The Blurred Lines Between New Psychoactive Substances and Potential Chemical Weapons
Abstract
1. Introduction
2. Bibliographic Review
3. Results
3.1. Historical Review of Modern Chemical Weapons

3.2. From Incapacitating Agents to Neuro-Weapons
3.3. The Use of Neuro-Weapons in Possible Cognitive Warfare
3.4. The NPS Phenomenon
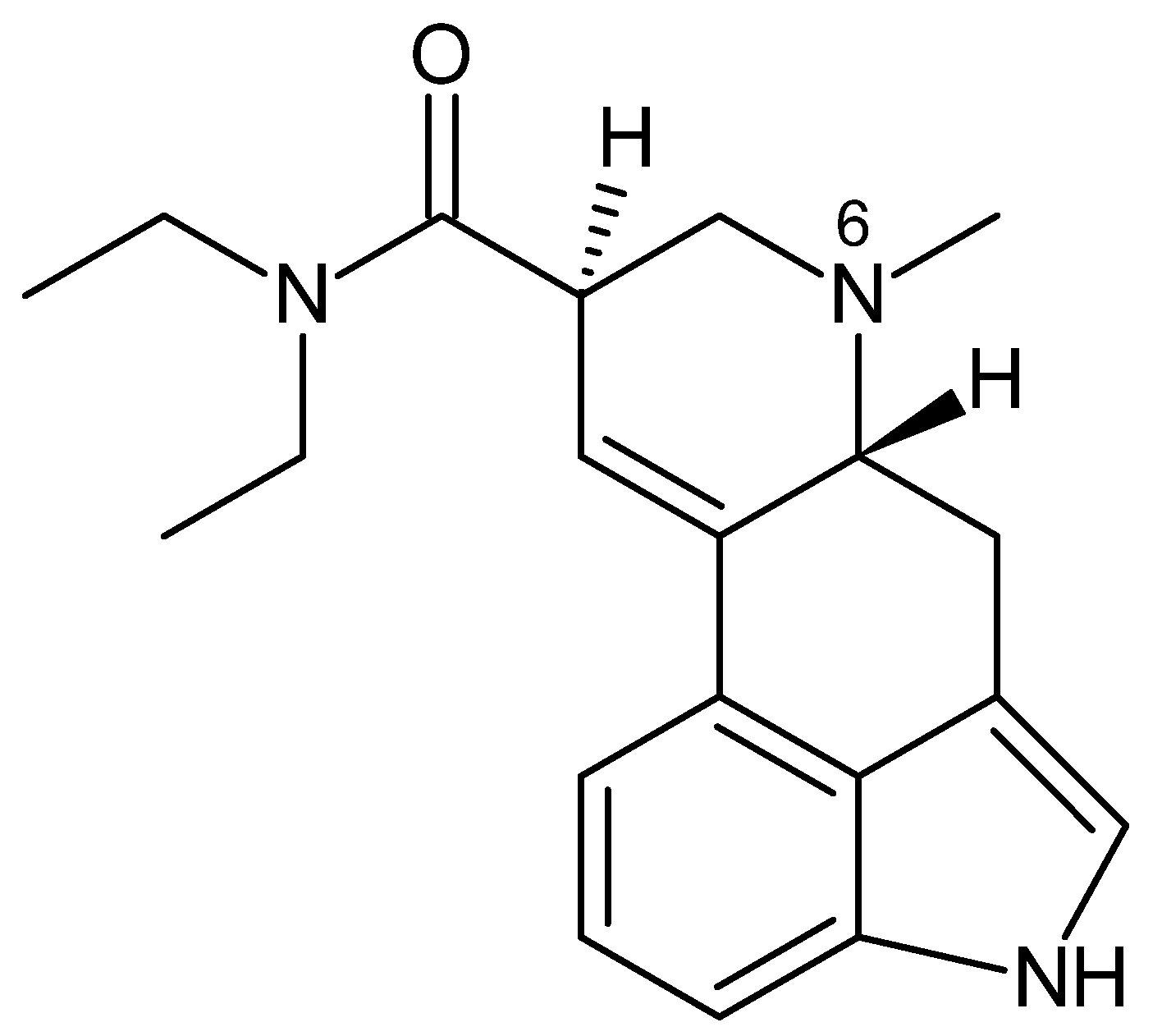
3.4.1. Lysergamides
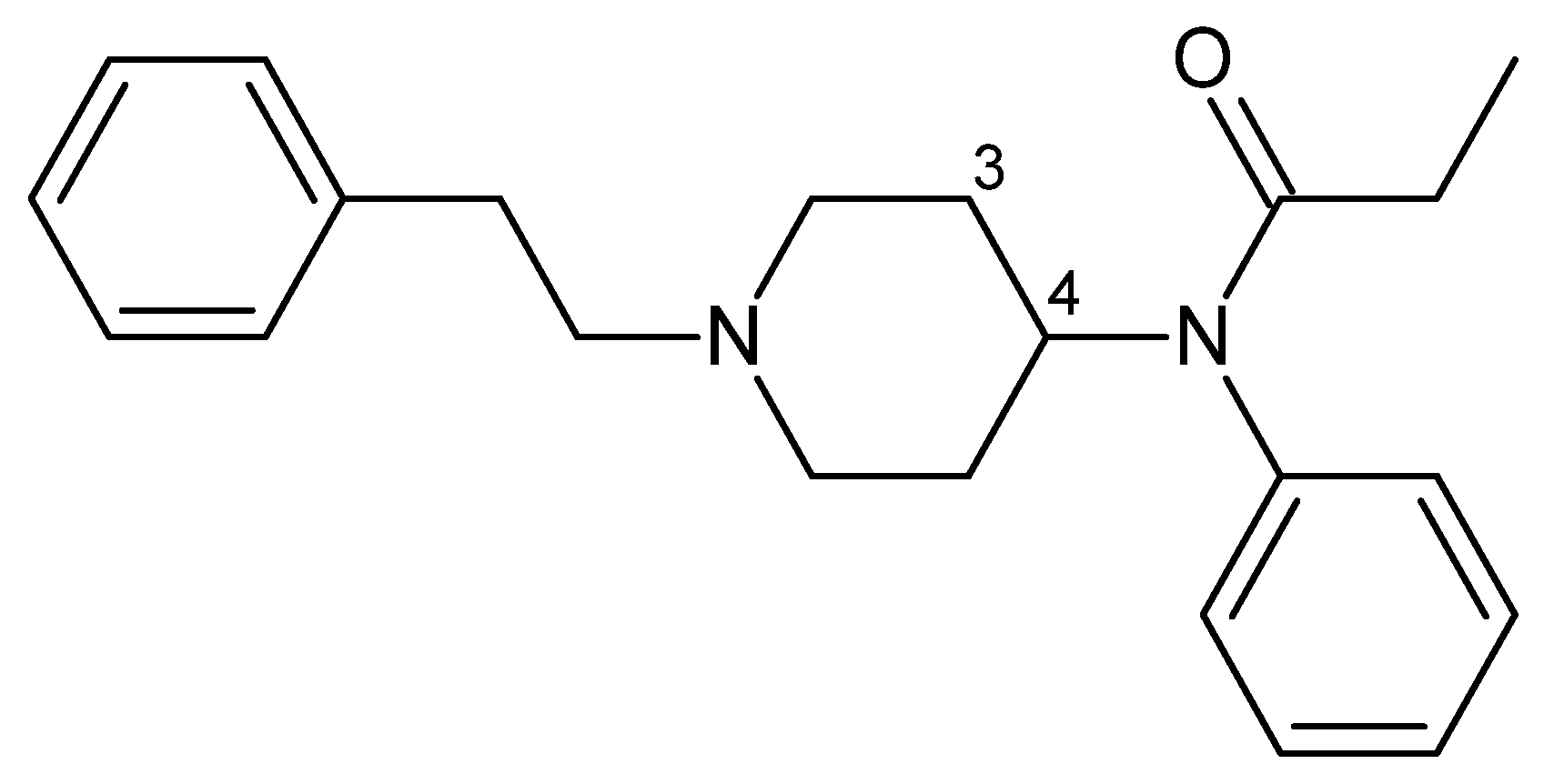
3.4.2. Phencyclidines
3.4.3. Fentanyl Derivatives
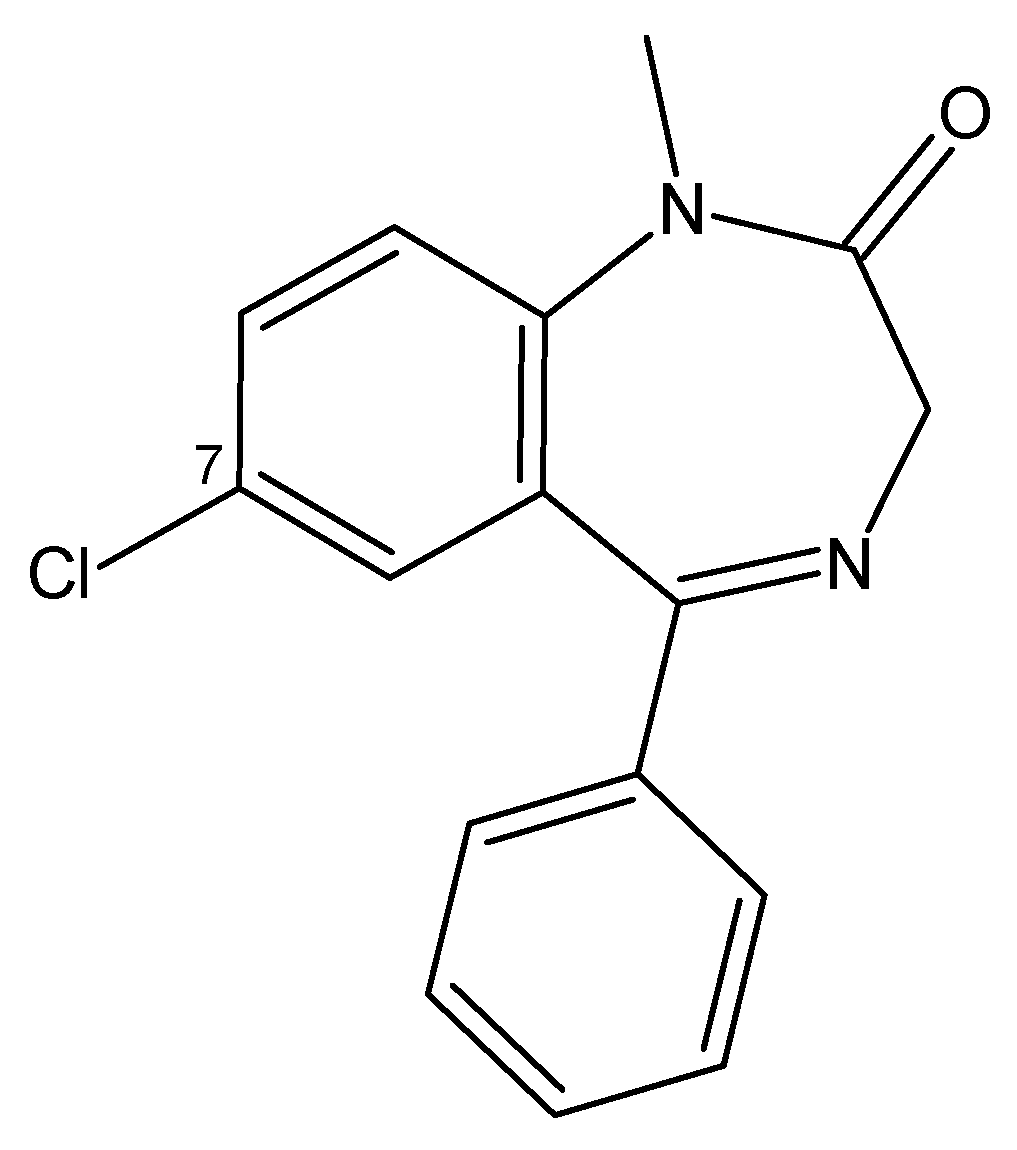
3.4.4. Benzodiazepines
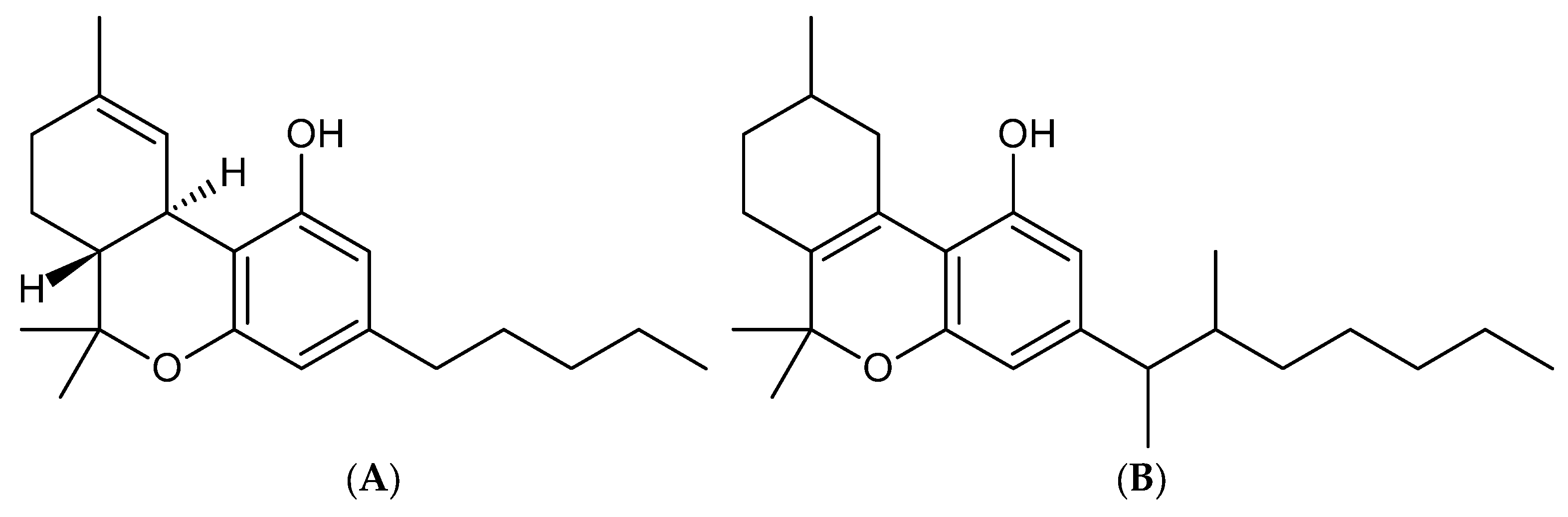
3.4.5. Synthetic Cannabinoids
| NPS Family | Chemical Agent | Species | ICt50 Inhal. mg·min/m3 | LD50 mg/kg | Sources |
|---|---|---|---|---|---|
| Lysergamides | LSD-25 | Human | 30 a 55 | ~0.2 (i.v.) | [66,67,69] |
| Phencyclidines | PCP | Rat | ≈1000 | 16 (i.v.) | [82,83] |
| Fentanyl derivatives | Fentanyl | Monkey | NA | 0.03 (i.v.) | [105] |
| Carfentanil | Mouse | NA | 18.75 (i.v.) | [110] | |
| Benzodiazepines | Midazolam | Rat | NA | 357 (i.v.) | [127] |
| Alprazolam | Rat | NA | 1200 (p.o.) | [128] | |
| Flunitrazepam | Rat | NA | 415 (p.o.) | [129] | |
| Clonazolam | Rat | NA | ~500 (p.o.) | [130] | |
| Cannabinoids | DMHP | Mouse | ≈0.075 | ~63 (i.v.) | [141,142] |
| AMB-FUBINACA | Rabbit | NA | ~300.1 (i.d.) | [156] | |
| ADB-FUBINACA | Rabbit | NA | ~1502 (i.d.) | [157] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Schedule | Description | Guidelines |
|---|---|---|
| Schedule 1 | Highly hazardous chemicals developed or used as chemical weapons. |
|
| Schedule 2 | Chemical substances with the potential to be used in the production of chemical weapons. |
|
| Schedule 3 | Chemicals and precursors that can be used for commercial purposes, but require control. |
|
| Toxic Chemicals | ||
|---|---|---|
| Number | Substance Name | Number CAS |
| (1) | O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Et, n-Pr or i-Pr)-phosphonofluoridates | |
| Example | Sarin: O-Isopropyl methylphosphonofluoridate | (107-44-8) |
| Soman: O-Pinacolyl methylphosphonofluoridate | (96-64-0) | |
| (2) | O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl (Me, Et, n-Pr or i-Pr) phosphoramidocyanidates | |
| Example | Tabun: O-Ethyl N,N-dimethyl phosphoramidocyanidate | (77-81-6) |
| (3) | O-Alkyl (H or ≤C10, incl. cycloalkyl) S-2-dialkyl (Me, Et, n-Pr or i-Pr)-aminoethyl alkyl (Me, Et, n-Pr or i-Pr) phosphonothiolates and corresponding alkylated or protonated salts | |
| Example | VX: O-Ethyl S-2-diisopropylaminoethyl methyl phosphonothiolate | (50782-69-9) |
| (4) | Sulfur mustards: | |
| 2-Chloroethylchloromethylsulfide | (2625-76-5) | |
| Mustard gas: Bis(2-chloroethyl)sulfide | (505-60-2) | |
| Bis(2-chloroethylthio)methane | (63869-13-6) | |
| Sesquimustard: 1,2-Bis(2-chloroethylthio)ethane | (3563-36-8) | |
| 1,3-Bis(2-chloroethylthio)-n-propane | (63905-10-2) | |
| 1,4-Bis(2-chloroethylthio)-n-butane | (142868-93-7) | |
| 1,5-Bis(2-chloroethylthio)-n-pentane | (142868-94-8) | |
| Bis(2-chloroethylthiomethyl)ether | (63918-90-1) | |
| O-Mustard: Bis(2-chloroethylthioethyl)ether | (63918-89-8) | |
| (5) | Lewisites: | |
| Lewisite 1: 2-Chlorovinyldichloroarsine | (541-25-3) | |
| Lewisite 2: Bis(2-chlorovinyl)chloroarsine | (40334-69-8) | |
| Lewisite 3: Tris(2-chlorovinyl)arsine | (40334-70-1) | |
| (6) | Nitrogen mustards: | |
| HN1: Bis(2-chloroethyl)ethylamine | (538-07-8) | |
| HN2: Bis(2-chloroethyl)methylamine | (51-75-2) | |
| HN3: Tris(2-chloroethyl)amine | (555-77-1) | |
| (7) | Saxitoxin | (35523-89-8) |
| (8) | Ricin | (9009-86-3) |
| (13) | P-alkyl (H or ≤C10, incl. cycloalkyl) N-(1-(dialkyl(≤C10, incl. cycloalkyl)amino))alkylidene(H or ≤C10, incl. cycloalkyl) phosphonamidic fluorides and corresponding alkylated or protonated salts | |
| Example | N-(1-(di-n-decylamino)-n-decylidene)-P-decylphosphonamidic fluoride | (2387495-99-8) |
| Methyl-(1-(diethylamino)ethylidene)phosphonamidofluoridate | (2387496-12-8) | |
| (14) | O-alkyl (H or ≤C10, incl. cycloalkyl) N-(1-(dialkyl(≤C10, incl. cycloalkyl)amino))alkylidene(H or ≤C10, incl. cycloalkyl) phosphoramidofluoridates and corresponding alkylated or protonated salts | |
| Example | O-n-Decyl N-(1-(di-n-decylamino)-n-decylidene)phosphoramidofluoridate | (2387496-00-4) |
| Methyl (1-(diethylamino)ethylidene)phosphoramidofluoridate | (2387496-04-8) | |
| Ethyl (1-(diethylamino)ethylidene)phosphoramidofluoridate | (2387496-06-0) | |
| (15) | Methyl-(bis(diethylamino)methylene)phosphonamidofluoridate | (2387496-14-0) |
| (16) | Carbamates (quaternaries and bisquaternaries of dimethylcarbamoyloxypyridines) | |
| Quaternaries of dimethylcarbamoyloxypyridines: | ||
| 1-[N,N-dialkyl(≤C10)-N-(n-(hydroxyl, cyano, acetoxy)alkyl(≤C10)) ammonio]-n-[N-(3-dimethylcarbamoxy-α-picolinyl)-N,N-dialkyl(≤C10) ammonio]decane dibromide (n = 1–8) | ||
| Example | 1-[N,N-dimethyl-N-(2-hydroxy)ethylammonio]-10-[N-(3-dimethylcarbamoxy-α-picolinyl)-N,N-dimethylammonio]decane dibromide | (77104-62-2) |
| Bisquaternaries of dimethylcarbamoyloxypyridines: | ||
| 1,n-Bis[N-(3-dimethylcarbamoxy-α-picolyl)-N,N-dialkyl(≤C10) ammonio]-alkane-(2,(n-1)-dione) dibromide (n = 2–12) | ||
| Example | Dibromuro de 1,10-bis[N-(3-dimetilcarbamoxi-α-picolil)-N-etil-N-metilamonio]decano-2,9-diona) | (77104-00-8) |
| Precursors | ||
| Number | Precursor name | Number CAS |
| (9) | Alkyl (Me, Et, n-Pr or i-Pr) phosphonyldifluorides | |
| Example | DF: Methylphosphonyldifluoride | (676-99-3) |
| (10) | O-Alkyl (H or ≤C10, incl. cycloalkyl) O-2-dialkyl (Me, Et, n-Pr or i-Pr)-aminoethyl alkyl (Me, Et, n-Pr or i-Pr) phosphonites and corresponding alkylated or protonated salts | |
| Example | QL: O-Ethyl O-2-diisopropylaminoethyl methylphosphonite | (57856-11-8) |
| (11) | Chlorosarin: O-Isopropyl methylphosphonochloridate | (1445-76-7) |
| (12) | Chlorosoman: O-Pinacolyl methylphosphonochloridate | (7040-57-5) |
| Toxic Chemicals | ||
|---|---|---|
| Number | Substance Name | Number CAS |
| (1) | Amiton: O,O-Diethyl S-[2-(diethylamino)ethyl] phosphorothiolate | (78-53-5) |
| and corresponding alkylated or protonated salts | ||
| (2) | PFIB: 1,1,3,3,3-Pentafluoro-2-(trifluoromethyl)-1-propene | (382-21-8) |
| (3) | BZ: 3-Quinuclidinyl benzilate | (6581-06-2) |
| Precursors | ||
| Number | Precursor name | Number CAS |
| (4) | Chemicals, except for those listed in Schedule 1, containing a phosphorus atom to which is bonded one methyl, ethyl, or propyl (normal or iso) group, but not further carbon atoms | |
| Example | Methylphosphonyl dichloride | (676-97-1) |
| Dimethyl methylphosphonate | (756-79-6) | |
| Exemption: Fonofos | O-Ethyl S-phenyl ethylphosphonothiolothionate | (944-22-9) |
| (5) | N,N-Dialkyl (Me, Et, n-Pr or i-Pr) phosphoramidic dihalides | — |
| (6) | Dialkyl (Me, Et, n-Pr or i-Pr) N,N-dialkyl (Me, Et, n-Pr or i-Pr)-phosphoramidates | — |
| (7) | Arsenic trichloride | (7784-34-1) |
| (8) | 2,2-Diphenyl-2-hydroxyacetic acid | (76-93-7) |
| (9) | Quinuclidin-3-ol | (1619-34-7) |
| (10) | N,N-Dialkyl (Me, Et, n-Pr or i-Pr) aminoethyl-2-chlorides and corresponding protonated salts | — |
| (11) | N,N-Dialkyl (Me, Et, n-Pr or i-Pr) aminoethane-2-ols and corresponding protonated salts | |
| Exemptions: | N,N-Dimethylaminoethanol | (108-01-0) |
| and corresponding protonated salts | ||
| N,N-dietilaminoetanol | (100-37-8) | |
| and corresponding protonated salts | ||
| (12) | N,N-Dialkyl (Me, Et, n-Pr or i-Pr) aminoethane-2-thiols and corresponding protonated salts | — |
| (13) | Thiodiglycol: Bis(2-hydroxyethyl)sulfide | (111-48-8) |
| (14) | Pinacolyl alcohol: 3,3-Dimethylbutan-2-ol | (464-07-3) |
| Toxic Chemicals | ||
|---|---|---|
| Number | Substance Name | Number CAS |
| (1) | Phosgene: Carbonyl dichloride | (75-44-5) |
| (2) | Cyanogen chloride | (506-77-4) |
| (3) | Hydrogen cyanide | (74-90-8) |
| (4) | Chloropicrin: Trichloronitromethane | (76-06-2) |
| Precursors | ||
| Number | Precursor name | Number CAS |
| (5) | Phosphorus oxychloride | (10025-87-3) |
| (6) | Phosphorus trichloride | (7719-12-2) |
| (7) | Phosphorus pentachloride | (10026-13-8) |
| (8) | Trimethyl phosphite | (121-45-9) |
| (9) | Triethyl phosphite | (122-52-1) |
| (10) | Dimethyl phosphite | (868-85-9) |
| (11) | Diethyl phosphite | (762-04-9) |
| (12) | Sulfur monochloride | (10025-67-9) |
| (13) | Sulfur dichloride | (10545-99-0) |
| (14) | Thionyl chloride | (7719-09-7) |
| (15) | Ethyldiethanolamine | (139-87-7) |
| (16) | Methyldiethanolamine | (105-59-9) |
| (17) | Triethanolamine | (102-71-6) |
References
- BBC Mundo. ¿Cómo Surgieron Y Dónde Se Siguen Usando Las Armas Químicas? BBC News Mundo, 18 April 2018. Available online: https://www.bbc.com/mundo/noticias-internacional-43798506 (accessed on 5 February 2025).
- Comité Internacional de la Cruz Roja (CICR). Convención de 1993 Sobre la Prohibición de Armas Químicas; CICR: Geneva, Switzerland, 2015; Available online: https://www.icrc.org/sites/default/files/document/file_list/final_chemical_weapons_esp_rev_gv_.pdf (accessed on 10 February 2025).
- United Nations Office for Disarmament Affairs. Armas Químicas; UNODA: New York, NY, USA, 2025; Available online: https://disarmament.unoda.org/es/adm/armas-quimicas/ (accessed on 8 June 2025).
- Organization for the Prohibition of Chemical Weapons. Nobel Peace Prize—2013 Awarded for Extensive Efforts to Eliminate Chemical Weapons; OPCW: La Haya, The Netherlands, 2025; Available online: https://www.opcw.org/about-us/nobel-peace-prize (accessed on 8 June 2025).
- National Institute on Drug Abuse. Información Básica Sobre el Fentanilo. NIH Medline Plus Magazine, 8 October 2024. Available online: https://magazine.medlineplus.gov/es/art%C3%ADculo/informacion-basica-sobre-el-fentanilo (accessed on 1 May 2025).
- Repilado, Á.A.; Urquía, G.M.L.; Martínez, G.M.E.; Llorente, B.M.T. Carfentanilo: Una doble amenaza para la salud pública. Sanid. Mil. 2023, 79, 118–124. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1887-85712023000200009 (accessed on 10 March 2025).
- Oficina de las Naciones Unidas contra la Droga y el Delito (UNODC). Amenazas Actuales de Las NSP, Volumen VI. 2023. Available online: https://www.unodc.org/res/scientists/ewa/NPS_threats_VI_SP.pdf (accessed on 10 March 2025).
- Östin, A.; Nyitrai, V.P.; Grub, C.; Ježić, L.; Deconinck, E.; Jones, J. Literature Review of the State-of-the-Art of Synthetic Opioids. JaTerror. 2024. Available online: https://www.jaterror.eu/wp-content/uploads/2024/11/8.3-Literature-review-of-state-of-the-art-of-synthetic-opioids.pdf (accessed on 8 December 2024).
- Sistema de Alerta Temprana (SAT) Drogas, Servicio Nacional para la Prevención y Rehabilitación del Consumo de Drogas y Alcohol (SENDA). Nuevas Sustancias Psicoactivas. Available online: https://satdrogas.gob.cl/nuevas-sustancias-psicoactivas/ (accessed on 5 January 2025).
- Pitschmann, V.; Hon, Z. Drugs as Chemical Weapons: Past and Perspectives. Toxics 2023, 11, 52. [Google Scholar] [CrossRef]
- Szinicz, L. History of chemical and biological warfare agents. Toxicology 2005, 214, 167–181. [Google Scholar] [CrossRef]
- Maria, A.C.; Geanpool, A.C.; Fabiana, C.Q.; Mary, C.G.; Mariapaula, C.Z.; Lisbeth, C.H.; Gianella, D.V.; Daniela, F.T.; Miguel, G.T.; Andrea, O.A.; et al. Armas Químicas a Través del Tiempo; Universidad Nacional de San Agustín, Facultad de ciencia biológicas, Escuela Profesional de Biología: Arequipa, Perú, 2022; Available online: https://www.researchgate.net/profile/Anthony-Rosas/publication/362530715_ARMAS_QUIMICAS_A_TRAVES_DEL_TIEMPO/links/62ee87ab4532247693850735/ARMAS-QUIMICAS-A-TRAVES-DEL-TIEMPO.pdf (accessed on 7 January 2025).
- Pitschmann, V. Overall View of Chemical and Biochemical Weapons. Toxins 2014, 6, 1761–1784. [Google Scholar] [CrossRef]
- Johnson, N.H.; Larsen, J.C.; Meek, E.C. Historical Perspective of Chemical Warfare Agents. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Academic Press: San Diego, CA, USA, 2020; pp. 17–26. [Google Scholar]
- Jáuregui-Lobera, I. Guerra química en la I y II Guerras Mundiales. J. Negat. No Posit. Results 2020, 5, 218–235. [Google Scholar] [CrossRef]
- Organisation for the Prohibition of Chemical Weapons (OPAQ). Guía Práctica Para la Gestión Médica de Bajas de Guerra Química. 2016. Available online: https://www.opcw.org/sites/default/files/documents/2018/07/Practical%20Guide%20for%20Medical%20Management%20of%20Chemical%20Warfare%20Casualties%20-%20Spanish.pdf (accessed on 5 December 2024).
- Fitzgerald, G.J. Chemical Warfare and Medical Response during World War I. Am. J. Public Health 2008, 98, 611–625. [Google Scholar] [CrossRef]
- Pampalakis, G.; Kostoudi, S. Chemical, Physical, and Toxicological Properties of V-Agents. Int. J. Mol. Sci. 2023, 24, 8600. [Google Scholar] [CrossRef]
- Organización para la Prohibición de las Armas Químicas (OPAQ). Anexo sobre Sustancias Químicas. OPCW.org. 2025. Available online: https://www.opcw.org/es/convencion-sobre-las-armas-quimicas/anexos/annex-chemicals/anexo-sobre-sustancias-quimicas (accessed on 15 March 2025).
- Hrvat, N.M.; Kovarik, Z. Counteracting poisoning with chemical warfare nerve agents. Arch. Ind. Hyg. Toxicol. 2020, 71, 266–284. [Google Scholar] [CrossRef]
- Chai, P.R.; Hayes, B.D.; Erickson, T.B.; Boyer, E.W. Novichok agents: A historical, current, and toxicological perspective. Toxicol. Commun. 2018, 2, 45–48. [Google Scholar] [CrossRef]
- Franca, T.C.C.; Kitagawa, D.S.; De a Cavalcante, S.F.; Da Silva, J.V.; Nepovimova, E.; Kuca, K. Novichoks: The Dangerous Fourth Generation of Chemical Weapons. Int. J. Mol. Sci. 2019, 20, 1222. [Google Scholar] [CrossRef]
- UK Health Security Agency. Phosgene: Toxicological Overview. GOV.UK, November 2024. Available online: https://www.gov.uk/government/publications/phosgene-properties-incident-management-and-toxicology/phosgene-toxicological-overview (accessed on 2 February 2025).
- Committee on Acute Exposure Guideline Levels; Committee on Toxicology; Board on Environmental Studies and Toxicology; Division on Earth and Life Studies; National Research Council. Lewisite Acute Exposure Guideline Levels. In Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2013; Volume 15. Available online: https://www.ncbi.nlm.nih.gov/books/NBK201338/ (accessed on 15 March 2025).
- Augerson, W.S. A Review of the Scientific Literature as it Pertains to Gulf War Illnesses: Volume 5: Chemical and Biological Warfare Agents; RAND Corporation: Santa Monica, CA, USA, 2000; Available online: https://www.rand.org/pubs/monograph_reports/MR1018z5.html (accessed on 21 March 2025).
- National Research Council of National Academies. Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, USA, 2003; Volume 3. Available online: https://www.epa.gov/sites/default/files/2014-11/documents/tsd45.pdf (accessed on 15 March 2025).
- Opresko, D.M.; Faust, R. Health Risk Assessment for Sulfur Mustard (HD). In Review of the U.S. Army’s Health Risk Assessments for Oral Exposure to Six Chemical-Warfare Agents; Doull, J., Klaassen, C.D., Amdur, M.O., Eds.; National Academies Press: Washington, DC, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK208318/#ddd00322 (accessed on 29 March 2025).
- National Research Council (US) Committee on Toxicology. Review of Acute Human-Toxicity Estimates for GA (Tabun). In Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents; National Academies Press: Washington, DC, USA, 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233734/ (accessed on 29 March 2025).
- National Research Council (US) Committee on Toxicology. Review of Acute Human-Toxicity Estimates for GB (Sarin). In Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents; National Academies Press: Washington, DC, USA, 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233733/ (accessed on 29 March 2025).
- National Research Council (US) Committee on Toxicology. Review of acute human-toxicity estimates for GD (Soman). In Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents; National Academies Press: Washington, DC, USA, 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233731/ (accessed on 29 March 2025).
- National Research Council (US) Committee on Toxicology. Review of Acute Human-Toxicity Estimates for VX. In Review of Acute Human-Toxicity Estimates for Selected Chemical-Warfare Agents; National Academies Press: Washington, DC, USA, 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233724/ (accessed on 29 March 2025).
- Nepovimova, E.; Kuca, K. Chemical warfare agent NOVICHOK—Mini-review of available data. Food Chem. Toxicol. 2018, 121, 343–350. [Google Scholar] [CrossRef]
- Webb, S.; Coulon, F.; Temple, T. A critical review of liquid, low toxicity chemical warfare agent simulants: Enhancing accuracy, safety, and methodological approaches for sampling. J. Hazard. Mater. 2025, 492, 138021. [Google Scholar] [CrossRef]
- Noga, M.; Michalska, A.; Jurowski, K. The prediction of hydrolysis and biodegradation of organophosphorus-based chemical warfare agents (G-series and V-series) using toxicology in silico methods. Ecotoxicol. Environ. Saf. 2024, 272, 116018. [Google Scholar] [CrossRef]
- Noga, M.; Michalska, A.; Jurowski, K. The prediction of acute toxicity (LD50) for organophosphorus-based chemical warfare agents (V-series) using toxicology in silico methods. Arch. Toxicol. 2023, 98, 267–275. [Google Scholar] [CrossRef]
- Organization for the Prohibition of Chemical Weapons (OPCW). What is a Chemical Weapon? The Hague. OPCW. 2025. Available online: https://www.opcw.org/our-work/what-chemical-weapon (accessed on 20 April 2025).
- BBC Mundo. Alexei Navalny: El Opositor Ruso «Fue Envenenado Con NOVICHOK», Según Alemania. BBC News Mundo, 2 September 2020. Available online: https://www.bbc.com/mundo/noticias-internacional-54000515 (accessed on 20 January 2025).
- Granlund, C. Chemicals in Cognitive Warfare: A Peek Inside the Mind-Modifying Arsenal. In STO-MP-HFM-361 Symposium on Mitigating and Responding to Cognitive Warfare; NATO Science and Technology Organization: Madrid, Spain, 2024; Available online: https://www.sto.nato.int/publications/STO%20Meeting%20Proceedings/STO-MP-HFM-361/MP-HFM-361-P07.pdf (accessed on 11 April 2025).
- Granlund, C. The Use of Chemicals as Agents of War. Kjeller, Norway. Norwegian Defence Research Establishment (FFI). 2023. Available online: https://www.ffi.no/en/publications-archive/the-use-of-chemicals-as-agents-of-war (accessed on 17 March 2025).
- Crowley, M.; Dando, M. Down the Slippery Slope? A Study of Contemporary Dual-Use Chemical and Life Science Research Potentially Applicable to Incapacitating Chemical Agent Weapons; Bradford Non-Lethal Weapons Research Project: Bradford, UK, 2014; Available online: https://www.opcw.org/sites/default/files/documents/PDF/Down_the_Slippery_Slope_Final_LQ.pdf (accessed on 19 March 2025).
- Ketchum, J.S.; Sidell, F.R. Incapacitating Agents (From Medical Aspects of Chemical and Biological Warfare, P 287-306, 1997, Frederick R. Sidell, M.D., Ernest T. Takafuji, M.D., eds; et al.—See NCJ-190599); Office of the Surgeon General, Borden Institute: Washington, DC, USA, 1997; pp. 287–306. Available online: https://www.ojp.gov/ncjrs/virtual-library/abstracts/incapacitating-agents-medical-aspects-chemical-and-biological (accessed on 19 March 2025).
- Fulton, C. Analytical classes of cannabinol compounds in Marihuana resin. Ind. Eng. Chem. Anal. Ed. 1942, 14, 407–412. [Google Scholar] [CrossRef]
- Appendino, G. The early history of cannabinoid research. Rend. Lincei. Sci. Fis. E Nat. 2020, 31, 919–929. [Google Scholar] [CrossRef]
- National Research Council (US); Panel on Anticholinesterase Chemicals; National Research Council (US); Panel on Anticholinergic Chemicals. Possible Long-Term Health Effects of Short-Term Exposure to Chemical Agents: Volume 1 Anticholinesterases and Anticholinergics; National Academies Press: Washington, DC, USA, 1982. Available online: https://www.ncbi.nlm.nih.gov/books/NBK217771/ (accessed on 20 March 2025).
- United States Department of Veterans Affairs. Edgewood/Aberdeen Experiments—VA Public Health; U.S. Department of Veterans Affairs: Washington, DC, USA, 2025. Available online: https://www.publichealth.va.gov/exposures/edgewood-aberdeen/index.asp (accessed on 22 March 2025).
- Torbay, J. The work of Donald Ewen Cameron: From psychic driving to MK Ultra. Hist. Psychiatry 2023, 34, 320–330. [Google Scholar] [CrossRef]
- García-Repetto, R.; Soria, M.L. Sumisión química: Reto para el toxicólogo forense. Rev. Española Med. Leg. 2011, 37, 105–112. [Google Scholar] [CrossRef]
- The Royal Society. Brain Waves Module 3: Neuroscience, Conflict and Security; The Royal Society: London, UK, 2012; Available online: https://royalsociety.org/-/media/policy/projects/brain-waves/2012-02-06-bw3.pdf (accessed on 22 March 2025).
- Mörén, L.; Qvarnström, J.; Larsson, A.; Östin, A. Chemical profiling of dispersed fentanyl analogues sampled from indoor surfaces using multivariate data classification to determine synthesis methods. Forensic Chem. 2021, 24, 100328. [Google Scholar] [CrossRef]
- Madsen, J.M. Generalidades Sobre Agentes de Guerra Química. MSD Manual Professional Version. 2024. Available online: https://www.msdmanuals.com/es/professional/lesiones-y-envenenamientos/armas-que-provocan-v%C3%ADctimas-en-masa/generalidades-sobre-agentes-de-guerra-qu%C3%ADmica (accessed on 20 March 2025).
- Kmietowicz, Z. Doctors condemn use of drugs as weapons. BMJ 2007, 334, 1073. [Google Scholar] [CrossRef]
- National Research Council. Emerging Cognitive Neuroscience and Related Technologies; The National Academies Press: Washington, DC, USA, 2008. [Google Scholar]
- Claverie, B.; du Cluzel, F. The Cognitive Warfare Concept. InnovationHub ACT. 2023. Available online: https://innovationhub-act.org/wp-content/uploads/2023/12/CW-article-Claverie-du-Cluzel-final_0.pdf (accessed on 25 March 2025).
- Morelle, M.; Cegarra, J.; Marion, D.; André, J.-M. Towards a Definition of Cognitive Warfare. In Conference on Artificial Intelligence for Defense; DGA: Rennes, France, 7 December 2023; Available online: https://hal.science/hal-04328461v1 (accessed on 25 March 2025).
- United Nations Office on Drugs and Crime. What Are NPS? UNODC Early Warning Advisory on New Psychoactive Substances. 2024. Available online: https://www.unodc.org/LSS/Page/NPS (accessed on 12 August 2024).
- Ministerio del Interior y Seguridad Pública de Chile. NSP y Precursores; Departamento de Sustancias Químicas Controladas: Santiago, Chile, 2015. Available online: https://www.interior.gob.cl/media/2016/09/NSP-y-Precursores-Departamento-de-Sustancias-Qu%C3%ADmicas-Controladas.pdf (accessed on 28 July 2024).
- United Nations Office on Drugs and Crime. Octubre de 2024—SAT de UNODC: Importante Diversificación Entre Cannabinoides Sintéticos y Opioides en 2023. UNODC Early Warning Advisory on New Psychoactive Substances. 2024. Available online: https://www.unodc.org/LSS/Announcement/Details/a02e4558-b42b-474a-b86b-7bda2b2a033a (accessed on 29 July 2024).
- United States Department of Justice. AlphaBay, the Largest Online ‘Dark Market,’ Shut Down; Office of Public Affairs: Washington, DC, USA, 20 July 2017. Available online: https://www.justice.gov/archives/opa/pr/alphabay-largest-online-dark-market-shut-down (accessed on 11 November 2024).
- Nichols, D.E. Structure–activity relationships of serotonin 5-HT2A agonists. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 559–579. [Google Scholar] [CrossRef]
- Sejvar, J.J. Neurochemical and neurobiological weapons. Neurol. Clin. 2020, 38, 881–896. [Google Scholar] [CrossRef]
- Muñoz-Canales, V.; Rodríguez-López, J. Armas químicas: Descripción general de tipos, riesgos y tratamientos. DOAJ 2021, 38, 52–65. [Google Scholar]
- Guelman, L.R. El LSD y su Impacto en el Movimiento Hippie a 105 Años del Nacimiento de su Descubridor, el Químico Dr. Albert Hofmann. Editorial Sciens 74, 9–18 June 2012. Available online: https://sciens.ar/el-lsd-y-su-impacto-en-el-movimiento-hippie-a-105-anos-del-nacimiento-de-su-descubridor-el-quimico-dr-albert-hofmann/ (accessed on 30 May 2025).
- Beharry, S.; Gibbons, S. An overview of emerging and new psychoactive substances in the United Kingdom. Forensic Sci. Int. 2016, 267, 25–34. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. NPS Data Visualisations; UNODC Early Warning Advisory on NPS: Vienna, Austria, 2025; Available online: https://www.unodc.org/LSS/Page/NPS/DataVisualisations (accessed on 31 October 2024).
- Nichols, D.E. Chemistry and Structure–Activity Relationships of Psychedelics. Curr. Top. Behav. Neurosci. 2017, 36, 1–43. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud. Respuesta de la Salud Pública a Las Armas Biológicas y Químicas: Guía de la OMS, 2nd ed.; Organización Panamericana de la Salud: Washington, DC, USA, 2003; Available online: https://iris.paho.org/handle/10665.2/764 (accessed on 5 September 2024).
- Ketchum, J.S.; Sidell, F.R. Incapacitating Agents. In Medical Aspects of Chemical and Biological Warfare; Sidell, F.R., Takafuji, E.T., Eds.; Borden Institute: Washington, DC, USA, 1997; pp. 287–306. Available online: https://archive.org/details/MedicalAspectsOfChemicalAndBiologicalWarfare_201804/page/n5/mode/2up (accessed on 28 September 2024).
- Jerome, L. Investigator’s Brochure: Lysergic Acid Diethylamide; Multidisciplinary Association for Psychedelic Studies: Sarasota, FL, USA, 2008; Available online: https://maps.org/research-archive/lsd/swisslsd/IB_LSD.pdf (accessed on 28 September 2024).
- Nichols, D.E.; Grob, C.S. Is LSD toxic? Forensic Sci. Int. 2018, 284, 141–145. [Google Scholar] [CrossRef]
- López Muñoz, F. Panacea Encadenada: La Farmacología Alemana Bajo el Yugo de la Esvástica; Real Academia de Doctores: Barcelona, Spain, 2015; Available online: https://raed.academy/wp-content/uploads/2015/11/Discurso-Dr.-L%C3%B3pez.pdf (accessed on 13 August 2024).
- Sánchez de Miguel, M.; Iturbide, L.M.; Lizaso, I. La Inteligencia Militar Norteamericana y el Uso Ambivalente de la Psicología Desde Una Perspectiva Histórica: El Programa Handicrafts (1941) y el Proyecto MKUltra (1953). Rev. Hist. Psicol. 2012, 33, 37–57. Available online: https://journals.copmadrid.org/historia/art/79a49b3e3762632813f9e35f4ba53d6c (accessed on 30 November 2024).
- Belvís, R.; Ezpeleta, D. Stranger things. Neurocientíficos militares y de la CIA en proyectos secretos de experimentación humana en los Estados Unidos (1945–1975). Kranion 2023, 18, 72–87. [Google Scholar] [CrossRef]
- Belouin, S.J.; Henningfield, J.E. Psychedelics: Where we are now, why we got here, what we must do. Neuropharmacology 2018, 142, 7–19. [Google Scholar] [CrossRef]
- Nichols, D.E. Dark classics in chemical neuroscience: Lysergic acid diethylamide (LSD). ACS Chem. Neurosci. 2018, 9, 2331–2343. [Google Scholar] [CrossRef]
- Nofil, B. MK-Ultra: Operation Midnight Climax and CIA LSD Experiments. History. 2024. Available online: https://www.history.com/mkultra-operation-midnight-climax-cia-lsd-experiments (accessed on 22 August 2024).
- United Nations Office on Drugs and Crime. Terminología e Información Sobre Drogas—Tercera Edición; United Nations: New York, NY, USA, 2018; Available online: https://www.unodc.org/documents/scientific/Terminology_Spanish.pdf (accessed on 22 April 2025).
- Bey, T.; Patel, A. Phencyclidine Intoxication and Adverse Effects: A Clinical and Pharmacological Review of an Illicit Drug. Cal. J. Emerg. Med. 2007, 8, 9–14. [Google Scholar]
- Bertron, J.L.; Seto, M.; Lindsley, C.W. DARK Classics in Chemical Neuroscience: Phencyclidine (PCP). ACS Chem. Neurosci. 2018, 9, 2459–2474. [Google Scholar] [CrossRef]
- Petersen, R.C.; Stillman, R.C. (Eds.) Phencyclidine (PCP) Abuse: An Appraisal; NIDA Research Monograph 21. National Institute on Drug Abuse: Rockville, MD, USA, 1978; Available online: http://www.thevespiary.org/rhodium/Rhodium/pdf/nida.monograph.21.pcp-overview.pdf (accessed on 25 May 2025).
- Aniline, O.; Pitts, F.N. Phencyclidine (PCP): A Review and Perspectives. CRC Crit. Rev. Toxicol. 1982, 10, 145–177. [Google Scholar] [CrossRef]
- Burns, R.S.; Lerner, S.E.; Corrado, R.; James, S.H.; Schnoll, S.H. Phencyclidine—States of Acute Intoxication and Fatalities. West. J. Med. 1975, 123, 345–349. [Google Scholar]
- Sirchie. NARK2009 Phencyclidine (PCP) Reagent—Safety Data Sheet; Sirchie: Youngsville, NC, USA, 2020; Available online: https://www.sirchie.com/media/resourcecenter/item/n/a/nark2009_phencyclidine_pcp_reagent_1.pdf (accessed on 2 March 2025).
- LookChem. Phencyclidine Hydrochloride (CAS No. 956-90-1)—Product Information; LookChem: Hangzhou, China, 2008; Available online: https://www.lookchem.com/cas-956/956-90-1.html (accessed on 5 May 2025).
- Becker, R.; Myall, S. Rapper Killed His Friend’s Girlfriend and Then Chewed Up and Swallowed Her Lungs; The Mirror: London, UK, 2017; Available online: https://www.mirror.co.uk/news/real-life-stories/rapper-killed-girlfriend-chewed-up-11566075 (accessed on 5 May 2025).
- Szalavitz, M. Myths and Facts About Angel Dust: Did PCP Drive Aaron Hernandez to Commit Murder? Time, 28 August 2013. Available online: https://healthland.time.com/2013/08/28/myths-and-facts-about-angel-dust-did-pcp-drive-aaron-hernandez-to-commit-murder/ (accessed on 7 May 2025).
- Serrano, B. La Noche Que el Equipo de Titanic Fue Drogado con Polvo de Ángel en la Sopa. El País, 17 April 2024. Available online: https://elpais.com/gente/2024-04-18/la-noche-que-el-equipo-de-titanic-fue-drogado-con-polvo-de-angel-en-la-sopa.html (accessed on 2 January 2025).
- National Drug Intelligence Center. PCP Fast Facts. Johnstown. Available online: https://www.justice.gov/archive/ndic/pubs4/4440/index.htm (accessed on 3 January 2025).
- Han, Y.; Yan, W.; Zheng, Y.; Khan, M.Z.; Yuan, K.; Lu, L. The rising crisis of illicit fentanyl use, overdose, and potential therapeutic strategies. Transl. Psychiatry 2019, 9, 282. [Google Scholar] [CrossRef]
- Armenian, P.; Vo, K.T.; Barr-Walker, J.; Lynch, K.L. Fentanyl, fentanyl analogs and novel synthetic opioids: A Comprehensive Review. Neuropharmacology 2017, 134, 121–132. [Google Scholar] [CrossRef]
- Hamilton, G.R.; BAskett, T.F. In the arms of Morpheus: The development of morphine for postoperative pain relief. Can. J. Anesth. J. Can. D Anesthésie 2000, 47, 367–374. [Google Scholar] [CrossRef]
- Anguiano Arreola, B.; Partido Lara, O. La Crisis del Fentanilo: Una Aproximación Preliminar. Epikeia, Revista del Departamento de Ciencias Sociales y Humanidades, Núm. 46. 2023. Available online: https://epikeia.iberoleon.mx/numeros/46/la-crisis-del-fentanilo.pdf (accessed on 2 January 2025).
- Gummin, D.D. Potent opioids and implications for national defense. Toxicol. Lett. 2019, 321, 90–94. [Google Scholar] [CrossRef]
- Institute for Human Data Science. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023; IQVIA: Durham, NC, USA, 2019; Available online: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023 (accessed on 26 August 2024).
- Patocka, J.; Wu, W.; Oleksak, P.; Jelinkova, R.; Nepovimova, E.; Spicanova, L.; Springerova, P.; Alomar, S.; Long, M.; Kuca, K. Fentanyl and its derivatives: Pain-killers or man-killers? Heliyon 2024, 10, e28795. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Magnusson, P.; LeQuang, J.A.K.; Breve, F. Illicitly manufactured fentanyl entering the United States. Cureus 2021, 13, 8. [Google Scholar] [CrossRef]
- Spencer, M.R.; Miniño, A.M.; Warner, M. Drug Overdose Deaths in the United States, 2001–2021; NCHS Data Brief, No. 457; National Center for Health Statistics: Hyattsville, MD, USA, 2022. [Google Scholar] [CrossRef]
- U.S. Drug Enforcement Administration. 2020 National Drug Threat Assessment; U.S. Department of Justice: Washington, DC, USA, 2021. Available online: https://www.dea.gov/sites/default/files/2021-02/DIR-008-21%202020%20National%20Drug%20Threat%20Assessment_WEB.pdf (accessed on 27 July 2025).
- U.S. Drug Enforcement Administration. Fentanyl Flow in the United States; U.S. Department of Justice: Washington, DC, USA, 2020. Available online: https://www.dea.gov/sites/default/files/2020-03/DEA_GOV_DIR-008-20%20Fentanyl%20Flow%20in%20the%20United%20States_0.pdf (accessed on 27 July 2025).
- Felbab-Brown, V. The Fentanyl Pipeline and China’s Role in the US Opioid Crisis. Brookings Institution, 1 October 2024. Available online: https://www.brookings.edu/articles/the-fentanyl-pipeline-and-chinas-role-in-the-us-opioid-crisis/ (accessed on 28 July 2025).
- Burns, S.M.; Cunningham, C.W.; Mercer, S.L. DARK Classics in Chemical Neuroscience: Fentanyl. ACS Chem. Neurosci. 2018, 9, 2428–2437. [Google Scholar] [CrossRef]
- Vuckovic, S.; Prostran, M.; Ivanovic, M.; Dosen-Micovic, L.J.; Todorovic, Z.; Nesic, Z.; Stojanovic, R.; Divac, N.; Mikovic, Z. Fentanyl Analogs: Structure-Activity-Relationship Study. Curr. Med. Chem. 2009, 16, 2468–2474. [Google Scholar] [CrossRef]
- Caves, J.P., Jr. Fentanyl as a Chemical Weapon; National Defense University Press: Washington, DC, USA, 2019; Available online: https://digitalcommons.ndu.edu/wmd-proceedings/3/ (accessed on 4 January 2025).
- Spriggs, S.M.; Platoff, G.E.; Jett, D.A. Research on Medical Countermeasures for Chemical Attacks on Civilians. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Academic Press: San Diego, CA, USA, 2020; pp. 1135–1144. [Google Scholar]
- Manral, L.; Muniappan, N.; Gupta, P.K.; Ganesan, K.; Malhotra, R.C.; Vijayaraghavan, R. Effect of exposure to fentanyl aerosol in mice on breathing pattern and respiratory variables. Drug Chem. Toxicol. 2009, 32, 108–113. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. Fentanyl-related compounds and derivatives: Current status and future prospects for pharmaceutical applications. Future Med. Chem. 2014, 6, 385–412. [Google Scholar] [CrossRef]
- U.S. Department of Homeland Security. S&T Master Question List for Synthetic Opioids. 2024. Available online: https://www.dhs.gov/publication/st-master-question-list-synthetic-opioids (accessed on 11 May 2025).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Fentanyl Drug Profile. Available online: https://www.euda.europa.eu/publications/drug-profiles/fentanyl_en (accessed on 11 May 2025).
- U.S. Drug Enforcement Administration (DEA). Facts About Fentanyl. Available online: https://www.dea.gov/resources/facts-about-fentanyl (accessed on 11 May 2025).
- Gao, X.; Zhuang, J.; Chen, Z.; Shi, S.; Xu, F. Sudden death induced by acute inhalation of aerosolized carfentanil. Arch. Clin. Biomed. Res. 2025, 9, 96–105. [Google Scholar] [CrossRef]
- Lent, E.M.; Maistros, K.J.; Oyler, J.M. In vitro dermal absorption of carfentanil. Toxicol. Vitr. 2019, 62, 104696. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Kuczyńska, K.; Kosmal, W.; Markiewicz, K.; Adamowicz, P. Carfentanil—From an animal anesthetic to a deadly illicit drug. Forensic Sci. Int. 2021, 320, 110715. [Google Scholar] [CrossRef]
- Lent, E.M.; Maistros, K.J.; Oyler, J.M. In Vitro Dermal Absorption of Carfentanil; Toxicology Directorate, Army Public Health Center: Aberdeen Proving Ground, MD, USA, 2018; Toxicity Report No. S.0055513-18; Available online: https://ph.health.mil/PHC%20Resource%20Library/InVitroDermalAbsorptionofCarfentanil.pdf (accessed on 28 July 2025).
- Tella, S.R. Statement Before the United States Sentencing Commission for a Public Hearing on Fentanyl and Synthetic Cannabinoid; U.S. Sentencing Commission: Washington, DC, USA, 2017. Available online: https://www.ussc.gov/sites/default/files/pdf/amendment-process/public-hearings-and-meetings/20171205/Tella.pdf (accessed on 28 July 2025).
- Feasel, M.G.; Lawrence, R.J.; Kristovich, R.L.; Wohlfarth, A.; Huestis, M.A. Translational Human Health Assessment of Carfentanil Using an Experimentally Refined PBPK Model; ECBC-TR-1528; U.S. Army Edgewood Chemical Biological Center: Baltimore, MD, USA, 2018; Available online: https://apps.dtic.mil/sti/trecms/pdf/AD1060142.pdf (accessed on 27 July 2025).
- Science and Technology Directorate, U.S. Department of Homeland Security. Master Question List (MQL) for Synthetic Opioids—Version 1, 30 September 2021; Chemical Security Analysis Center & Science and Technology Directorate, U.S. Department of Homeland Security: Washington, DC, USA, 2021. Available online: https://www.dhs.gov/sites/default/files/publications/21_1110_st_csac_mql_synthetic_opioids_30sep2021_pr_508.pdf (accessed on 27 July 2025).
- Ralston, M.S.A.; Murray, M.B.P.; Vela-Duarte, D.; Orjuela, K.D.; Pastula, D.M. Neuroterrorism preparedness for the neurohospitalist. Neurohospitalist 2018, 9, 151–159. [Google Scholar] [CrossRef]
- Ringuette, A.E.; Spock, M.; Lindsley, C.W.; Bender, A.M. DARK classics in chemical neuroscience: Carfentanil. ACS Chem. Neurosci. 2020, 11, 3955–3967. [Google Scholar] [CrossRef]
- O’Brien, C.P. Benzodiazepine Use, Abuse, and Dependence. J. Clin. Psychiatry 2005, 66 (Suppl. S2), 28–33. Available online: https://www.psychiatrist.com/jcp/benzodiazepine-abuse-dependence-2/ (accessed on 27 July 2025).
- Sanabria, E.; Cuenca, R.E.; Esteso, M.Á.; Maldonado, M. Benzodiazepines: Their Use Either as Essential Medicines or as Toxics Substances. Toxics 2021, 9, 25. [Google Scholar] [CrossRef]
- Schmitz, A. Benzodiazepine Benzodiazepine use, misuse, and abuse: A review. Ment. Health Clin. 2016, 6, 120–126. [Google Scholar] [CrossRef]
- Ramadan, A.S.E.; Wenanu, O.; Cock, A.D.E.; Maes, V.; Lheureux, P.; Mols, P. Chemical submission to commit robbery: A series of involuntary intoxications with flunitrazepam in Asian travellers in Brussels. J. Forensic Leg. Med. 2013, 20, 918–921. [Google Scholar] [CrossRef]
- Missliwetz, J.; Reiter, C. ’Oral hygiene’—A method of killing in a medical setting. Med. Law 1995, 14, 45–51. [Google Scholar]
- United Nations Office on Drugs and Crime. The Non-Medical Use of Prescription Drugs: Policy Direction Issues; United Nations: New York, NY, USA, 2011; Available online: https://www.unodc.org/documents/drug-prevention-and-treatment/nonmedical-use-prescription-drugs.pdf (accessed on 24 April 2025).
- Brunetti, P.; Giorgetti, R.; Tagliabracci, A.; Huestis, M.; Busardò, F. Designer Benzodiazepines: A review of toxicology and public health risks. Pharmaceuticals 2021, 14, 560. [Google Scholar] [CrossRef]
- Cayman Chemical. Safety Data Sheet: Flunitrazepam (Item No. 16188). Cayman Chemical, 28 August 2024. Available online: https://cdn.caymanchem.com/cdn/msds/16188m.pdf (accessed on 15 April 2025).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 134664, Benzodiazepine; National Library of Medicine: Bethesda, MD, USA, 2025. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/benzodiazepine (accessed on 1 June 2025).
- Megarbane, B.; Lesguillons, N.; Galliot-Guilley, M.; Borron, S.W.; Trout, H.; Declèves, X.; Risède, P.; Monier, C.; Boschi, G.; Baud, F.J. Cerebral and plasma kinetics of a high dose of midazolam and correlations with its respiratory effects in rats. Toxicol. Lett. 2005, 159, 22–31. [Google Scholar] [CrossRef]
- Fermion. Alprazolam—Safety Data Sheet; Fermion: Espoo, Finland, 2017; Available online: https://www.fermion.fi/siteassets/documents/material-safety-data-sheets/alprazolam.pdf (accessed on 23 May 2025).
- Gable, R.S. Acute toxic effects of club drugs. J. Psychoact. Drugs 2004, 36, 303–313. [Google Scholar] [CrossRef]
- Cayman Chemical. Safety Data Sheet: Clonazolam (Item No. 18173). Cayman Chemical, 2 April 2024. Available online: https://cdn.caymanchem.com/cdn/msds/18173m.pdf (accessed on 15 April 2025).
- Ketchum, J.S.; Salem, H. Incapacitating Agents. In Medical Aspects of Chemical Warfare; Zajtchuk, R., Bellamy, R.F., Eds.; Borden Institute: Washington, DC, USA, 2008; Chapter 12; pp. 411–440. Available online: https://medcoeckapwstorprd01.blob.core.usgovcloudapi.net/pfw-images/borden/chemwarfare/Ch12_pgs411-440.pdf (accessed on 16 April 2025).
- Dubovsky, S.L.; Marshall, D. Benzodiazepines Remain Important Therapeutic Options in Psychiatric Practice. Psychother. Psychosom. 2022, 91, 307–334. [Google Scholar] [CrossRef]
- Okada, A.; Sera, S.; Nagai, N. Appropriate Use of Triazolam in Elderly Patients Considering a Quantitative Benefit-Risk Assessment Based on the Pharmacokinetic-Pharmacodynamic Modeling and Simulation Approach Supported by Real-World Data. BMC Pharmacol. Toxicol. 2024, 25, 60. [Google Scholar] [CrossRef]
- Boisselier, R.L.; Alexandre, J.; Lelong-Boulouard, V.; Debruyne, D. Focus on cannabinoids and synthetic cannabinoids. Clin. Pharmacol. Ther. 2016, 101, 220–229. [Google Scholar] [CrossRef]
- Worob, A.; Wenthur, C. DARK classics in chemical neuroscience: Synthetic cannabinoids (Spice/K2). ACS Chem. Neurosci. 2019, 11, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Spaderna, M.; Addy, P.H.; D’Souza, D.C. Spicing things up: Synthetic cannabinoids. Psychopharmacology 2013, 228, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Pease, D.C.; Clark, J.H. Isolation of cannabinol, cannabidiol and quebrachitol from red oil of Minnesota wild hemp. J. Am. Chem. Soc. 1940, 62, 2194–2196. [Google Scholar] [CrossRef]
- Lee, M.A. High Spy. Rolling Stone, 1 September 1983. Available online: https://www.cia.gov/readingroom/document/cia-rdp90-01208r000100090027-5 (accessed on 17 May 2025).
- Hinojosa Becerra, M.; Marín Gutiérrez, I. El Descubrimiento del Cannabidiol, el Principal Componente del Cannabis. Cannabis Magazine. June 2017. Available online: https://www.researchgate.net/publication/317237790_El_descubrimiento_del_cannabidiol_el_principal_componente_del_cannabis (accessed on 24 May 2025).
- Kamieński, Ł. Intoxicants in Warfare. In Routledge Handbook of Intoxicants and Intoxication; Hunt, G., Antin, T.M.J., Frank, V.A., Eds.; Routledge: London, UK, 2023; pp. 239–257. Available online: https://library.oapen.org/handle/20.500.12657/75522 (accessed on 17 May 2025).
- Ketchum, J.S. Chemical Warfare: Secrets Almost Forgotten: A Personal Story of Medical Testing of Army Volunteers with Incapacitating Chemical Agents During the Cold War (1955–1975); ChemBooks Inc.: Santa Rosa, CA, USA, 2006; Available online: https://medicinthegreentime.com/wp-content/uploads/2019/06/ARMY-GUINEA-PIGS-KETCHUMS-BOOK.pdf (accessed on 17 May 2025).
- National Research Council. Possible Long-Term Health Effects of Short-Term Exposure to Chemical Agents, Volume 2: Cholinesterase Reactivators, Psychochemicals and Irritants and Vesicants; The National Academies Press: Washington, DC, USA, 1984; pp. 47–100. [Google Scholar]
- Saint Brand Cannabis Co. Cannabinoid Formulation for the Sedation of a Human or Animal. U.S. Patent 9,585,867 B2, 7 March 2017. [Google Scholar]
- Lobato-Freitas, C.; Brito-Da-Costa, A.M.; Dinis-Oliveira, R.J.; Carmo, H.; Carvalho, F.; Silva, J.P.; Dias-Da-Silva, D. Overview of Synthetic Cannabinoids ADB-FUBINACA and AMB-FUBINACA: Clinical, Analytical, and Forensic Implications. Pharmaceuticals 2021, 14, 186. [Google Scholar] [CrossRef]
- Adams, A.J.; Banister, S.D.; Irizarry, L.; Trecki, J.; Schwartz, M.; Gerona, R. “Zombie” Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York. N. Engl. J. Med. 2016, 376, 235–242. [Google Scholar] [CrossRef]
- World Health Organization. Critical Review Report: FUB-AMB (MMB-FUBINACA, AMB-FUBINACA); Expert Committee on Drug Dependence, Forty-first Meeting; WHO: Geneva, Switzerland, 2018; Available online: https://ecddrepository.org/sites/default/files/2023-04/fub_amb.pdf (accessed on 25 May 2025).
- Lam, R.P.K.; Tang, M.H.Y.; Leung, S.C.; Chong, Y.K.; Tsui, M.S.H.; Mak, T.W.L. Supraventricular tachycardia and acute confusion following ingestion of e-cigarette fluid containing AB-FUBINACA and ADB-FUBINACA: A case report with quantitative analysis of serum drug concentrations. Clin. Toxicol. 2017, 55, 662–667. [Google Scholar] [CrossRef]
- Horth, R.Z.; Crouch, B.; Horowitz, B.Z.; Prebish, A.; Slawson, M.; McNair, J.; Elsholz, C.; Gilley, S.; Robertson, J.; Risk, I.; et al. Notes from the field: Acute poisonings from a synthetic cannabinoid sold as cannabidiol—Utah, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 587–588. [Google Scholar] [CrossRef]
- Panons, J.; PA Media. Prison Staff ‘Deliberately Poisoned’ with Drug. BBC News. South East, 16 May 2024. Available online: https://www.bbc.com/news/articles/c14k4knwgk9o (accessed on 20 May 2025).
- Pandey, N. Prison Guards in UK Hospitalised After Inmates Spike Staff Curry with Dangerous Drug; NDTV: New Delhi, India, 2024; Available online: https://www.ndtv.com/world-news/prison-guards-in-uk-hospitalised-after-inmates-spike-staff-curry-with-dangerous-drug-5681326 (accessed on 22 May 2025).
- Angerer, V.; Möller, C.; Auwärter, V. Synthetic cannabinoids in prisons—Invisibly impregnated paper sheets as a Trojan horse. In Proceedings of the the 56th Annual Meeting of the International Association of Forensic Toxicologists (TIAFT), Ghent, Belgium, 26–30 August 2018; Available online: https://www.uniklinik-freiburg.de/fileadmin/mediapool/08_institute/rechtsmedizin/pdf/Poster_2018/Angerer_V_-_Tiaft_2018.pdf (accessed on 26 May 2025).
- Kuai, D.; Blanco, L.E.R.; Krotulski, A.; Walton, S.; Denn, M.; Kelly, B.; Kiernan, E.; Steck, A.; Carpenter, J. Identification and health risks of an emerging means of drug use in correctional facilities. JAMA Netw. Open 2024, 7, e2451951. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Synthetic Cannabinoids in Herbal Products; UNODC: Vienna, Austria, 2011; Available online: https://www.unodc.org/documents/scientific/Synthetic_Cannabinoids.pdf (accessed on 26 May 2025).
- United Nations. Commission on Narcotic Drugs. Report on the Reconvened Sixty-Third Session (2–4 December 2020) (Economic and Social Council Official Records, Supplement No. 8A), New York, NY, USA, 2–4 December 2020. Available online: https://documents.un.org/doc/undoc/gen/v20/076/33/pdf/v2007633.pdf (accessed on 25 May 2025).
- European Monitoring Centre for Drugs and Drug Addiction. Synthetic Cannabinoids in Europe—A Review; Publications Office of the European Union: Luxembourg, 2021; Available online: https://www.euda.europa.eu/system/files/publications/14035/Synthetic-cannabinoids-in-Europe-EMCDDA-technical-report.pdf (accessed on 22 May 2025).
- Cayman Chemical. Safety Data Sheet: MMB-FUBINACA (Item No. 31090). Cayman Chemical, 22 January 2024. Available online: https://cdn.caymanchem.com/cdn/msds/31090m.pdf (accessed on 15 April 2025).
- Cayman Chemical. Safety Data Sheet: ADB-FUBINACA (Item No. 14292). Cayman Chemical, 11 December 2024. Available online: https://cdn.caymanchem.com/cdn/msds/14292m.pdf (accessed on 15 April 2025).
- Centenera, M. Tragedia en Argentina: Al Menos 20 Muertos y Más de 70 Hospitalizados Por Consumir Cocaína Envenenada. El País, 2 February 2022. Available online: https://elpais.com/internacional/2022-02-02/tragedia-en-argentina-varios-muertos-y-decenas-de-hospitalizados-por-consumir-cocaina-envenenada.html (accessed on 15 January 2025).
- Molina, F.R. Cocaína Mortal en Argentina: Los Peritos Encuentran Carfentanilo, un Opiáceo Para Dormir Elefantes, en la Droga Que Mató a 24 Personas. El País, 10 February 2022. Available online: https://elpais.com/internacional/2022-02-10/cocaina-mortal-en-argentina-los-peritos-encuentran-carfentanilo-un-opioide-para-dormir-elefantes-en-la-droga-que-mato-a-24-personas.html (accessed on 15 January 2025).
- Oficina de las Naciones Unidas contra la Droga y el Delito. Los Tratados de Fiscalización Internacional de Drogas; UNODC: Vienna, Austria, 2013; Available online: https://www.unodc.org/documents/commissions/CND/Int_Drug_Control_Conventions/Ebook/The_International_Drug_Control_Conventions_S.pdf (accessed on 27 May 2025).
- Organización para la Prohibición de las Armas Químicas. ¿Qué es un Arma Química? OPAQ: The Hague, The Netherlands, 2025; Available online: https://www.opcw.org/es/nuestra-labor/que-es-un-arma-quimica (accessed on 17 March 2025).
- Bernacchi, A. Armas Químicas y el Marco Internacional. In Desarme y no Proliferación: Un Enfoque Multidisciplinario. Universidad de la Defensa Nacional, Ed.; UNDEF Libros: Buenos Aires, Argentina, 2022; pp. 11–27. Available online: https://cefadigital.edu.ar/handle/1847939/2278 (accessed on 23 April 2025).
- United Nations. Generic Legislation. Available online: https://syntheticdrugs.unodc.org/syntheticdrugs/en/legal/national/genericcontrols.html (accessed on 19 March 2025).
- Dorocka, W. Cómo la IA Está Mejorando los Diagnósticos y los Resultados Terapéuticos, Transformando la Asistencia Médica. World Economic Forum, 30 September 2024. Available online: https://es.weforum.org/stories/2024/09/como-la-ia-esta-mejorando-los-diagnosticos-y-los-resultados-terapeuticos-transformando-la-asistencia-medica/ (accessed on 12 January 2025).
- Craig, J. Widely Available AI Could Have Deadly Consequences. Wired, 17 May 2022. Available online: https://www.wired.com/story/ai-dr-evil-drug-discovery/ (accessed on 21 March 2025).
- J.L.G. Todo un Anticipo de los Medicamentos del Futuro. El País, 27 June 2024. Available online: https://elpais.com/tecnologia/branded/inteligencia-artificial/2024-06-27/todo-un-anticipo-de-los-medicamentos-del-futuro.html (accessed on 7 February 2025).
- Urbina, F.; Lentzos, F.; Invernizzi, C.; Ekins, S. Dual use of artificial-intelligence-powered drug discovery. Nat. Mach. Intell. 2022, 4, 189–191. [Google Scholar] [CrossRef]
- Mikulak-Klucznik, B.; Klucznik, T.; Beker, W.; Moskal, M.; Grzybowski, B.A. Catalyst: Curtailing the scalable supply of fentanyl by using chemical AI. Chem 2024, 10, 1319–1326. [Google Scholar] [CrossRef]

| Generation | Chemical Agent | LCt50 (Inhal.) mg·min/m3 | LD50 p.c. mg/kg (Liquid) | LCt50 p.c. (Vapor) mg·min/m3 | Sources |
|---|---|---|---|---|---|
| First | Phosphogen | 2000 | NA | NA | [23] |
| Lewisite | 1200 | 35–40 | 99,000 | [24,25] | |
| Mustard Gas (HD) | 1500 | 100 | 10,000 | [26,27] | |
| Second | Tabun (GA) | 70 | ~21.4 | 15,000 | [28] |
| Sarin (GB) | 35 | ~24 | 10,000 | [29] | |
| Soman (GD) | 35 | ~5 | 2500 | [30] | |
| Third | VX | 30 | 0.07 | 150 | [31] |
| Fourth | A-230 | 1.9–3 | 0.6 | NA | [22] |
| A-232 (Novichok 5) | 7 | 5 | NA | [32] |
| Type of Chemical Agent | Examples of Agents | Method of Dispersion | Mode of Action | Effects |
|---|---|---|---|---|
| Asphyxiants | Chlorine (Cl2), Phosgene (CG), Diphosgene (DP), Chloropicrin (PS). | Gas | Absorption through the lungs. | Accumulation of fluid in the lungs that causes irritation to the nose, throat and airways, making breathing difficult, which can lead to asphyxia and death. |
| Vesicants | Sulfur Mustard (HHD), Nitrogen Mustard (HN), Lewisite (L), Phosgene Oxime (CX). | Liquid, Aerosol, Vapor, and Dust | Absorption through the lungs and skin. | Irritation of the skin and mucous membranes, causing blistering of the exposed surface, and respiratory tract, with the risk of being life threatening. |
| Hemotoxicants | Hydrogen Cyanide (AC), Cyanogen Chloride (CK), Arsine (SA). | Gas | Absorption through the lungs and skin; inhibition of the enzyme, cytochrome C oxidase. | They interfere with the ability to transport oxygen in the blood, causing hypoxia. |
| Neurotoxicants | Sarin (GB), Soman (GD), Tabun (GA), VX, Novichok (A-230). | Liquid, Aerosol, Vapor, and Dust | Absorption through the lungs (G-series); skin contact (V-series); inhibition of acetyl cholinesterase. | They generate hyperstimulation of the central nervous system, causing convulsions, muscle paralysis, respiratory distress, dizziness, confusion, and, in extreme cases, death. |
| Riot Control Agents (RCAs) | Pepper Gas (OC), Tear Gas (CS). | Liquid, Aerosol, Gas | Absorption through lungs, skin, and eyes. | Irritation of mucous membranes and activation of pain receptors in eyes, nose, and throat. Causes excessive tearing, shortness of breath, coughing, excessive salivation, and nasal discharge. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela-Tapia, L.N.; Quintul, C.A.; Rubio-Concha, N.D.; Toledo-Ríos, L.; Salas-Kuscevic, C.; Leisewitz, A.V.; Cámpora-Oñate, P.; Campanini-Salinas, J. The Blurred Lines Between New Psychoactive Substances and Potential Chemical Weapons. Toxics 2025, 13, 659. https://doi.org/10.3390/toxics13080659
Valenzuela-Tapia LN, Quintul CA, Rubio-Concha ND, Toledo-Ríos L, Salas-Kuscevic C, Leisewitz AV, Cámpora-Oñate P, Campanini-Salinas J. The Blurred Lines Between New Psychoactive Substances and Potential Chemical Weapons. Toxics. 2025; 13(8):659. https://doi.org/10.3390/toxics13080659
Chicago/Turabian StyleValenzuela-Tapia, Loreto N., Cristóbal A. Quintul, Nataly D. Rubio-Concha, Luis Toledo-Ríos, Catalina Salas-Kuscevic, Andrea V. Leisewitz, Pamela Cámpora-Oñate, and Javier Campanini-Salinas. 2025. "The Blurred Lines Between New Psychoactive Substances and Potential Chemical Weapons" Toxics 13, no. 8: 659. https://doi.org/10.3390/toxics13080659
APA StyleValenzuela-Tapia, L. N., Quintul, C. A., Rubio-Concha, N. D., Toledo-Ríos, L., Salas-Kuscevic, C., Leisewitz, A. V., Cámpora-Oñate, P., & Campanini-Salinas, J. (2025). The Blurred Lines Between New Psychoactive Substances and Potential Chemical Weapons. Toxics, 13(8), 659. https://doi.org/10.3390/toxics13080659








