Accumulation Patterns and Health Risk Assessment of Trace Elements in Intermuscular Bone-Free Crucian Carp
Highlights
- Gene-edited WUCI carp showed a unique pattern of trace element accumulation versus other varieties, with elevated Mo in the liver and Rb in the muscle/liver.
- All tested crucian carp varieties showed trace element levels within safe thresholds (THQ < 1, HI < 0.25, TCR < 10−6), confirming their safety as food.
- Muscle arsenic levels exceeded hepatic concentrations, suggesting differential detoxification mechanisms between tissues.
- This study provides critical data for evaluating the safety of gene-edited aquatic products, supporting their potential commercialization.
- The findings highlight genotype-specific metabolic adaptations, offering insights for future breeding programs aimed at optimizing elemental profiles in fish.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Sample Pretreatment and Analysis
2.3. Heavy Metal Pollution Evaluation
2.4. Health Risk Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Comparative Analysis of Trace Element Profiles in Four Crucian Carp Strains
3.2. Correlation Analysis of Trace Elements in Crucian Carp Tissues
3.3. Assessment of Heavy Metal Contamination Levels
3.4. Potential Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Yang, Y.; Rizwan, M.; Yue, D.D.; Khan, I.M.; Yao, F.; Zhong, J.; Huang, S.J. Compensatory growth in gibel carp (Carassius auratus gibelio) after the stress of stocking density. Aquac. Res. 2021, 52, 1697–1704. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.Q.; Jiang, Y.; Chu, W. Diazinon exposure produces histological damage, oxidative stress, immune disorders and gut microbiota dysbiosis in crucian carp (Carassius auratus gibelio). Environ. Pollut. 2021, 269, 116129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, P.J.; Xia, C.G.; Cheng, Y.; Guo, X.Y.; Li, Y.H. Effects of malic acid and citric acid on growth performance, antioxidant capacity, haematology and immune response of Carassius auratus gibelio. Aquac. Res. 2020, 51, 2766–2776. [Google Scholar] [CrossRef]
- Lyall, B.A.; Witten, P.E.; Carter, C.G.; Perrott, M.R.; Symonds, J.E.; Walker, S.P.; Waddington, Z.; Amoroso, G. The problems with pin bones: Intermuscular bone development and function in salmonids and their implications for aquaculture. Rev. Aquac. 2024, 16, 1981–1995. [Google Scholar] [CrossRef]
- Zhang, X.B.; Fu, J.J.; Luo, M.K.; Cai-rang, Z.M.; Min, Q.W.; Hu, J.L.; Zeng, S.; Luo, T.X.; Zhu, W.B.; Liu, T.; et al. Genetic and reproductive mode analyses of a golden-back mutant of crucian carp from a rice-fish integrated farming system. Aquac. Rep. 2022, 24, 101146. [Google Scholar] [CrossRef]
- Olivier, L.C.-B.; Louise, V.; Joseph, C.; Gilles-Éric, S. Factors to consider before production and commercialization of aquatic genetically modified organisms: The case of transgenic salmon. Environ. Sci. Policy 2009, 12, 170–189. [Google Scholar] [CrossRef]
- Xu, H.; Tong, G.X.; Yan, T.; Dong, L.; Yang, X.X.; Dou, D.Y.; Sun, Z.P.; Liu, T.Q.; Zheng, H.X.; Yang, J.; et al. Transcriptomic analysis provides insights to reveal the bmp6 function related to the development of intermuscular bones in zebrafish. Front. Cell Dev. Biol. 2022, 10, 821471. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.Y.; Zheng, H.X.; Cao, D.C.; Sun, Z.P.; Tong, G.X.; Xu, H.; Yan, T.; Tang, Z.S.; Chen, Z.X.; Zhang, T.T.; et al. Generate a new crucian carp (Carassius auratus) strain without intermuscular bones by knocking out bmp6. Aquaculture 2023, 569, 739407. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.W.; Liu, X.; Ma, L.; Yang, J.X. Molecular mechanisms of intermuscular bone development in fish: A review. Zool. Res. 2021, 42, 362–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ye, L.; Wang, C.; Xiong, X.F.; Li, Y.Y.; Li, P.J.; Zhang, X.T.; Yu, H.B. Toxic effect of combined exposure of microplastics and copper on goldfish (Carassius auratus): Insight from oxidative stress, inflammation, apoptosis and autophagy in hepatopancreas and intestine. Bull. Environ. Contam. Toxicol. 2022, 109, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Mousavi-Sabet, H.; Hedayati, A.; Zargari, A.; Multisanti, C.R.; Faggio, C. Copper-oxide nanoparticles effects on goldfish (Carassius auratus): Lethal toxicity, haematological, and biochemical effects. Vet. Res. Commun. 2024, 48, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Ghafarifarsani, H.; Hedayati, S.A.; Yousefi, M.; Hoseinifar, S.H.; Yarahmadi, P.; Mahmoudi, S.S.; Doan, H.V. Toxic and bioaccumulative effects of zinc nanoparticle exposure to goldfish, Carassius auratus (Linnaeus, 1758). Drug Chem. Toxicol. 2023, 46, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M.; Vijayaram, S.; Rohani, M.F.; Doan, H.V.; Sun, Y.Z. Comparative effect of chemical and green zinc nanoparticles on the growth, hematology, serum biochemical, antioxidant parameters, and immunity in serum and mucus of goldfish, Carassius auratus (Linnaeus,1758). Biol. Trace Elem. Res. 2024, 202, 1264–1278. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Z.; Zhang, Y.; Zhang, P.; Liu, J.; Cheng, Y.; Zhang, L.; Li, Y. Effect on intestinal microbiota, bioaccumulation, and oxidative stress of Carassius auratus gibelio under waterborne cadmium exposure. Fish Physiol. Biochem. 2020, 46, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Yadrenkina, E.N.; Bortnikova, S.B.; Yurkevich, N.V.; Korneeva, T.V.; Shevko, A.Y.; Olenchenko, V.V.; Khvachevskaya, A.A. Transfer and distribution of metals and metalloids in Carassius auratus organs from tailings pond and their influence on morphological characteristics. Appl. Sci. 2022, 12, 12446. [Google Scholar] [CrossRef]
- Mmanda, F.P. Importance of Minerals and Their Bioavailability in Boosting Aquaculture: A Systematic Review. Aquaculture Fish Fish. 2025, 5, e70067. [Google Scholar] [CrossRef]
- Bai, S.Y.; Qin, D.L.; Chen, Z.X.; Wu, S.; Tang, Z.S.; Gao, L.; Wang, P. Geographic origin discrimination of red swamp crayfish Procambarus clarkii from different Chinese regions using mineral element analysis assisted by machine learning techniques. Food Control 2022, 138, 109047. [Google Scholar] [CrossRef]
- Tang, Z.S.; Song, D.; Bai, S.Y.; Huang, X.L.; Chen, Z.X.; Wang, P.; Qin, D.L. Trace elements accumulation and health risk assessment of Chinese mitten crab (Eriocheir sinensis) from monoculture and rice-crab co-culture mode. J. Food Compos. Anal. 2023, 123, 105640. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.J.; Yuan, Z.J.; Ge, M.; Wang, S.S.; Liu, Y.; Da, C.N. Occurrence, potential health risk of heavy metals in aquatic organisms from Laizhou Bay, China. Mar. Pollut. Bull. 2019, 140, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Fan, Z.H.; Qiu, L.P.; Liu, X.L.; Yin, T.Y.; Jamus, I.M.I.; Song, C.; Chen, J.Z. Occurrence and health risk assessment of residual heavy metals in the Chinese mitten crab (Eriocheir sinensis). J. Food Compos. Anal. 2021, 97, 103787. [Google Scholar] [CrossRef]
- Zhang, C.C.; Miao, X.; Du, S.; Zhang, T.; Chen, L.Z.; Liu, Y.; Zhang, L. Effects of culinary procedures on concentrations and bioaccessibility of Cu, Zn, and As in different food ingredients. Foods 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.P. The definition and classification of metabolic liver disease. Natl. Med. J. China 2020, 100, 401–406. [Google Scholar]
- Tran, T.T.; Prakash, H.; Nagasawa, T.; Nakao, M.; Somanoto, T. Characterization of CD83 homologs differently expressed during monocytes differentiation in ginbuna crucian carp, Carassius auratus langsdorfii. Dev. Comp. Immunol. 2024, 159, 105212. [Google Scholar] [CrossRef] [PubMed]
- Sergio, F.T.; Jhon, J.M.P.; María, J.M.; Rosa, C.R.M.D.; Rafael, M. Metals and metalloids in freshwater fish from the floodplain of Tablas de Daimiel National Park, Spain. Ecotoxicol. Environ. Saf. 2021, 208, 111602. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Y.; Wu, X.G.; Cong, J.J.; Wu, J.H.; Diao, H.F.; He, B.; Han, Z. Comparison of biological indicators and umami-related compounds in the gonad and abdomen meats of Eriocheir sinensis during different fattening periods under salinity. Aquac. Res. 2021, 52, 142–151. [Google Scholar] [CrossRef]
- Luczynska, J.; Pietrzak-Fiećko, R.; Purkiewicz, A.; Luczyński, M.J. Assessment of fish quality based on the content of heavy metals. Int. J. Environ. Res. Public Health 2022, 19, 2307. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yin, H.; Zhu, M.H.; Yu, X.L.; Shao, P.L.; Dang, Z. MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil. Chemosphere 2022, 294, 133733. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.L.; Tang, Z.S.; Bai, S.Y.; Zheng, M.; Wang, H.T.; Chen, Z.X.; Wu, S.; Mou, Z.B. Heavy Metal Concentrations in Muscle of Fishes from the Northeast China. J. Agro-Environ. Sci. 2014, 2, 264–270. [Google Scholar]
- Hasan, G.M.M.A.; Das, A.K.; Satter, M.A.; Asif, M. Distribution of Cr, Cd, Cu, Pb and Zn in organs of three selected local fish species of Turag river, Bangladesh and impact assessment on human health. Emerg. Contam. 2023, 9, 100197. [Google Scholar] [CrossRef]
- Tang, Z.S.; Gao, L.; Qin, D.L.; Wang, H.T.; Huang, L.; Wu, S.; Bai, S.Y.; Du, N.N.; Sun, Y.C.; Wang, P.; et al. Toxic Effects of Arsenic on Four Freshwater Aquatic Species and Its Transformation Metabolism in Crucian Carp (Carassius auratus). Toxics 2024, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.S.; Luan, P.X.; Chen, F.; Wang, P.; Qin, D.L.; Chen, Z.X.; Hu, G. Determination and safety evaluation of metal element contents of Oviductus Ranae produced from Rana dybowskii by pond culture–forest grazing relay model in Heilongjiang Province, China. Qual. Assur. Saf. Crops Foods 2024, 16, 111–126. [Google Scholar] [CrossRef]
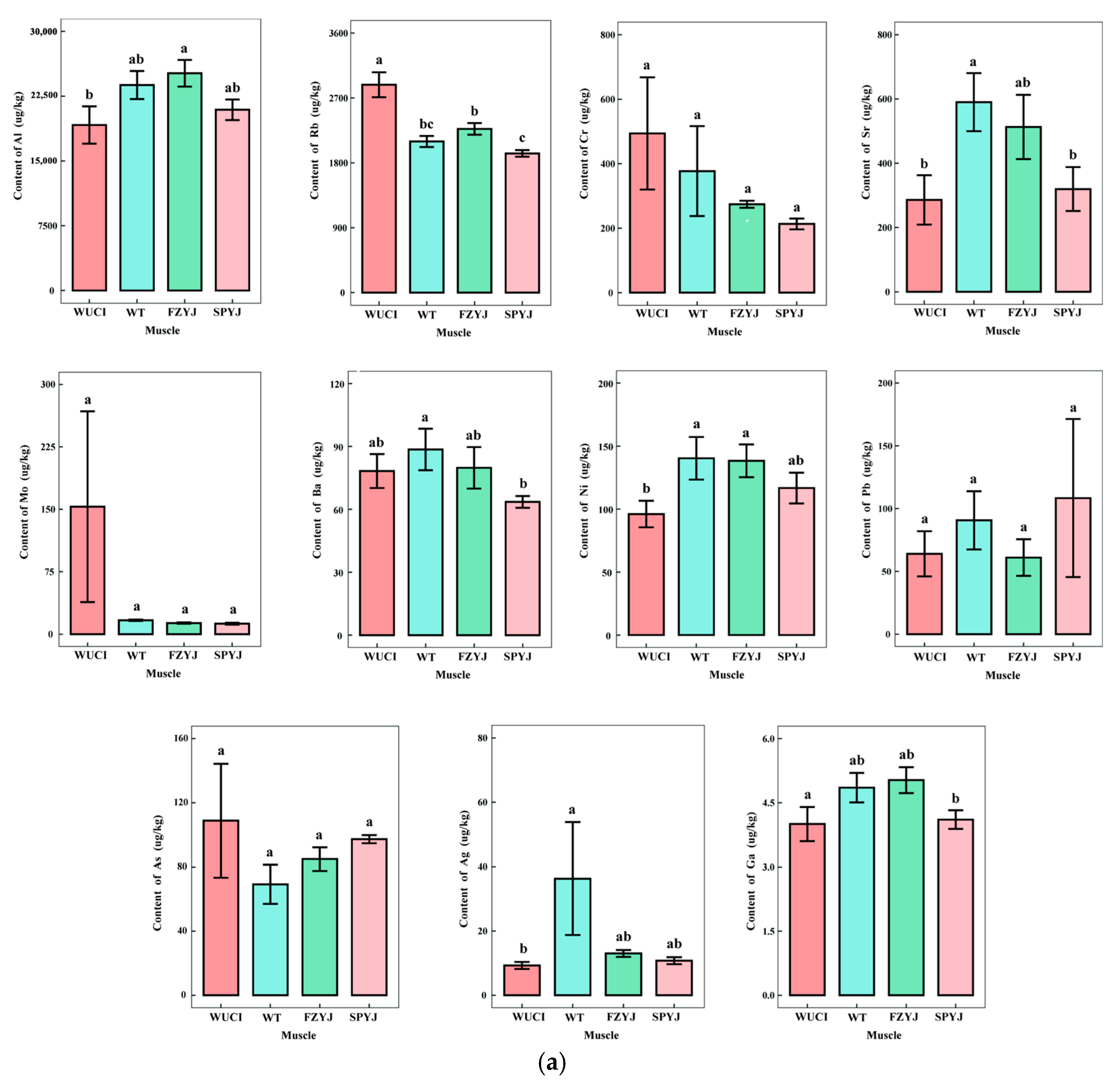
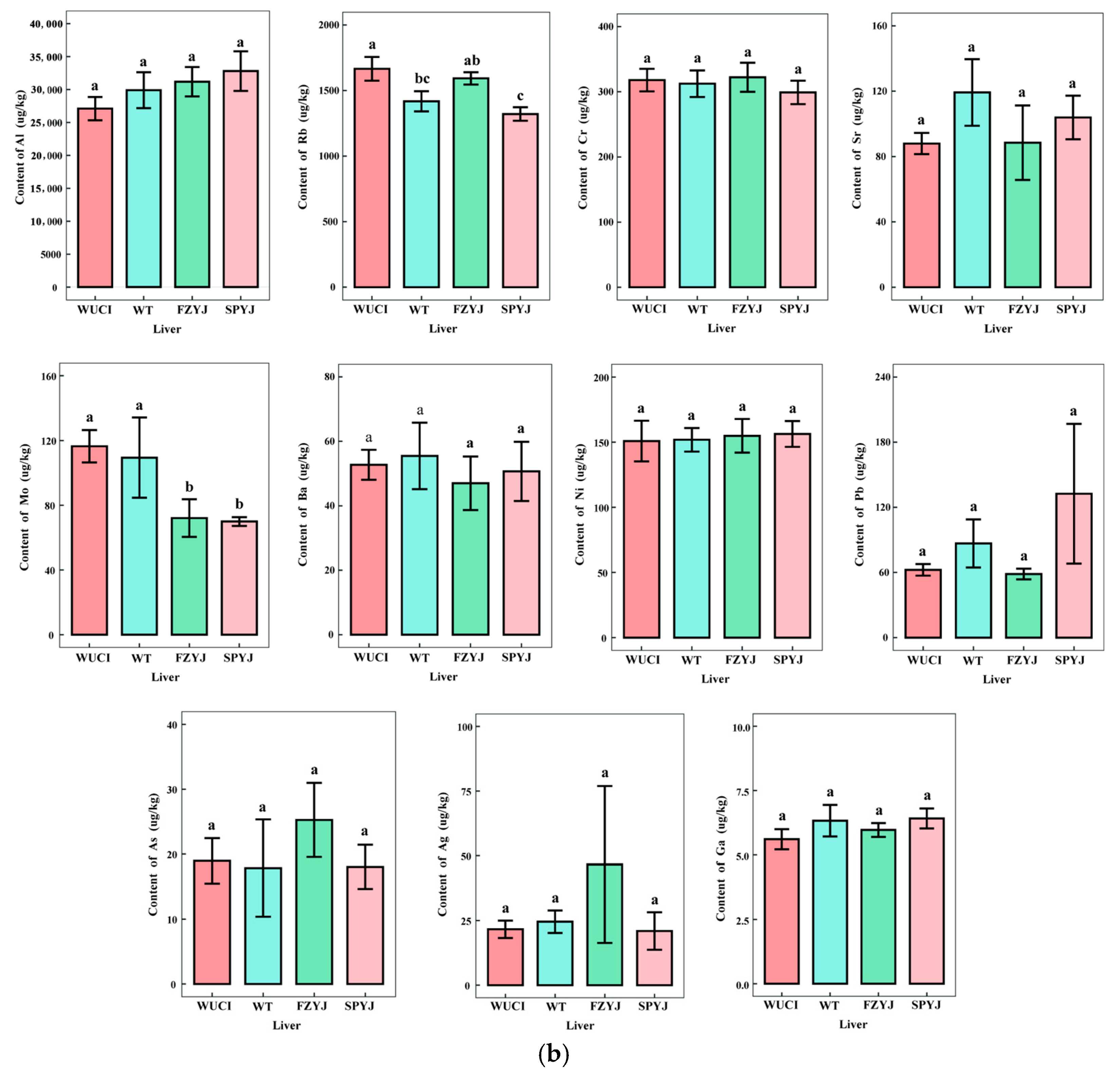
| Strain | Body Weight (g) | Body Length (mm) |
|---|---|---|
| WUCI | 87.70 ± 19.74 | 134.69 ± 9.67 |
| WT | 70.60 ± 12.43 | 124.29 ± 6.82 |
| FZYJ | 75.51 ± 17.67 | 128.54 ± 10.50 |
| SPYJ | 82.09 ± 18.19 | 132.31 ± 8.52 |
| (a) | |||||||||||
| Element | Al | Cr | Ni | Ga | As | Rb | Sr | Mo | Ag | Ba | Pb |
| Al | 1 | ||||||||||
| Cr | 0.091 | 1 | |||||||||
| Ni | 0.952 ** | −0.018 | 1 | ||||||||
| Ga | 0.830 ** | −0.079 | 0.915 ** | 1 | |||||||
| As | −0.455 | −0.152 | −0.358 | −0.527 | 1 | ||||||
| Rb | −0.576 | 0.006 | −0.588 | −0.624 | 0.648 * | 1 | |||||
| Sr | −0.503 | −0.176 | −0.37 | −0.406 | 0.830 ** | 0.782 ** | 1 | ||||
| Mo | 0.006 | 0.770 ** | −0.079 | 0.018 | −0.491 | −0.236 | −0.455 | 1 | |||
| Ag | 0.709 * | 0.164 | 0.721 * | 0.806 ** | −0.709 * | −0.818 ** | −0.709 * | 0.479 | 1 | ||
| Ba | −0.564 | 0.091 | −0.43 | −0.442 | 0.745 * | 0.479 | 0.624 | −0.248 | −0.636 * | 1 | |
| Pb | −0.03 | 0.842 ** | −0.091 | −0.079 | −0.176 | −0.236 | −0.345 | 0.673 * | 0.188 | 0.273 | 1 |
| (b) | |||||||||||
| Element | Al | Cr | Ni | Ga | As | Rb | Sr | Mo | Ag | Ba | Pb |
| Al | 1 | ||||||||||
| Cr | 0.588 | 1 | |||||||||
| Ni | 0.673 * | 0.333 | 1 | ||||||||
| Ga | 0.200 | 0.103 | 0.333 | 1 | |||||||
| As | −0.782 ** | −0.479 | −0.515 | −0.309 | 1 | ||||||
| Rb | −0.648 * | −0.358 | −0.394 | −0.176 | 0.782 ** | 1 | |||||
| Sr | −0.261 | −0.297 | −0.091 | −0.248 | 0.539 | 0.818 ** | 1 | ||||
| Mo | 0.855 ** | 0.818 ** | 0.673 * | 0.358 | −0.806 ** | −0.721 * | −0.503 | 1 | |||
| Ag | 0.467 | 0.539 | 0.527 | 0.37 | −0.745 * | −0.345 | −0.164 | 0.661 * | 1 | ||
| Ba | −0.479 | −0.455 | −0.212 | −0.224 | 0.442 | 0.709 * | 0.673 * | −0.624 | −0.139 | 1 | |
| Pb | 0.333 | 0.127 | 0.624 | 0.358 | −0.552 | −0.164 | 0.152 | 0.394 | 0.842 ** | 0.127 | 1 |
| (c) | |||||||||||
| Element | Al | Cr | Ni | Ga | As | Rb | Sr | Mo | Ag | Ba | Pb |
| Al | 1 | ||||||||||
| Cr | 0.697 * | 1 | |||||||||
| Ni | 0.721 * | 0.879 ** | 1 | ||||||||
| Ga | 0.818 ** | 0.891 ** | 0.758 * | 1 | |||||||
| As | −0.418 | −0.321 | −0.127 | −0.394 | 1 | ||||||
| Rb | −0.527 | −0.479 | −0.394 | −0.648 * | 0.515 | 1 | |||||
| Sr | −0.115 | −0.091 | 0.042 | −0.285 | 0.733 * | 0.758 * | 1 | ||||
| Mo | 0.673 * | 0.661 * | 0.564 | 0.648 * | −0.709 * | −0.697 * | −0.503 | 1 | |||
| Ag | 0.624 | 0.685 * | 0.770 ** | 0.527 | −0.006 | −0.321 | 0.212 | 0.515 | 1 | ||
| Ba | 0.345 | 0.309 | 0.418 | 0.176 | 0.406 | 0.418 | 0.806 ** | −0.03 | 0.394 | 1 | |
| Pb | 0.636 * | 0.6 | 0.673 * | 0.624 | 0.345 | −0.055 | 0.503 | 0.091 | 0.673 * | 0.697 * | 1 |
| (d) | |||||||||||
| Element | Al | Cr | Ni | Ga | As | Rb | Sr | Mo | Ag | Ba | Pb |
| Al | 1 | ||||||||||
| Cr | 0.939 ** | 1 | |||||||||
| Ni | 0.818 ** | 0.939 ** | 1 | ||||||||
| Ga | 0.842 ** | 0.770 ** | 0.588 | 1 | |||||||
| As | −0.733 * | −0.830 ** | −0.782 ** | −0.709 * | 1 | ||||||
| Rb | −0.855 ** | −0.806 ** | −0.770 ** | −0.685 * | 0.782 ** | 1 | |||||
| Sr | −0.733 * | −0.697 * | −0.552 | −0.733 * | 0.745 * | 0.758 * | 1 | ||||
| Mo | 0.794 ** | 0.818 ** | 0.721 * | 0.842 ** | −0.867 ** | −0.794 ** | −0.867 ** | 1 | |||
| Ag | 0.588 | 0.673 * | 0.612 | 0.576 | −0.6 | −0.527 | −0.261 | 0.588 | 1 | ||
| Ba | −0.042 | 0.006 | 0.091 | −0.139 | 0.394 | 0.212 | 0.576 | −0.321 | 0.273 | 1 | |
| Pb | 0.721 * | 0.673 * | 0.624 | 0.564 | −0.37 | −0.673 * | −0.394 | 0.624 | 0.697 * | 0.382 | 1 |
| Elements | Limit Standards | Tissues | WUCI | WT | FZYJ | SPYJ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pi | Pollution Level | Pi | Pollution Level | Pi | Pollution Level | Pi | Pollution Level | |||
| Cr a | 2 a | Muscle | 0.247 | Mild | 0.189 | Clean | 0.137 | Clean | 0.107 | Clean |
| As a | 0.5 a | Muscle | 0.218 | Mild | 0.139 | Clean | 0.170 | Clean | 0.195 | Clean |
| Pb a | 0.5 a | Muscle | 0.128 | Clean | 0.181 | Clean | 0.122 | Clean | 0.217 | Mild |
| Cu b | 50 b | Muscle | 0.014 | Clean | 0.022 | Clean | 0.016 | Clean | 0.047 | Clean |
| Cd a | 0.5 a | Muscle | 0.001 | Clean | 0.001 | Clean | 0.001 | Clean | 0.001 | Clean |
| Cr a | 2 a | Liver | 0.159 | Clean | 0.156 | Clean | 0.161 | Clean | 0.150 | Clean |
| As a | 0.5 a | Liver | 0.038 | Clean | 0.042 | Clean | 0.051 | Clean | 0.036 | Clean |
| Pb a | 0.5 a | Liver | 0.125 | Clean | 0.174 | Clean | 0.117 | Clean | 0.265 | Mild |
| Cu b | 50 b | Liver | 0.246 | Mild | 0.153 | Clean | 0.108 | Clean | 0.051 | Clean |
| Cd a | 0.5 a | Liver | 0.001 | Clean | 0.001 | Clean | 0.001 | Clean | 0.001 | Clean |
| Fish Sample | Target Hazard Quotient (THQ) | Hazard Index (HI) | Target Carcinogenic Risk (TCR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Cr | Ni | As | Sr | Ag | Ba | Pb | Cr | Ni | As | Pb | ||
| Liver | |||||||||||||
| WUCI | 0.0373 | 0.0417 | 0.0030 | 0.0249 | 0.0001 | 0.0017 | 0.0001 | 0.0061 | 0.1149 | 1.01 × 10−6 | 1.63 × 10−6 | 1.81 × 10−7 | 3.40 × 10−9 |
| WT | 0.0430 | 0.0423 | 0.0030 | 0.0331 | 0.0001 | 0.0037 | 0.0001 | 0.0058 | 0.1310 | 1.03 × 10−6 | 1.68 × 10−6 | 2.42 × 10−7 | 3.19 × 10−9 |
| FZYJ | 0.0412 | 0.0410 | 0.0030 | 0.0234 | 0.0001 | 0.0019 | 0.0001 | 0.0085 | 0.1192 | 9.95 × 10−7 | 1.65 × 10−6 | 1.71 × 10−7 | 4.73 × 10−9 |
| SPYJ | 0.0452 | 0.0392 | 0.0031 | 0.0237 | 0.0001 | 0.0016 | 0.0001 | 0.0130 | 0.1260 | 9.53 × 10−7 | 1.69 × 10−6 | 1.73 × 10−7 | 7.22 × 10−9 |
| Muscle | |||||||||||||
| WUCI | 0.0264 | 0.0648 | 0.0019 | 0.1427 | 0.0002 | 0.0007 | 0.0002 | 0.0063 | 0.2432 | 1.57 × 10−6 | 1.04 × 10−6 | 1.04 × 10−6 | 3.49 × 10−9 |
| WT | 0.0346 | 0.0360 | 0.0027 | 0.1114 | 0.0003 | 0.0010 | 0.0002 | 0.0060 | 0.1922 | 8.74 × 10−7 | 1.50 × 10−6 | 8.11 × 10−7 | 3.32 × 10−9 |
| FZYJ | 0.0328 | 0.0495 | 0.0028 | 0.0909 | 0.0004 | 0.0029 | 0.0002 | 0.0089 | 0.1882 | 1.20 × 10−6 | 1.52 × 10−6 | 6.62 × 10−7 | 4.94 × 10−9 |
| SPYJ | 0.0288 | 0.0280 | 0.0023 | 0.1276 | 0.0002 | 0.0008 | 0.0001 | 0.0107 | 0.1986 | 6.79 × 10−7 | 1.26 × 10−6 | 9.30 × 10−7 | 5.91 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, S.; Li, N.; Sun, Z.; Yan, T.; Zhang, T.; Xu, H.; Chen, Z.; Qin, D.; Kuang, Y. Accumulation Patterns and Health Risk Assessment of Trace Elements in Intermuscular Bone-Free Crucian Carp. Toxics 2025, 13, 595. https://doi.org/10.3390/toxics13070595
Tang S, Li N, Sun Z, Yan T, Zhang T, Xu H, Chen Z, Qin D, Kuang Y. Accumulation Patterns and Health Risk Assessment of Trace Elements in Intermuscular Bone-Free Crucian Carp. Toxics. 2025; 13(7):595. https://doi.org/10.3390/toxics13070595
Chicago/Turabian StyleTang, Shizhan, Na Li, Zhipeng Sun, Ting Yan, Tingting Zhang, Huan Xu, Zhongxiang Chen, Dongli Qin, and Youyi Kuang. 2025. "Accumulation Patterns and Health Risk Assessment of Trace Elements in Intermuscular Bone-Free Crucian Carp" Toxics 13, no. 7: 595. https://doi.org/10.3390/toxics13070595
APA StyleTang, S., Li, N., Sun, Z., Yan, T., Zhang, T., Xu, H., Chen, Z., Qin, D., & Kuang, Y. (2025). Accumulation Patterns and Health Risk Assessment of Trace Elements in Intermuscular Bone-Free Crucian Carp. Toxics, 13(7), 595. https://doi.org/10.3390/toxics13070595







