Effects of Diethylstilbestrol on Uterus Structure and Immunological Function in Mice During Early Pregnancy
Highlights
- In pregnant mice, DES prolongs the estrous cycle and decreases the number of embryos, additionally inducing imbalances in estrogen and progesterone levels.
- It disrupts the local immune balance of the uterus in pregnant rats, causing endometrial histological changes.
- It reduces the antioxidant capacity of uterine and spleen tissues and intensifies oxidative stress and inflammatory responses.
- DES suppresses murine reproduction by interfering with early pregnancy processes.
- By reducing fertility, it can limit population growth and density in mice.
- A decrease in mouse population density may lower the risk of plague transmission within wild populations.
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry and Tissue Collection
2.2. Serum Hormone and Cytokine Measurement
2.3. Hematoxylin and Eosin Staining
2.4. Lymphocyte Proliferation Assay
2.5. Immunohistochemistry
2.6. Antioxidant Index Assays
2.7. Statistical Analysis
3. Results
3.1. DES-Induced Changes in Maternal Body Weight, Organ Development, and Embryo Number
3.2. DES-Induced Changes in Uterine Histology and Serum Hormone Levels
3.3. DES-Induced Changes in Local Immune Responses in the Uterus and Spleen
3.4. DES-Induced Changes in Cell Proliferation, Apoptosis, and Oxidative Stress in the Uterus
3.5. DES-Induced Changes in Antioxidant Capacity of Uterine and Splenic Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahmoudi, A.; Kryštufek, B.; Sludsky, A.; Schmid, B.V.; De Almeida, A.M.; Lei, X.; Ramasindrazana, B.; Bertherat, E.; Yeszhanov, A.; Stenseth, N.C. Plague reservoir species throughout the world. Integr. Zool. 2021, 16, 820–833. [Google Scholar] [CrossRef]
- Rahelinirina, S.; Scobie, K.; Ramasindrazana, B.; Andrianaivoarimanana, V.; Rasoamalala, F.; Randriantseheno, L.N.; Rakotoniaina, J.S.; Gorge, O.; Lambin, X.; Valade, E. Rodent control to fight plague: Field assessment of methods based on rat density reduction. Integr. Zool. 2021, 16, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Singleton, G.R. Ecologically-based rodent management integrating new developments in biotechnology. Proc. Vertebr. Pest Conf. 2000, 19, 221–227. [Google Scholar] [CrossRef]
- Singleton, G.R. Ecologically-based rodent management 15 years on: A pathway to sustainable agricultural production. Proc. Vertebr. Pest Conf. 2014, 26, 176–179. [Google Scholar] [CrossRef]
- Cowan, D.P.; Massei, G. Wildlife contraception, individuals, and populations: How much fertility control is enough? Proc. Vertebr. Pest Conf. Pest Conf. 2008, 23, 220–228. [Google Scholar] [CrossRef]
- Fagerstone, K.A. Wildlife Fertility Control. In USDA National Wildlife Research Center Staff Publications, Proceedings of the Wildlife Fertility Control Conference, Fort Collins, CO, USA, 2002; USDA National Wildlife Research Center: Fort Collins, CO, USA, 2002; p. 489. [Google Scholar]
- Massei, G.; Cowan, D. Fertility control to mitigate human–wildlife conflicts: A review. Wildl. Res. 2014, 41, 1–21. [Google Scholar] [CrossRef]
- Clements, D.R.; Shen, S. Ecological management of invasive alien plants. Front. Environ. Sci. 2023, 14, 1345472. [Google Scholar] [CrossRef]
- Wiersma, A.; Hirsch, B.; Tsafriri, A.; Hanssen, R.; Van de Kant, M.; Kloosterboer, H.; Conti, M.; Hsueh, A. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J. Clin. Investig. 1998, 102, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Song, R.; Zhang, L. Effect of oxidative stress on the estrogen-NOS-NO-KCa channel pathway in uteroplacental dysfunction: Its implication in pregnancy complications. Oxid. Med. Cell. Longev. 2019, 1, 9194269. [Google Scholar] [CrossRef]
- Sultana, Z.; Qiao, Y.; Maiti, K.; Smith, R. Involvement of oxidative stress in placental dysfunction, the pathophysiology of fetal death and pregnancy disorders. Reproduction 2023, 166, R25–R38. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martinez, O.; De Leon-Oliva, D.; Boaru, D.L.; Garcia-Puente, L.M.; De León-Luis, J.A.; Bravo, C.; Diaz-Pedrero, R.; Lopez-Gonzalez, L.; Álvarez-Mon, M. Exploring the role of Mediterranean and Westernized diets and their main nutrients in the modulation of oxidative stress in the placenta: A narrative review. Antioxidants 2023, 12, 1918. [Google Scholar] [CrossRef]
- Schjenken, J.E.; Green, E.S.; Overduin, T.S.; Mah, C.Y.; Russell, D.L.; Robertson, S.A. Endocrine disruptor compounds—A cause of impaired immune tolerance driving inflammatory disorders of pregnancy? Front. Endocrinol. 2021, 12, 607539. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine disrupting chemicals and disease susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef]
- Zama, A.M.; Bhurke, A.; Uzumcu, M. Effects of endocrine-disrupting chemicals on female reproductive health. Open Biotechnol. J. 2016, 10, 205–229. [Google Scholar] [CrossRef]
- Araújo, R.S. Profilaxia da peste. Rev. Soc. Bras. Med. Trop. 1967, 1, 327–336. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Li, Y.; Li, H.; Song, X.; Ma, Z.; Lu, H.; Liu, S.; Zhao, Y.; Tan, M.; Wang, S. Identification of Toxoplasma gondii tyrosine hydroxylase (TH) activity and molecular immunoprotection against toxoplasmosis. Vaccines 2020, 8, 158. [Google Scholar] [CrossRef]
- Chen, F.; Reheman, A.; Cao, J.; Wang, Z.; Dong, Y.; Zhang, Y.; Chen, Y. Effect of melatonin on monochromatic light-induced T-lymphocyte proliferation in the thymus of chickens. J. Photochem. Photobiol. B 2016, 161, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Shuster, S.M.; Pyzyna, B.; Mayer, L.P.; Dyer, C.A. The opportunity for sexual selection and the evolution of non-responsiveness to pesticides, sterility inducers and contraceptives. Heliyon 2018, 4, e00900. [Google Scholar] [CrossRef]
- Page, L.; Younge, N.; Freemark, M. Hormonal determinants of growth and weight gain in the human fetus and preterm infant. Nutrients 2023, 15, 4041. [Google Scholar] [CrossRef] [PubMed]
- Limones, M.; Sevillano, J.; Sánchez-Alonso, M.G.; Herrera, E.; Ramos-Álvarez, M.P. Metabolic alterations associated with maternal undernutrition during the first half of gestation lead to a diabetogenic state in the rat. Eur. J. Nutr. 2019, 58, 2521–2533. [Google Scholar] [CrossRef] [PubMed]
- Sammin, D.; Markey, B.; Bassett, H.; Buxton, D. The ovine placenta and placentitis—A review. Vet. Microbiol. 2009, 135, 90–97. [Google Scholar] [CrossRef]
- Aréchiga-Flores, C.; Cortés-Vidauri, Z.; Hernández-Briano, P.; Flores-Flores, G.; Rochín-Berumen, F.; Ruiz-Fernández, E. Function and regression of the corpus luteum during the estrous cycle. Abanico Vet. 2019, 9, 1–21. [Google Scholar]
- Parisi, F.; Fenizia, C.; Introini, A.; Zavatta, A.; Scaccabarozzi, C.; Biasin, M.; Savasi, V. The pathophysiological role of estrogens in the initial stages of pregnancy: Molecular mechanisms and clinical implications for pregnancy outcome from the periconceptional period to end of the first trimester. Hum. Reprod. Update 2023, 29, 699–720. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; LeBlanc, S.J. Graduate Student Literature Review: Implications of transition cow health for reproductive function and targeted reproductive management. J. Dairy Sci. 2024, 10, 8234–8246. [Google Scholar] [CrossRef]
- Liao, B.; Qi, X.; Yun, C.; Qiao, J.; Pang, Y. Effects of androgen excess-related metabolic disturbances on granulosa cell function and follicular development. Front. Endocrinol. 2022, 13, 815968. [Google Scholar] [CrossRef]
- Suthaporn, S.; Jayaprakasan, K.; Thornton, J.; Walker, K.; Medrano, J.H.; Castellanos, M.; May, S.; Polanski, L.; Raine-Fenning, N.; Maalouf, W.E. Suboptimal mid-luteal progesterone concentrations are associated with aberrant endometrial gene expression, potentially resulting in implantation failure. Reprod. Biomed. Online 2021, 42, 595–608. [Google Scholar] [CrossRef]
- Massimiani, M.; Lacconi, V.; La Civita, F.; Ticconi, C.; Rago, R.; Campagnolo, L. Molecular signaling regulating endometrium–blastocyst crosstalk. Int. J. Mol. Sci. 2019, 21, 23. [Google Scholar] [CrossRef]
- Mantena, S.R.; Kannan, A.; Cheon, Y.-P.; Li, Q.; Johnson, P.F.; Bagchi, I.C.; Bagchi, M.K. C/EBPβ is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc. Natl. Acad. Sci. USA 2006, 103, 1870–1875. [Google Scholar] [CrossRef]
- Reis, F.M.; Petraglia, F.; Taylor, R.N. Endometriosis: Hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 2013, 19, 406–418. [Google Scholar] [CrossRef]
- Kong, L.; Tang, M.; Zhang, T.; Wang, D.; Hu, K.; Lu, W.; Wei, C.; Liang, G.; Pu, Y. Nickel nanoparticles exposure and reproductive toxicity in healthy adult rats. Int. J. Mol. Sci. 2014, 15, 21253–21269. [Google Scholar] [CrossRef]
- Segerer, S.; Sonntag, B.; Gutensohn, K.; Keck, C. Hormonanalytik–was der Frauenarzt wissen muss. Gynäkologe 2018, 51, 891–909. [Google Scholar] [CrossRef]
- Sellix, M.T.; Menaker, M. Circadian clocks in the ovary. Trends Endocrinol. Metab. 2010, 21, 628–636. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, T.; Li, C.; Zhang, X.; Shen, H. Immunoregulatory therapy improves reproductive outcomes in elevated Th1/Th2 women with embryo transfer failure. Biomed. Res. Int. 2022, 1, 4990184. [Google Scholar] [CrossRef]
- Warning, J.C.; McCracken, S.A.; Morris, J.M. A balancing act: Mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 2011, 141, 715–724. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Wang, C.; Yang, Q.; Zhou, X. SB-273005, an antagonist of αvβ3 integrin, reduces the production of Th2 cells and cytokine IL-10 in pregnant mice. Exp. Ther. Med. 2014, 7, 1677–1682. [Google Scholar] [CrossRef][Green Version]
- Walker, C.G.; Meier, S.; Littlejohn, M.D.; Lehnert, K.; Roche, J.R.; Mitchell, M.D. Modulation of the maternal immune system by the pre-implantation embryo. BMC Genom. 2010, 11, 474. [Google Scholar] [CrossRef]
- Martínez-Varea, A.; Pellicer, B.; Perales-Marín, A.; Pellicer, A. Relationship between maternal immunological response during pregnancy and onset of preeclampsia. J. Immunol. Res. 2014, 2014, 210241. [Google Scholar] [CrossRef]
- Morelli, S.S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 2015, 171–189. [Google Scholar] [CrossRef]
- Ratthé, C.; Ennaciri, J.; Garcês Gonçalves, D.M.; Chiasson, S.; Girard, D. Interleukin (IL)-4 induces leukocyte infiltration in vivo by an indirect mechanism. Mediat. Inflamm. 2009, 2009, 193970. [Google Scholar] [CrossRef]
- Ticconi, C.; Pietropolli, A.; Di Simone, N.; Piccione, E.; Fazleabas, A. Endometrial immune dysfunction in recurrent pregnancy loss. Int. J. Mol. Sci. 2019, 20, 5332. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Zhang, L. Hypoxia and mitochondrial dysfunction in pregnancy complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Matsubara, K.; Higaki, T.; Matsubara, Y.; Nawa, A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int. J. Mol. Sci. 2015, 16, 4600–4614. [Google Scholar] [CrossRef]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of oxidative stress on pregnancy. Oxid. Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef]
- Ashok, A.; Gupta, S.; Malhotra, N.; Sharma, D. Oxidative stress and its role in female infertility and assisted reproduction: Clinical implications. Int. J. Fertil. Steril. 2009, 2, 147–164. [Google Scholar]
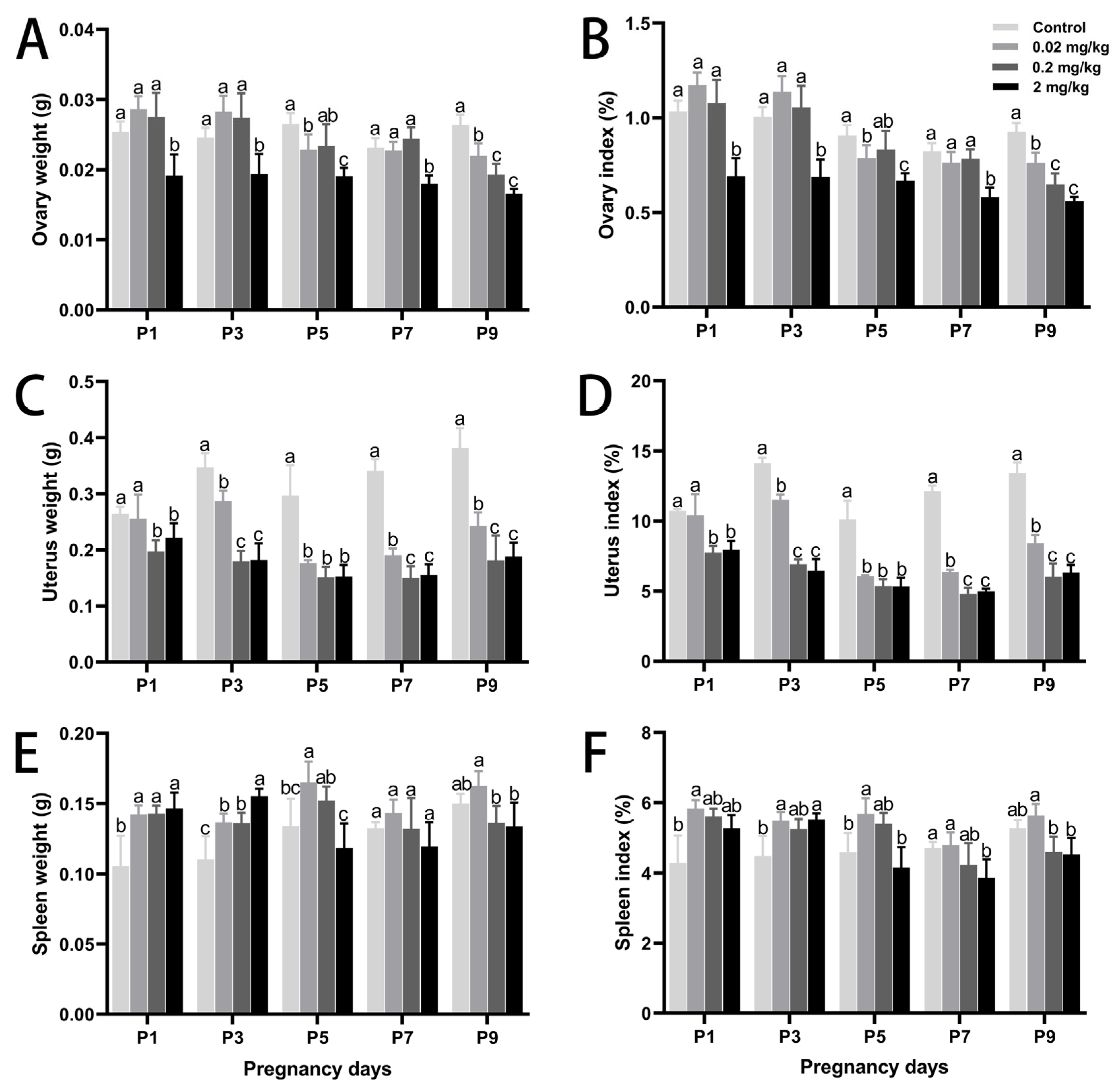

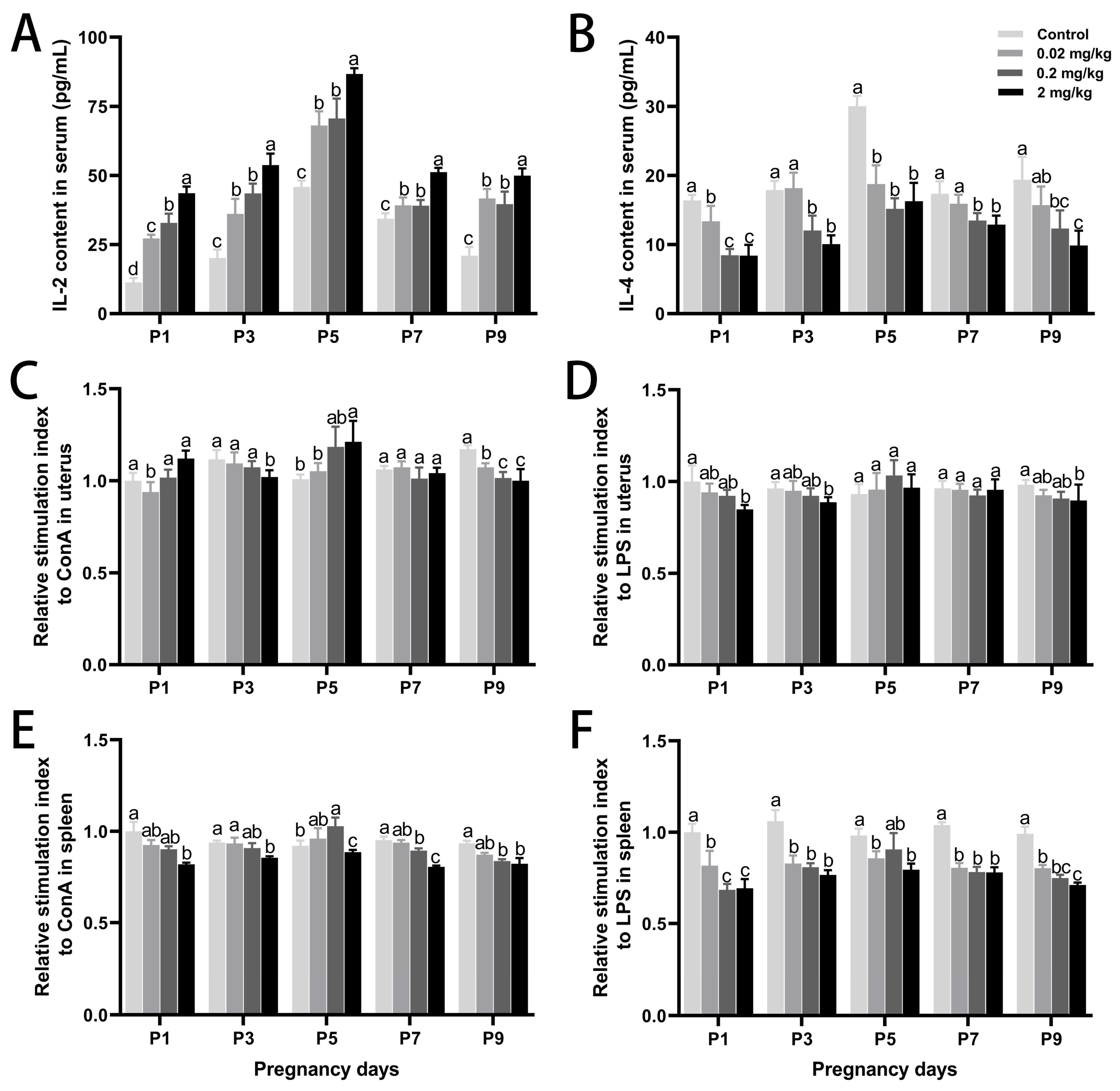
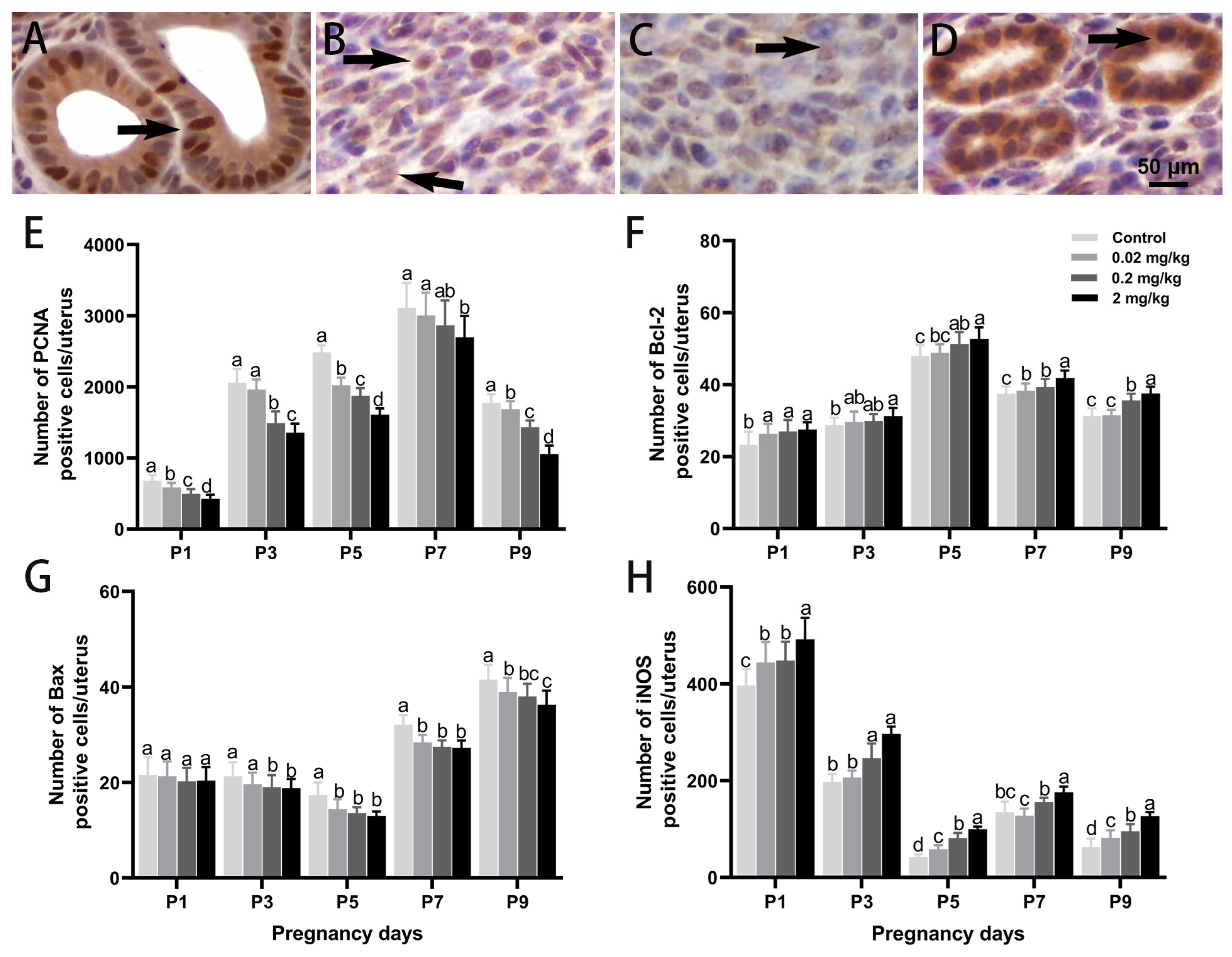
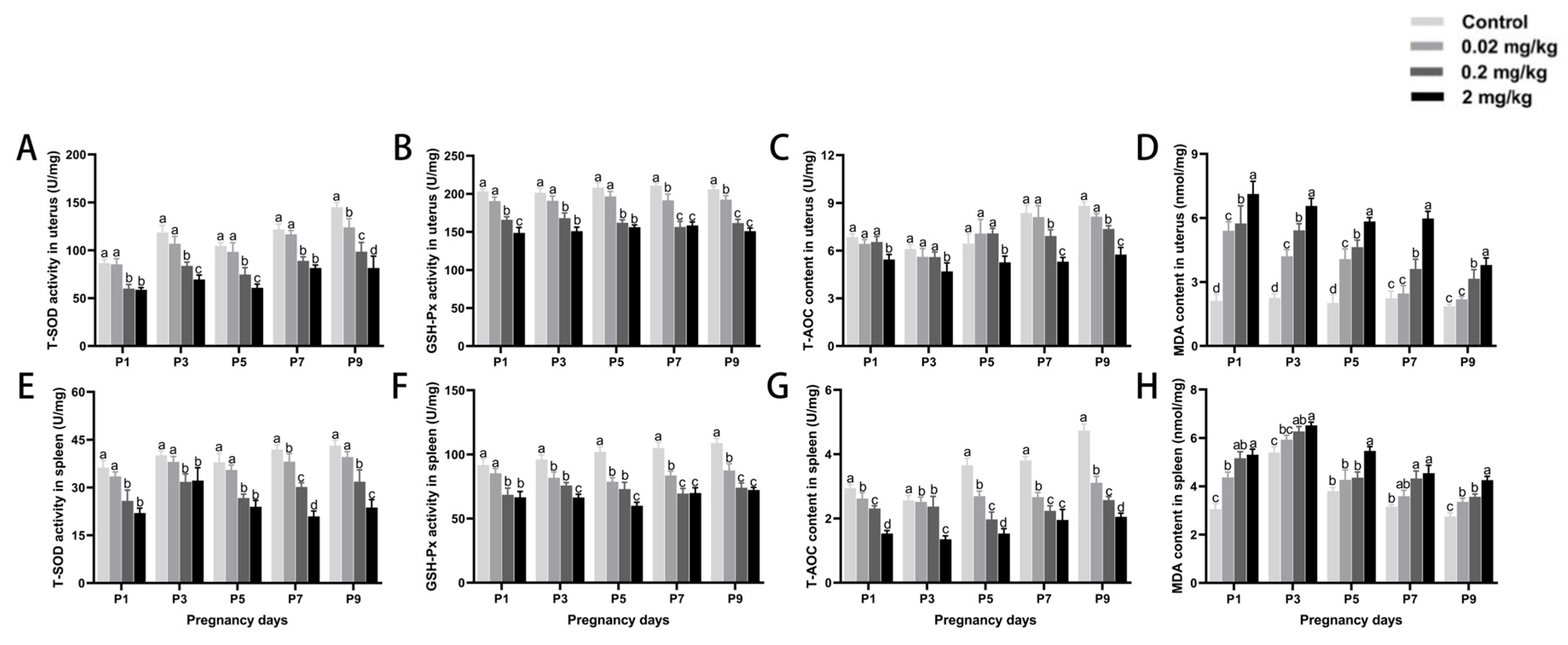
| DES Dose (mg/kg·BW) | Control | 0.02 mg/kg | 0.2 mg/kg | 2 mg/kg | |
| Body Weight (g) | P1 | 24.61 ± 1.20 b | 24.41 ± 0.72 b | 25.51 ± 0.93 b | 27.72 ± 1.08 a |
| P3 | 24.58 ± 1.10 b | 24.87 ± 0.86 b | 25.95 ± 1.34 b | 28.14 ± 0.92 a | |
| P5 | 29.23 ± 1.47 a | 29.04 ± 1.15 a | 28.10 ± 0.92 a | 28.49 ± 0.56 a | |
| P7 | 28.12 ± 0.74 a | 29.93 ± 2.66 a | 31.19 ± 1.59 a | 31.00 ± 2.63 a | |
| P9 | 28.42 ± 1.05 a | 28.84 ± 0.70 a | 29.81 ± 2.80 a | 29.62 ± 1.44 a | |
| Estrous Cycle | 4.60 ± 0.55 a | 5.20 ± 0.76 ab | 6.10 ± 0.34 b | 7.30 ± 0.48 c | |
| Early-Pregnancy Embryo Number | 12.50 ± 1.34 a | 9.50 ± 1.16 b | 6.00 ± 2.15 c | 3.30 ± 0.59 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Xu, R.; Wang, G.; Su, Y.; Chen, Y.; Cao, J. Effects of Diethylstilbestrol on Uterus Structure and Immunological Function in Mice During Early Pregnancy. Toxics 2025, 13, 672. https://doi.org/10.3390/toxics13080672
Li J, Xu R, Wang G, Su Y, Chen Y, Cao J. Effects of Diethylstilbestrol on Uterus Structure and Immunological Function in Mice During Early Pregnancy. Toxics. 2025; 13(8):672. https://doi.org/10.3390/toxics13080672
Chicago/Turabian StyleLi, Jian, Ruiping Xu, Guan Wang, Yanhua Su, Yaoxing Chen, and Jing Cao. 2025. "Effects of Diethylstilbestrol on Uterus Structure and Immunological Function in Mice During Early Pregnancy" Toxics 13, no. 8: 672. https://doi.org/10.3390/toxics13080672
APA StyleLi, J., Xu, R., Wang, G., Su, Y., Chen, Y., & Cao, J. (2025). Effects of Diethylstilbestrol on Uterus Structure and Immunological Function in Mice During Early Pregnancy. Toxics, 13(8), 672. https://doi.org/10.3390/toxics13080672







