Association of 6:2 Fluorotelomer Ethoxylate Exposure with Serum Lipids in General Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Standards and Reagents
2.2. Subjects and Human Serum Collection
2.3. Analysis of 6:2 FTEOs in Human Serum
2.4. Determination of Lipids in Human Serum
2.5. QA/QC
2.6. Statistical Analysis
3. Results and Discussion
3.1. Demographic Characteristics of Study Subjects
3.2. Detection of 6:2 FTEOs in Human Serum
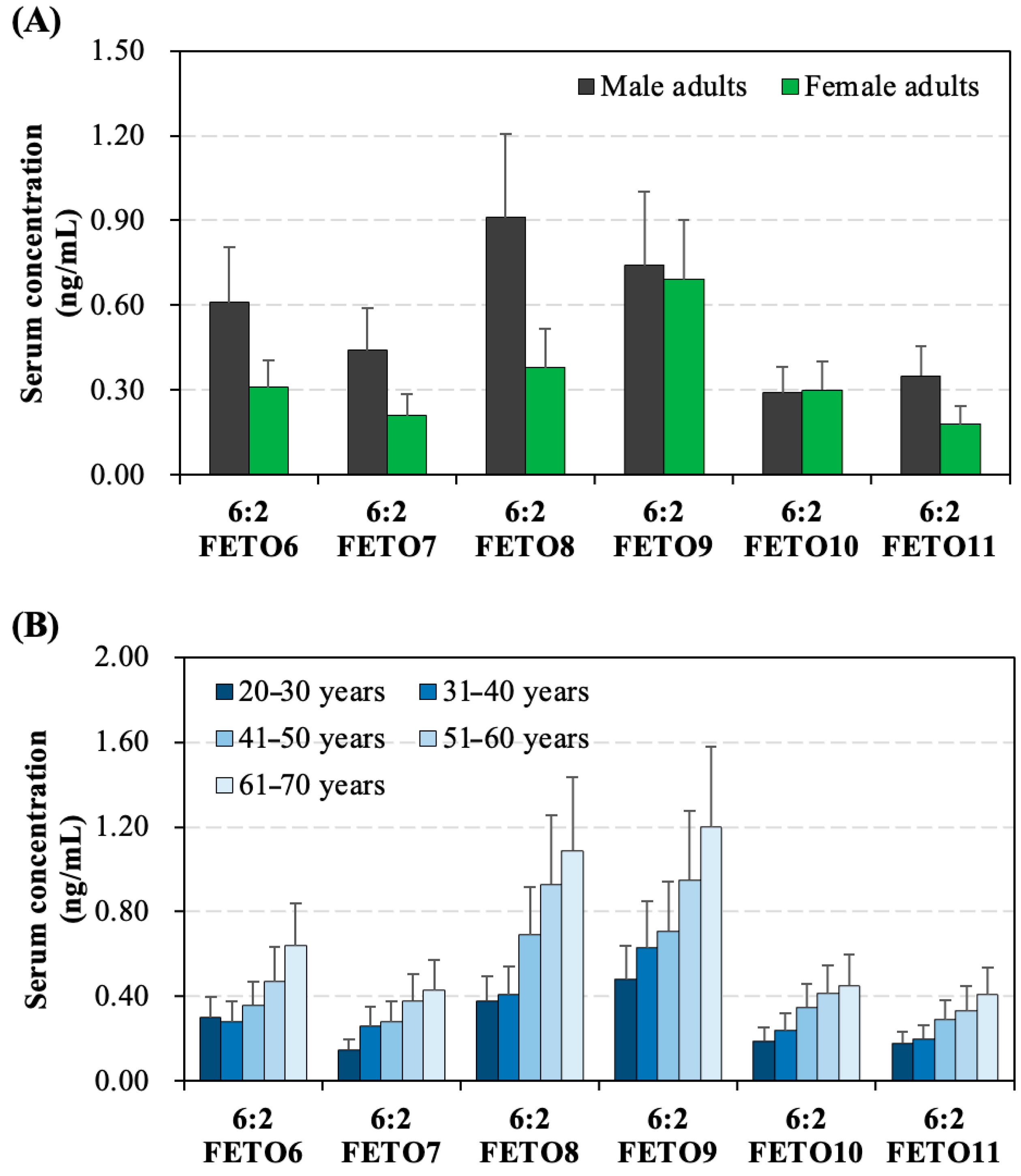
3.3. Gender- and Age-Specific Distinctions
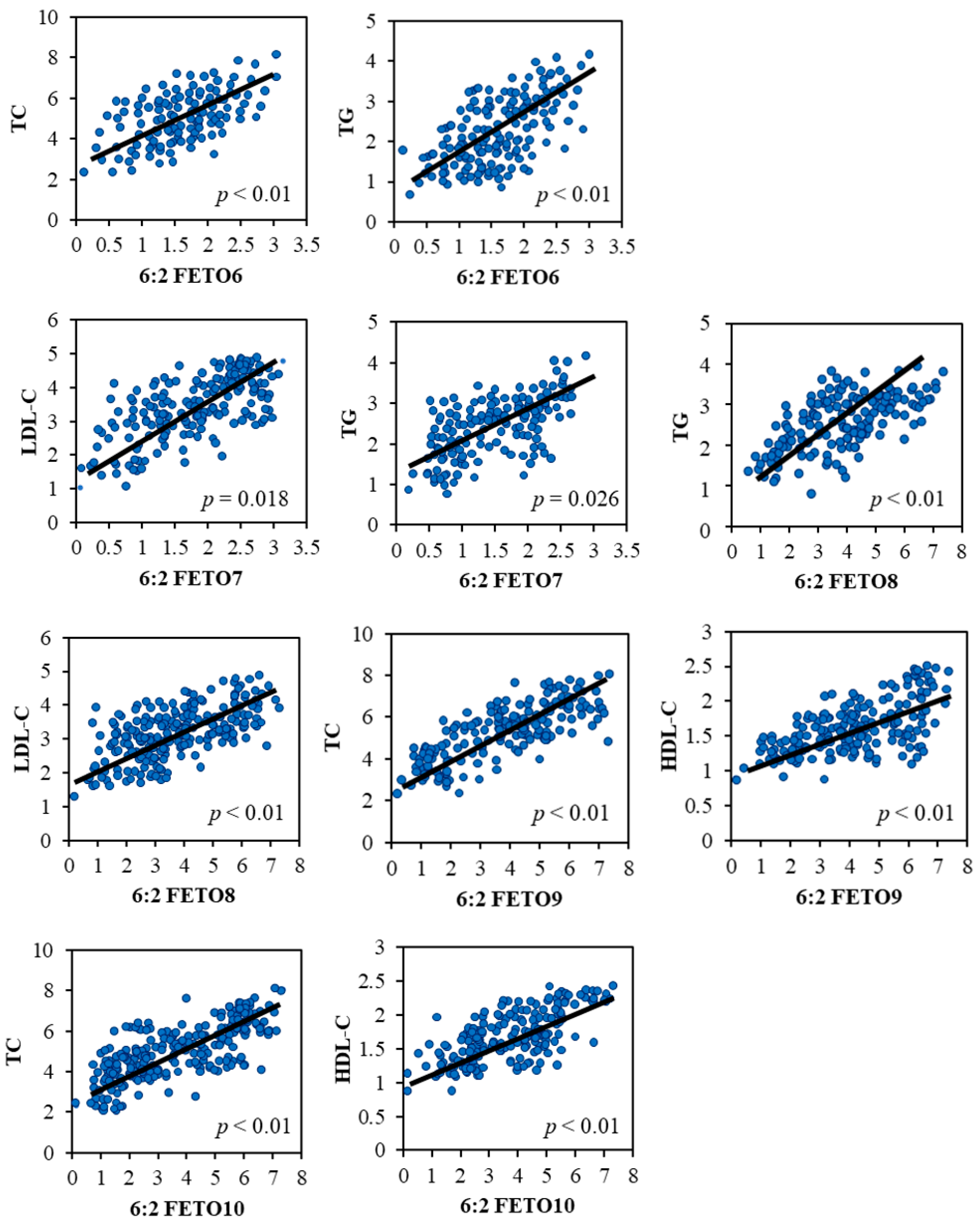
3.4. Associations Between 6:2 FETO Exposure and Dyslipidemia
3.5. Limitations of the Present Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per-and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Gaines, L.G. Historical and current usage of per- and polyfluoroalkyl substances (PFAS): A literature review. Am. J. Ind. Med. 2023, 66, 353–378. [Google Scholar] [CrossRef]
- Panieri, E.; Baralic, K.; Djukic-Cosic, D.; Buha Djordjevic, A.; Saso, L. PFAS molecules: A major concern for the human health and the environment. Toxics 2022, 10, 44. [Google Scholar] [CrossRef]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Evich, M.G.; Davis, M.J.; McCord, J.P.; Acrey, B.; Awkerman, J.A.; Knappe, D.R.; Lindstrom, A.B.; Speth, T.F.; Tebes-Stevens, C.; Strynar, M.J. Per-and polyfluoroalkyl substances in the environment. Science 2022, 375, eabg9065. [Google Scholar] [CrossRef]
- Herkert, N.J.; Kassotis, C.D.; Zhang, S.; Han, Y.; Pulikkal, V.F.; Sun, M.; Ferguson, P.L.; Stapleton, H.M. Characterization of per-and polyfluorinated alkyl substances present in commercial anti-fog products and their in vitro adipogenic activity. Environ. Sci. Technol. 2022, 56, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, L.; Hu, S.; Fang, F. Prevention of fogging of protective eyewear for medical staff during the COVID-19 pandemic. J. Emerg. Nurs. 2020, 46, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Simalti, A.; Bansal, A.; Gupta, N.; Patki, V.; Sood, A.K.; Sachdev, A.; Parekh, B.J. Use of personal protective equipments during COVID-19 pandemic in resource limited settings-the barest minimum needed. Indian J. Pract. Pediatr. 2020, 22, 83. [Google Scholar]
- Dattani, S.; Mohr, H. Personal protective equipment: Suggested best practices for pharmacies during the COVID-19 pandemic. Can. Pharm. J. 2020, 153, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Y.; Wang, X.; Lv, Z.; Zhou, F.; Huang, B.; Liu, X.; Chen, D. 6:2 Fluorotelomer Ethoxylates in Human Serum and Residential Homes: A Growing Environmental Health Concern. Environ. Sci. Technol. 2025, 59, 5182–5190. [Google Scholar] [CrossRef]
- Steeves, K.L.; Bissram, M.J.; Kleywegt, S.; Stevens, D.; Dorman, F.L.; Simpson, A.J.; Simpson, M.J.; Cahill, L.S.; Jobst, K.J. Nontargeted screening reveals fluorotelomer ethoxylates in indoor dust and industrial wastewater. Environ. Int. 2023, 171, 107634. [Google Scholar] [CrossRef]
- Frömel, T.; Knepper, T.P. Fluorotelomer ethoxylates: Sources of highly fluorinated environmental contaminants part I: Biotransformation. Chemosphere 2010, 80, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, R.; Singh, A.K.; Pradhan, S.; Unnikumar, K. Lipids: Structure, function and biotechnology aspects. In A Textbook of Molecular Biotechnology; Chauhan, A.K., Ed.; I.K. International Publishing House Pvt. Ltd.: New Delhi, India, 2009; pp. 173–209. [Google Scholar]
- Pandey, M.K. Uncovering the Lipid Web: Discovering the Multifaceted Roles of Lipids in Human Diseases and Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 13223. [Google Scholar] [CrossRef]
- Adorni, M.P.; Ronda, N.; Bernini, F.; Zimetti, F. High density lipoprotein cholesterol efflux capacity and atherosclerosis in cardiovascular disease: Pathophysiological aspects and pharmacological perspectives. Cells 2021, 10, 574. [Google Scholar] [CrossRef]
- Dakal, T.C.; Xiao, F.; Bhusal, C.K.; Sabapathy, P.C.; Segal, R.; Chen, J.; Bai, X. Lipids dysregulation in diseases: Core concepts, targets and treatment strategies. Lipids Health Dis. 2025, 24, 61. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids 2015, 2015, 971453. [Google Scholar] [CrossRef] [PubMed]
- Boullart, A.; De Graaf, J.; Stalenhoef, A. Serum triglycerides and risk of cardiovascular disease. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 867–875. [Google Scholar] [CrossRef]
- Ye, X.; Kong, W.; Zafar, M.I.; Chen, L.-L. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: A systematic review and meta-analysis of prospective studies. Cardiovasc. Diabetol. 2019, 18, 48. [Google Scholar] [CrossRef]
- Fragki, S.; Dirven, H.; Fletcher, T.; Grasl-Kraupp, B.; Bjerve Gützkow, K.; Hoogenboom, R.; Kersten, S.; Lindeman, B.; Louisse, J.; Peijnenburg, A. Systemic PFOS and PFOA exposure and disturbed lipid homeostasis in humans: What do we know and what not? Crit. Rev. Toxicol. 2021, 51, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Janssen, A.; Staats, M.; Hoogenboom, R.; Kersten, S.; Peijnenburg, A. Perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorononanoic acid (PFNA) increase triglyceride levels and decrease cholesterogenic gene expression in human HepaRG liver cells. Arch. Toxicol. 2020, 94, 3137–3155. [Google Scholar] [CrossRef]
- Song, X.; Ye, T.; Jing, D.; Wei, K.; Ge, Y.; Bei, X.; Qi, Y.; Wang, H.; Li, J.; Zhang, Y. Association between exposure to per-and polyfluoroalkyl substances and levels of lipid profile based on human studies. Rev. Environ. Health 2025, 40, 133–145. [Google Scholar] [CrossRef]
- Batzella, E.; Zare Jeddi, M.; Pitter, G.; Russo, F.; Fletcher, T.; Canova, C. Associations between mixture of perfluoroalkyl substances and lipid profile in a highly exposed adult community in the Veneto Region. Int. J. Environ. Res. Public Health 2022, 19, 12421. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Nicolescu, A.; Haug, L.S.; Husøy, T.; Deleanu, C.; Dirven, H.; Lindeman, B. Lipoprotein profiles associated with exposure to poly-and perfluoroalkyl substances (PFASs) in the EuroMix human biomonitoring study. Environ. Pollut. 2022, 308, 119664. [Google Scholar] [CrossRef]
- Geiger, S.D.; Xiao, J.; Ducatman, A.; Frisbee, S.; Innes, K.; Shankar, A. The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 2014, 98, 78–83. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, L.; Wang, M.; Sun, Q. Associations between per-and polyfluoroalkyl substances exposures and blood lipid levels among adults—A meta-analysis. Environ. Health Perspect. 2023, 131, 056001. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Mao, W.; Chen, M.; Jin, H. Prediction of p-Phenylenediamine Antioxidant Concentrations in Human Urine Using Machine Learning Models. J. Hazard. Mater. 2025, 487, 137184. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-W.; Qian, Z.; Emo, B.; Vaughn, M.; Bao, J.; Qin, X.-D.; Zhu, Y.; Li, J.; Lee, Y.L.; Dong, G.-H. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci. Total Environ. 2015, 512, 364–370. [Google Scholar] [CrossRef]
- Eriksen, K.T.; Raaschou-Nielsen, O.; McLaughlin, J.K.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS ONE 2013, 8, e56969. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M. Sex differences in human and animal toxicology: Toxicokinetics. Toxicol. Pathol. 2017, 45, 172–189. [Google Scholar] [CrossRef]
- Niu, S.; Cao, Y.; Chen, R.; Bedi, M.; Sanders, A.P.; Ducatman, A.; Ng, C. A state-of-the-science review of interactions of per-and polyfluoroalkyl substances (PFAS) with renal transporters in health and disease: Implications for population variability in PFAS toxicokinetics. Environ. Health Perspect. 2023, 131, 076002. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Katz, R.; Mozaffarian, D.; Dalrymple, L.S.; De Boer, I.; Sarnak, M.; Shlipak, M.; Siscovick, D.; Kestenbaum, B. Physical activity and rapid decline in kidney function among older adults. Arch. Intern. Med. 2009, 169, 2116–2123. [Google Scholar] [CrossRef]
- Denic, A.; Glassock, R.J.; Rule, A.D. Structural and functional changes with the aging kidney. Adv. Chronic Kidney Dis. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Tan, N.-S.; Shaw, N.S.; Vinckenbosch, N.; Liu, P.; Yasmin, R.; Desvergne, B.; Wahli, W.; Noy, N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol. Cell. Biol. 2002, 22, 5114–5127. [Google Scholar] [CrossRef] [PubMed]
- Fruchart, J.-C. Peroxisome proliferator-activated receptor-α activation and high-density lipoprotein metabolism. Am. J. Cardiol. 2001, 88, 24–29. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Sambasiva Rao, M. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol.—Gastrointest. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, K.; Peng, X.; Kan, Y.; Li, H.; Zhu, Y.; Wang, Z.; Li, Z.; Liu, H.-Y.; Cai, D. Nuclear receptor PPARα as a therapeutic target in diseases associated with lipid metabolism disorders. Nutrients 2023, 15, 4772. [Google Scholar] [CrossRef]
- Sheng, N.; Cui, R.; Wang, J.; Guo, Y.; Wang, J.; Dai, J. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Arch. Toxicol. 2018, 92, 359–369. [Google Scholar] [CrossRef]
- Jones, P.D.; Hu, W.; De Coen, W.; Newsted, J.L.; Giesy, J.P. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem. 2003, 22, 2639–2649. [Google Scholar] [CrossRef]
- Luebker, D.J.; Hansen, K.J.; Bass, N.M.; Butenhoff, J.L.; Seacat, A.M. Interactions of flurochemicals with rat liver fatty acid-binding protein. Toxicology 2002, 176, 175–185. [Google Scholar] [CrossRef]
- Beggs, K.M.; McGreal, S.R.; McCarthy, A.; Gunewardena, S.; Lampe, J.N.; Lau, C.; Apte, U. The role of hepatocyte nuclear factor 4-alpha in perfluorooctanoic acid-and perfluorooctanesulfonic acid-induced hepatocellular dysfunction. Toxicol. Appl. Pharmacol. 2016, 304, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Scharmach, E.; Buhrke, T.; Lichtenstein, D.; Lampen, A. Perfluorooctanoic acid affects the activity of the hepatocyte nuclear factor 4 alpha (HNF4α). Toxicol. Lett. 2012, 212, 106–112. [Google Scholar] [CrossRef] [PubMed]

| Males (n = 115) | Females (n = 122) | |||
|---|---|---|---|---|
| n | Percentage | n | Percentage | |
| Age (Years) | ||||
| 20–30 | 19 | 17% | 18 | 15% |
| 31–40 | 25 | 22% | 31 | 25% |
| 41–50 | 20 | 17% | 19 | 16% |
| 51–60 | 28 | 24% | 32 | 26% |
| 61–70 | 23 | 20% | 22 | 18% |
| Body mass index (kg/m2) | ||||
| <18.5 | 24 | 21% | 30 | 25% |
| 18.5–24.5 | 60 | 52% | 78 | 64% |
| >24.5 | 31 | 27% | 14 | 11% |
| Educational level | ||||
| Lower than high school | 35 | 30% | 37 | 30% |
| High school | 47 | 41% | 39 | 32% |
| College | 33 | 29% | 46 | 38% |
| Occupational status | ||||
| Employed | 86 | 75% | 78 | 64% |
| Unemployed | 29 | 25% | 44 | 36% |
| Marital status | ||||
| Married | 101 | 88% | 92 | 75% |
| Unmarried | 14 | 12% | 30 | 25% |
| Residence | ||||
| Urban region | 93 | 81% | 98 | 80% |
| Rural region | 22 | 19% | 24 | 20% |
| Alcohol consumption | ||||
| No | 70 | 61% | 107 | 88% |
| Yes | 45 | 39% | 15 | 12% |
| Tobacco smoker | ||||
| No | 78 | 68% | 109 | 89% |
| Yes | 37 | 32% | 13 | 11% |
| Percentile | ||||||
|---|---|---|---|---|---|---|
| Detection Frequency | Mean | 25th | 50th | 75th | Max | |
| 6:2 FETO2 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO3 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO4 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO5 | 30% | NC | <LOD | <LOD | 0.26 | 1.17 |
| 6:2 FETO6 | 71% | 0.44 | <LOD | 0.43 | 1.16 | 3.03 |
| 6:2 FETO7 | 79% | 0.30 | 0.10 | 0.41 | 1.17 | 3.15 |
| 6:2 FETO8 | 81% | 0.69 | 0.41 | 0.45 | 4.02 | 7.36 |
| 6:2 FETO9 | 76% | 0.71 | 0.098 | 0.80 | 4.11 | 8.12 |
| 6:2 FETO10 | 63% | 0.29 | <LOD | 0.31 | 1.49 | 2.90 |
| 6:2 FETO11 | 63% | 0.24 | <LOD | 0.17 | 1.10 | 4.35 |
| 6:2 FETO12 | 44% | NC | <LOD | <LOD | 0.28 | 1.77 |
| 6:2 FETO13 | 22% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO14 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO15 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO16 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO17 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| 6:2 FETO18 | 0% | NC | <LOD | <LOD | <LOD | <LOD |
| Detection Frequency | Mean | Median | Range | |
|---|---|---|---|---|
| TC | 100% | 5.26 | 5.39 | 2.30–8.07 |
| HDL-C | 100% | 1.68 | 1.78 | 0.89–2.45 |
| LDL-C | 100% | 2.72 | 3.01 | 1.05–4.77 |
| TG | 100% | 2.25 | 2.60 | 0.62–4.14 |
| Beta, (95% CI), p-Value | ||||
|---|---|---|---|---|
| TC | HDL-C | LDL-C | TG | |
| 6:2 FETO6 | 0.81 ** (0.23–1.40) | −0.21 (−0.43–0.05) | 0.082 (−0.29–0.28) | 0.99 * (0.49–1.47) |
| 6:2 FETO7 | 2.42 (−1.6–6.09) | 3.29 (−2.14–5.56) | 1.16 * (1.04–2.29) | 2.13 * (1.01–3.46) |
| 6:2 FETO8 | −1.27 (−2.79–0.33) | −2.23 (−3.53–0.99) | 3.17 ** (1.21–4.25) | 1.95 ** (4.26–4.24) |
| 6:2 FETO9 | 0.94 * (0.084–2.08) | 2.46 ** (1.01–3.79) | 0.45 (−2.80–3.51) | −0.53 (3.71–4.27) |
| 6:2 FETO10 | 2.07 ** (1.03–3.11) | 1.01 * (0.34–1.73) | −0.79 (−1.46–0.13) | −2.06 (−2.44–0.38) |
| 6:2 FETO11 | 0.63 (−4.20–5.46) | 0.77 (−1.45–3.03) | 0.12 (−0.47–0.66) | 0.089 (−1.38–1.43) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Li, Q.; Deng, R.; Wang, R.; Fu, J.; Ren, F.; Jin, H. Association of 6:2 Fluorotelomer Ethoxylate Exposure with Serum Lipids in General Adults. Toxics 2025, 13, 664. https://doi.org/10.3390/toxics13080664
Wu Y, Li Q, Deng R, Wang R, Fu J, Ren F, Jin H. Association of 6:2 Fluorotelomer Ethoxylate Exposure with Serum Lipids in General Adults. Toxics. 2025; 13(8):664. https://doi.org/10.3390/toxics13080664
Chicago/Turabian StyleWu, Yan, Qianjin Li, Rendi Deng, Rui Wang, Junfen Fu, Fangfang Ren, and Hangbiao Jin. 2025. "Association of 6:2 Fluorotelomer Ethoxylate Exposure with Serum Lipids in General Adults" Toxics 13, no. 8: 664. https://doi.org/10.3390/toxics13080664
APA StyleWu, Y., Li, Q., Deng, R., Wang, R., Fu, J., Ren, F., & Jin, H. (2025). Association of 6:2 Fluorotelomer Ethoxylate Exposure with Serum Lipids in General Adults. Toxics, 13(8), 664. https://doi.org/10.3390/toxics13080664






