Cognitive Functions Among Pupils in Schools Near and Around an Electronic Waste Recycling Site at Agbogbloshie in Accra, Ghana
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
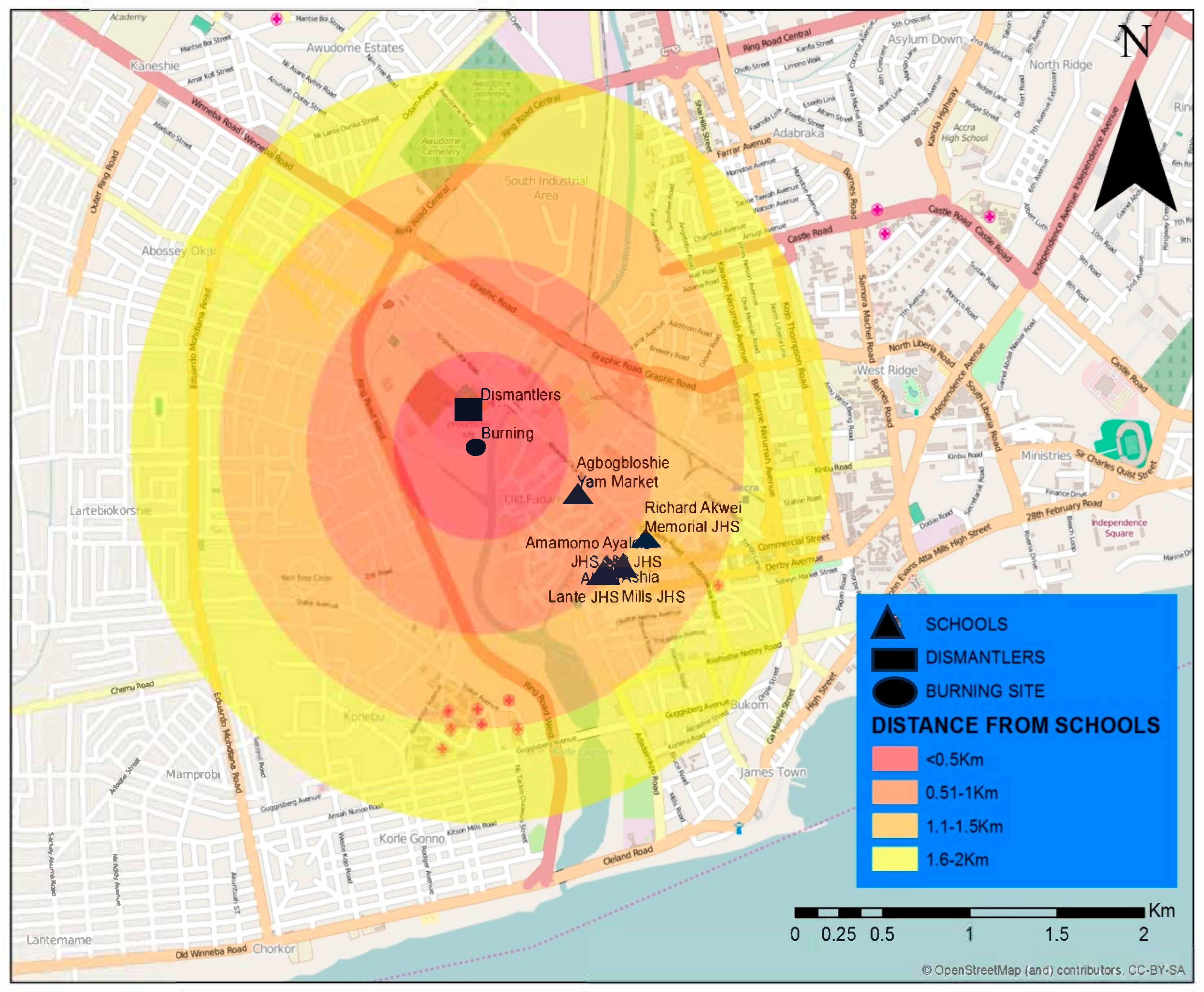
2.2. Study Area
2.3. Participant Recruitment
2.4. Data Collection
2.4.1. Questionnaire Survey
2.4.2. Blood and Urine Sample Collection
2.4.3. Laboratory Analysis
2.4.4. Cognitive Function Analysis
2.5. Statistical Analysis
3. Results
3.1. Socio-Demographic Characteristics of Pupils
3.2. Prevalence of Cognitive Dysfunction Symptoms
3.3. Metal Concentrations in Blood and Urine
3.4. Association Between Metals and Full-Scale IQ
3.5. Associations Between Metal Exposure and Cognitive Functions
3.6. Correlation Heatmap of Metal Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acquah, A.A.; D’Souza, C.; Martin, B.; Arko-Mensah, J.; Nti, A.A.; Kwarteng, L.; Takyi, S.; Quakyi, I.A.; Robins, T.G.; Fobil, J.N. Processes and challenges associated with informal electronic waste recycling at Agbogbloshie, a suburb of Accra, Ghana. In Proceedings of the Human Factors and Ergonomics Society Annual Meeting 2019, Seattle, WA, USA, 28 October–1 November 2019; Volume 63, pp. 938–942. [Google Scholar] [CrossRef]
- Lebbie, T.S.; Moyebi, O.D.; Asante, K.A.; Fobil, J.; Brune-Drisse, M.N.; Suk, W.A.; Sly, P.D.; Gorman, J.; Carpenter, D.O. E-Waste in Africa: A Serious Threat to the Health of Children. Int. J. Environ. Res. Public Health 2021, 18, 8488. [Google Scholar] [CrossRef] [PubMed]
- Bora, J.; Kumari, M.; Panda, I.; Gupta, S.; Priya, S.; Mondal, S.; Malik, S.; Lata, S. Health Effects of Heavy Metals Contamination in Children. In Nanotechnology Applications and Innovations for Improved Soil Health; IGI Global: Hershey, PA, USA, 2024; pp. 254–275. [Google Scholar]
- Püschel, P.; Agbeko, K.M.; Amoabeng-Nti, A.A.; Arko-Mensah, J.; Bertram, J.; Fobil, J.N.; Waldschmidt, S.; Löhndorf, K.; Schettgen, T.; Lakemeyer, M.; et al. Lead exposure by E-waste disposal and recycling in Agbogbloshie, Ghana. Int. J. Hyg. Environ. Health 2024, 259, 114375. [Google Scholar] [CrossRef]
- Acka, M. Metals in Agbogbloshie e-Waste Recycling Site, Accra, Ghana: Distribution, Bioaccessibility and Health Risk Assessment. Ph.D. Thesis, Macquarie University, Sydney, Australia, 2020. [Google Scholar]
- Dodd, M.; Amponsah, L.O.; Grundy, S.; Darko, G. Human health risk associated with metal exposure at Agbogbloshie e-waste site and the surrounding neighbourhood in Accra, Ghana. Environ. Geochem. Health 2023, 45, 4515–4531. [Google Scholar] [CrossRef]
- Brumatti, L.V.; Rosolen, V.; Mariuz, M.; Piscianz, E.; Valencic, E.; Bin, M.; Athanasakis, E.; D’Adamo, P.; Fragkiadoulaki, E.; Calamandrei, G.; et al. Impact of Methylmercury and Other Heavy Metals Exposure on Neurocognitive Function in Children Aged 7 Years: Study Protocol of the Follow-up. J. Epidemiol. 2021, 31, 157–163. [Google Scholar] [CrossRef]
- Kim, H.; Harrison, F.E.; Aschner, M.; Bowman, A.B. Exposing the role of metals in neurological disorders: A focus on manganese. Trends Mol. Med. 2022, 28, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.Y.; Asad, I.; Coleman, B.; Menard, L.; Benki-Nugent, S.; Hussein Were, F.; Karr, C.J.; McHenry, M.S. Heavy metals and neurodevelopment of children in low and middle-income countries: A systematic review. PLoS ONE 2022, 17, e0265536. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Soetrisno, F.N.; Delgado-Saborit, J.M. Chronic exposure to heavy metals from informal e-waste recycling plants and children’s attention, executive function and academic performance. Sci. Total Environ. 2020, 717, 137099. [Google Scholar] [CrossRef]
- Parvez, S.M.; Jahan, F.; Brune, M.N.; Gorman, J.F.; Rahman, M.J.; Carpenter, D.; Islam, Z.; Rahman, M.; Aich, N.; Knibbs, L.D.; et al. Health consequences of exposure to e-waste: An updated systematic review. Lancet Planet. Health 2021, 5, e905–e920. [Google Scholar] [CrossRef]
- Angerer, J.; Schaller, K.H.; Fleischer, M. Nickel in urine. In Analyses of Hazardous Substances in Biological Materials; German Science Foundation: Weinheim, Germany, 1985; Volume 1, pp. 177–188. [Google Scholar]
- Angerer, J.; Schaller, K.H. Analyses of Hazardous Substances in Biological Materials. Fresenius J. Anal. Chem. 1997, 5. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; The Psychological Corporation: San Antonio, TX, USA, 2003; Volume WISC-IV. [Google Scholar]
- Wasserman, G.A.; Liu, X.; Parvez, F.; Ahsan, H.; Levy, D.; Factor-Litvak, P.; Kline, J.; van Geen, A.; Slavkovich, V.; LoIacono, N.J.; et al. Water Manganese Exposure and Children’s Intellectual Function in Araihazar, Bangladesh. Environ. Health Perspect. 2006, 114, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, M.; Laforest, F.; Vandelac, L.; Bellinger, D.; Mergler, D. Hair Manganese and Hyperactive Behaviors: Pilot Study of School-Age Children Exposed through Tap Water. Environ. Health Perspect. 2007, 115, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xin, Y.; Li, Q.; Shang, Y.; Ping, Z.; Min, J.; Cahill, C.M.; Rogers, J.T.; Wang, F. Biomarkers of environmental manganese exposure and associations with childhood neurodevelopment: A systematic review and meta-analysis. Environ. Health 2020, 19, 104. [Google Scholar] [CrossRef]
- Neuwirth, L.S.; Lopez, O.E.; Schneider, J.S.; Markowitz, M.E. Low-level lead exposure impairs fronto-executive functions: A call to update the DSM–5 with lead poisoning as a neurodevelopmental disorder. Psychol. Neurosci. 2020, 13, 299–325. [Google Scholar] [CrossRef] [PubMed]
- Islam, G.M.R.; Rahman, M.M.; Hasan, M.I.; Tadesse, A.W.; Hamadani, J.D.; Hamer, D.H. Hair, serum and urine chromium levels in children with cognitive defects: A systematic review and meta-analysis of case control studies. Chemosphere 2022, 291, 133017. [Google Scholar] [CrossRef]
- Ajibo, D.N.; Orish, C.N.; Ruggieri, F.; Bocca, B.; Battistini, B.; Frazzoli, C.; Orish, F.C.; Orisakwe, O.E. An Update Overview on Mechanistic Data and Biomarker Levels in Cobalt and Chromium-Induced Neurodegenerative Diseases. Biol. Trace Elem. Res. 2024, 202, 3538–3564. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Wise, J.P.; Young, J.L.; Cai, J.; Cai, L. Current understanding of hexavalent chromium [Cr(VI)] neurotoxicity and new perspectives. Environ. Int. 2022, 158, 106877. [Google Scholar] [CrossRef] [PubMed]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Murphy, A.; Sun, H.; Costa, M. Molecular and epigenetic mechanisms of Cr(VI)-induced carcinogenesis. Toxicol. Appl. Pharmacol. 2019, 377, 114636. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Zhang, J. Neuroinflammation, memory, and depression: New approaches to hippocampal neurogenesis. J. Neuroinflamm. 2023, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.; Guan, Q.; Liu, Q.; Mo, X.; Luo, L.; Rong, J.; Zhang, T.; Jin, W.; Zhao, L.; Wu, S.; et al. Association Between Urinary Metal Levels and Chronic Kidney Dysfunction in Rural China: A Study on Sex-Specific Differences. Toxics 2025, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, A. Exploring the Association Between Heavy Metal Exposure and Neurodevelopmental Disorders in Children. Afr. J. Biomed. Res. 2025, 28, 1009–1016. [Google Scholar] [CrossRef]
- de Souza, M.R.; Rohr, P.; Kahl, V.F.S.; Kvitko, K.; Cappetta, M.; Lopes, W.M.; Simon, D.; da Silva, J. The influence of polymorphisms of xenobiotic-metabolizing and DNA repair genes in DNA damage, telomere length and global DNA methylation evaluated in open-cast coal mining workers. Ecotoxicol. Environ. Saf. 2020, 189, 109975. [Google Scholar] [CrossRef]
- Vikas, A.M. The Role of Nutrition in Modulating Neurodevelopmental and Mental Health Outcomes in Pediatric Populations: A Review. Bachelor’s Thesis, University of South Carolina, Columbia, SC, USA, 2024. [Google Scholar]
- Singh, O.; Mahajan, M.; Juneja, D.; Mohammed, F. Severe Lead Poisoning Managed with Combination Chelation Therapy: Report from a Resource-limited Country. J. Assoc. Physicians India 2025, 73, e21–e23. [Google Scholar] [CrossRef]

| Characteristics | Frequency (n = 56) | Percentage (%) | Mean (±SD) |
|---|---|---|---|
| Gender of respondents | |||
| Male | 25 | 44.6 | |
| Female | 31 | 55.4 | |
| Age (years) | 14.6 (1.20) | ||
| Weight (kg) | 52.1 (16.8) | ||
| Height (cm) | 158.6 (8.2) | ||
| Years in present school compound | |||
| 1 year | 19 | 33.9 | |
| 2 years | 3 | 5.4 | |
| 3 years | 3 | 5.4 | |
| 5 years | 4 | 7.1 | |
| 6 years | 6 | 10.7 | |
| 7 years | 13 | 23.2 | |
| 8 years | 7 | 12.5 | |
| 10 years | 1 | 1.8 | |
| Mother’s highest education | |||
| Never schooled | 17 | 30.4 | |
| Primary school | 14 | 25 | |
| Middle school/junior high school | 17 | 30.4 | |
| Senior high school | 5 | 8.9 | |
| Institute/tertiary | 1 | 1.8 | |
| Marital status of parents | |||
| Married | 34 | 60.7 | |
| Divorced | 10 | 17.9 | |
| Separated | 6 | 10.7 | |
| Widowed | 6 | 10.7 | |
| Amount spent at school in a day | |||
| 1 cedi | 12 | 21.4 | |
| 2 cedis | 21 | 37.5 | |
| 3 cedis and above | 23 | 41.1 | |
| School attended | |||
| Ayalolo 1&2 JHS | 12 | 21 | |
| Ashia Mills JHS | 20 | 36 | |
| Amamomo JHS | 12 | 22 | |
| Richard AM JHS | 12 | 21 |
| Cognitive Function | Never (%) | Occasionally (Not Severe) (%) | Occasionally (Severe) (%) | Frequently (Not Severe) (%) | Frequently (Severe) (%) | Total Prevalence (%) |
|---|---|---|---|---|---|---|
| Poor Memory | 32.1 | 46.4 | 10.7 | 9.0 | 1.8 | 67.9 |
| Confusion | 25.0 | 62.5 | 10.7 | 1.8 | 0.0 | 75.0 |
| Poor Concentration | 40.0 | 57.1 | 5.3 | 3.6 | 0.0 | 66.0 |
| Poor Coordination | 73.2 | 19.6 | 3.6 | 1.8 | 1.8 | 26.8 |
| Difficulty Making Decisions | 64.2 | 25.0 | 3.6 | 3.6 | 3.6 | 35.8 |
| Stuttering/Stammering | 87.5 | 5.3 | 3.6 | 1.8 | 1.8 | 12.5 |
| Slurred Speech | 80.4 | 3.6 | 7.1 | 5.3 | 3.6 | 19.6 |
| Learning Disability | 66.1 | 16.1 | 7.1 | 10.7 | 0.0 | 33.9 |
| Inattentiveness | 62.5 | 23.2 | 3.6 | 7.1 | 3.6 | 37.5 |
| Metal (μg/L) | Exposure Medium | Mean Concentration (μg/L) | Standard Deviation (±SD) |
|---|---|---|---|
| Pb | Blood | 60.43 | 45.7 |
| Mn | Blood | 5.51 | 6.5 |
| Cd | Urine | 0.13 | 0.3 |
| Cr | Urine | 0.15 | 0.3 |
| Ni | Urine | 5.32 | 4.1 |
| As | Urine | 21.50 | 43.9 |
| Metal | β | 95% CI | p-Value |
|---|---|---|---|
| B-Pb | 0.01 | (−0.06, 0.09) | 0.783 |
| B-Mn | −0.48 | (−1.16, 0.21) | 0.166 |
| U-Cd | −5.12 | (−21.01, 10.77) | 0.520 |
| U-Cr | −18.42 | (2.01, 34.84) | 0.029 * |
| U-Ni | −0.38 | (−0.82, 1.57) | 0.529 |
| U-As | −0.01 | (−0.11, −0.08) | 0.770 |
| Adjusted coefficient of determination is 3% | |||
| Poor Memory | Confusion | Poor Concentration | Poor Coordination | Difficulty Making Decisions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metal | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| B-Pb | 1.01 (0.98, 1.02) | 0.446 | 1.02 (0.99–1.06) | 0.200 | 1.01 (0.98–1.02) | 0.414 | 1.01 (0.99–1.02) | 0.478 | 1.02 (0.98–1.05) | 0.223 |
| B-Mn | 1.01 (0.88, 1.15) | 0.917 | 1.01 (0.88–1.17) | 0.847 | 1.02 (0.89–1.16) | 0.798 | 1.08 (0.93–1.25) | 0.334 | 1.14 (0.98–1.32) | 0.090 |
| U-Cd | 10.14 (0.14, 71.2) | 0.287 | 1.78 (0.03–118.46) | 0.787 | 1.49 (0.06–39.34) | 0.809 | 0.69 (0.00–166.45) | 0.898 | 47.07 (0.56–3967.91) | 0.089 |
| U-Cr | 2.95 (0.10, 85.64) | 0.530 | 1.26 (0.04–35.60) | 0.893 | 5.54 (0.13–234.28) | 0.370 | 0.00 (5.49–2.13) | 0.084 | 0.00 (1.77 × 10−8–0.19) | 0.018 |
| U-As | 0.98 (0.95, 1.00) | 0.103 | 0.99 (0.97–1.02) | 0.675 | 0.99 (0.96–1.01) | 0.160 | 1.02 (0.98–1.05) | 0.239 | 1.00 (0.98–1.03) | 0.843 |
| U-Ni | 0.89 (0.71, 1.12) | 0.341 | 0.94 (0.73–1.21) | 0.613 | 0.91 (0.73–1.13) | 0.401 | 0.84 (0.59–1.18) | 0.309 | 1.13 (0.89–1.43) | 0.328 |
| Stuttering/Stammering | Slurred Speech | Learning Disability | Inattentiveness | |||||||
| Metal | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI | p-value | ||
| B-Pb | 0.99 (0.97–1.03) | 0.872 | 0.99 (0.98–1.02) | 0.978 | 1.02 (0.99–1.04) | 0.202 | 0.99 (0.97–1.01) | 0.285 | ||
| B-Mn | 1.04 (0.84–1.29) | 0.719 | 0.93 (0.77–1.13) | 0.468 | 1.01 (0.88–1.15) | 0.926 | 0.96 (0.83–1.11) | 0.568 | ||
| U-Cd | 4.69 (0.12–190.49) | 0.413 | 1.42 (0.04–46.18) | 0.842 | 5.55 (0.22–137.48) | 0.295 | 21.04 (0.25–1801.43) | 0.180 | ||
| U-Cr | 1.58 (0.02–154.49) | 0.845 | 1.19 (0.02–93.45) | 0.938 | 0.49 (0.01–17.75) | 0.701 | 0.54 (0.01–23.32) | 0.751 | ||
| U-As | 0.99 (0.97–1.02) | 0.592 | 1.00 (0.98–1.03) | 0.655 | 0.99 (0.97–1.01) | 0.384 | 0.98 (0.94–1.01) | 0.201 | ||
| U-Ni | 1.02 (0.72–1.44) | 0.919 | 1.04 (0.81–1.32) | 0.770 | 1.02 (0.82–1.27) | 0.843 | 1.18 (0.92–1.52) | 0.195 | ||
| Abbreviations: OR—odds ratio; 95% CI—95% confidence interval. Models were adjusted for age and maternal smoking. | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bawua, S.A.; Agbeko, K.M.; Issah, I.; Amoabeng-Nti, A.A.; Waldschmidt, S.; Löhndorf, K.; Küpper, T.; Hogarh, J.; Fobil, J.N. Cognitive Functions Among Pupils in Schools Near and Around an Electronic Waste Recycling Site at Agbogbloshie in Accra, Ghana. Toxics 2025, 13, 615. https://doi.org/10.3390/toxics13080615
Bawua SA, Agbeko KM, Issah I, Amoabeng-Nti AA, Waldschmidt S, Löhndorf K, Küpper T, Hogarh J, Fobil JN. Cognitive Functions Among Pupils in Schools Near and Around an Electronic Waste Recycling Site at Agbogbloshie in Accra, Ghana. Toxics. 2025; 13(8):615. https://doi.org/10.3390/toxics13080615
Chicago/Turabian StyleBawua, Serwaa A., Kwame M. Agbeko, Ibrahim Issah, Afua A. Amoabeng-Nti, Saskia Waldschmidt, Katja Löhndorf, Thomas Küpper, Jonathan Hogarh, and Julius N. Fobil. 2025. "Cognitive Functions Among Pupils in Schools Near and Around an Electronic Waste Recycling Site at Agbogbloshie in Accra, Ghana" Toxics 13, no. 8: 615. https://doi.org/10.3390/toxics13080615
APA StyleBawua, S. A., Agbeko, K. M., Issah, I., Amoabeng-Nti, A. A., Waldschmidt, S., Löhndorf, K., Küpper, T., Hogarh, J., & Fobil, J. N. (2025). Cognitive Functions Among Pupils in Schools Near and Around an Electronic Waste Recycling Site at Agbogbloshie in Accra, Ghana. Toxics, 13(8), 615. https://doi.org/10.3390/toxics13080615









