Forty Years After Chernobyl: Radiocaesium in Wild Edible Mushrooms from North-Eastern Poland and Its Relevance for Dietary Exposure and Food Safety †
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Instruments and Equipment
- Relative efficiency: 30% (34.8% at 1.33 MeV for 60Co, per the manufacturer’s calibration certificate);
- Energy resolution: 1.72 keV at 1.33 MeV (60Co) and 0.883 keV at 122 keV (57Co);
- Peak-to-Compton ratio: 68.4:1 at 1.33 MeV;
- Energy range: 50 keV—10 MeV;
- Power supply: 3000 V DC;
- Cryostat: Cryo-Cycle Hybrid Cryostat;
- Spectrum analysis: Digital Spectrum Analyzer DSA-1000.
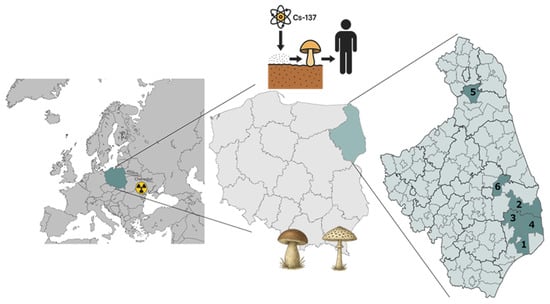
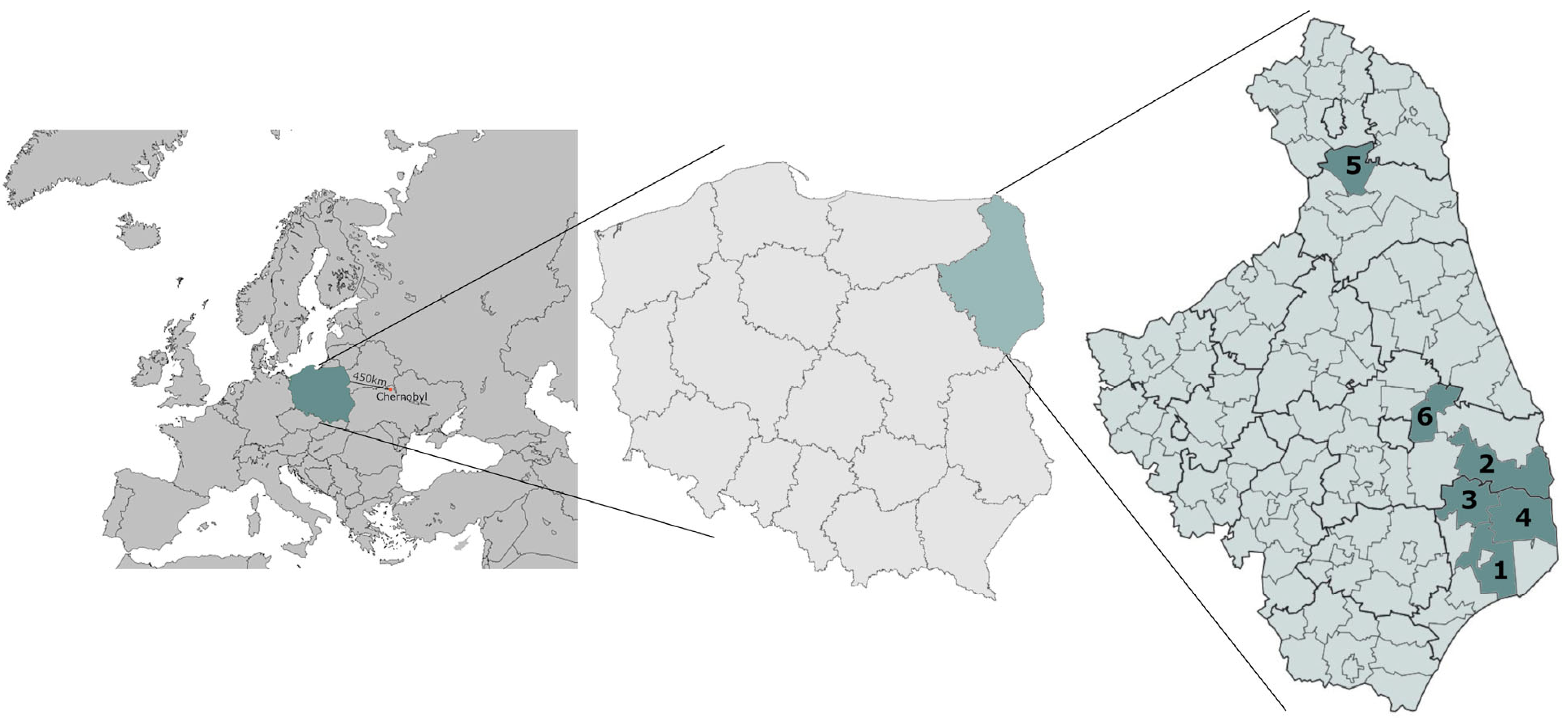
2.3. Sampling Locations
2.4. Analytical Methods
2.5. Calculation of Transfer Factors
- Am = activity concentration of the radionuclide in mushrooms (Bq/kg d.m.);
- As = activity concentration of the radionuclide in soil (Bq/kg d.m.) [35].
2.6. Effective Dose Estimation
- X—average activity concentration of 137Cs or 40K in mushrooms (Bq·kg−1);
- Z—annual mushroom consumption by an adult (kg·year−1);
- dc—effective dose conversion coefficients for 137Cs and 40K ingested with food by adults (aged 17 and older), with values of 1.3×10−8 Sv·Bq−1 and 6.2×10−9 Sv·Bq−1, respectively, as recommended by the International Commission on Radiological Protection (ICRP) [36,37] and the Regulation of the Council of Ministers of 9 September 2021 [38].
- −
- Consumption of 5 kg/year of fresh mushrooms—representing high-end, regionally elevated consumption.
- −
- Consumption of 1 kg/year of dried mushrooms—representing preserved product intake. In this case, activity concentrations were used on a dry mass basis (d.m.), assuming direct consumption without rehydration, thus representing a conservative upper-bound exposure scenario.
- −
- These three intake levels enable a more nuanced and realistic assessment of potential radiological exposure from the consumption of wild mushrooms in both household and commercial contexts.
2.7. Statistical Analysis
3. Results
3.1. Radioactivity of Soil Samples
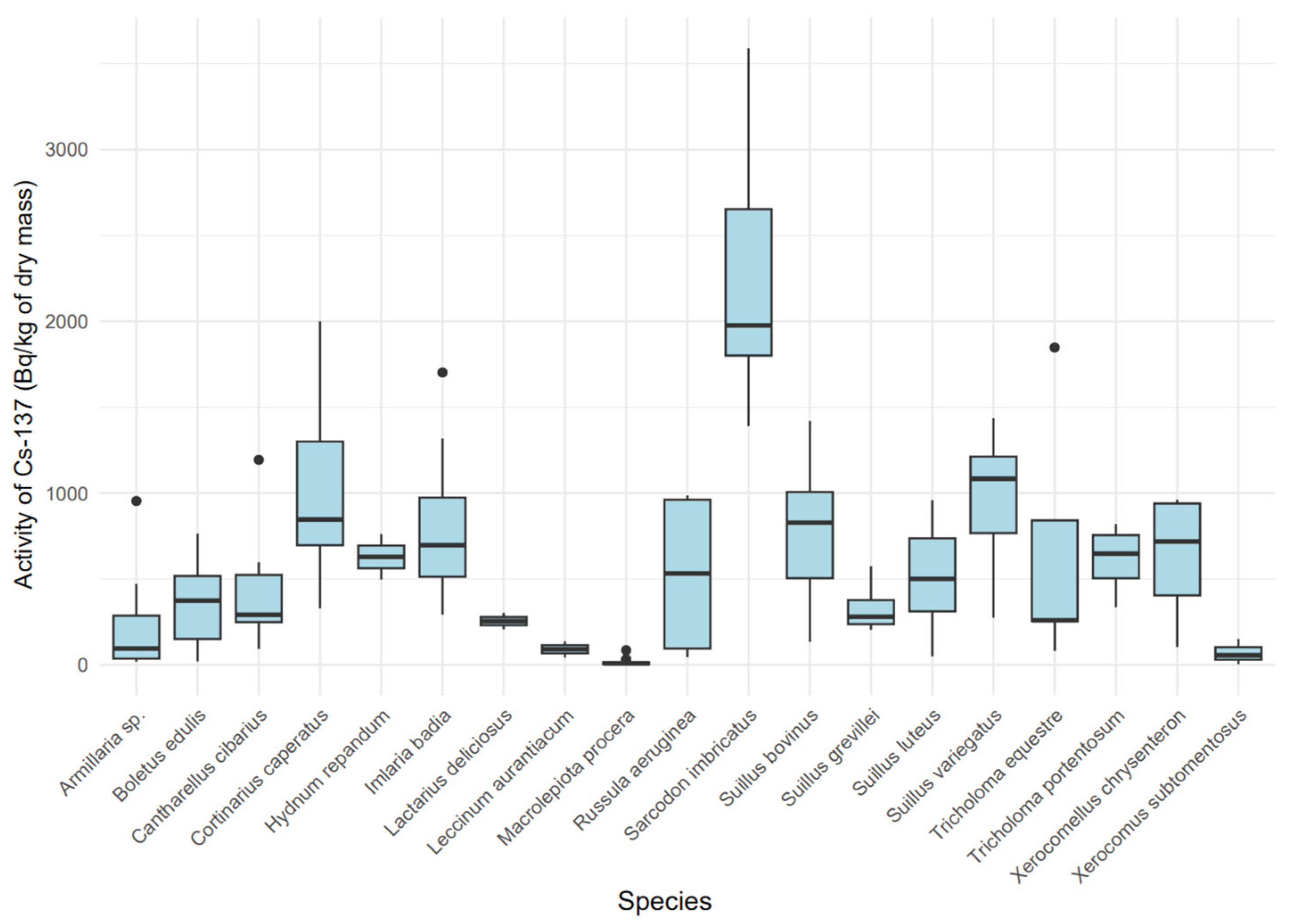
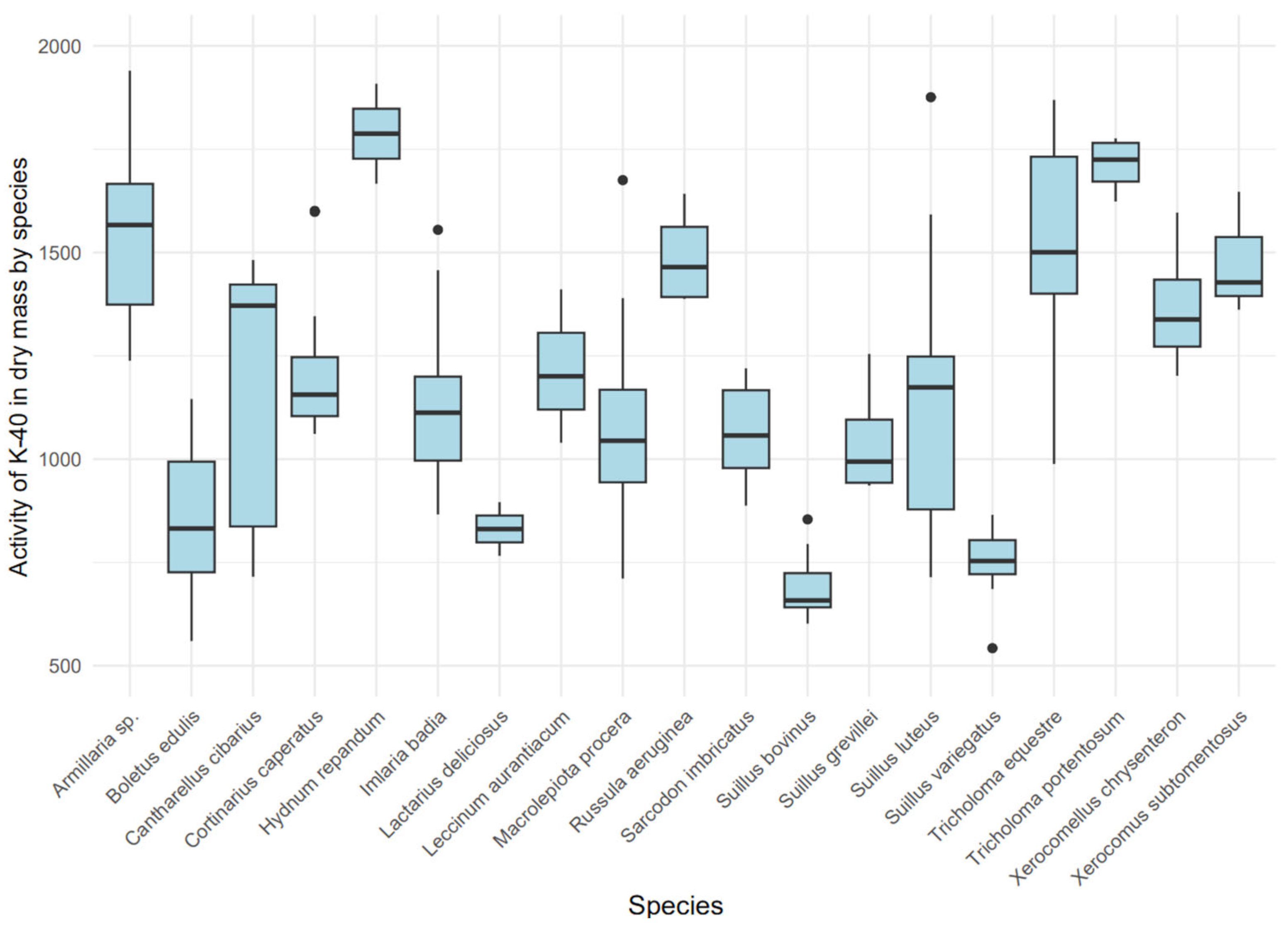
3.2. Radioactivity Concentration of Mushroom Samples
3.3. Soil-to-Mushroom Transfer Factors
3.4. Mean Effective Doses Calculation
3.5. Assessment of 137Cs Activity Concentration in Dry Mass Across Species
3.6. Assessment of 40K Activity in Dry Mass Across Species
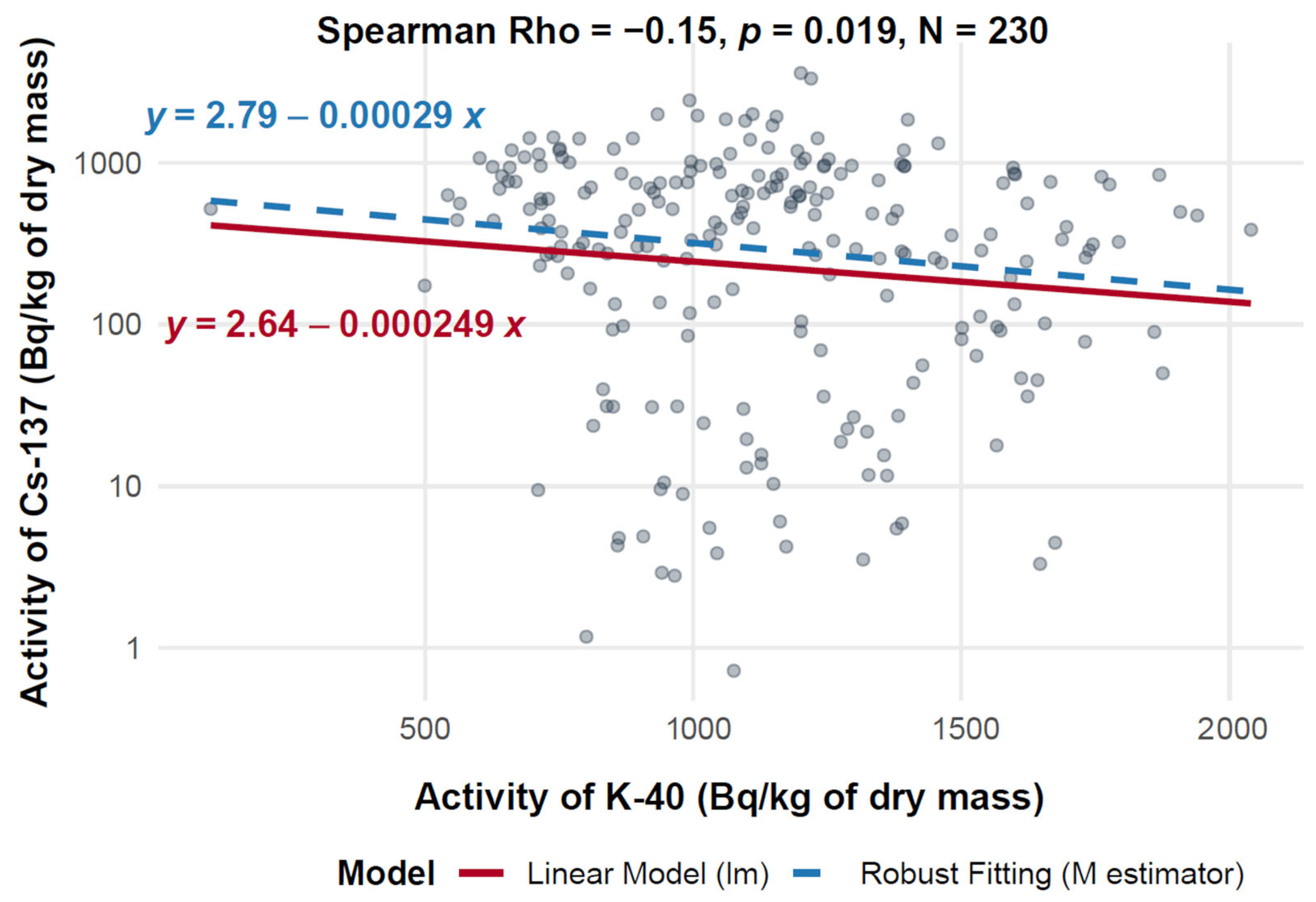
3.7. Assessment of Relationships Between 40K and 137Cs Activity Concentrations
3.7.1. Assessment of the Relationship Between 40K and 137Cs Activity in Mushroom d.m.
3.7.2. Assessment of the Relationship Between 40K and 137Cs Activity in f.m.
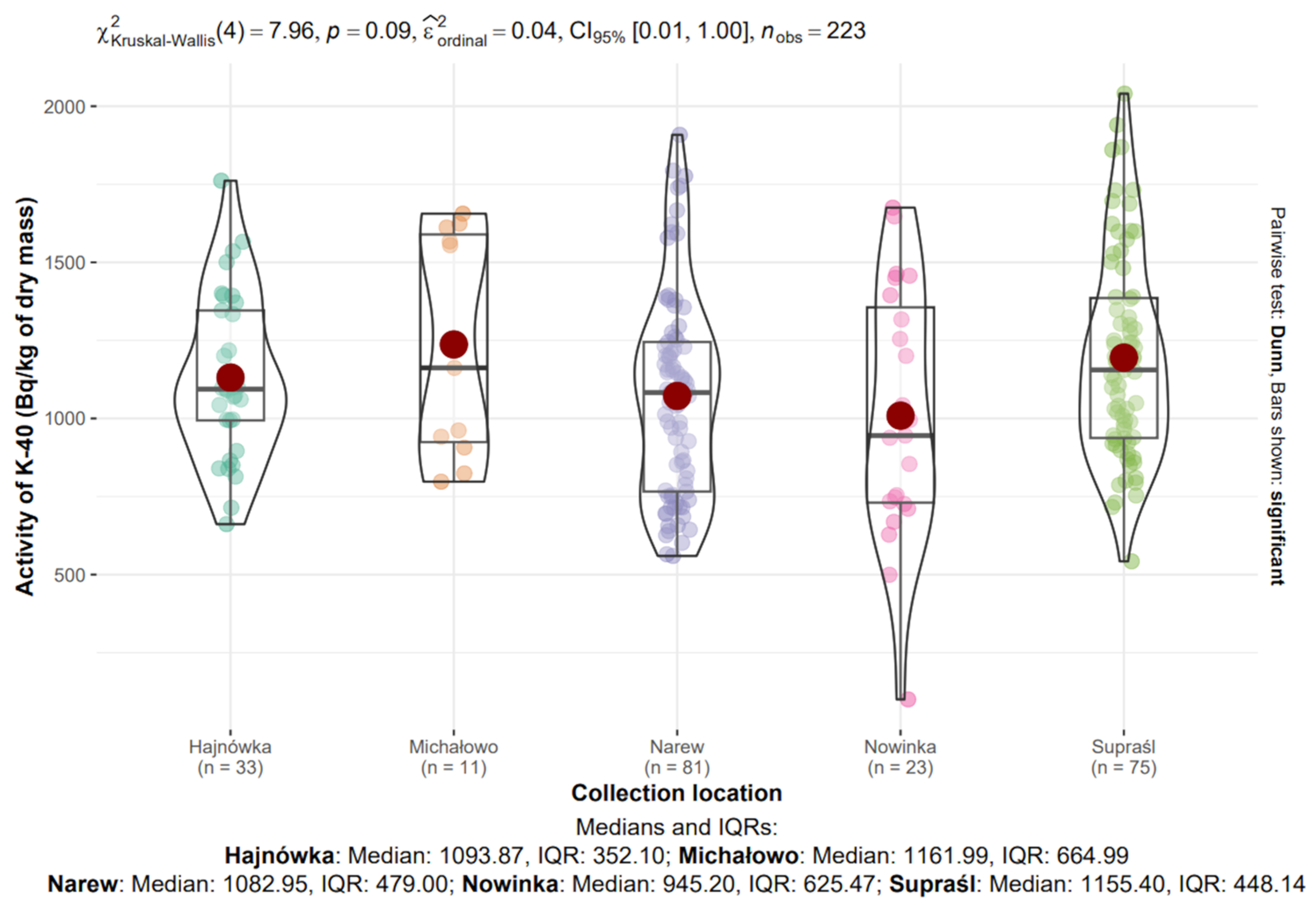
3.8. Assessment of the Effect of Collection Location on 40K and 137Cs Activity
3.8.1. 40K in Mushrooms from Different Locations
3.8.2. 137Cs in Mushrooms from Different Locations
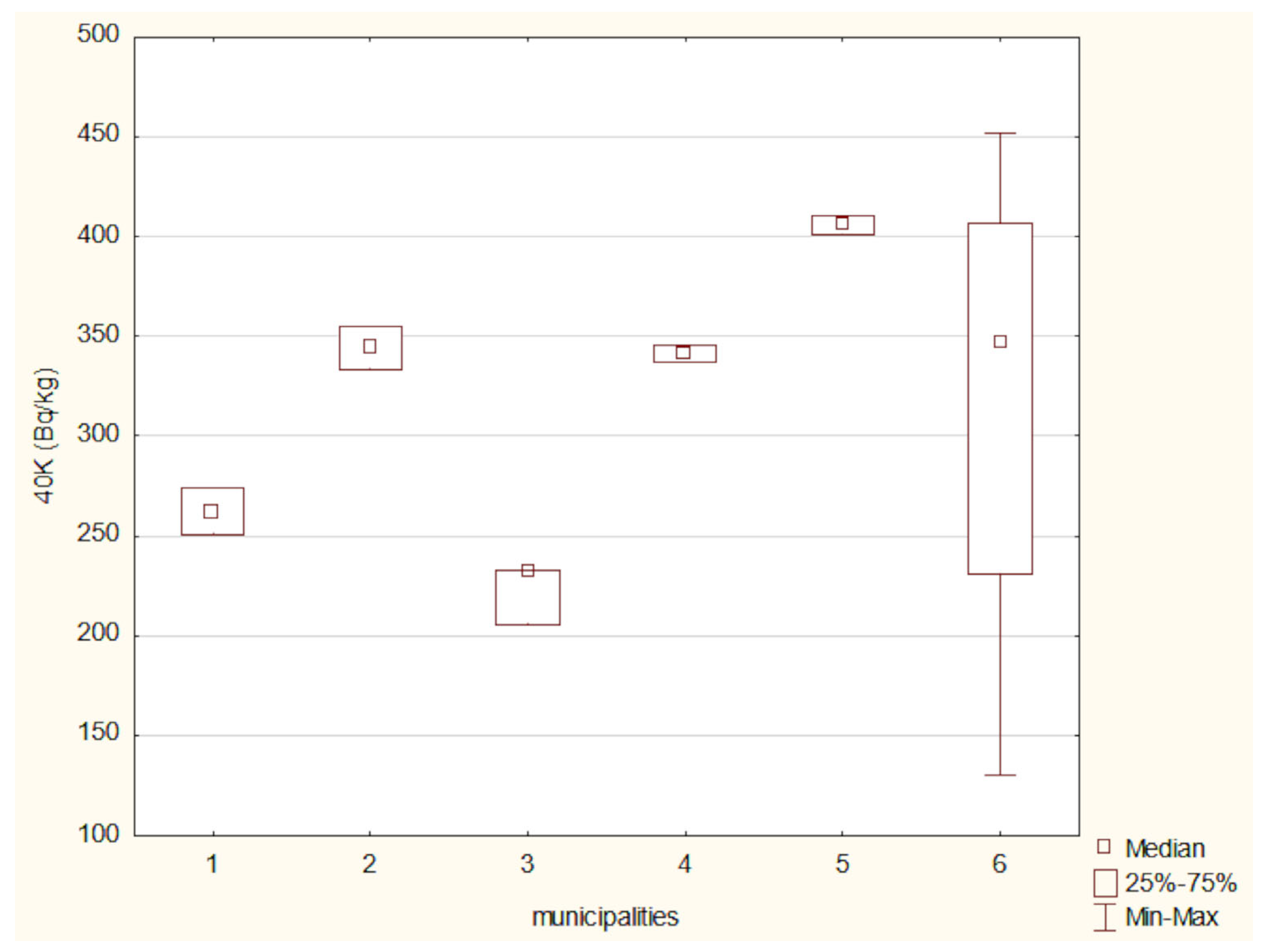
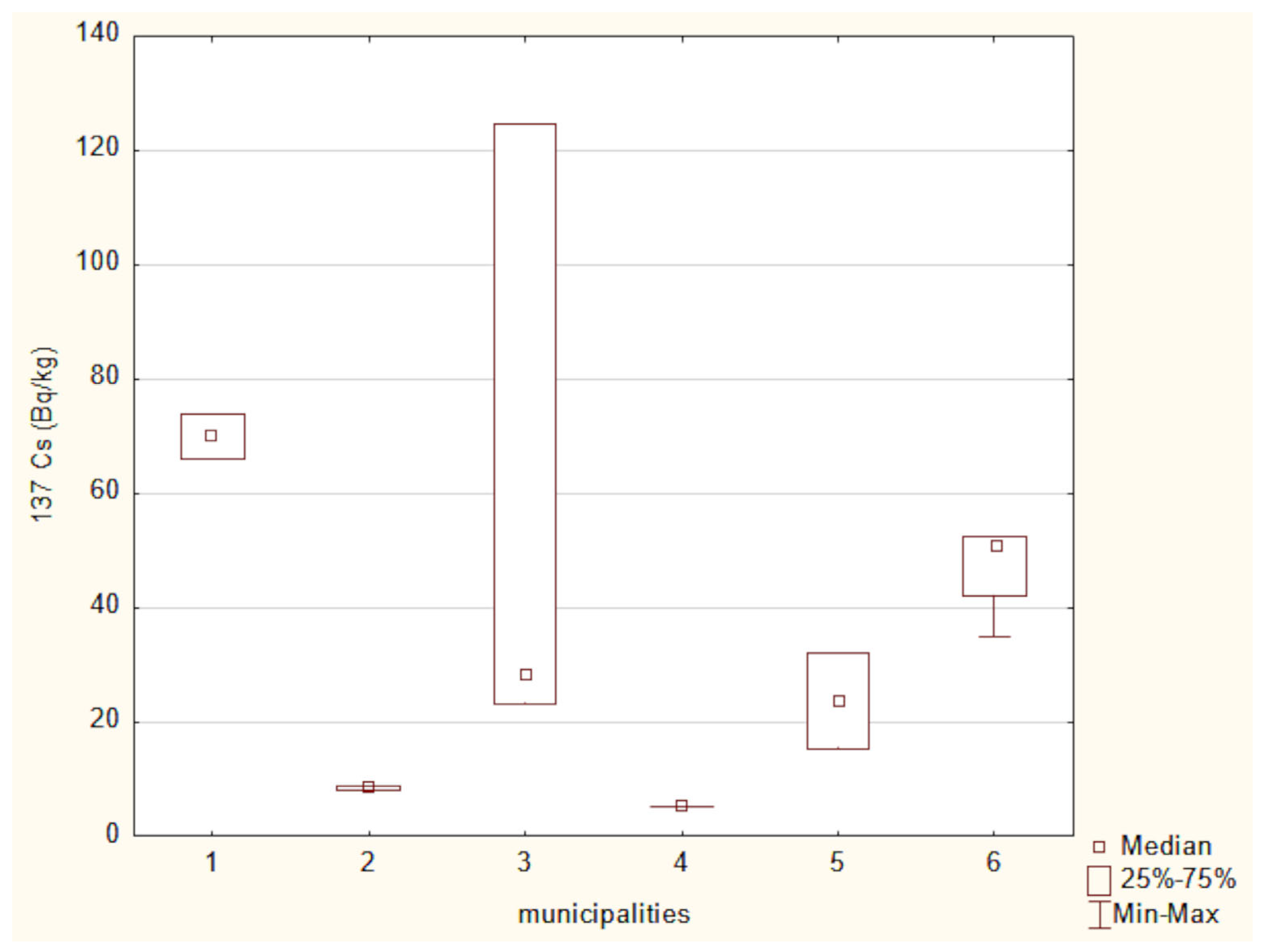
3.9. Difference Analysis of the Activity Concentrations of 40K and 137Cs in Soil Across Different Regions
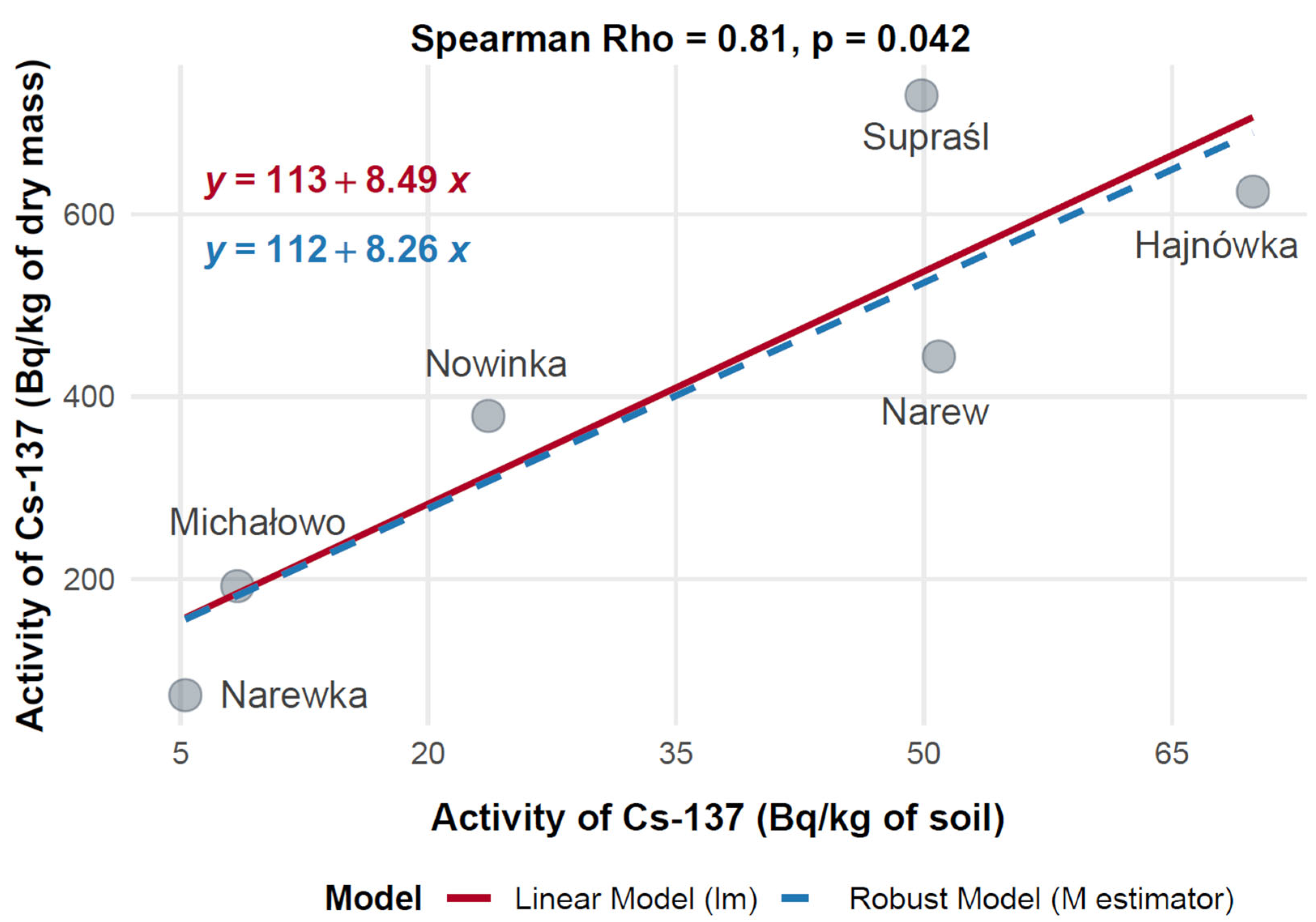
3.10. Correlation of 137Cs and 40K Activity in Soil and Mushroom Dry Mass
3.11. Assessment of 40K Activity Concentration in Dry Mass Across Nutritional Strategies
3.12. Assessment of 137Cs Activity Concentration in Dry Mass Across Nutritional Strategies
4. Discussion
4.1. Activity of the Soil Samples
4.2. 137Cs in the Analyzed Mushroom and Soil Samples
4.3. 40K in the Analyzed Mushroom and Soil Samples
4.4. Reference Level
4.5. Effective Dose
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| d.m. | dry mass |
| f.m. | fresh mass |
| HPGe | High-purity germanium |
| ICRP | International Commission on Radiological Protection |
| MANOVA | multivariate analysis of variance |
| PCA | principal component analysis |
| Rho | rank correlation coefficient |
Appendix A
| No. | Species | Type of Nutritional Strategy | Location | Number of Samples n = 230 |
|---|---|---|---|---|
| 1. | Armillaria sp. | saprotrophic | Hajnówka | 2 |
| Narew | 4 | |||
| Supraśl | 19 | |||
| Michałowo | 4 | |||
| 2. | Boletus edulis | mycorrhizal | Hajnówka | 6 |
| Narew | 10 | |||
| Nowinka | 8 | |||
| Supraśl | 3 | |||
| 3. | Cantharellus cibarius | mycorrhizal | Hajnówka | 2 |
| Narew | 2 | |||
| Nowinka | 3 | |||
| Michałowo | 1 | |||
| Supraśl | 3 | |||
| 4. | Cortinarius caperatus | mycorrhizal | Hajnówka | 5 |
| Narew | 8 | |||
| Supraśl | 2 | |||
| 5. | Hydnum repandum | mycorrhizal | Narew | 2 |
| 6. | Imlaria badia | mycorrhizal | Hajnówka | 5 |
| Narew | 11 | |||
| Nowinka | 4 | |||
| Michałowo | 1 | |||
| Supraśl | 10 | |||
| 7. | Lactarius deliciosus | mycorrhizal | Hajnówka | 1 |
| Narew | 1 | |||
| 8. | Leccinum aurantiacum | mycorrhizal | Hajnówka | 1 |
| Narew | 1 | |||
| Narewka | 1 | |||
| 9. | Macrolepiota procera | saprotrophic | Hajnówka | 1 |
| Narew | 8 | |||
| Narewka | 2 | |||
| Nowinka | 3 | |||
| Michałowo | 3 | |||
| Supraśl | 14 | |||
| 10. | Russula aeruginea | saprotrophic | Hajnówka | 1 |
| Narew | 2 | |||
| Narewka | 1 | |||
| 11. | Sarcodon imbricatus | mycorrhizal | Narew | 2 |
| Supraśl | 6 | |||
| 12. | Suillus bovinus | mycorrhizal | Hajnówka | 1 |
| Narew | 7 | |||
| Nowinka | 1 | |||
| Supraśl | 2 | |||
| 13. | Suillus grevillei | mycorrhizal | Nowinka | 3 |
| Supraśl | 1 | |||
| 14. | Suillus luteus | mycorrhizal | Hajnówka | 3 |
| Narew | 6 | |||
| Michałowo | 2 | |||
| Supraśl | 9 | |||
| Narewka | 1 | |||
| 15. | Suillus variegatus | mycorrhizal | Hajnówka | 2 |
| Narew | 10 | |||
| Supraśl | 2 | |||
| 16. | Tricholoma equestre | mycorrhizal | Hajnówka | 2 |
| Narew | 1 | |||
| Supraśl | 2 | |||
| 17. | Tricholoma portentosum | mycorrhizal | Hajnówka | 1 |
| Narew | 1 | |||
| Supraśl | 2 | |||
| 18. | Xerocomellus chrysenteron | mycorrhizal | Narew | 4 |
| 19. | Xerocomus subtomentosus | mycorrhizal | Narew | 1 |
| Narewka | 1 | |||
| Nowinka | 1 |
References
- de Frutos, P. Changes in world patterns of wild edible mushrooms use measured through international trade flows. For. Policy Econ. 2020, 112, 102093. [Google Scholar] [CrossRef]
- Regulation of the Minister of Health of 19 November 2024 on Mushrooms Admitted for Trade, Production of Mushroom Products and Foodstuffs Containing Mushrooms (in Polish). Available online: https://eli.gov.pl/api/acts/DU/2024/1686/text/O/D20241686.pdf (accessed on 13 June 2025).
- Carrasco-Gonzalez, J.A.; Serna-Saldivar, S.O.; Gutierrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Comp. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Zujko, M.E.; Mirończuk-Chodakowska, I. Comparative study of wild edible mushrooms as sources of antioxidants. Int. J. Med. Mush 2011, 13, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.P.; Liu, Q.M.; Bao, C.J.; Fan, J. Comparison of free total amino acid compositions and their functional classifications in 13 wild edible mushrooms. Molecules 2017, 22, 350. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M.; Gutierrez-Orozco, F.; Moreno-Perez, M.A. Identification of phenolic compounds by liquid chromatography-mass spectrometry in seventeen species of wild mushrooms in Central Mexico and determination of their antioxidant activity and bioactive compounds. Food Chem. 2017, 226, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R. The bioactive agent ergothioneine, a key component of dietary mushrooms, inhibits monocyte binding to endothelial cells characteristic of early cardiovascular disease. J. Med. Food 2010, 13, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E.; Terlikowska, K.M. Quantitative evaluation of 1,3,1,6 β-D-glucan contents in wild-growing species of edible Polish mushrooms. Rocz. Panstw. Zakl. Hig. 2017, 68, 281–290. [Google Scholar] [PubMed]
- Mirończuk-Chodakowska, I.; Socha, K.; Witkowska, A.M.; Zujko, M.E.; Borawska, M.H. Cadmium and lead in wild edible mushrooms from the eastern of Poland’s ‘Green Lungs’. Polish J. Environ. Stud. 2013, 22, 1759–1765. [Google Scholar]
- Cocchi, L.; Vescovi, L.; Petrini, L.E.; Petrini, O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006, 98, 277–284. [Google Scholar] [CrossRef]
- Eckl, P.; Hofmann, W.; Türk, R. Uptake of natural and man-made radionuclides by lichens and mushrooms. Radiat. Environ. Bioph 1986, 25, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, S.; Solecki, J.; Szczypa, J.; Suprynowicz, R. Study of radioactive contamination of Eastern Poland. Sci. Total Environ. 1994, 158, 71–77. [Google Scholar] [CrossRef]
- Abbasi, A.; Mirekhtiary, F. 137Cs and 40K concentration ratios (CRs) in annual and perennial plants in the Caspian coast. Mar. Pollut. Bull. 2019, 146, 671–677. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. Annual Report; International Atomic Energy Agency: Vienna, Austria, 2005; Available online: https://www.iaea.org/sites/default/files/anrep2005_full.pdf (accessed on 15 April 2025).
- National Atomic Energy Agency. Activities of the President of National Atomic Energy Agency and Assessment of Nuclear Safety and Radiological Protection in Poland in 2012. National Atomic Energy Agency: Warsaw, Poland, 2013. Available online: https://www.gov.pl/attachment/cd7b4697-930a-432c-9465-c26b6c58d5d2 (accessed on 15 April 2025).
- Steinhauser, G.; Brandl, A.; Johnson, T.E. Comparison of the Chernobyl and Fukushima nuclear accidents: A review of the environmental impacts. Sci. Total Environ. 2014, 470–471, 800–817. [Google Scholar] [CrossRef] [PubMed]
- De Meutter, P.; Gueibe, C.; Tomasc, J.; den Outer, P.; Apituley, A.; Bruggeman, M.; Camps, J.; Delcloo, A.; Knetsch, G.J.; Roobol, L.; et al. The assessment of the April 2020 Chernobyl wildfires and their impact on Cs-137 levels in Belgium and The Netherlands. J. Environ. Radioact. 2021, 237, 106688. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Zalewska, T.; Krasińska, G.; Apanel, A.; Wang, Y.; Pankavec, S. Evaluation of the radioactive contamination in fungi genus Boletus in the region of Europe and Yunnan Province in China. Appl. Microbiol. Biotechnol. 2015, 99, 8217–8224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Čadová, M.; Havránková, R.; Havránek, J.; Zölzer, F. Radioactivity in mushrooms from selected locations in the Bohemian Forest, Czech Republic. Radiat. Environ. Biophys. 2017, 56, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Fawaris, B.H.; Johanson, K.J. Radiocaesium in soil and plants in a forest in central Swed. Sci. Total Environ. 1994, 157, 133–138. [Google Scholar] [CrossRef]
- Fuma, S.; Kubota, Y.; Ihara, S.; Takahashi, H.; Watanabe, Y.; Aono, T.; Soeda, H.; Yoshida, S. Radiocaesium contamination of wild boars in Fukushima and surrounding regions after the Fukushima nuclear accident. J. Environ. Radioact. 2016, 164, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Zakaly, H.M.H.; Badawi, A. The anthropogenic radiotoxic element of 137Cs accumulate to biota in the Mediterranean Sea. Mar. Pollut. Bull. 2021, 164, 112043. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Almousa, N.; Zakaly, H.M.H. The distribution of radiotoxic 137Cs concentrations in seaweed and mussel species in the Mediterranean Sea. Mar. Pollut. Bull. 2023, 197, 115737. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A. 137Cs distribution in the South Caspian region, transfer to biota and dose rate assessment. Inter. J. Environ. Analyt Chem. 2019, 100, 576–590. [Google Scholar] [CrossRef]
- Abbasi, A. 210Pb and 137Cs based techniques for the estimation of sediment chronologies and sediment rates in the Anzali Lagoon, Caspian Sea. J. Radioanal. Nucl. Chem. 2019, 322, 319–330. [Google Scholar] [CrossRef]
- Falandysz, J.; Zalewska, T.; Saniewski, M.; Fernandes, A.R. An evaluation of the occurrence and trends in 137Cs and 40K radioactivity in King Bolete (Boletus edulis) mushrooms in Poland during 1995–2019. Environ. Sci. Poll. Res. 2021, 28, 32405–32415. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Nishina, K.; Hashimoto, S. Extensive analysis of radiocesium concentrations in wild mushrooms in eastern Japan affected by the Fukushima nuclear accident: Use of open accessible monitoring data. Environ. Pollut. 2019, 255, 113236. [Google Scholar] [CrossRef] [PubMed]
- Orita, M.; Nakashima, K.; Taira, Y.; Fukuda, T.; Fukushima, Y.; Kudo, T.; Endo, Y.; Yamashita, S.; Takamura, N. Radiocesium concentrations in wild mushrooms after the accident at the Fukushima Daiichi Nuclear Power Station: Follow-up study in Kawauchi village. Sci. Rep. 2017, 7, 6744. [Google Scholar] [CrossRef] [PubMed]
- Krolak, E.; Kwapulinski, J.; Fischer, A. (137)Cs and (40)K isotopes in forest and wasteland soils in a selected region of eastern Poland 20 years after the Chernobyl accident. Radiat. Environ. Biophys. 2010, 49, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Senila, M.; Resz, M.A.; Torok, I.; Senila, L. Nutritional composition and health risk of toxic metals of some edible wild mushrooms growing in a mining area of Apuseni Mountains, Western Carpathians. J. Food Compos. Anal. 2024, 128, 106061. [Google Scholar] [CrossRef]
- Malinowska, E.; Szefer, P.; Bojanowski, R. Radionuclides content in Xerocomus badius and other commercial mushrooms from several regions of Poland. Food Chem. 2006, 97, 19–24. [Google Scholar] [CrossRef]
- Skotniczna, M. Technical Meeting on the Harmonization of Reference Levels for Foodstuffs and Drinking Water Contaminated Following a Nuclear Accident National Atomic Energy Agency (PAA), Vienna, Austria, 8–12 September 2014. Available online: https://gnssn.iaea.org/RTWS/general/Shared%20Documents/Radiation%20Protection/TM%20on%20Harmonization%20of%20Reference%20Levels%20for%20Foodstuffs%20and%20Drinking%20Water%20Contaminated%20Following%20a%20Nuclear%20Accident/Item%20%209d%20-%20POLAND%20-%20M.Skotniczna.pdf (accessed on 16 June 2025).
- Mirończuk-Chodakowska, I.; Kapała, J.; Kujawowicz, K.; Cyuńczyk, M.; Witkowska, A.M. 137Cs and 40K radioactivity of wild edible mushrooms from Podlaskie Voivodeship, Poland. In Proceedings of the The 5th International Electronic Conference on Foods, Online, 28–30 October 2024. [Google Scholar]
- Currie, L.A. Limits of qualitative detection and quantitative determination. Anal. Chem. 1968, 40, 586–593. [Google Scholar] [CrossRef]
- Baeza, A.; Guillén, J.; Bernedo, J.M. Soil–fungi transfer coefficients: Importance of the location of mycelium in soil and of the differential availability of radionuclides in soil fractions. J. Environ. Radioact. 2005, 81, 89–106. [Google Scholar] [CrossRef] [PubMed]
- International Atomic Energy Agency. International Basic Safety Standards for Protection Against Ionizing Radiation and for the Safety of Radiation Sources (1996) Safety Series No. 115 Vienne. Available online: https://gnssn.iaea.org/Superseded%20Safety%20Standards/Safety_Series_115_1996_Pub996_EN.pdf (accessed on 15 April 2025).
- ICRP. Age-Dependent Doses to Members of the Public from Intake of Radionuclides—Part 5 Compilation of Ingestion and Inhalation Coefficients, ICRP Publication 72, Ann. ICRP 26; ICRP: Ottawa, ON, Canada, 1995; Available online: https://journals.sagepub.com/doi/pdf/10.1177/ANIB_26_1 (accessed on 15 June 2025).
- Regulation of the Council of Ministers of 9 September 2021 (in Polish). Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20210001657/O/D20211657.pdf (accessed on 14 June 2025).
- Central Statistical Office (GUS). Demographic Data of the Podlaskie Voivodeship, 2024 (in Polish). Available online: https://bialystok.stat.gov.pl/publikacje-i-foldery/roczniki-statystyczne/rocznik-statystyczny-wojewodztwa-podlaskiego-2024,7,21.html (accessed on 15 June 2025).
- Selected Aspects of Agri-Food Markets in Poland (in Polish). Available online: https://www.ieif.sggw.pl/wp-content/uploads/2021/02/WYBRANE-ASPEKTY-RYNK%C3%93W-ROLNO-%C5%BBYWNO%C5%9ACIOWYCH-W-POLSCE_Stolarska-1.pdf (accessed on 15 June 2025).
- Kapała, J.; Mnich, K.; Mnich, S.; Karpińska, M.; Bielawska, A. Time-dependence of 137Cs activity concentration in wild game meat in Knyszyn Primeval Forest (Poland). J. Environ. Radioact. 2015, 141, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, B.M.; Vitorović, O.; Ajtić, J.; Vranjes, B. Radioactivity in the Environment and food chain at Mt. Maljen, Serbia. Rom. Rep. Phys. 2020, 72, 1–11. [Google Scholar]
- UNSCEAR. United Nations Scientific Committee on the Effects of Atomic Radiation, Sources and Effects of Ionizing Radiation. Exposures from Natural Radiation Sources. New York. 2000. Available online: https://www.unscear.org/unscear/en/publications/2020_2021_1.html (accessed on 15 April 2025).
- Orita, M.; Kimura, Y.; Taira, Y.; Fukuda, T.; Takahashi, J.; Gutevych, O.; Chornyi, S.; Kudo, T.; Yamashita, S.; Takamura, N. Activities concentration of radiocesium in wild mushroom collected in Ukraine 30 years after the Chernobyl power plant accident. Peer J. 2018, 6, e4222. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Hong, G.-H.; Kim, S.H.; Lee, H. Annual Effective Dose of 210Po from Sea Food Origin (Oysters and Mussels) in Korea. J. Radiat. Prot. Res. 2016, 41, 245–252. [Google Scholar] [CrossRef]
- Tagami, K.; Yasutaka, T.; Takada, M.; Uchida, S. Aggregated transfer factor of 137Cs in wild edible mushrooms collected in 2016–2020 for long-term internal dose assessment use. J. Environ. Radioact. 2021, 237, 106664. [Google Scholar] [CrossRef] [PubMed]
- Cipáková, A. 137Cs content in mushrooms from localities in eastern Slovakia. Nukleonika 2004, 49 (Suppl. 1), S25–S29. [Google Scholar]
- Nakashima, K.; Orita, M.; Fukuda, N.; Taira, Y.; Hayashida, N.; Matsuda, N.; Takamura, N. Radiocesium concentrations in wild mushrooms collected in Kawauchi Village after the accident at the Fukushima Daiichi Nuclear Power Plant. Peer J. 2015, 3, e1427. [Google Scholar] [CrossRef] [PubMed]
- Zalewska, T.; Cocchi, L.; Falandysz, J. Radiocaesium in Cortinarius spp. mushrooms in the regions of the Reggio Emilia in Italy and Pomerania in Poland. Environ. Sci. Pollut. Res. Int. 2016, 23, 23169–23174. [Google Scholar] [CrossRef] [PubMed]
- Saniewski, M.; Zalewska, T.; Falandysz, J. 137Cs and 40K activity concentrations in edible mushrooms from China regions during the 2014–2016 period. Food Raw Material 2022, 10, 85–96. [Google Scholar] [CrossRef]
- Koarashi, J.; Atarashi-Andoh, M.; Nishimura, S. Effect of soil organic matter on the fate of 137Cs vertical distribution in forest soils. Ecotoxicol. Environ. Safety 2023, 262, 115177. [Google Scholar] [CrossRef] [PubMed]
- Duff, M.C.; Ramsey, M.L. Accumulation of radiocesium by mushrooms in the environment: A literature review. J. Environ. Radioact. 2008, 99, 912–932. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Saba, M.; Strumińska-Parulska, D. 137Caesium, 40K and total K in Boletus edulis at different maturity stages: Effect of braising and estimated radiation dose intake. Chemosphere 2021, 268, 129336. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Zalewska, T.; Fernandes, A.R. 137Cs and 40K in Cortinarius caperatus mushrooms (1996–2016) in Poland—Bioconcentration and estimated intake: 137Cs in Cortinarius spp. from the Northern Hemisphere from 1974 to 2016. Environ. Pollut. 2019, 255, 113208. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.L.; Reiter, G.; Piepenbring, M.; Bässler, C. Spatial risk assessment of radiocesium contamination of edible mushrooms—Lessons from a highly frequented recreational area. Sci. Total Environ. 2022, 807, 150861. [Google Scholar] [CrossRef] [PubMed]
- Ronda, O.; Grządka, E.; Ostolska, I.; Orzeł, J.; Cieślik, B.M. Accumulation of radioisotopes and heavy metals in selected species of mushrooms. Food Chem. 2022, 367, 130670. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.J.; García, M.Á. Natural radioactivity and total K content in wild-growing or cultivated edible mushrooms and soils from Galicia (NW, Spain). Environ. Sci. Pollut. Res. Int. 2021, 28, 52925–52935. [Google Scholar] [CrossRef] [PubMed]
- Guillén, J.; Baeza, A. Radioactivity in mushrooms: A health hazard? Food Chem 2014, 154, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.L.; Inouye, R.S.; McGonigle, T.P.; White, G.J. The distribution of stable cesium in soils and plants of the eastern Snake River Plain in southern Idaho. J. Arid. Environ. 2007, 69, 40–64. [Google Scholar] [CrossRef]
- Wang, S.; Yang, B.; Zhou, Q.; Li, Z.; Li, W.; Zhang, J.; Tuo, F. Radionuclide content and risk analysis of edible mushrooms in northeast China. Rad. Med. Prot. 2021, 2, 165–170. [Google Scholar] [CrossRef]
- Petrovic, N.; Kosanic, M.; Tosti, T.; Srbljak, I.; Đurić, A. Chemical Characterization and Bioactive Properties of the Edible and Medicinal Honey Mushroom Armillaria mellea (Agaricomycetes) from Serbia. Int. J. Med. Mushrooms 2023, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mietelski, J.W.; Dubchak, S.; Błażej, S.; Anielska, T.; Turnau, K. 137Cs and 40K in fruiting bodies of different fungal species collected in a single forest in southern Poland. J. Environ. Radioact. 2010, 101, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, H.; Uchida, T.; Kobayashi, H.; Ota, T.; Shinano, T. Comparative dynamics of potassium and radiocesium in soybean with different potassium application levels. J. Environ. Radioact. 2021, 233, 106609. [Google Scholar] [CrossRef] [PubMed]
- Marčiulionienė, D.; Lukšienė, B.; Jefanova, O. Accumulation and translocation peculiarities of 137Cs and 40K in the soil—Plant system. J. Environ. Radioact. 2015, 150, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Council Regulation (Euratom) 2016/52 of 15 January 2016 Laying Down Maximum Permitted Levels of Radioactive Contamination of Food and Feed Following a Nuclear Accident or Any Other Case of Radiological Emergency, and Repealing. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:32016R0052 (accessed on 15 June 2025).
- Regulation of the Council of Ministers of 18 January 2005 on Dose Limits of Ionizing Radiation. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20050200168/O/D20050168.pdf (accessed on 3 July 2025).
- Škrkal, J.; Rulík, P.; Fantínová, K.; Mihalík, J.; Timková, J. Radiocaesium levels in game in the Czech Republic. J. Environ. Radioact. 2015, 139, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Mietelski, J.W.; Jasińska, M.; Kubica, B.; Kozak, K.; Macharski, P. Radioactive contamination of Polish mushrooms. Sci. Total Environ. 1994, 157, 217–226. [Google Scholar] [CrossRef]
- Sartayev, Y.; Matsuu-Matsuyama, M.; Yamaguchi, I.; Takahashi, J.; Gutevich, A.; Hayashida, N. Internal radiation exposure from 137Cs and its association with the dietary habits of residents from areas affected by the Chernobyl nuclear accident, Ukraine: 2016–2018. PLoS ONE 2023, 18, e0291498. [Google Scholar] [CrossRef] [PubMed]
- Falandysz, J.; Meloni, D.; Fernandes, A.R.; Saniewski, M. Effect of drying, blanching, pickling and maceration on the fate of 40K, total K and 137Cs in bolete mushrooms and dietary intake. Environ. Sci. Pollut. Res. Int. 2022, 29, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Beresford, N.A.; Voigt, G.; Wright, S.M.; Howard, B.J.; Bennett, C.L.; Prister, B. Self-help countermeasure strategies for populations living within contaminated areas Belarus, Russia and Ukraine. J. Environ. Radioact. 2001, 56, 215–239. [Google Scholar] [CrossRef] [PubMed]





| No. | Species of Mushrooms | n | Water Content | |
|---|---|---|---|---|
| Median | Min–Max (%) | |||
| 1. | Armillaria sp. | 29 | 92.83 | 84.59–94.05 |
| 2. | Boletus edulis | 27 | 89.46 | 85.75–91.58 |
| 3. | Cantharellus cibarius | 11 | 91.27 | 84.77–93.08 |
| 4. | Cortinarius caperatus | 15 | 92.50 | 91.96–93.85 |
| 5. | Hydnum repandum | 2 | 93.99 | 92.20–95.77 |
| 6. | Imlaria badia | 31 | 92.00 | 74.29–94.78 |
| 7. | Lactarius deliciosus | 2 | 85.45 | 80.96–89.95 |
| 8. | Leccinum aurantiacum | 3 | 93.50 | 87.91–94.19 |
| 9. | Macrolepiota procera | 31 | 88.42 | 66.48–93.05 |
| 10. | Russula aeruginea | 4 | 93.20 | 90.12–93.44 |
| 11. | Sarcodon imbricatus | 8 | 91.87 | 88.18–95.37 |
| 12. | Suillus bovinus | 11 | 90.45 | 86.10–96.56 |
| 13. | Suillus grevillei | 4 | 93.56 | 89.34–94.77 |
| 14. | Suillus luteus | 21 | 92.54 | 85.14–96.21 |
| 15. | Suillus variegatus | 14 | 91.09 | 89.41–93.53 |
| 16. | Tricholoma equestre | 5 | 91.33 | 85.20–92.10 |
| 17. | Tricholoma portentosum | 4 | 92.42 | 90.79–93.45 |
| 18. | Xerocomellus chrysenteron | 4 | 91.55 | 88.88–93.60 |
| 19. | Xerocomus subtomentosus | 3 | 91.00 | 90.48–91.45 |
| No. | Species | n | Activity in f.m. | Activity in d.m. | ||
|---|---|---|---|---|---|---|
| 40K (Bq/kg) | 137Cs (Bq/kg) | 40K (Bq/kg) | 137Cs (Bq/kg) | |||
| 1 | Armillaria sp. | 29 | 109.9 (103.2; 122.9) | 7.36 (2.89; 20.4) | 1567.0 (1382.6; 1696.3) | 95.2 (2.89; 253.6) |
| 2 | Boletus edulis | 27 | 85.9 (74.5; 105.0) | 40.9 (21.1; 62.2) | 813.8 (725.0; 1010.8) | 373.8 (150.3; 516.9) |
| 3 | Cantharellus cibarius | 11 | 104.6 (75.9; 155.3) | 29.2 (17.1; 36.0) | 1371.3 (850.0; 1435.5) | 291.2 (97.8; 372.4) |
| 4 | Cortinarius caperatus | 15 | 85.6 (82.3; 95.6) | 63.6 (49.9; 99.8) | 1156.0 (1097.1; 1239.8) | 845.9 (646.9; 1250.4) |
| 5 | Hydnum repandum | 2 | 109.6 (90.1; 129.2) | 35.5 (33.8; 37.1) | 1787.5 (1666.5; 1908.5) | 628.8 (496.4; 761.2) |
| 6 | Imlaria badia | 31 | 91.7 (78.6; 102.4) | 61.9 (39.8; 79.8) | 1112.5 (938.2; 1141.4) | 696.4 (393.9; 855.2) |
| 7 | Lactarius deliciosus | 2 | 118.0 (90.0; 146.0) | 34.9 (32.7; 37.2) | 830.9 (765.9; 895.9) | 255.0 (206.9; 303.1) |
| 8 | Leccinum aurantiacum | 3 | 82.0 (67.6; 106.4) | 8.92 (5.73; 9.94) | 1200.6 (1039.5; 1225.1) | 90.6 (43.5; 90.4) |
| 9 | Macrolepiota procera | 31 | 119.0 (106.2; 179.0) | 0.94 (0.56; 1.30) | 1044.6 (923.3; 1147.4) | 8.94 (5.46; 14.49) |
| 10 | Russula aeruginea | 4 | 107.5 (98.6; 120.1) | 36.5 (6.3; 72.8) | 1464.6 (1387.8; 1557.6) | 532.5 (112.0; 978.2) |
| 11 | Sarcodon imbricatus | 8 | 89.3 (57.2; 109.4) | 159.0 (135.1; 175.3) | 1057.2 (933.7; 1122.1) | 1976.7 (1414.7; 2267.0) |
| 12 | Suillus bovinus | 11 | 64.4 (59.4; 68.8) | 84.1 (55.0; 99.6) | 657.9 (626.1; 708.9) | 827.8 (326.5; 827.8) |
| 13 | Suillus grevillei | 4 | 66.1 (64.4; 75.1) | 23.2 (17.6; 29.2) | 993.9 (935.8; 1088.6) | 279.8 (203.8; 343.9) |
| 14 | Suillus luteus | 22 | 74.9 (60.5; 98.2) | 38.4 (25.8; 58.1) | 1173.6 (873.5; 1243.5) | 501.0 (297.0; 723.1) |
| 15 | Suillus variegatus | 14 | 66.6 (58.7; 77.5) | 90.2 (71.9; 107.6) | 753.5 (711.9; 794.3) | 1083.5 (704.1; 1150.2) |
| 16 | Tricholoma equestre | 5 | 150.2 (119.2; 151.2) | 30.7 (22.5; 68.1) | 1500.6 (1400.4; 1731.9) | 259.2 (81.0; 667.5) |
| 17 | Tricholoma portentosum | 4 | 134.1 (106.3; 143.5) | 45.7 (32.3; 53.6) | 1724.7 (1623.2; 1716.7) | 647.3 (560.7; 811.7) |
| 18 | Xerocomellus chrysenteron | 4 | 126.1 (103.5; 140.9) | 61.4 (32.5; 88.0) | 1338.0 (1201.6; 1363.7) | 718.7 (504.4; 1039.8) |
| 19 | Xerocomus subtomentosus | 3 | 129.6 (129.6; 135.8) | 5.02 (3.84; 9.68) | 1427.6 (1361.6; 1504.4) | 55.8 (31.0; 90.9) |
| No. | Species | n | TF | |
|---|---|---|---|---|
| Me Min–Max | ||||
| 40K | 137Cs | |||
| 1 | Armillaria sp. | 29 | 5.20 3.40–8.02 | 4.17 0.31–13.65 |
| 2 | Boletus edulis | 27 | 3.20 0.25–5.12 | 9.53 0.28–32.45 |
| 3 | Cantharellus cibarius | 11 | 3.44 2.17–5.31 | 10.17 2.13–34.46 |
| 4 | Cortinarius caperatus | 15 | 5.06 4.04–5.64 | 16.26 5.60–28.61 |
| 5 | Hydnum repandum | 2 | 7.99 7.45–8.53 | 10.70 8.45–12.96 |
| 6 | Imlaria badia | 31 | 3.87 2.31–5.60 | 14.07 4.75–55.80 |
| 7 | Lactarius deliciosus | 2 | 3.42 3.41–3.42 | 3.93 3.52–4.33 |
| 8 | Leccinum aurantiacum | 3 | 4.58 4.14–4.65 | 2.34 1.30–8.21 |
| 9 | Macrolepiota procera | 31 | 3.85 1.75–6.17 | 0.24 0.02–2.21 |
| 10 | Russula aeruginea | 4 | 6.03 4.81–6.23 | 12.37 1.60–16.81 |
| 11 | Sarcodon imbricatus | 8 | 3.32 2.79–5.45 | 41.86 29.43–61.11 |
| 12 | Suillus bovinus | 11 | 2.80 2.03–3.11 | 14.09 5.65–24.17 |
| 13 | Suillus grevillei | 4 | 2.75 2.33–3.09 | 11.33 8.62- 13.18 |
| 14 | Suillus luteus | 22 | 3.60 1.83–6.82 | 12.27 3.30–77.17 |
| 15 | Suillus variegatus | 14 | 3.21 1.38–3.65 | 18.44 3.92–24.45 |
| 16 | Tricholoma equestre | 5 | 5.44 4.23–5.87 | 5.49 1.16–26.43 |
| 17 | Tricholoma portentosum | 4 | 6.01 5.10–7.61 | 11.80 7.10–12.49 |
| 18 | Xerocomellus chrysenteron | 4 | 5.73 5.14–6.84 | 12.23 1.78–16.35 |
| 19 | Xerocomus subtomentosus | 3 | 4.19 4.06–5.83 | 2.57 1.31–10.53 |
| No. | Species | Mean Effective Doses | |||||
|---|---|---|---|---|---|---|---|
| 137Cs | 40K | ||||||
| 1 kg of Fresh Mushrooms | 5 kg of Fresh Mushrooms | 1 kg of Dry Mushrooms | 1 kg of Fresh Mushrooms | 5 kg of Fresh Mushrooms | 1 kg of Dry Mushrooms | ||
| µ Sv | |||||||
| 1. | Armillaria sp. | 0.17 | 0.85 | 2.37 | 0.76 | 3.78 | 9.64 |
| 2. | Boletus edulis Bull. | 0.47 | 2.35 | 4.48 | 0.54 | 2.70 | 5.02 |
| 3. | Cantharellus cibarius Fr. | 0.56 | 2.80 | 5.26 | 0.70 | 3.50 | 7.07 |
| 4. | Cortinarius caperatus (Pers.) Fr. | 1.02 | 5.1 | 13.58 | 0.56 | 0.53 | 7.58 |
| 5. | Hydnum repandum L. | 0.46 | 2.30 | 8.17 | 0.55 | 2.75 | 11.08 |
| 6. | Imleria badia (Fr.) Fr. | 0.85 | 4.25 | 9.8 | 0.61 | 3.05 | 6.95 |
| 7. | Lactarius deliciosus (L.) Pers. | 0.45 | 2.25 | 3.32 | 0.73 | 3.65 | 5.15 |
| 8. | Leccinum aurantiacum (Bull.) Gray | 0.10 | 0.5 | 1.18 | 0.61 | 3.05 | 7.54 |
| 9. | Macrolepiota procera (Scop.) Singer | 0.02 | 0.1 | 0.16 | 0.88 | 4.40 | 6.74 |
| 10. | Russula aeruginea Lindblad ex Fr. | 0.55 | 2.75 | 6.82 | 0.68 | 3.40 | 9.23 |
| 11. | Sarcodon imbricatus (L.) P. Karst. | 2.32 | 11.6 | 29.30 | 0.55 | 2.75 | 6.59 |
| 12. | Suillus bovinus (L.) Roussel | 0.98 | 4.9 | 10.17 | 0.40 | 2.00 | 4.27 |
| 13. | Suillus grevillei (Klotzsch) Singer | 0.31 | 1.55 | 4.35 | 0.46 | 2.30 | 6.48 |
| 14. | Suillus luteus (L.) Roussel | 0.54 | 2.70 | 6.86 | 0.52 | 2.60 | 6.95 |
| 15. | Suillus variegatus (Sw.) Richon & Roze | 1.11 | 5.55 | 12.70 | 0.39 | 1.95 | 4.68 |
| 16. | Tricholoma equestre (L.) P. Kumm. | 0.73 | 3.65 | 8.53 | 0.93 | 4.65 | 9.29 |
| 17. | Tricholoma portentosum (Fr.) Quel. | 0.60 | 3.00 | 7.96 | 0.82 | 4.10 | 10.61 |
| 18. | Xerocomellus chrysenteron (Bull.) Šutara | 0.77 | 3.85 | 8.130 | 0.73 | 3.65 | 8.48 |
| 19. | Xerocomus subtomentosus (L.) Quél. | 0.10 | 0.50 | 1.03 | 0.82 | 4.10 | 9.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirończuk-Chodakowska, I.; Kapała, J.; Kujawowicz, K.; Sejbuk, M.; Witkowska, A.M. Forty Years After Chernobyl: Radiocaesium in Wild Edible Mushrooms from North-Eastern Poland and Its Relevance for Dietary Exposure and Food Safety. Toxics 2025, 13, 601. https://doi.org/10.3390/toxics13070601
Mirończuk-Chodakowska I, Kapała J, Kujawowicz K, Sejbuk M, Witkowska AM. Forty Years After Chernobyl: Radiocaesium in Wild Edible Mushrooms from North-Eastern Poland and Its Relevance for Dietary Exposure and Food Safety. Toxics. 2025; 13(7):601. https://doi.org/10.3390/toxics13070601
Chicago/Turabian StyleMirończuk-Chodakowska, Iwona, Jacek Kapała, Karolina Kujawowicz, Monika Sejbuk, and Anna Maria Witkowska. 2025. "Forty Years After Chernobyl: Radiocaesium in Wild Edible Mushrooms from North-Eastern Poland and Its Relevance for Dietary Exposure and Food Safety" Toxics 13, no. 7: 601. https://doi.org/10.3390/toxics13070601
APA StyleMirończuk-Chodakowska, I., Kapała, J., Kujawowicz, K., Sejbuk, M., & Witkowska, A. M. (2025). Forty Years After Chernobyl: Radiocaesium in Wild Edible Mushrooms from North-Eastern Poland and Its Relevance for Dietary Exposure and Food Safety. Toxics, 13(7), 601. https://doi.org/10.3390/toxics13070601