Evaluation of Fly Ash Composition from Municipal Solid Waste Incinerators: The Role of the Incinerator Type and Flue Gas Deacidification Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Chemical Composition Analysis
2.3. Heavy Metal Analysis
2.4. Statistical Analysis
2.5. Risk Assessment
3. Results
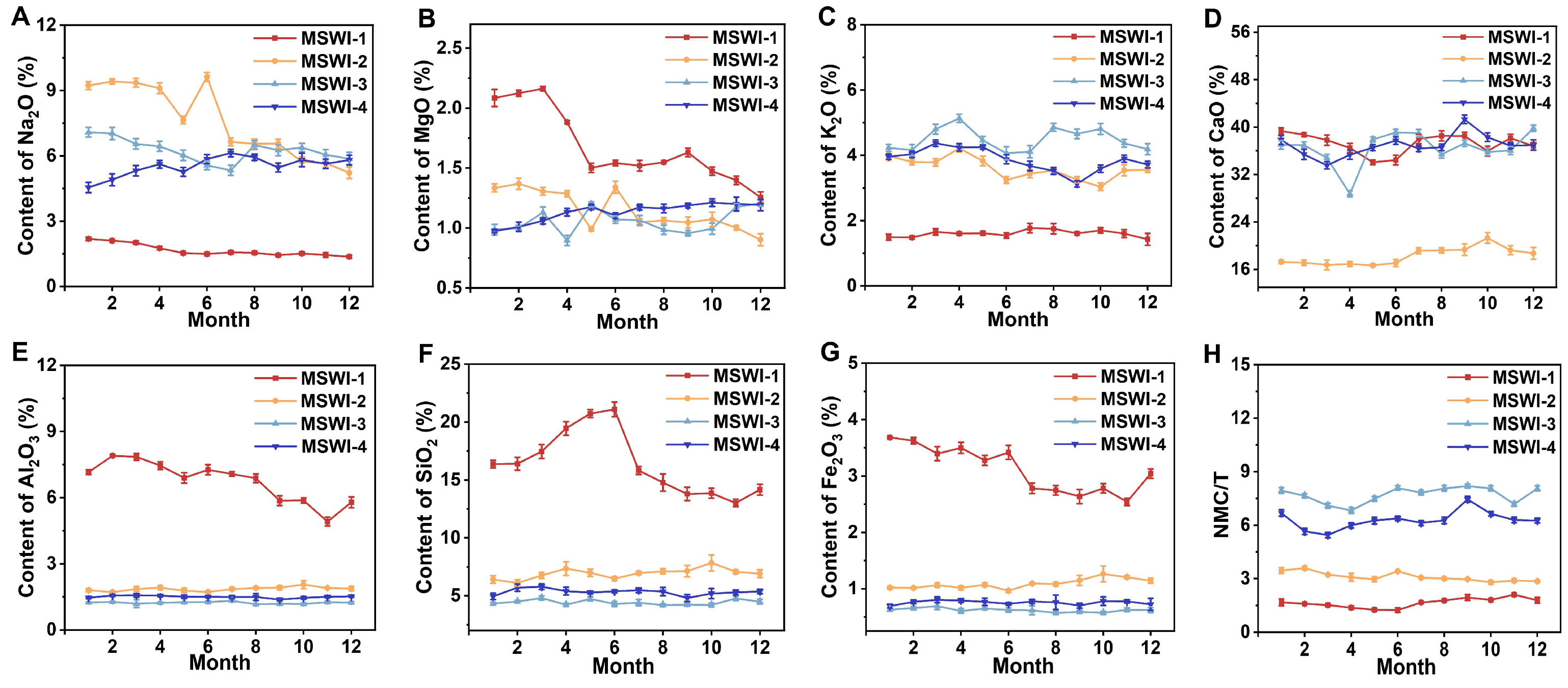
3.1. Oxide Analysis
3.2. Heavy Metal Analysis
3.3. Correlation Analysis
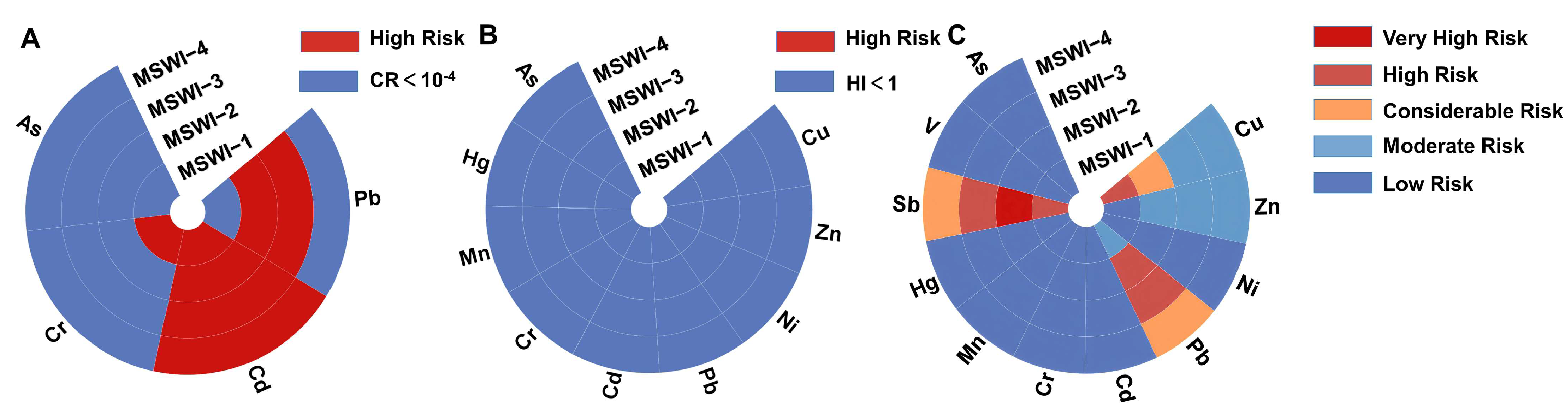
3.4. Health and Ecological Risk Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Woerden, F.V. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development Series Washington, D.C.: World Bank Group. Available online: http://documents.worldbank.org/curated/en/697271544470229584 (accessed on 26 May 2025).
- Vukovic, N.; Makogon, E. Waste-to-Energy Generation: Complex Efficiency Analysis of Modern Technologies. Sustainability 2022, 14, 13814. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. A technical review of bioenergy and resource recovery from municipal solid waste. J. Hazard. Mater. 2021, 403, 123970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C.W. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics of China. 2023. Available online: https://data.stats.gov.cn/easyquery.htm?cn=C01&zb=A0C06&sj=2023 (accessed on 26 May 2025).
- Ghouleh, Z.; Shao, Y. Turning municipal solid waste incineration into a cleaner cement production. J. Clean. Prod. 2018, 195, 268–279. [Google Scholar] [CrossRef]
- Yan, D.; Peng, Z.; Yu, L.; Sun, Y.; Yong, R.; Helge Karstensen, K. Characterization of heavy metals and PCDD/Fs from water-washing pretreatment and a cement kiln co-processing municipal solid waste incinerator fly ash. Waste Manag. 2018, 76, 106–116. [Google Scholar] [CrossRef]
- Sarmiento, L.M.; Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Critical examination of recycled municipal solid waste incineration ash as a mineral source for portland cement manufacture—A case study. Resour. Conserv. Recycl. 2019, 148, 1–10. [Google Scholar] [CrossRef]
- Medici, F.; Piga, L.; Rinaldi, G. Behaviour of polyaminophenolic additives in the granulation of lime and fly-ash. Waste Manag. 2000, 20, 491–498. [Google Scholar] [CrossRef]
- Wu, K.; Shi, H.; Schutter, G.D.; Guo, X.; Ye, G. Preparation of alinite cement from municipal solid waste incineration fly ash. Cem. Concr. Compos. 2012, 34, 322–327. [Google Scholar] [CrossRef]
- Moon, G.D.; Oh, S.; Choi, Y.C. Effects of the physicochemical properties of fly ash on the compressive strength of high-volume fly ash mortar. Constr. Build. Mater. 2016, 124, 1072–1080. [Google Scholar] [CrossRef]
- Lou, Y.; Jiang, S.; Du, B.; Dai, X.; Wang, T.; Wang, J.; Zhang, Y. Leaching morphology characteristics and environmental risk assessment of 13 hazardous trace elements from municipal solid waste incineration fly ash. Fuel 2023, 346, 128374. [Google Scholar] [CrossRef]
- Hwang, I.-H.; Matsuo, T.; Matsuto, T.; Tojo, Y.; Sameshima, R. Dry scrubbing of municipal solid waste incineration flue gas using porous sodium carbonate produced via vacuum thermal treatment of sodium bicarbonate. J. Mater. Cycles Waste Manag. 2021, 23, 1609–1616. [Google Scholar] [CrossRef]
- Ma, X.; He, T.; Da, Y.; Su, F.; Yang, R. The Toxicity Leaching and the Cement Admixtures Properties with Incineration Fly Ash of Different Furnace Types. Langmuir 2024, 40, 24870–24881. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Tang, R.; Li, C.; Gu, X.; Gong, Z.; Zhu, C.; Li, J.; Wang, K.; Yu, J. Recent advances in process and materials for dry desulfurization of industrial flue gas: An overview. Sep. Purif. Technol. 2025, 353, 128425. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Ai, H.; Liu, Z. A comparative study on characteristics and leaching toxicity of fluidized bed and grate furnace MSWI fly ash. J. Environ. Manag. 2022, 305, 114345. [Google Scholar] [CrossRef]
- Wang, W.; Tian, S.; Long, J.; Liu, J.; Ma, Q.; Xu, K.; Zhang, Z. Investigation and Evaluation of Flue Gas Pollutants Emission in Waste-to-Energy Plant with Flue Gas Recirculation. Atmosphere 2022, 13, 1016. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, L.; Gu, X.; Luo, J.; Liu, Y.; Guo, L.; Shi, Y.; Huang, T.; Cheng, S. Levels and ecological risk assessment of metals in soils from a typical e-waste recycling region in southeast China. Ecotoxicology 2015, 24, 1947–1960. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.B.; Zhu, M.M.; Cheng, F.Q.; Lu, X.F.; Zhang, Z.Z.; Zhang, D.K. An experimental investigation into the effect of flue gas recirculation on ash deposition and Na migration behaviour in circulating fluidized bed during combustion of high sodium Zhundong lignite. Fuel Process. Technol. 2020, 199, 106300. [Google Scholar] [CrossRef]
- Yang, S.; Song, G.; Na, Y.; Yang, Z. Alkali metal transformation and ash deposition performance of high alkali content Zhundong coal and its gasification fly ash under circulating fluidized bed combustion. Appl. Therm. Eng. 2018, 141, 29–41. [Google Scholar] [CrossRef]
- Cho, Y.K.; Jung, S.H.; Choi, Y.C. Effects of chemical composition of fly ash on compressive strength of fly ash cement mortar. Constr. Build. Mater. 2019, 204, 255–264. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Y.; Kuo, J.H.; Lin, C.L. Agglomeration-influenced transformation of heavy metals in gas-solid phases during simulated sewage sludge co-incineration: Effects of phosphorus and operating temperature. Sci. Total Environ. 2023, 858, 159759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, X.; Liu, B.; Lv, G.; Jin, Y.; Yan, J. Co-combustion of Bituminous Coal and Pickling Sludge in a Drop-Tube Furnace: Thermodynamic Study and Experimental Data on the Distribution of Cr, Ni, Mn, As, Cu, Sb, Pb, Cd, Zn, and Sn. Energy Fuels 2017, 31, 3019–3028. [Google Scholar] [CrossRef]
- Liu, Z.; Yue, Y.; Lu, M.; Zhang, J.; Sun, F.; Huang, X.; Zhou, J.; Qian, G. Comprehension of heavy metal stability in municipal solid waste incineration fly ash with its compositional variety: A quick prediction case of leaching potential. Waste Manag. 2019, 84, 329–339. [Google Scholar] [CrossRef]
- Kalisz, S.; Wejkowski, R.; Maj, I.; Garbacz, P. A novel approach to the dry desulfurization process by means of sodium bicarbonate: A full-scale study on SO2 emission and geochemistry of fly ash. Energy 2023, 279, 128046. [Google Scholar] [CrossRef]
- Zhao, S.; Duan, Y.; Lu, J.; Liu, S.; Pudasainee, D.; Gupta, R.; Liu, M.; Lu, J. Enrichment characteristics, thermal stability and volatility of hazardous trace elements in fly ash from a coal-fired power plant. Fuel 2018, 225, 490–498. [Google Scholar] [CrossRef]
- Hailu, S.L.; McCrindle, R.I.; Seopela, M.P.; Combrinck, S. Speciation of major and trace elements leached from coal fly ash and the kinetics involved. J. Environ. Sci. Health 2019, 54, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Marczak, G.M.; Piersa, P.; Karczewski, M.; Szufa, S.; Ünyay, H.; Kędzierska, S.A.; Bochenek, P. Modified Fly Ash-Based Adsorbents (MFA) for Mercury and Carbon Dioxide Removal from Coal-Fired Flue Gases. Energies 2021, 14, 7101. [Google Scholar] [CrossRef]
- Zhou, C.C.; Liu, G.J.; Xu, Z.Y.; Sun, H.; Kwan Sing Lam, P. Retention mechanisms of ash compositions on toxic elements (Sb, Se and Pb) during fluidized bed combustion. Fuel 2018, 213, 98–105. [Google Scholar] [CrossRef]
- Mao, L.Q.; Deng, N.; Liu, L.; Cui, H.; Zhang, W.Y. Effects of Al2O3, Fe2O3, and SiO2 on Cr(VI) formation during heating of solid waste containing Cr(III). Chem. Eng. J. 2016, 304, 216–222. [Google Scholar] [CrossRef]
- Xing, Y.Q.; Wang, B.M. Chemical speciation, distribution, and leaching behaviors of heavy metals in alkali-activated converter steel slag-based stabilization/solidification of MSWI FA. Constr. Build. Mater. 2024, 417, 135209. [Google Scholar] [CrossRef]
- Li, Y.K.; Feng, D.D.; Bai, C.X.; Sun, S.Z.; Zhang, Y.; Zhao, Y.J.; Li, Y.Z.; Zhang, F.; Chang, G.Z.; Qin, Y.K. Thermal synergistic treatment of municipal solid waste incineration (MSWI) fly ash and fluxing agent in specific situation: Melting characteristics, leaching characteristics of heavy metals. Fuel Process. Technol. 2022, 233, 107311. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Y.; Yi, Y.; Fang, M. Carbonation treatment of gasification fly ash from municipal solid waste using sodium carbonate and sodium bicarbonate solutions. Environ. Pollut. 2022, 299, 118906. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, C.; Zhang, Y.; Su, Z.; Jiang, T. Influence of Al2O3-induced MnO2–SiO2 smelting on silicate phase and consolidation behavior of manganese ore sinters. Ceram. Int. 2022, 48, 34332–34340. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Vanadium geochemistry in the biogeosphere–speciation, solid-solution interactions, and ecotoxicity. Appl. Geochem. 2019, 102, 1–25. [Google Scholar] [CrossRef]
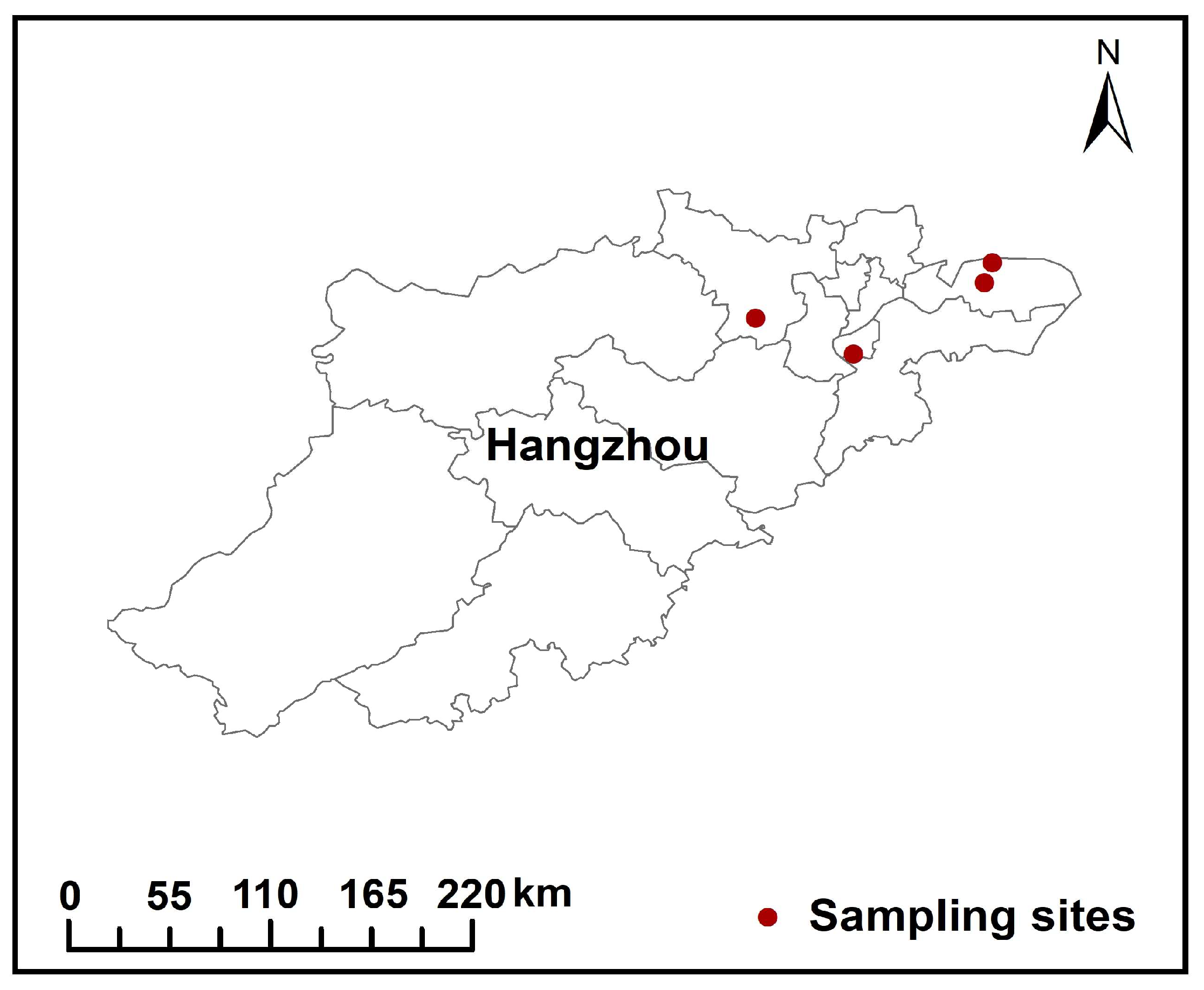


| Sample No. | Incinerator Type | Flue Gas Deacidification | Capacity (t/d) | Operating Hours (h) |
|---|---|---|---|---|
| MSWI-1 | Fluidized bed | Dry and semi-dry | 600 | 82,080 |
| MSWI-2 | Grate furnace | Sodium bicarbonate dry | 150 | 178,560 |
| MSWI-3 | Grate furnace | Dry and semi-dry | 870 | 38,880 |
| MSWI-4 | Grate furnace | Dry and semi-dry | 750 | 65,520 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, X.; Wang, Y.; Chen, F.; Li, C.; He, Y.; Dou, J.; Zhang, S.; Ding, J.; Zhang, H.; Zhong, Y. Evaluation of Fly Ash Composition from Municipal Solid Waste Incinerators: The Role of the Incinerator Type and Flue Gas Deacidification Process. Toxics 2025, 13, 588. https://doi.org/10.3390/toxics13070588
Qu X, Wang Y, Chen F, Li C, He Y, Dou J, Zhang S, Ding J, Zhang H, Zhong Y. Evaluation of Fly Ash Composition from Municipal Solid Waste Incinerators: The Role of the Incinerator Type and Flue Gas Deacidification Process. Toxics. 2025; 13(7):588. https://doi.org/10.3390/toxics13070588
Chicago/Turabian StyleQu, Xuetong, Yanan Wang, Feifei Chen, Chuqiao Li, Yunfei He, Jibo Dou, Shuai Zhang, Jiafeng Ding, Hangjun Zhang, and Yuchi Zhong. 2025. "Evaluation of Fly Ash Composition from Municipal Solid Waste Incinerators: The Role of the Incinerator Type and Flue Gas Deacidification Process" Toxics 13, no. 7: 588. https://doi.org/10.3390/toxics13070588
APA StyleQu, X., Wang, Y., Chen, F., Li, C., He, Y., Dou, J., Zhang, S., Ding, J., Zhang, H., & Zhong, Y. (2025). Evaluation of Fly Ash Composition from Municipal Solid Waste Incinerators: The Role of the Incinerator Type and Flue Gas Deacidification Process. Toxics, 13(7), 588. https://doi.org/10.3390/toxics13070588







