 117892. [Google Scholar] [CrossRef]
- Dasgupta, S.; Peng, X.; Xu, H.; Ta, K.; Chen, S.; Li, J.; Du, M. Deep Seafloor Plastics as the Source and Sink of Organic Pollutants in the Northern South China Sea. Sci. Total Environ. 2021, 765, 144228. [Google Scholar] [CrossRef]
- Barco-Bonilla, N.; Romero-González, R.; Plaza-Bolaños, P.; Fernández-Moreno, J.L.; Garrido Frenich, A.; Martínez Vidal, J.L. Comprehensive Analysis of Polycyclic Aromatic Hydrocarbons in Wastewater Using Stir Bar Sorptive Extraction and Gas Chromatography Coupled to Tandem Mass Spectrometry. Anal. Chim. Acta 2011, 693, 62–71. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Atmospheric Pressure Gas Chromatography-Time-of-Flight-Mass Spectrometry (APGC-ToF-MS) for the Determination of Regulated and Emerging Contaminants in Aqueous Samples after Stir Bar Sorptive Extraction (SBSE). Anal. Chim. Acta 2014, 851, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Aizezi, N.; Feng, J.; Han, B.; Li, X.; Su, Z.; Li, L.; Liu, Y. Advanced Characterization of Industrial Smoke: Particle Composition and Size Analysis with Single Particle Aerosol Mass Spectrometry and Optimized Machine Learning. Anal. Chem. 2025, 97, 5554–5562. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.C.; Park, B.J.; Palace, V.P. Microplastics in Aquatic Environments: Implications for Canadian Ecosystems. Environ. Pollut. 2016, 218, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Federigi, I.; Verani, M.; Carducci, A. Organic Pollutants Associated with Plastic Debris in Marine Environment: A Systematic Review of Analytical Methods, Occurrence, and Characteristics. Int. J. Environ. Res. Public Health 2023, 20, 4892. [Google Scholar] [CrossRef]
- Ciufegni, E.; Asensio-Montesinos, F.; Castle, C.R.; Anfuso, G. Fresh Versus Beach Users’ Deposited Litter in El Puerto De Santa Maria (Cádiz, SW Spain). J. Mar. Sci. Eng. 2025, 13, 258. [Google Scholar] [CrossRef]
- Anfuso, G.; Rangel-Buitrago, N.; Cortés-Useche, C.; Iglesias Castillo, B.; Gracia, F.J. Characterization of Storm Events along the Gulf of Cadiz (Eastern Central Atlantic Ocean). Int. J. Climatol. 2016, 36, 3690–3707. [Google Scholar] [CrossRef]
- Williams, A.T.; Randerson, P.; Di Giacomo, C.; Anfuso, G.; Macias, A.; Perales, J.A. Distribution of Beach Litter along the Coastline of Cádiz, Spain. Mar. Pollut. Bull. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- INE Estadística de Movimientos Turísticos en Fronteras (FRONTUR). 2024. Available online: https://www.ine.es/dyngs/Prensa/FRONTUR1224.htm (accessed on 10 May 2025).
- Somerville, S.E.; Miller, K.L.; Mair, J.M. Assessment of the Aesthetic Quality of a Selection of Beaches in the Firth of Forth, Scotland. Mar. Pollut. Bull. 2003, 46, 1184–1190. [Google Scholar] [CrossRef]
- Asensio-Montesinos, F.; Anfuso, G.; Ramírez, M.O.; Smolka, R.; Sanabria, J.G.; Enríquez, A.F.; Arenas, P.; Bedoya, A.M. Beach Litter Composition and Distribution on the Atlantic Coast of Cádiz (SW Spain). Reg. Stud. Mar. Sci. 2020, 34, 101050. [Google Scholar] [CrossRef]
- David, F.; Thomais, V.; Georg, H. Joint List of Litter Categories for Marine Macro-Litter Monitoring: Manual for the Application of the Classification System; Publications Office of the European Union: Luxembourg, 2021; ISBN 9789276214458. [Google Scholar]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.J.; et al. Validation of ATR FT-IR to Identify Polymers of Plastic Marine Debris, Including Those Ingested by Marine Organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-09307-8. [Google Scholar]
- Kumar, A.; Naumenko, D.; Cozzarini, L.; Barba, L.; Cassetta, A.; Pedio, M. Influence of Substrate on Molecular Order for Self-assembled Adlayers of CoPc and FePc. J. Raman Spectrosc. 2018, 49, 1015–1022. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 978-0-12-386984-5. [Google Scholar]
- Zielinski, S.; Anfuso, G.; Botero, C.M.; Milanes, C.B. Beach Litter Assessment: Critical Issues and the Path Forward. Sustainability 2022, 14, 11994. [Google Scholar] [CrossRef]
- EA/NALG. Assessment of Aesthetic Quality of Coastal and Bathing Beaches. In Monitoring Protocol and Classification Scheme; Environment Agency and The National Aquatic Litter Group: Bristol, UK, 2000; p. 15. [Google Scholar]
- Maria, A.A.; Georg, H.; Perrine, L. Top Marine Beach Litter Items in Europe: A Review and Synthesis Based on Beach Litter Data; Publications Office: Luxembourg, 2017; ISBN 9789279877117. [Google Scholar]
- Nachite, D.; Maziane, F.; Anfuso, G.; Williams, A.T. Spatial and Temporal Variations of Litter at the Mediterranean Beaches of Morocco Mainly Due to Beach Users. Ocean. Coast. Manag. 2019, 179, 104846. [Google Scholar] [CrossRef]
- Munari, C.; Corbau, C.; Simeoni, U.; Mistri, M. Marine Litter on Mediterranean Shores: Analysis of Composition, Spatial Distribution and Sources in North-Western Adriatic Beaches. Waste Manag. 2016, 49, 483–490. [Google Scholar] [CrossRef]
- Fernández-Enríquez, A.; Anfuso, G.; Asensio-Montesinos, F.; Bakaj, A.; Ismailaj, M.; Cobaj, G. Distribution and Composition of Beach Litter along the Ionian Coastline of Albania. Water 2024, 16, 2370. [Google Scholar] [CrossRef]
- Topçu, E.N.; Tonay, A.M.; Dede, A.; Öztürk, A.A.; Öztürk, B. Origin and Abundance of Marine Litter along Sandy Beaches of the Turkish Western Black Sea Coast. Mar. Environ. Res. 2013, 85, 21–28. [Google Scholar] [CrossRef]
- Thiel, M.; Hinojosa, I.A.; Miranda, L.; Pantoja, J.F.; Rivadeneira, M.M.; Vásquez, N. Anthropogenic Marine Debris in the Coastal Environment: A Multi-Year Comparison between Coastal Waters and Local Shores. Mar. Pollut. Bull. 2013, 71, 307–316. [Google Scholar] [CrossRef]
- Chitaka, T.Y.; von Blottnitz, H. Accumulation and Characteristics of Plastic Debris along Five Beaches in Cape Town. Mar. Pollut. Bull. 2019, 138, 451–457. [Google Scholar] [CrossRef]
- Driedger, A.G.J.; Dürr, H.H.; Mitchell, K.; Van Cappellen, P. Plastic Debris in the Laurentian Great Lakes: A Review. J. Great Lakes Res. 2015, 41, 9–19. [Google Scholar] [CrossRef]
- Anfuso, G.; Álvarez, O.; Dilauro, G.; Sabato, G.; Scardino, G.; Sozio, A.; Rizzo, A. A First Attempt to Describe the Real-Time Behavior and Fate of Marine Litter Items in the Nearshore and Foreshore under Low Energetic Marine Conditions. Water 2024, 16, 409. [Google Scholar] [CrossRef]
- UNEP/MAP. Marine Litter Assessment in the Mediterranean; UNEP/MAP: Athens, Greece, 2015; ISBN 9789280735642. [Google Scholar]
- Mira Veiga, J.; Fleet, D.; Kinsey, S.; Nilsson, P.; Vlachogianni, T.; Werner, S.; Galgani, F.; Thompson, R.C.; Dagevos, J.; Gago, J.; et al. Identifying Sources of Marine Litter; The Joint Research Centre (JRC): Brussels, Belgium, 2016. [Google Scholar] [CrossRef]
- Vlachogianni, T.; Fortibuoni, T.; Ronchi, F.; Zeri, C.; Mazziotti, C.; Tutman, P.; Varezić, D.B.; Palatinus, A.; Trdan, Š.; Peterlin, M.; et al. Marine Litter on the Beaches of the Adriatic and Ionian Seas: An Assessment of Their Abundance, Composition and Sources. Mar. Pollut. Bull. 2018, 131, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, A.; Chuturkova, R. Marine Litter Accumulation along the Bulgarian Black Sea Coast: Categories and Predominance. Waste Manag. 2019, 84, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Ocean Conservancy. Available online: https://oceanconservancy.org (accessed on 2 July 2025).
- Surfrider Foundation Environmental Report of the Ocean Initiatives 2016. Available online: https://www.surfrider.org/ (accessed on 2 July 2025).
- Yousefi Nasab, A.; Oskoei, V.; Rezanasab, M.; Alinejad, N.; Hosseinzadeh, A.; Kashi, G. Cigarette Butt Littering Consequences: A Study of Pollution Rate on Beaches and Urban Environments. Environ. Sci. Pollut. Res. 2022, 29, 45396–45403. [Google Scholar] [CrossRef] [PubMed]
- Kungskulniti, N.; Charoenca, N.; Hamann, S.L.; Pitayarangsarit, S.; Mock, J. Cigarette Waste in Popular Beaches in Thailand: High Densities That Demand Environmental Action. Int. J. Environ. Res. Public Health 2018, 15, 630. [Google Scholar] [CrossRef]
- Loizidou, X.I.; Loizides, M.I.; Orthodoxou, D.L. Persistent Marine Litter: Small Plastics and Cigarette Butts Remain on Beaches after Organized Beach Cleanups. Environ. Monit. Assess. 2018, 190, 414. [Google Scholar] [CrossRef]
- Araújo, M.C.B.; Costa, M.F. A Critical Review of the Issue of Cigarette Butt Pollution in Coastal Environments. Environ. Res. 2019, 172, 137–149. [Google Scholar] [CrossRef]
- De Araújo, M.C.B.; da Costa, M.F. Cigarette Butts in Beach Litter: Snapshot of a Summer Holiday. Mar. Pollut. Bull. 2021, 172, 112858. [Google Scholar] [CrossRef]
- Asensio-Montesinos, F.; Anfuso, G.; Randerson, P.; Williams, A.T. Seasonal Comparison of Beach Litter on Mediterranean Coastal Sites (Alicante, SE Spain). Ocean. Coast. Manag. 2019, 181, 104914. [Google Scholar] [CrossRef]
- Asensio-Montesinos, F.; Ramírez, M.O.; Aguilar-Torrelo, M.T.; Anfuso, G. Abundance and Distribution of Cigarette Butts on Coastal Environments: Examples from Southern Spain. J. Mar. Sci. Eng. 2021, 9, 129. [Google Scholar] [CrossRef]
- Fernández García, G.; Asensio-Montesinos, F.; Anfuso, G.; Arenas-Granados, P. Beach Litter Variability According to the Number of Visitors in Cádiz Beaches, SW Spain. J. Mar. Sci. Eng. 2024, 12, 201. [Google Scholar] [CrossRef]
- Ciufegni, E.; Anfuso, G.; Gutiérrez Romero, J.C.; Asensio-Montesinos, F.; Rodríguez Castle, C.; González, C.J.; Álvarez, O. Spatial and Temporal Deposition Rate of Beach Litter in Cadiz Bay (Southwest Spain). Sustainability 2024, 16, 1010. [Google Scholar] [CrossRef]
- Allison, N.L.; Dale, A.C.; Narayanaswamy, B.E.; Turrell, W.R. Investigating Local Trawl Fishing as a Source of Plastic Beach Litter. Mar. Pollut. Bull. 2024, 205, 116627. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R.; Andrady, A.L. Plastics in the Marine Environment. In Plastics and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 379–401. ISBN 0471095206. [Google Scholar]
- Soares, J.B.P.; McKenna, T.F.L. Introduction to Polyolefins. In Polyolefin Reaction Engineering; John Wiley & Sons: Weinheim, Germany, 2012; ISBN 978-3-527-64694-4. [Google Scholar]
- Pelsmaekers, E.P.B.; Janssen, C.R.; Cannicci, S.; Munga, C.N.; Dahdouh-Guebas, F. Assessing Plastic Pollution in Kenyan Mangroves: Distribution, Sources, and Social Impact in Gazi Bay. Estuaries Coasts 2025, 48, 135. [Google Scholar] [CrossRef]
- Schmid, C.; Cozzarini, L.; Zambello, E. A Critical Review on Marine Litter in the Adriatic Sea: Focus on Plastic Pollution. Environ. Pollut. 2021, 273, 116430. [Google Scholar] [CrossRef]
- Gajšt, T.; Bizjak, T.; Palatinus, A.; Liubartseva, S.; Kržan, A. Sea Surface Microplastics in Slovenian Part of the Northern Adriatic. Mar. Pollut. Bull. 2016, 113, 392–399. [Google Scholar] [CrossRef]
- Korez, Š.; Gutow, L.; Saborowski, R. Microplastics at the Strandlines of Slovenian Beaches. Mar. Pollut. Bull. 2019, 145, 334–342. [Google Scholar] [CrossRef]
- Oluniyi Solomon, O.; Palanisami, T. Microplastics in the Marine Environment: Current Status, Assessment Methodologies, Impacts and Solutions. J. Pollut. Eff. Control 2016, 4, 161. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Chen, W.; Zhu, B.; Qu, S.; Xu, M. Critical Review of Global Plastics Stock and Flow Data. J. Ind. Ecol. 2021, 25, 1300–1317. [Google Scholar] [CrossRef]
- Cozzarini, L.; Buoninsegni, J.; Corbau, C.; Lughi, V. Characterization of Large Microplastic Debris in Beach Sediments in the Po Delta Area. Microplastics 2023, 2, 147–157. [Google Scholar] [CrossRef]
- Das, T.; Das, N.; Zuthi, M.F.R.; Pal, S.K.; Kraft, E.; Haupt, T.; Kuehlewindt, S. Plastic Waste in Marine Ecosystems: Identification Techniques and Policy Interventions. Water Air Soil. Pollut. 2025, 236, 478. [Google Scholar] [CrossRef]
- Schwarz, A.E.; Ligthart, T.N.; Boukris, E.; van Harmelen, T. Sources, Transport, and Accumulation of Different Types of Plastic Litter in Aquatic Environments: A Review Study. Mar. Pollut. Bull. 2019, 143, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.T.; Randerson, P.; Allen, C.; Cooper, J.A.G. Beach Litter Sourcing: A Trawl along the Northern Ireland Coastline. Mar. Pollut. Bull. 2017, 122, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Willis, K.; Denise Hardesty, B.; Kriwoken, L.; Wilcox, C. Differentiating Littering, Urban Runoff and Marine Transport as Sources of Marine Debris in Coastal and Estuarine Environments. Sci. Rep. 2017, 7, 44479. [Google Scholar] [CrossRef]
- Buoninsegni, J.; Anfuso, G.; Asensio-Montesinos, F.; Marrocchino, E.; Vaccaro, C. The Seasonal and Cross-Shore Distribution of Beach Litter Along Four Sites on the Northern Adriatic Coast (Ferrara, Italy). Water 2025, 17, 2173. [Google Scholar] [CrossRef]
- Trapletti-Lanti, Y.; Expósito-Granados, M.; Álvarez-Ruiz, S.; López-Martínez, S.; Ansoar-Rodríguez, Y.; Bertrand, L.; Rimondino, G.N.; Rivas, M.L. Characterisation of Plastic Debris (Macro-, Meso-, and Microplastics) from Stranded Alcids in Southern Spain. J. Hazard. Mater. 2025, 492, 138128. [Google Scholar] [CrossRef]
- Gunaalan, K.; Fabbri, E.; Capolupo, M. The Hidden Threat of Plastic Leachates: A Critical Review on Their Impacts on Aquatic Organisms. Water Res. 2020, 184, 116170. [Google Scholar] [CrossRef]
- Mandal, S.; Bolan, N.S.; Sarkar, B.; Wijesekara, H.; Bradney, L.; Kirkham, M.B. Environmentally Toxic Components of Particulate Plastics. In Particulate Plastics in Terrestrial and Aquatic Environments, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 165–179. [Google Scholar]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Rowland, S.J.; Thompson, R.C.; Galloway, T.S.; Yamashita, R.; et al. Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef]
- Frigione, M.; Marini, G.; Pinna, M.A. Thermal Analysis-Based Approach to Identify Different Waste Macroplastics in Beach Litter: The Case Study of Aquatina Di Frigole NATURA 2000 Site (IT9150003, Italy). Sustainability 2021, 13, 3186. [Google Scholar] [CrossRef]
- Browne, M.A.; Galloway, T.; Thompson, R. Microplastic—An Emerging Contaminant of Potential Concern? Integr. Environ. Assess. Manag. 2007, 3, 559–561. [Google Scholar] [CrossRef]
- Andrady, A.L. Plastics and the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 0471095206. [Google Scholar]
- Nowak, B.; Pająk, J.; Drozd-Bratkowicz, M.; Rymarz, G. Microorganisms Participating in the Biodegradation of Modified Polyethylene Films in Different Soils under Laboratory Conditions. Int. Biodeterior. Biodegrad. 2011, 65, 757–767. [Google Scholar] [CrossRef]
- Gómez, E.F.; Michel, F.C. Biodegradability of Conventional and Bio-Based Plastics and Natural Fiber Composites during Composting, Anaerobic Digestion and Long-Term Soil Incubation. Polym. Degrad. Stab. 2013, 98, 2583–2591. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A Critical Analysis of the Biological Impacts of Plasticizers on Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An Overview of Chemical Additives Present in Plastics: Migration, Release, Fate and Environmental Impact during Their Use, Disposal and Recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-Fragmentation of Marine Plastic Waste and Their Environmental Implications: A Critical Review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Godoy, V.; Martín-Lara, M.A.; Calero, M.; Blázquez, G. The Relevance of Interaction of Chemicals/Pollutants and Microplastic Samples as Route for Transporting Contaminants. Process Saf. Environ. Prot. 2020, 138, 312–323. [Google Scholar] [CrossRef]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The Ecological Impacts of Marine Debris: Unraveling the Demonstrated Evidence from What Is Perceived. Ecology 2016, 97, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Rochman, C.M.; Browne, M.A.; Halpern, B.S.; Hentschel, B.T.; Hoh, E.; Karapanagioti, H.K.; Rios-Mendoza, L.M.; Takada, H.; Teh, S.; Thompson, R.C. Classify Plastic Waste as Hazardous. Nature 2013, 494, 169–171. [Google Scholar] [CrossRef]
- Wang, J.; Tan, Z.; Peng, J.; Qiu, Q.; Li, M. The Behaviors of Microplastics in the Marine Environment. Mar. Environ. Res. 2016, 113, 7–17. [Google Scholar] [CrossRef]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic Transfer of Microplastics and Mixed Contaminants in the Marine Food Web and Implications for Human Health. Environ. Int. 2018, 115, 400–409. [Google Scholar] [CrossRef]
- Wang, W.; Gao, H.; Jin, S.; Li, R.; Na, G. The Ecotoxicological Effects of Microplastics on Aquatic Food Web, from Primary Producer to Human: A Review. Ecotoxicol. Environ. Saf. 2019, 173, 110–117. [Google Scholar] [CrossRef]
- Sridharan, S.; Kumar, M.; Saha, M.; Kirkham, M.B.; Singh, L.; Bolan, N.S. The Polymers and Their Additives in Particulate Plastics: What Makes Them Hazardous to the Fauna? Sci. Total Environ. 2022, 824, 153828. [Google Scholar] [CrossRef] [PubMed]
- Bester, K.; Klasmeier, J.; Kupper, T. Emissions of OTNE (Iso-E-Super)—Mass Flows in Sewage Treatment Plants. Chemosphere 2008, 71, 2003–2010. [Google Scholar] [CrossRef] [PubMed]
- Ehiguese, F.O.; Alam, M.R.; Pintado-Herrera, M.G.; Araújo, C.V.M.; Martin-Diaz, M.L. Potential of Environmental Concentrations of the Musks Galaxolide and Tonalide to Induce Oxidative Stress and Genotoxicity in the Marine Environment. Mar. Environ. Res. 2020, 160, 105019. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.R.; de la Luz Tovar Salvador, M.; Pintado-Herrera, M.G.; Albergaria-Barbosa, A.C.R.; Martins, C.C.; Lourenço, R.A.; Combi, T. Legacy and Novel Contaminants in Surface Sediments of Admiralty Bay, Antarctica Peninsula. Sci. Total Environ. 2024, 951, 175551. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.D.; Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Sandstrom, M.W. Is There a Risk Associated with the Insect Repellent DEET (N,N-Diethyl-m-Toluamide) Commonly Found in Aquatic Environments? Sci. Total Environ. 2007, 384, 214–220. [Google Scholar] [CrossRef]
- Mena, E.; Rey, A.; Rodríguez, E.M.; Beltrán, F.J. Reaction Mechanism and Kinetics of DEET Visible Light Assisted Photocatalytic Ozonation with WO3 Catalyst. Appl. Catal. B 2017, 202, 460–472. [Google Scholar] [CrossRef]
- Dvorská, A.; Lammel, G.; Klánová, J. Use of Diagnostic Ratios for Studying Source Apportionment and Reactivity of Ambient Polycyclic Aromatic Hydrocarbons over Central Europe. Atmos. Environ. 2011, 45, 420–427. [Google Scholar] [CrossRef]
- Gbeddy, G.; Egodawatta, P.; Goonetilleke, A.; Akortia, E.; Glover, E.T. Influence of Photolysis on Source Characterization and Health Risk of Polycyclic Aromatic Hydrocarbons (PAHs), and Carbonyl-, Nitro-, Hydroxy-PAHs in Urban Road Dust. Environ. Pollut. 2021, 269, 116103. [Google Scholar] [CrossRef]
- Haleem, A.M.; Amin, S.; Mahmood, U.H. Heavy Metal and Polycyclic Aromatic Hydrocarbons in Cigarettes: An Analytical Assessment. Popul. Med. 2020, 2, 19. [Google Scholar] [CrossRef]




| Item(s) | Sample | Fragment | FTIR Group | Search Library | Search Best Hit Description |
|---|---|---|---|---|---|
| Dust wipes | A | 1 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Wet wipes | A | 2 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Sanitary towels | A | 3 | PE | W | W01468.SP W01468 PETROTHENE LR 923 POLYETHYLENE RESIN |
| Credit card | A | 4 | PVC | W | W01532.SP W01532 DURAL 600 POLYVINYL CHLORIDE |
| Cable tie | A | 5 | PA | POLIMERI | PA 6/6—NYLON 6/6 (POLY HEXAMETHYLENE ADIPAMIDE) |
| Transparent wardrobe freshener package | A | 6 | PVC | polyatr | POLY(VINYL CHLORIDE), CARBOXYLATED 1.8% CARBOXYLATED |
| Clear blue glass | A | 7 | PP | W | W01161.SP W01161 REXENE PP11S5 |
| Medicine blister pack | A | 8 | PVC | polyatr | POLY(VINYL CHLORIDE) INHERENT VISCOSITY 1.26 |
| Fork | A | 9 | PS | POLIMERI | POLYSTYRENE—UATR SPECTRUM |
| Green clothes peg | A | 10 | PP | W | W01448.SP W01448 FORTILENE 3251 POLYPROPYLENE |
| Blue hard plastic tube | A | 11 | ABS | POLIMERI | ABS—ACRYLONITRILE-STYRENE-BUTADIENE COPOLYMER—UNIVERSAL ATR |
| White piece of plastic | A | 12 | PP | POLIMERI | POLYPROPYLENE + CALCIUM CARBONATE |
| Corrugated tube | A | 13 | PVC | W | W01534.SP W01534 DURAL 602 NATURAL POLYVINYL CHLORIDE |
| Black piece of plastic | A | 14 | PP | POLIMERI | POLYPROPYLENE + CALCIUM CARBONATE |
| Creamy white soft tube | A | 15 | PVC | W | W01543.SP W01543 PVC 2214-85 CLEAR POLYVINYL CHLORIDE EXTRUSION COMPOUN |
| Inner layer of tetrapack package | B | 1 | PE | W | W02032.SP W02032 NEOPOLEN 1720 MOLDABLE POLY-ETHYLENE FOAM |
| Starlight package for fishing | B | 2 | PE | W | W01468.SP W01468 PETROTHENE LR 923 POLYETHYLENE RESIN |
| Green and black sweet wrapper | B | 3 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Condom package | B | 4 | PA | polyatr | POLYAMIDE RESIN MELTING PT 95DEG C |
| Small gray bag | B | 5 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Yellow piece of plastic | B | 7 | PE | polyatr | POLYETHYLENE HIGH DENSITY |
| Blue adhesive label | B | 8 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Transparent film (n. 1, in Figure 2) | B | 9 | PET | polyatr | POLY(ETHYLENE THEEPHTHALATE) |
| Transparent and purple sweet wrapper | B | 10 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| White and blue sweet wrapper | B | 11 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Transparent film (n. 2, in Figure 2) | B | 12 | PP | W | W01972.SP W01972 NOVA-PAC POLYPROPYLENE SEED BAG REINFORCED WITH POLYOL |
| Fragment of white bag | B | 13 | PE | polyatr | POLYETHYLENE, CHLOROSULFONATED |
| Transparent straw wrapper | B | 14 | PP | W | W01972.SP W01972 NOVA-PAC POLYPROPYLENE SEED BAG REINFORCED WITH POLYOL |
| Brown piece of plastic | B | 15 | PVC | W | W01543.SP W01543 PVC 2214-85 CLEAR POLYVINYL CHLORIDE EXTRUSION COMPOUN |
| Transparent film (n. 3, in Figure 2) | B | 16 | PET | W | W01904.SP W01904 MELINEX 377/200 POLYESTER FILM TRANSLUCENT MATTE FILM |
| Fragment of green bag | B | 17 | Noplastic | POLIMERI | CELLULOSE |
| Outer layer of tetrapak | B | 18 | PE | W | W01468.SP W01468 PETROTHENE LR 923 POLYETHYLENE RESIN |
| Fragment of blue bag | B | 19 | PVC | W | W01215.SP W01215 ALPHA PVC 2212-100 CLEAR POLY- VINYL CHLORIDE |
| Black electrical tape | B | 20 | PET | POLIMERI | THERMOPLASTIC ELASTOMER POLYESTER BASE—HYTREL |
| Site | Concentrations (ng m−1) | ||
|---|---|---|---|
| PAHs | Pesticides | Fragrances | |
| La Jara | 0.01 | 0.31 | 1.03 |
| Bajo de Guía | 0.07 | 7.66 | 28.47 |
| La Puntilla | 11.61 | 26.84 | 100.50 |
| Site | Concentrations (ng m−1) | ||
|---|---|---|---|
| PAHs | Pesticides | Fragrances | |
| Bajo de Guía | 1.06 | 4.52 | 26.66 |
| La Puntilla | 2.75 | 19.03 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traverso-Soto, J.M.; Figueredo, M.; Punta-Sánchez, I.; Campana, O.; Ciufegni, E.; Hampel, M.; Buoninsegni, J.; Manzano Quiñones, M.A.; Anfuso, G. Assessment of Organic Pollutants Desorbed from Plastic Litter Items Stranded on Cadiz Beaches (SW Spain). Toxics 2025, 13, 673. https://doi.org/10.3390/toxics13080673
Traverso-Soto JM, Figueredo M, Punta-Sánchez I, Campana O, Ciufegni E, Hampel M, Buoninsegni J, Manzano Quiñones MA, Anfuso G. Assessment of Organic Pollutants Desorbed from Plastic Litter Items Stranded on Cadiz Beaches (SW Spain). Toxics. 2025; 13(8):673. https://doi.org/10.3390/toxics13080673
Chicago/Turabian StyleTraverso-Soto, Juan Manuel, Manuel Figueredo, Irene Punta-Sánchez, Olivia Campana, Elisabetta Ciufegni, Miriam Hampel, Joana Buoninsegni, Manuel A. Manzano Quiñones, and Giorgio Anfuso. 2025. "Assessment of Organic Pollutants Desorbed from Plastic Litter Items Stranded on Cadiz Beaches (SW Spain)" Toxics 13, no. 8: 673. https://doi.org/10.3390/toxics13080673
APA StyleTraverso-Soto, J. M., Figueredo, M., Punta-Sánchez, I., Campana, O., Ciufegni, E., Hampel, M., Buoninsegni, J., Manzano Quiñones, M. A., & Anfuso, G. (2025). Assessment of Organic Pollutants Desorbed from Plastic Litter Items Stranded on Cadiz Beaches (SW Spain). Toxics, 13(8), 673. https://doi.org/10.3390/toxics13080673











