Presence of Thioxanthones and Their Metabolites in Human Urine and Human Exposure Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Chemicals
2.2. Study Participants and Urine Sample Collection
2.3. Extraction of Human Urine Samples
2.4. Instrumental Analysis
2.5. Human Daily Excretion Estimation
2.6. QA/QC
2.7. Statistical Analysis
3. Results and Discussion
3.1. TXs in Human Urine
3.2. Metabolites of 2-ITX and DETX in Human Urine
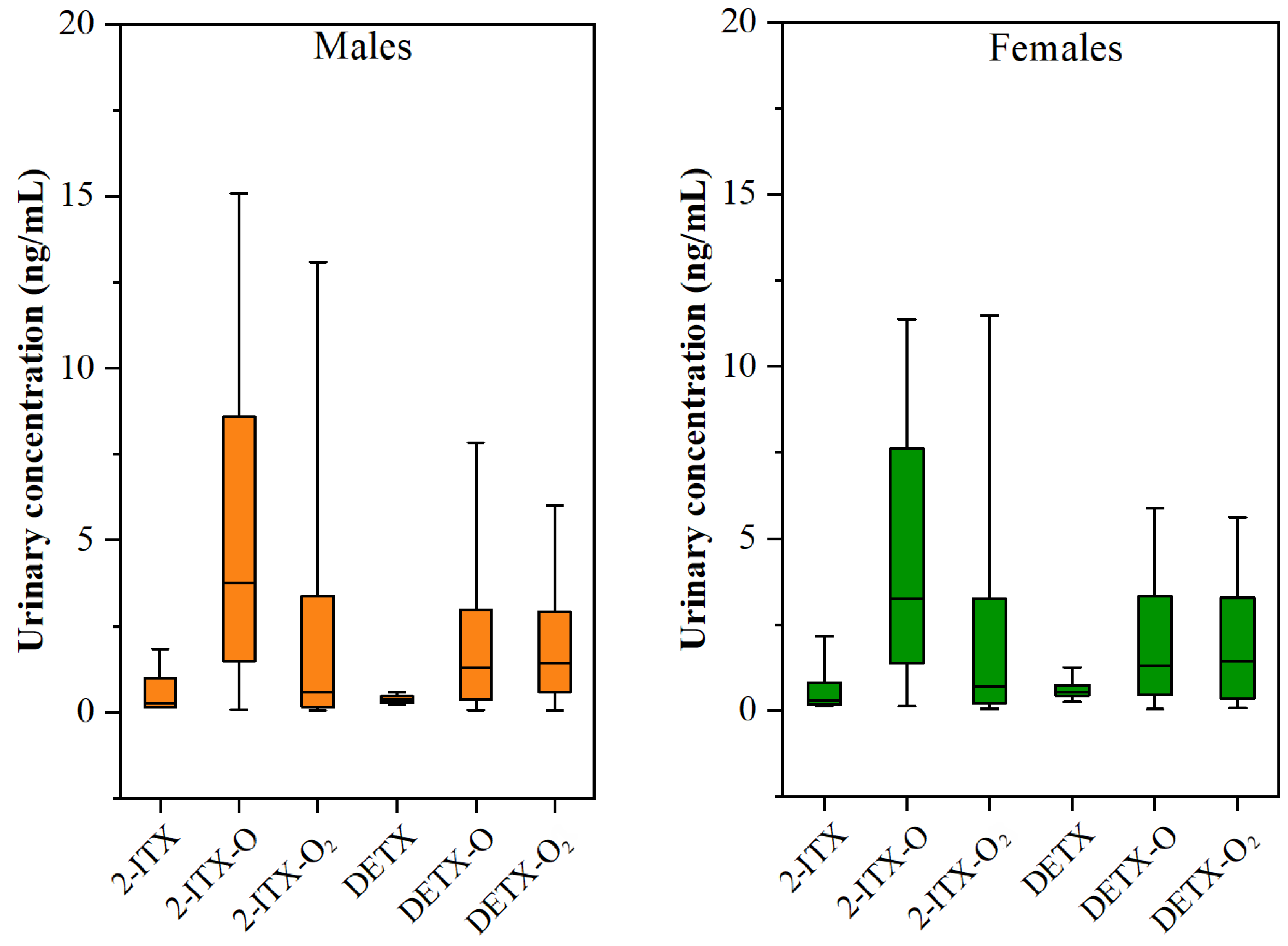
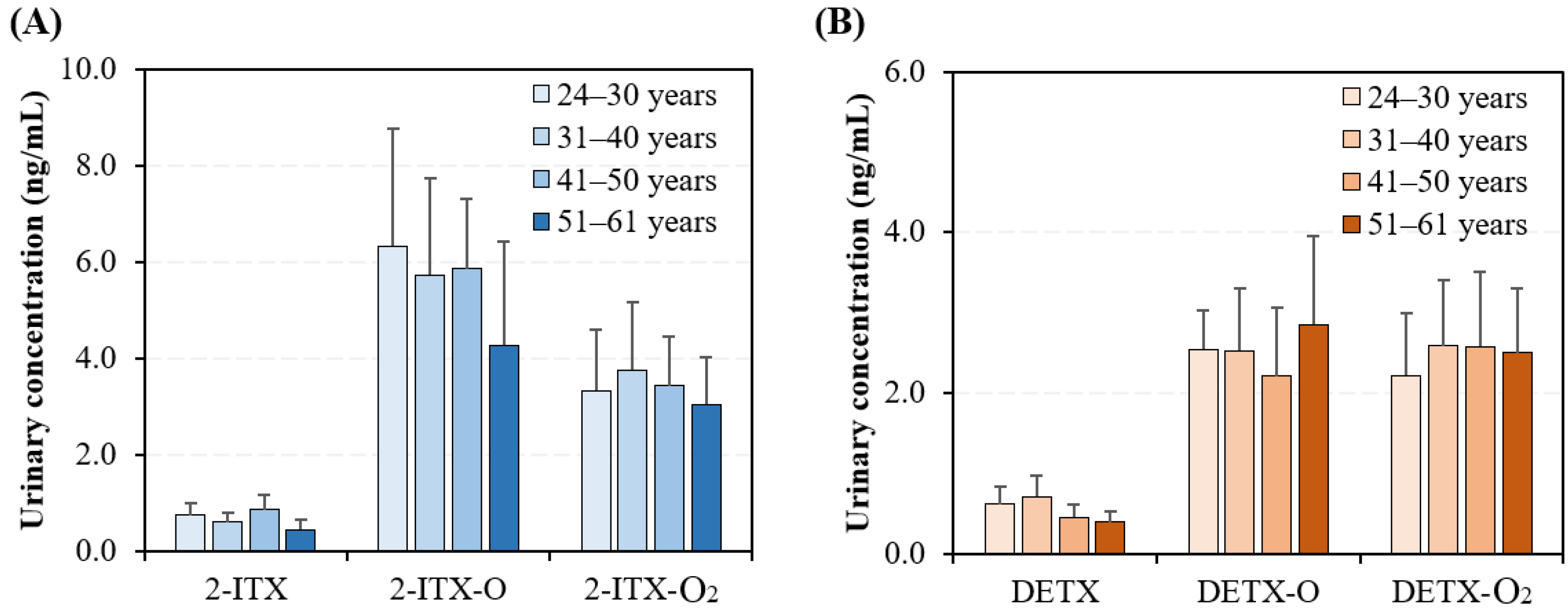
3.3. Gender-Specific and Age-Specific Differences
3.4. Daily Exposure (DE) to 2-Cl-TX and DETX
3.5. Limitations of This Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagtap, A.; More, A. A review on self-initiated and photoinitiator-free system for photopolymerization. Polym. Bull. 2022, 79, 8057–8091. [Google Scholar] [CrossRef]
- Vazquez-Martel, C.; Mainik, P.; Blasco, E. Natural and naturally derived photoinitiating systems for light-based 3D printing. Org. Mater. 2022, 4, 281–291. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on perylene-based photoinitiators of polymerization. Eur. Polym. J. 2021, 159, 110734. [Google Scholar] [CrossRef]
- Müller, S.M.; Schlögl, S.; Wiesner, T.; Haas, M.; Griesser, T. Recent advances in type I photoinitiators for visible light induced photopolymerization. ChemPhotoChem 2022, 6, e202200091. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Lalevée, J. Photochemical production of interpenetrating polymer networks; simultaneous initiation of radical and cationic polymerization reactions. Polymers 2014, 6, 2588–2610. [Google Scholar] [CrossRef]
- Azani, M.-R.; Hassanpour, A. UV-Curable Polymer Nanocomposites: Material Selection, Formulations, and Recent Advances. J. Compos. Sci. 2024, 8, 441. [Google Scholar] [CrossRef]
- Yildiz, Z.; Kocak, E.D. Emerging Applications of Photocurable Polymers. In Specialty Polymers; CRC Press: Boca Raton, FL, USA, 2023; pp. 97–111. [Google Scholar]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A review on modeling cure kinetics and mechanisms of photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef]
- Jin, Z.Y. Adcance in photointiators. Image Technol. 2011, 23, 8–18. [Google Scholar]
- Liu, R.; Lin, Y.; Hu, F.; Liu, R.; Ruan, T.; Jiang, G. Observation of emerging photoinitiator additives in household environment and sewage sludge in China. Environ. Sci. Technol. 2016, 50, 97–104. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Gao, X.; Liu, L.; Shen, M.; Chen, H.; Zhu, M.; Zeng, L.; Zeng, E.Y. Occurrence of multiple classes of emerging photoinitiators in indoor dust from E-waste recycling facilities and adjacent communities in South China and implications for human exposure. Environ. Int. 2020, 136, 105462. [Google Scholar] [CrossRef]
- Ji, X.; Liang, J.; Liu, J.; Shen, J.; Li, Y.; Wang, Y.; Jing, C.; Mabury, S.A.; Liu, R. Occurrence, fate, human exposure, and toxicity of commercial photoinitiators. Environ. Sci. Technol. 2023, 57, 11704–11717. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chen, P.; Fu, H.; Li, Y.; Li, L.; Li, W.; Song, S.; Xu, R.; Pan, Q.; Xin, Y. Single-component thioxanthone-based photoinitiator for free-radical photopolymerization of acrylates via UV-LED irradiation. Dye. Pigment. 2025, 235, 112640. [Google Scholar] [CrossRef]
- Macherey, M.; Schuhmacher-Wolz, U.; Belz, H.; Delbanco, E.H.; Mohr, K.; Gude, T.; Kaiser, E. Curing of UV prints–Assessment of possible toxicological hazard for consumers. Regul. Toxicol. Pharmacol. 2021, 124, 104965. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-D.; Hong, J.-W. Photo-curing kinetics for the UV-initiated cationic polymerization of a cycloaliphatic diepoxide system photosensitized by thioxanthone. Eur. Polym. J. 2005, 41, 367–374. [Google Scholar] [CrossRef]
- Hola, E.; Pilch, M.; Ortyl, J. Thioxanthone derivatives as a new class of organic photocatalysts for photopolymerisation processes and the 3D printing of photocurable resins under visible light. Catalysts 2020, 10, 903. [Google Scholar] [CrossRef]
- Qian, C.; Bai, L.; Wang, W.; Luo, Y.; Li, J.; Wang, Y. Occurrences and migration characteristics of photoinitiators in paper food packaging: Implication for human exposure. J. Environ. Sci. 2024, S1001074224004765. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. First detection of photoinitiators and metabolites in human sera from United States donors. Environ. Sci. Technol. 2018, 52, 10089–10096. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Identification of photoinitiators, including novel phosphine oxides, and their transformation products in food packaging materials and indoor dust in Canada. Environ. Sci. Technol. 2019, 53, 4109–4118. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Ji, X.; Liang, J.; Feng, X.; Liu, X.; Wang, Y.; Zhang, Q.; Zhang, Q.; Qu, G. Nail salon dust reveals alarmingly high photoinitiator levels: Assessing occupational risks. J. Hazard. Mater. 2024, 475, 134913. [Google Scholar] [CrossRef]
- Reitsma, M.; Bovee, T.F.; Peijnenburg, A.A.; Hendriksen, P.J.; Hoogenboom, R.L.; Rijk, J.C. Endocrine-disrupting effects of thioxanthone photoinitiators. Toxicol. Sci. 2013, 132, 64–74. [Google Scholar] [CrossRef]
- Zhan, T.; Pan, L.; Liu, Z.; Chen, J.; Ge, Z.; Lu, L.; Zhang, X.; Cui, S.; Zhang, C.; Liu, W. Metabolic susceptibility of 2-chlorothioxanthone and its toxic effects on mRNA and protein expression and activities of human CYP1A2 and CYP3A4 enzymes. Environ. Sci. Technol. 2018, 52, 11904–11912. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Inomata, A.; Moriyasu, T.; Suzuki, T. Cytotoxic effects of thioxanthone derivatives as photoinitiators on isolated rat hepatocytes. J. Appl. Toxicol. 2020, 40, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Genuis, S.J.; Beesoon, S.; Birkholz, D.; Lobo, R.A. Human excretion of bisphenol A: Blood, urine, and sweat (BUS) study. J. Environ. Public Health 2012, 2012, 185731. [Google Scholar] [CrossRef]
- Masereeuw, R.; Mutsaers, H.A.; Toyohara, T.; Abe, T.; Jhawar, S.; Sweet, D.H.; Lowenstein, J. The kidney and uremic toxin removal: Glomerulus or tubule? In Seminars in Nephrology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 191–208. [Google Scholar]
- Lee, E.J.; Arbuckle, T.E. Urine-sampling methods for environmental chemicals in infants and young children. J. Expo. Sci. Environ. Epidemiol. 2009, 19, 625–633. [Google Scholar] [CrossRef]
- Elliott, P.; Peakman, T.C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef]
- Tasoglu, S. Toilet-based continuous health monitoring using urine. Nat. Rev. Urol. 2022, 19, 219–230. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental chemicals in urine and blood: Improving methods for creatinine and lipid adjustment. Environ. Health Persp. 2016, 124, 220–227. [Google Scholar] [CrossRef]
- Alves, A.; Kucharska, A.; Erratico, C.; Xu, F.; Den Hond, E.; Koppen, G.; Vanermen, G.; Covaci, A.; Voorspoels, S. Human biomonitoring of emerging pollutants through non-invasive matrices: State of the art and future potential. Anal. Bioanal. Chem. 2014, 406, 4063–4088. [Google Scholar] [CrossRef]
- Maitre, L.; Robinson, O.; Martinez, D.; Toledano, M.B.; Ibarluzea, J.; Marina, L.S.; Sunyer, J.; Villanueva, C.M.; Keun, H.C.; Vrijheid, M.; et al. Urine Metabolic Signatures of Multiple Environmental Pollutants in Pregnant Women: An Exposome Approach. Environ. Sci. Technol. 2018, 52, 13469–13480. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Liu, F.; Yu, G. Occurrence and Removal of Pharmaceutical Contaminants in Urine: A Review. Water 2023, 15, 1517. [Google Scholar] [CrossRef]
- Li, J.; Lam, J.C.; Li, W.; Du, B.; Chen, H.; Zeng, L. Occurrence and distribution of photoinitiator additives in paired maternal and cord plasma in a South China population. Environ. Sci. Technol. 2019, 53, 10969–10977. [Google Scholar] [CrossRef] [PubMed]
- Loskutov, V.; Shelkovnikov, V. Synthesis of hexafluorophosphates of 9-oxo-10-(4-heptoxyphenyl) thioxanthenium. Russ. J. Org. Chem. 2006, 42, 298–301. [Google Scholar] [CrossRef]
- Jala, A.; Varghese, B.; Dutta, R.; Adela, R.; Borkar, R.M. Levels of parabens and bisphenols in personal care products and urinary concentrations in Indian young adult women: Implications for human exposure and health risk assessment. Chemosphere 2022, 297, 134028. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, R.; Liang, W.; Wei, S.; Zhou, Y.; Wang, Z.; Lan, L.; Chen, J.; Zeng, F. Large-scale biomonitoring of bisphenol analogues and their metabolites in human urine from Guangzhou, China: Implications for health risk assessment. Chemosphere 2023, 338, 139601. [Google Scholar] [CrossRef]
- Hyland, C.; Kogut, K.; Gunier, R.B.; Castorina, R.; Curl, C.; Eskenazi, B.; Bradman, A. Organophosphate pesticide dose estimation from spot and 24-hr urine samples collected from children in an agricultural community. Environ. Int. 2021, 146, 106226. [Google Scholar] [CrossRef]
- Frederiksen, H.; Kranich, S.K.; Jørgensen, N.; Taboureau, O.; Petersen, J.H.; Andersson, A.-M. Temporal variability in urinary phthalate metabolite excretion based on spot, morning, and 24-h urine samples: Considerations for epidemiological studies. Environ. Sci. Technol. 2013, 47, 958–967. [Google Scholar] [CrossRef]
- Qu, J.; Mao, W.; Chen, M.; Jin, H. Prediction of p-Phenylenediamine Antioxidant Concentrations in Human Urine Using Machine Learning Models. J. Hazard. Mater. 2025, 487, 137184. [Google Scholar] [CrossRef]
- Li, Z.M.; Kannan, K. Determination of 1,3-Diphenylguanidine, 1,3-Di-o-tolylguanidine, and 1,2,3-Triphenylguanidine in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Environ. Sci. Technol. 2023, 57, 8883–8889. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Mu, Y.; He, Y.; Qiu, T.; Li, W.; Zeng, L. Determination of 21 photoinitiators in human plasma by using high-performance liquid chromatography coupled with tandem mass spectrometry: A systemically validation and application in healthy volunteers. J. Chromatogr. A 2021, 1643, 462079. [Google Scholar] [CrossRef]
- Shen, J.; Ji, X.; Liang, J.; Feng, X.; Liu, X.; Wang, Y.; Zhang, Q.; Zhang, Q.; Qu, G.; Yan, B. Elevated Levels of Photoinitiators in Nail Salon Workers’ Hand Wipes and Occupational Risk Estimation. Environ. Sci. Technol. Lett. 2024, 11, 1082–1089. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; James-Todd, T.; Sun, Q. Urinary polycyclic aromatic hydrocarbon excretion and regional body fat distribution: Evidence from the US National Health and Nutrition Examination Survey 2001–2016. Environ. Health 2022, 21, 75. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.N.; Curhan, G.C. Body size and 24-hour urine composition. Am. J. Kidney Dis. 2006, 48, 905–915. [Google Scholar] [CrossRef]
- Galloway, T.; Cipelli, R.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; Corsi, A.M.; Money, C.; McCormack, P.; Melzer, D. Daily bisphenol A excretion and associations with sex hormone concentrations: Results from the InCHIANTI adult population study. Environ. Health Perspect. 2010, 118, 1603–1608. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, D.E.; Newell, K.M. Changing complexity in human behavior and physiology through aging and disease. Neurobiol. Aging 2002, 23, 1–11. [Google Scholar] [CrossRef]
- Amarya, S.; Singh, K.; Sabharwal, M. Ageing process and physiological changes. Gerontology 2018, 32, 137–144. [Google Scholar]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Cao, W.J.; Chen, C.S.; Hua, Y.; Li, Y.M.; Xu, Y.Y.; Hua, Q.Z. Factor analysis of a health-promoting lifestyle profile (HPLP): Application to older adults in Mainland China. Arch. Gerontol. Geriatr. 2012, 55, 632–638. [Google Scholar] [CrossRef]


| Detection Frequency | |||||||
|---|---|---|---|---|---|---|---|
| Mean | Median | Min | 25th | 75th | Max | ||
| 2-ITX | 82% | 0.66 | 0.72 | <LOD | 0.17 | 0.93 | 3.1 |
| 2-ITX-O | 76% | 5.5 | 3.8 | <LOD | 1.3 | 7.8 | 29 |
| 2-ITX-O2 | 77% | 3.4 | 2.9 | <LOD | 0.19 | 12 | 27 |
| DETX | 79% | 0.51 | 0.45 | <LOD | 0.29 | 3.9 | 5.9 |
| DETX-O | 82% | 2.5 | 1.9 | <LOD | 0.37 | 8.4 | 18 |
| DETX-O2 | 85% | 2.4 | 2.4 | <LOD | 0.45 | 4.3 | 18 |
| 2-TF-TX | 19% | NC a | <LOD | <LOD | <LOD | <LOD | 8.6 |
| 2-Cl-TX | 8.1% | NC a | <LOD | <LOD | <LOD | <LOD | 2.9 |
| TX | 5.1% | NC a | <LOD | <LOD | <LOD | <LOD | 0.60 |
| Mean | Median | Min | 25th | 75th | Max | |
|---|---|---|---|---|---|---|
| All participants | ||||||
| 2-ITX | 240 | 176 | <1.0 | 51 | 324 | 915 |
| DETX | 151 | 147 | <1.3 | 19 | 295 | 732 |
| Female participants | ||||||
| 2-ITX | 248 | 195 | <1.0 | 50 | 347 | 915 |
| DETX | 158 | 152 | <1.3 | 20 | 302 | 731 |
| Male participants | ||||||
| 2-ITX | 219 | 192 | <1.2 | 44 | 284 | 870 |
| DETX | 148 | 144 | <1.3 | 14 | 328 | 708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Zhang, L.; Zhou, L.; Ren, F.; Jin, H.; Wu, X. Presence of Thioxanthones and Their Metabolites in Human Urine and Human Exposure Assessment. Toxics 2025, 13, 535. https://doi.org/10.3390/toxics13070535
Gao L, Zhang L, Zhou L, Ren F, Jin H, Wu X. Presence of Thioxanthones and Their Metabolites in Human Urine and Human Exposure Assessment. Toxics. 2025; 13(7):535. https://doi.org/10.3390/toxics13070535
Chicago/Turabian StyleGao, Lin, Ling Zhang, Lisha Zhou, Fangfang Ren, Hangbiao Jin, and Xiaoyu Wu. 2025. "Presence of Thioxanthones and Their Metabolites in Human Urine and Human Exposure Assessment" Toxics 13, no. 7: 535. https://doi.org/10.3390/toxics13070535
APA StyleGao, L., Zhang, L., Zhou, L., Ren, F., Jin, H., & Wu, X. (2025). Presence of Thioxanthones and Their Metabolites in Human Urine and Human Exposure Assessment. Toxics, 13(7), 535. https://doi.org/10.3390/toxics13070535






