Nano-Curcumin Mitigates Doxorubicin-Induced Reproductive Toxicity via Antioxidant, Anti-Apoptosis, and SIRT1-Modulating Effects in Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Animals and Experimental Design
2.3. Sperm Count, Viability, and Morphology
2.4. Assay of Testosterone, Gonadotropin Hormones, and Inflammatory Biomarkers
2.5. Assay of Antioxidants
2.6. Gene Expression
2.7. Histopathological and Immunohistochemistry (IHC) Examination
2.8. Statistical Analysis
3. Results
3.1. NCR Significantly Mitigates Decreased Body Weight Caused by DOX, and Neither Affect Testicular Weight
3.2. NCR Attenuates the Effect of DOX on Sperm Morphology, Count, Abnormality, and Rapid Progressive Mobility
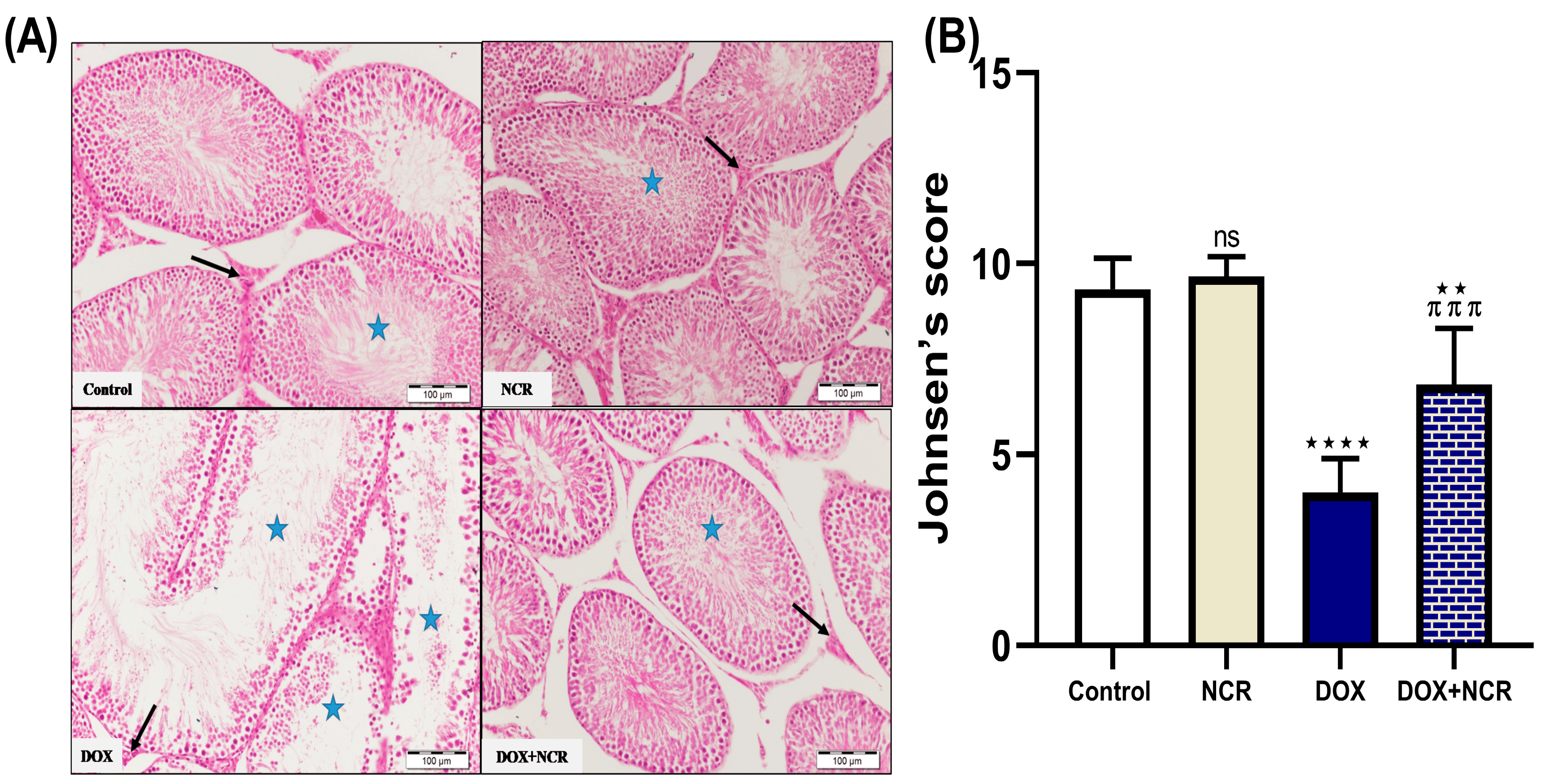
3.3. NCR Modulates the Effect of DOX on Seminiferous Tubules—Normal; Detached and Sloughed; And Vacuolated Histology in Testicular Tissue
3.4. NCR Effects on DOX-Induced Testicular Histopathological Changes
3.5. NCR Attenuates the Effect of DOX on Reproductive Hormones
3.6. NCR Attenuated the Oxidative Stress and Inflammatory Biomarkers in Testicular Tissues in Rats Treated with DOX
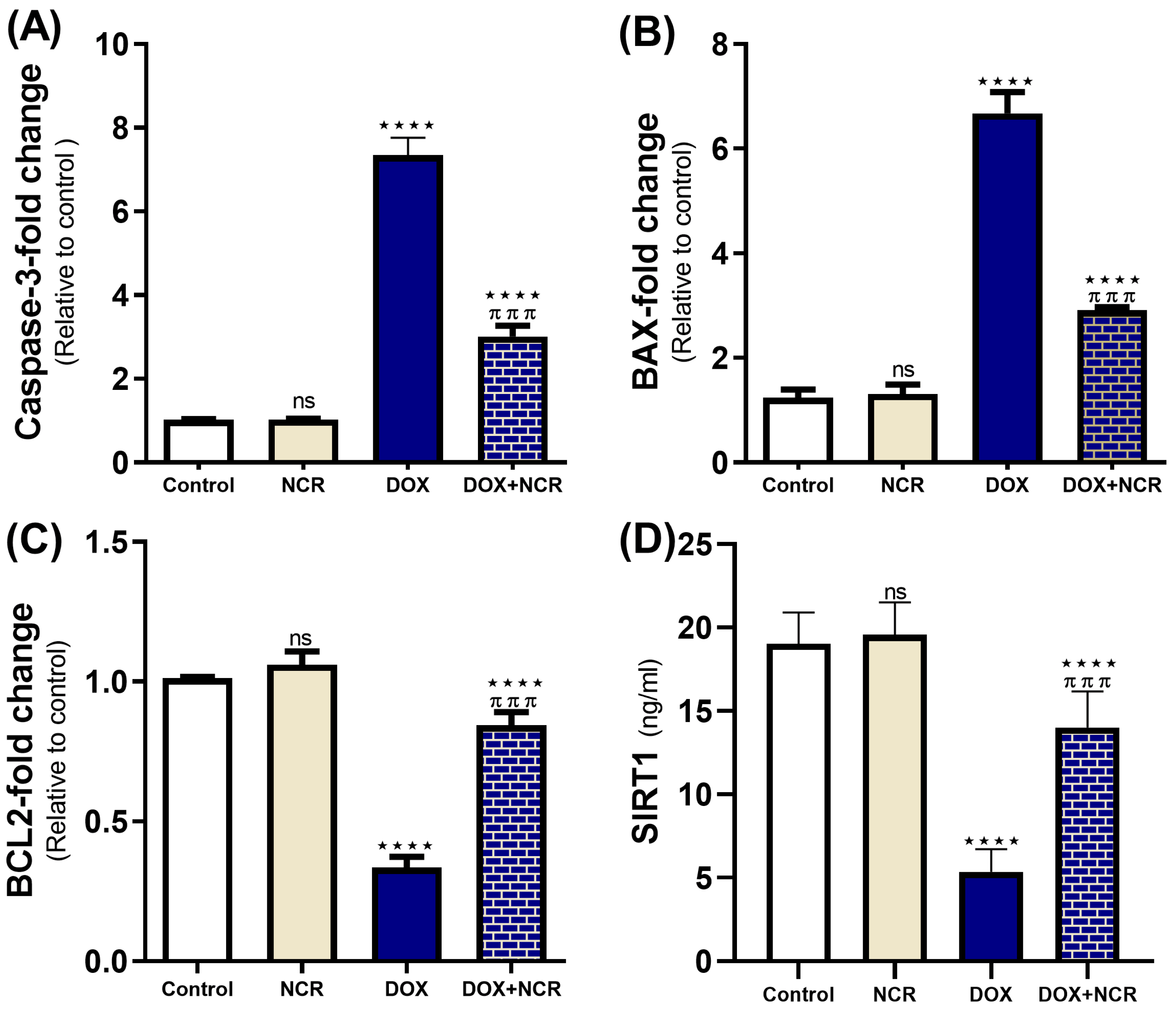
3.7. NCR Attenuated the Caspase-3, Bax, Bcl2 Gene Expression, and SIRT1 Level in DOX-Testicular Toxicity
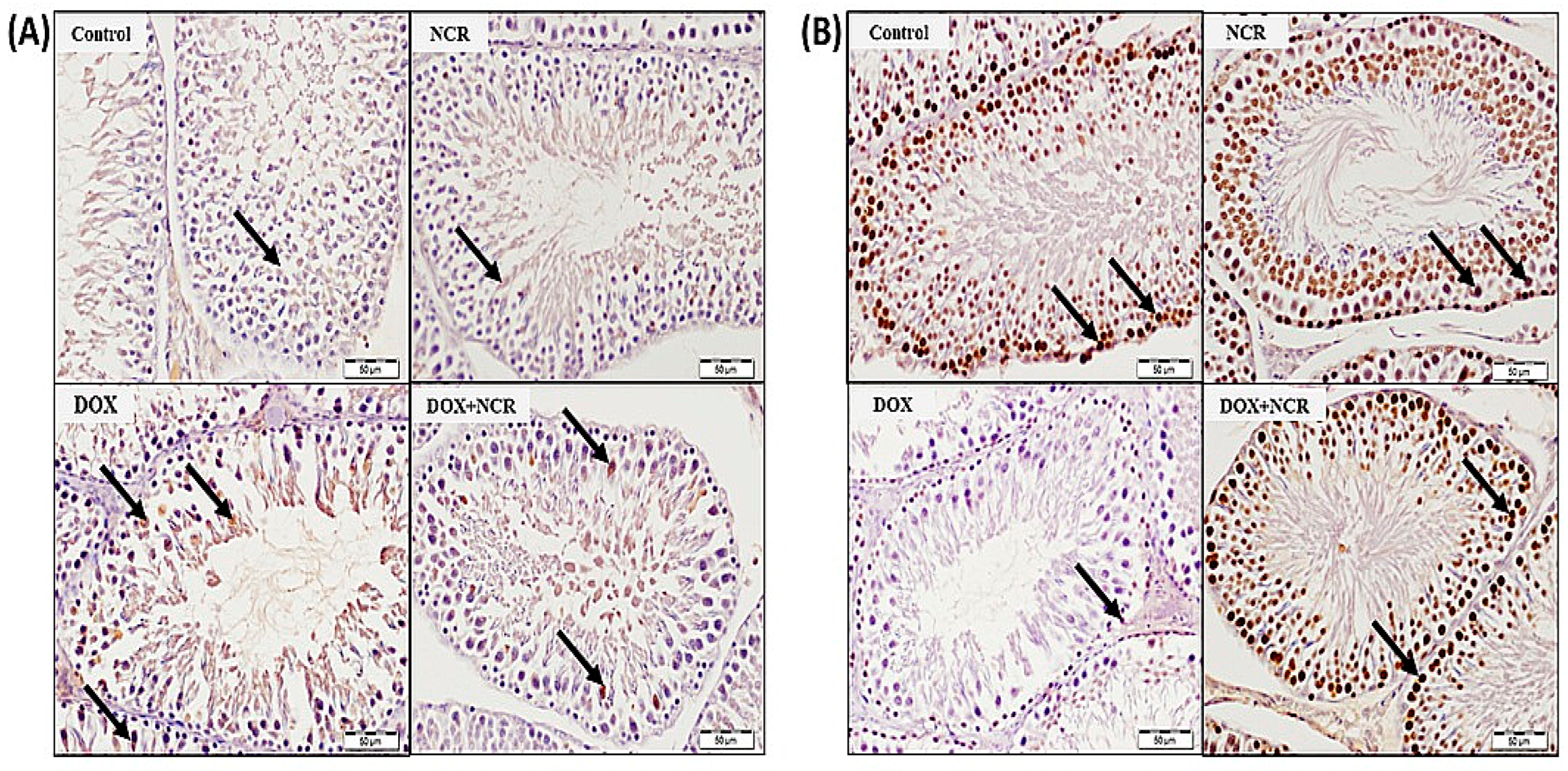
3.8. NCR Attenuated the DOX-Induced Change in iNOS and PCNA Expression
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peter, S.; Alven, S.; Maseko, R.B.; Aderibigbe, B.A. Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review. Molecules 2022, 27, 4478. [Google Scholar] [CrossRef] [PubMed]
- Alsyaad, K.M. Ameliorative impacts of propolis against testicular toxicity promoted by doxorubicin. Vet. World 2024, 17, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Makipour, A.; Hosseinifar, S.; Khazaeel, K.; Tabandeh, M.R.; Jamshidian, J. Protective effect of Chlorella vulgaris on testicular damage, sperm parameters, androgen production, apoptosis and oxidative stress index in male rats following doxorubicin administration. Reprod. Toxicol. 2024, 128, 108653. [Google Scholar] [CrossRef] [PubMed]
- Mohan, U.P.; PB, T.P.; Iqbal, S.T.A.; Arunachalam, S. Mechanisms of doxorubicin-mediated reproductive toxicity—A review. Reprod. Toxicol. 2021, 102, 80–89. [Google Scholar] [CrossRef]
- Renu, K.; Valsala Gopalakrishnan, A. Deciphering the molecular mechanism during doxorubicin-mediated oxidative stress, apoptosis through Nrf2 and PGC-1α in a rat testicular milieu. Reprod. Biol. 2019, 19, 22–37. [Google Scholar] [CrossRef]
- Ağan, K.; Kaya, S.T.; Ağan, A.F.; Ağyar-Yoldaş, P.; Yoldaş, T.; İkinci-Keleş, A.; Çaprazlı, T.; Arıca, E.; Kekeçoglu, M. Alleviating doxorubicin-induced reproductive toxicity: Protective and androgenic effects of drone larvae on sperm morphology and hormonal balance. Toxicol. Res. 2025, 41, 149–165. [Google Scholar] [CrossRef]
- Kanter, M.; Aktas, C.; Erboga, M. Curcumin attenuates testicular damage, apoptotic germ cell death, and oxidative stress in streptozotocin-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 1578–1585. [Google Scholar] [CrossRef]
- Tian, M.P.; Song, R.X.; Wang, T.; Sun, M.J.; Liu, Y.; Chen, X.G. Inducing sustained release and improving oral bioavailability of curcumin via chitosan derivatives-coated liposomes. Int. J. Biol. Macromol. 2018, 120, 702–710. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin delivery mediated by bio-based nanoparticles: A review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Bertoncini-Silva, C.; Vlad, A.; Ricciarelli, R.; Giacomo Fassini, P.; Suen, V.M.M.; Zingg, J.M. Enhancing the bioavailability and bioactivity of curcumin for disease prevention and treatment. Antioxidants 2024, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.W.; Gad-Allah, H.N.; Kaooh, S.M.; Hussein, H.A.M.; El-Galil, T.I.A. Protective Role of Nanocurcumin in Cyclophosphamide-induced Cardiac Toxicity in Adult Male Albino Rat: A Histological, Immunohistochemical, Biochemical Study. J. Adv. Zool. 2023, 44, 124. [Google Scholar]
- Morsy, M.M.; Ahmed, R.G.; Abdel-Gabbar, M. Nano-Curcumin improves caffeine-induced cerebral alterations in male Wistar rats by modifying oxidative stress, inflammation, and COX-2/NF-κB/Nrf2 signaling. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 93. [Google Scholar] [CrossRef]
- Moshari, S.; Nejati, V.; Najafi, G. Nanomicelle curcumin-induced DNA fragmentation in testicular tissue; Correlation between mitochondria dependent apoptosis and failed PCNA-related hemostasis. Acta Histochem. 2017, 119, 372–381. [Google Scholar] [CrossRef]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Alghibiwi, H.K.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Nano-curcumin prevents copper reproductive toxicity by attenuating oxidative stress and inflammation and improving Nrf2/HO-1 signaling and pituitary-gonadal axis in male rats. Toxics 2022, 10, 356. [Google Scholar] [CrossRef]
- Elbassiouni, F.E.; El-Kholy, W.M.; Elhabibi, E.S.M.; Albogami, S.; Fayad, E. Comparative Study between Curcumin and Nanocurcumin Loaded PLGA on Colon Carcinogenesis Induced Mice. Nanomaterials 2022, 12, 324. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Hu, L.; Wang, Y.; Huang, L.; Liang, A.; Wang, X.; Zhang, Q.; Chen, Y.; Cao, Y.; et al. Icariin improves testicular dysfunction via enhancing proliferation and inhibiting mitochondria-dependent apoptosis pathway in high-fat diet and streptozotocin-induced diabetic rats. Reprod. Biol. Endocrinol. 2021, 19, 168. [Google Scholar] [CrossRef]
- Ujah, G.A.; Nna, V.U.; Suleiman, J.B.; Eleazu, C.; Nwokocha, C.; Rebene, J.A.; Imowo, M.U.; Obi, E.O.; Amachree, C.; Udechukwu, E.C.; et al. Tert-butylhydroquinone attenuates doxorubicin-induced dysregulation of testicular cytoprotective and steroidogenic genes, and improves spermatogenesis in rats. Sci. Rep. 2021, 11, 5522. [Google Scholar] [CrossRef]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J. Cell Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Lv, X.; Zhou, X.; Zhao, W.; Meng, L.; Xu, H.; Zhu, S.; Wang, Y. Doxorubicin-Induced Cardiotoxicity Through SIRT1 Loss Potentiates Overproduction of Exosomes in Cardiomyocytes. Int. J. Mol. Sci. 2024, 25, 12376. [Google Scholar] [CrossRef]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Nagamori, I.; Williams, E.O.; Del Rosario, A.M.; Bryson, B.D.; Watson, N.; White, F.M.; Sassone-Corsi, P.; Guarente, L. SirT1 is required in the male germ cell for differentiation and fecundity in mice. Development 2014, 141, 3495–3504. [Google Scholar] [CrossRef]
- Khawar, M.B.; Liu, C.; Gao, F.; Gao, H.; Liu, W.; Han, T.; Wang, L.; Li, G.; Jiang, H.; Li, W. Sirt1 regulates testosterone biosynthesis in Leydig cells via modulating autophagy. Protein Cell 2021, 12, 67–75. [Google Scholar] [CrossRef]
- Khawar, M.B.; Sohail, A.M.; Li, W. SIRT1: A Key Player in Male Reproduction. Life 2022, 12, 318. [Google Scholar] [CrossRef]
- Khodavysi, M.; Kheiripour, N.; Ghasemi, H.; Soleimani-Asl, S.; Jouzdani, A.F.; Sabahi, M.; Ganji, Z.; Azizi, Z.; Ranjbar, A. How can nanomicelle-curcumin modulate aluminum phosphideinduced neurotoxicity?: Role of SIRT1/FOXO3 signaling pathway. AIMS Neurosci. 2023, 10, 56–74. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Lin, Y.; Yi, W.; Liang, Z.; Yan, J.; Wang, N.; Deng, C.; Zhang, S.; Li, Y.; et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free. Radic. Biol. Med. 2013, 65, 667–679. [Google Scholar] [CrossRef]
- Alam, F.; Syed, H.; Amjad, S.; Baig, M.; Khan, T.A.; Rehman, R. Interplay between oxidative stress, SIRT1, reproductive and metabolic functions. Curr. Res. Physiol. 2021, 4, 119–124. [Google Scholar] [CrossRef]
- Podyacheva, E.; Shmakova, T.; Kushnareva, E.; Onopchenko, A.; Martynov, M.; Andreeva, D.; Toropov, R.; Cheburkin, Y.; Levchuk, K.; Goldaeva, A.; et al. Modeling doxorubicin-induced cardiomyopathy with fibrotic myocardial damage in Wistar rats. Cardiol. Res. 2022, 13, 339. [Google Scholar] [CrossRef]
- Omar, N.N.; Mosbah, R.A.; Sarawi, W.S.; Rashed, M.M.; Badr, A.M. Rifaximin Protects against Malathion-Induced Rat Testicular Toxicity: A Possible Clue on Modulating Gut Microbiome and Inhibition of Oxidative Stress by Mitophagy. Molecules 2022, 27, 4069. [Google Scholar] [CrossRef]
- Preuss, H.G.; Jarrell, S.T.; Scheckenbach, R.; Lieberman, S.; Anderson, R.A. Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr. 1998, 17, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Marklund, S.L. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1985, 148, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Grisham, M.B.; Johnson, G.G.; Lancaster, J.R., Jr. Quantitation of nitrate and nitrite in extracellular fluids. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 237–246. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Belhan, S.; Yıldırım, S.; Huyut, Z.; Özdek, U.; Oto, G.; Algül, S. Effects of curcumin on sperm quality, lipid profile, antioxidant activity and histopathological changes in streptozotocin-induced diabetes in rats. Andrologia 2020, 52, e13584. [Google Scholar] [CrossRef]
- Sudjarwo, S.A.; Sudjarwo, G.W. Protective effect of curcumin on lead acetate-induced testicular toxicity in Wistar rats. Res. Pharm. Sci. 2017, 12, 381–390. [Google Scholar] [CrossRef]
- Asanga, E.E.; Okokon, J.E.; Joseph, A.P.; Ekeleme, C.M.; Ilechukwu, S.B.; Anagboso, M.O.; Umoh, M.; Raymond, A.-E.M. The attenuation of doxorubicin-induced testicular toxicity with improved testicular histoarchitecture of mice by the bioactive compounds in Solanum anomalum leaves: Experimental and computational studies. Toxicol. Rep. 2024, 13, 101827. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Salah EL-Din, E.Y.; El-Dakdoky, M.H.; Ahmed, T.A. The impact of mesenchymal stem cells on doxorubicin-induced testicular toxicity and progeny outcome of male prepubertal rats. Birth Defects Res. 2019, 111, 906–919. [Google Scholar] [CrossRef]
- Salimi, A.; Kheiripour, N.; Fathi Jouzdani, A.; Ghasemi, H.; Soleimani Asl, S.; Ghafouri-Khosrowshahi, A.; Ranjbar, A. Nanocurcumin Improves Lipid Status, Oxidative Stress, and Function of the Liver in Aluminium Phosphide-Induced Toxicity: Cellular and Molecular Mechanisms. Biomed. Res. Int. 2022, 2022, 7659765. [Google Scholar] [CrossRef]
- El-Desoky, G.E.; Wabaidur, S.M.; AlOthman, Z.A.; Habila, M.A. Regulatory role of nano-curcumin against tartrazine-induced oxidative stress, apoptosis-related genes expression, and genotoxicity in rats. Molecules 2020, 25, 5801. [Google Scholar] [CrossRef]
- Alqrad, M.A.I.; El-Agamy, D.S.; Ibrahim, S.R.M.; Sirwi, A.; Abdallah, H.M.; Abdel-Sattar, E.; El-Halawany, A.M.; Elsaed, W.M.; Mohamed, G.A. SIRT1/Nrf2/NF-κB Signaling Mediates Anti-Inflammatory and Anti-Apoptotic Activities of Oleanolic Acid in a Mouse Model of Acute Hepatorenal Damage. Medicina 2023, 59, 1351. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Fikry, E.M.; Alsufyani, S.E.; Ashour, A.M.; El-Sheikh, A.A.K.; Darwish, H.W.; Al-Hossaini, A.M.; Saad, M.A.; Al-Shorbagy, M.Y.; Eid, A.H. Stimulation of Autophagy by Dapagliflozin Mitigates Cadmium-Induced Testicular Dysfunction in Rats: The Role of AMPK/mTOR and SIRT1/Nrf2/HO-1 Pathways. Pharmaceuticals 2023, 16, 1006. [Google Scholar] [CrossRef] [PubMed]
- Hamidie, R.D.R.; Patriasih, R.; Sulastri, A. Potential of Nanocurcumin on Cytokine Storm through Decreased IL-6 and TNF-a Expression. Malays. J. Med. Health Sci. 2021, 17, 115–119. [Google Scholar]
- Farag, M.R.; Moselhy, A.A.A.; El-Mleeh, A.; Aljuaydi, S.H.; Ismail, T.A.; Di Cerbo, A.; Crescenzo, G.; Abou-Zeid, S.M. Quercetin alleviates the immunotoxic impact mediated by oxidative stress and inflammation induced by doxorubicin exposure in rats. Antioxidants 2021, 10, 1906. [Google Scholar] [CrossRef]
- Aly, H.A.A.; Eid, B.G. Cisplatin induced testicular damage through mitochondria mediated apoptosis, inflammation and oxidative stress in rats: Impact of resveratrol. Endocr. J. 2020, 67, 969–980. [Google Scholar] [CrossRef]
- Abdel Fattah, H.S.; Omar, E.M. The protective role of curcumin nanoparticles on the submandibular salivary gland toxicity induced by methotrexate in male rats. Arch. Oral Biol. 2023, 152, 105717. [Google Scholar] [CrossRef]
- Theas, M.S. Germ cell apoptosis and survival in testicular inflammation. Andrologia 2018, 50, e13083. [Google Scholar] [CrossRef]
- Kim, J.M.; Ghosh, S.R.; Weil, A.C.P.; Zirkin, B.R. Caspase-3 and caspase-activated deoxyribonuclease are associated with testicular germ cell apoptosis resulting from reduced intratesticular testosterone. Endocrinology 2001, 142, 3809–3816. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Hosny, E.N.; Mohammed, H.S. Protective effect of nanocurcumin against neurotoxicity induced by doxorubicin in rat’s brain. Neurotoxicology 2021, 85, 1–9. [Google Scholar] [CrossRef]



| Gene | Primers (5′-3′) | Size (bp) |
|---|---|---|
| BCL-2 | F: ACTCTTCAGGGATGGGGTGA R: TGACATCTCCCTGTTGACGC | 94 |
| Bax | F: AGGACGCATCCACCAAGAAG R: CAGTTGAAGTTGCCGTCTGC | 166 |
| Caspase-3 | F: GGAGCTTGGAACGCGAAGAA R: ACACAAGCCCATTTCAGGGT | 169 |
| β-actin | F: AGGAGTACGATGAGTCCGGC R: CGCAGCTCAGTAACAGTCCG | 1140 |
| Groups | Initial Body Weight (gm) | Final Body Weight (gm) | Testes Weight/Final B Wt. (g/100 gm B Wt) |
|---|---|---|---|
| Control | 186 ± 3.7 | 255 ± 8.7 | 0.94 ± 0.03 |
| NCR | 189 ± 2.9 | 207 ± 16.5 | 0.95 ± 0.01 |
| DOX | 196 ± 2.8 | 167 ± 3.3 *** | 0.82 ± 0.04 |
| DOX+NCR | 192 ± 3.2 | 205 ± 4.8 ** π | 0.88 ± 0.02 |
| Groups | Percentage of Tubules | ||
|---|---|---|---|
| Normal | Detached and Sloughed | Vacuolated | |
| Control | 84.78 ± 3.8 | 2.5 ± 0.07 | 2.67 ± 0.045 |
| NCR | 92.4 ± 2.09 | 2.67 ± 0.04 | 3.02 ± 0.02 |
| DOX | 12.5 ± 1.56 **** | 66.89 ± 4.8 **** | 47.7 ± 2.4 **** |
| DOX+NCR | 44.6 ± 3.7 π π π **** | 26.8 ± 4.6 π π π **** | 17.7 ± 4.09 π π π **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshuwayer, N.A.; Alqahtani, Q.H.; Hussein, M.H.; Mohammed, R.; Siyal, A.; Hasan, I.H. Nano-Curcumin Mitigates Doxorubicin-Induced Reproductive Toxicity via Antioxidant, Anti-Apoptosis, and SIRT1-Modulating Effects in Rat Model. Toxics 2025, 13, 574. https://doi.org/10.3390/toxics13070574
Alshuwayer NA, Alqahtani QH, Hussein MH, Mohammed R, Siyal A, Hasan IH. Nano-Curcumin Mitigates Doxorubicin-Induced Reproductive Toxicity via Antioxidant, Anti-Apoptosis, and SIRT1-Modulating Effects in Rat Model. Toxics. 2025; 13(7):574. https://doi.org/10.3390/toxics13070574
Chicago/Turabian StyleAlshuwayer, Noha A., Qamraa H. Alqahtani, Marwa H. Hussein, Raeesa Mohammed, Abdulaziz Siyal, and Iman H. Hasan. 2025. "Nano-Curcumin Mitigates Doxorubicin-Induced Reproductive Toxicity via Antioxidant, Anti-Apoptosis, and SIRT1-Modulating Effects in Rat Model" Toxics 13, no. 7: 574. https://doi.org/10.3390/toxics13070574
APA StyleAlshuwayer, N. A., Alqahtani, Q. H., Hussein, M. H., Mohammed, R., Siyal, A., & Hasan, I. H. (2025). Nano-Curcumin Mitigates Doxorubicin-Induced Reproductive Toxicity via Antioxidant, Anti-Apoptosis, and SIRT1-Modulating Effects in Rat Model. Toxics, 13(7), 574. https://doi.org/10.3390/toxics13070574






