Trace Metal and Metalloid Profiles in Hair Samples from Children in the Oil-Producing Region of Kazakhstan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Issues
2.3. Data Collection
2.4. Statistical Methods
3. Results
3.1. Content of Toxic Elements in the Scalp Hair of Children from Western Kazakhstan
3.2. Associations of Children’s Hair Toxic Element Level with Age and Gender
3.3. Association Between Distance to Oil and Gas Sites and Hair Levels of Toxic Trace Elements
4. Discussion
- As a cross-sectional study, the present research does not permit the assessment of changes over time.
- The contribution of food consumption to the elemental composition of hair has not been assessed, including the impact of fish and cereal consumption, which may influence cadmium and mercury levels in hair.
- The content of toxic elements in soil, water, and air was not assessed.
- This study did not include an assessment of toxic element concentrations in other biological matrices like blood or urine.
- The capacity of oil and gas fields and, accordingly, the intensity of pollution produced by them were not taken into account.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICP-MS | inductively coupled plasma mass spectrometry |
| BMI | body mass index |
| AM | arithmetic mean; |
| GM | geometric mean |
References
- Peña-Fernández, A.; González-Muñoz, M.J.; Lobo-Bedmar, M.C. Evaluating the effect of age and area of residence in the metal and metalloid contents in human hair and urban topsoils. Environ. Sci. Pollut. Res. 2016, 23, 21299–21312. [Google Scholar] [CrossRef] [PubMed]
- Nurkassimova, M.; Omarova, N.; Yushin, N.; Grozdow, D.; Vergel, K.; Zinicovscaia, I. Assessment of air pollution in South Kazakhstan using moss (Hylocomium splendens) biomonitoring technique and neutron activation analysis. J. Radioanal. Nucl. Chem. 2024, 333, 4367–4376. [Google Scholar] [CrossRef]
- Netalieva, I.; Wesseler, J.; Heijman, W. Health costs caused by oil extraction air emissions and the benefits from abatement: The case of Kazakhstan. Energy Policy 2005, 33, 1169–1177. [Google Scholar] [CrossRef]
- Hasan, M.Y.; Kosanovic, M.; Fahim, M.A.; Adem, A.; Petroianu, G. Trace metal profiles in hair samples from children in urban and rural regions of the United Arab Emirates. Vet. Hum. Toxicol. 2004, 46, 119–121. [Google Scholar]
- Peña-Fernández, A.; González-Muñoz, M.J.; Lobo-Bedmar, M.C. Establishing the importance of human health risk assessment for metals and metalloids in urban environments. Environ. Int. 2014, 72, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Molina-Villalba, I.; Lacasaña, M.; Rodríguez-Barranco, M.; Hernández, A.F.; Gonzalez-Alzaga, B.; Aguilar-Garduño, C.; Gil, F. Biomonitoring of arsenic, cadmium, lead, manganese and mercury in urine and hair of children living near mining and industrial areas. Chemosphere 2015, 124, 83–91. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Pietris, G.; Mazzei, C.; Marconi, E.; Canepari, S. Element Levels and Predictors of Exposure in the Hair of Ethiopian Children. Int. J. Environ. Res. Public Health 2020, 17, 8652. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). The Priority List of Hazardous Substances; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2022. Available online: https://www.atsdr.cdc.gov/programs/substance-priority-list.html (accessed on 16 February 2025).
- Zhou, C.C.; Gao, Z.Y.; He, Y.Q.; Wu, M.Q.; Chen, F.; Wang, J.; Liu, J.X.; Yan, C.H. Effects of lead, mercury, aluminium and manganese co-exposure on the serum BDNF concentration of pre-school children in Taizhou, China. Chemosphere 2019, 217, 158–165. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Boezen, H.M.; Huo, X. Children with health impairments by heavy metals in an e-waste recycling area. Chemosphere 2016, 148, 408–415. [Google Scholar] [CrossRef]
- Tietz, T.; Lenzner, A.; Kolbaum, A.E.; Zellmer, S.; Riebeling, C.; Gürtler, R.; Jung, C.; Kappenstein, O.; Tentschert, J.; Giulbudagian, M.; et al. Aggregated aluminium exposure: Risk assessment for the general population. Arch. Toxicol. 2019, 93, 3503–3521. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Saili, K.S.; Zurlinden, T.J.; Schwab, A.J.; Silvin, A.; Baker, N.C.; Hunter, E.S., 3rd; Ginhoux, F.; Knudsen, T.B. Blood-brain barrier development: Systems modeling and predictive toxicology. Birth Defects Res. 2017, 109, 1680–1710. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, R.; Estevan, C.; Estévez, J.; Alcaide, C.; Sogorb, M.A.; Vilanova, E. Reference Values on Children’s Hair for 28 Elements (Heavy Metals and Essential Elements) Based on a Pilot Study in a Representative Non-Contaminated Local Area. Int. J. Mol. Sci. 2023, 24, 8127. [Google Scholar] [CrossRef] [PubMed]
- Moya, J.; Bearer, C.F.; Etzel, R.A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 2004, 113 (Suppl. S4), 996–1006. [Google Scholar] [CrossRef]
- Xue, J.; Zartarian, V.; Tulve, N.; Moya, J.; Freeman, N.; Auyeung, W.; Beamer, P. A meta-analysis of children’s object-to-mouth frequency data for estimating non-dietary ingestion exposure. J. Expo. Sci. Environ. Epidemiol. 2010, 20, 536–545. [Google Scholar] [CrossRef]
- Moyebi, O.D.; Lebbie, T.; Carpenter, D.O. Standards for levels of lead in soil and dust around the world. Rev. Environ. Health 2024, 40, 185–196. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.B.; Jung, H.J.; Chung, M.; Park, S.E.; Lee, K.H.; Kim, W.S.; Moon, J.H.; Lee, J.W.; Shim, J.W.; et al. Environmental Health Committee of the Korean Pediatric Society. Protecting our future: Environmental hazards and children’s health in the face of environmental threats: A comprehensive overview. Clin. Exp. Pediatr. 2024, 67, 589–598. [Google Scholar] [CrossRef]
- Callan, A.C.; Winters, M.; Barton, C.; Boyce, M.; Hinwood, A.L. Children’s exposure to metals: A community-initiated study. Arch. Environ. Contam. Toxicol. 2012, 62, 714–722. [Google Scholar] [CrossRef][Green Version]
- Varrica, D.; Tamburo, E.; Dongarrà, G.; Sposito, F. Trace elements in scalp hair of children chronically exposed to volcanic activity (Mt. Etna, Italy). Sci. Total. Environ. 2014, 470–471, 117–126. [Google Scholar] [CrossRef]
- Barbosa, F., Jr.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef]
- Kosanovic, M.; Jokanovic, M. Quantitative analysis of toxic and essential elements in human hair. Clinical validity of results. Environ. Monit. Assess. 2011, 174, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Peña-Fernández, A.; González-Muñoz, M.J.; Lobo-Bedmar, M.C. "Reference values" of trace elements in the hair of a sample group of Spanish children (aged 6-9 years)—Are urban topsoils a source of contamination? Environ. Toxicol. Pharmacol. 2014, 38, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Varrica, D.; Tamburo, E.; Milia, N.; Vallascas, E.; Cortimiglia, V.; De Giudici, G.; Dongarrà, G.; Sanna, E.; Monna, F.; Losno, R. Metals and metalloids in hair samples of children living near the abandoned mine sites of Sulcis-Inglesiente (Sardinia, Italy). Environ. Res. 2014, 134, 366–374. [Google Scholar] [CrossRef]
- Ballesteros, M.T.L.; Serrano, I.N.; Álvarez, S.I. Reference levels of trace elements in hair samples from children and adolescents in Madrid, Spain. J. Trace Elements Med. Biol. 2017, 43, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Zhukovskaya, E.V.; Kireeva, G.N.; Skalnaya, M.G.; Grabeklis, A.R.; Radysh, I.V.; Shakieva, R.A.; Nikonorov, A.A.; Tinkov, A.A. Whole blood and hair trace elements and minerals in children living in metal-polluted area near copper smelter in Karabash, Chelyabinsk region, Russia. Environ. Sci. Pollut. Res. 2018, 25, 2014–2020. [Google Scholar] [CrossRef]
- Esplugas, R.; Mari, M.; Marquès, M.; Schuhmacher, M.; Domingo, J.L.; Nadal, M. Biomonitoring of Trace Elements in Hair of Schoolchildren Living Near a Hazardous Waste Incinerator-A 20 Years Follow-Up. Toxics 2019, 7, 52. [Google Scholar] [CrossRef]
- Ngure, V.; Kinuthia, G. Health risk implications of lead, cadmium, zinc, and nickel for consumers of food items in Migori Gold mines, Kenya. J. Geochem. Explor. 2020, 209, 106430. [Google Scholar] [CrossRef]
- Nadal, M.; Bocio, A.; Schuhmacher, M.; Domingo, J.L. Monitoring metals in the population living in the vicinity of a hazardous waste incinerator: Levels in hair of school children. Biol. Trace Element Res. 2005, 104, 203–213. [Google Scholar] [CrossRef]
- Ferré-Huguet, N.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Monitoring metals in blood and hair of the population living near a hazardous waste incinerator: Temporal trend. Biol. Trace Element Res. 2009, 128, 191–199. [Google Scholar] [CrossRef]
- Pino, A.; Chiarotti, F.; Calamandrei, G.; Gotti, A.; Karakitsios, S.; Handakas, E.; Bocca, B.; Sarigiannis, D.; Alimonti, A. Human biomonitoring data analysis for metals in an Italian adolescents cohort: An exposome approach. Environ. Res. 2017, 159, 344–354. [Google Scholar] [CrossRef]
- Noreen, F.; Sajjad, A.; Mahmood, K.; Anwar, M.; Zahra, M.; Waseem, A. Human Biomonitoring of Trace Elements in Scalp Hair from Healthy Population of Pakistan. Biol. Trace Elem. Res. 2020, 196, 37–46. [Google Scholar] [CrossRef]
- Vogel, N.; Murawski, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Doyle, U.; Kämpfe, A.; Höra, C.; Hildebrand, J.; Schäfer, M.; Drexler, H.; et al. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany—Human biomonitoring results of the German Environmental Survey 2014-2017 (GerES V). Int. J. Hyg. Environ. Health 2021, 237, 113822. [Google Scholar] [CrossRef] [PubMed]
- Kusanagi, E.; Takamura, H.; Hoshi, N.; Chen, S.J.; Adachi, M. Levels of Toxic and Essential Elements and Associated Factors in the Hair of Japanese Young Children. Int. J. Environ. Res. Public Health 2023, 20, 1186. [Google Scholar] [CrossRef] [PubMed]
- Batyrova, G.; Tlegenova, Z.S.; Umarova, G.; Kononets, V.; Umarov, Y.A.; Kudabayeva, K.I.; Aitmaganbet, P.; Amanzholkyzy, A. Microelement Status of the Adult Population in Western Kazakhstan. Ekol. Cheloveka (Hum. Ecol.) 2021, 11, 42–49. [Google Scholar] [CrossRef]
- Batyrova, G.; Tlegenova, Z.; Kononets, V.; Umarova, G.; Kudabayeva, K.; Bazargaliyev, Y.; Amanzholkyzy, A.; Umarov, Y. Hair Toxic Trace Elements of Residents across the Caspian Oil and Gas Region of Kazakhstan: Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 11158. [Google Scholar] [CrossRef] [PubMed]
- Umarova, G.; Batyrova, G.; Tlegenova, Z.; Kononets, V.; Balmagambetova, S.; Umarov, Y.; Yessengaliyeva, I.; Mamyrbayev, A. Essential Trace Elements in Scalp Hair of Residents across the Caspian Oil and Gas Region of Kazakhstan. Toxics 2022, 10, 364. [Google Scholar] [CrossRef]
- Demographic statistics Publications. Available online: https://stat.gov.kz/en/industries/social-statistics/demography/publications/ (accessed on 20 August 2022).
- Batyrova, G.; Umarova, G.; Umarov, Y.; Taskozhina, G.; Kononets, V.; Batyrov, R. Elemental Status of the Children’s Population of the Western region of the Republic of Kazakhstan: Research Protocol. JMIR Prepr. 2025, 72207. [Google Scholar] [CrossRef]
- McKenzie, L.M.; Crooks, J.; Peel, J.L.; Blair, B.D.; Brindley, S.; Allshouse, W.B.; Malin, S.; Adgate, J.L. Relationships between indicators of cardiovascular disease and intensity of oil and natural gas activity in Northeastern Colorado. Environ. Res. 2019, 170, 56–64. [Google Scholar] [CrossRef]
- Pragst, F.; Stieglitz, K.; Runge, H.; Runow, K.-D.; Quig, D.; Osborne, R.; Runge, C.; Ariki, J. High concentrations of lead and barium in hair of the rural population caused by water pollution in the Thar Jath oilfields in South Sudan. Forensic Sci. Int. 2017, 274, 99–106. [Google Scholar] [CrossRef]
- Barbieri, F.L.; Cournil, A.; Souza Sarkis, J.E.; Bénéfice, E.; Gardon, J. Hair trace elements concentration to describe polymetallic mining waste exposure in Bolivian Altiplano. Biol. Trace Element Res. 2011, 139, 10–23. [Google Scholar] [CrossRef]
- Drobyshev, E.J.; Solovyev, N.D.; Ivanenko, N.B.; Kombarova, M.Y.; Ganeev, A.A. Trace element biomonitoring in hair of school children from a polluted area by sector field inductively coupled plasma mass spectrometry. J. Trace Elements Med. Biol. 2017, 39, 14–20. [Google Scholar] [CrossRef]
- Dongarrà, G.; Varrica, D.; Tamburo, E.; D’Andrea, D. Trace elements in scalp hair of children living in differing environmental contexts in Sicily (Italy). Environ. Toxicol. Pharmacol. 2012, 34, 160–169. [Google Scholar] [CrossRef]
- Chłopicka, J.; Zachwieja, Z.; Zagrodzki, P.; Frydrych, J.; Słota, P.; Krośniak, M. Lead and cadmium in the hair and blood of children from a highly industrial area in Poland. Biol. Trace Elem. Res. 1998, 62, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Tamburo, E.; Varrica, D.; Dongarrà, G. Gender as a key factor in trace metal and metalloid content of human scalp hair. A multi-site study. Sci. Total Environ. 2016, 573, 996–1002. [Google Scholar] [CrossRef]
- Seifert, B.; Becker, K.; Helm, D.; Krause, C.; Schulz, C.; Seiwert, M. The German Environmental Survey 1990/1992 (GerES II): Reference concentrations of selected environmental pollutants in blood, urine, hair, house dust, drinking water and indoor air. J. Expo. Anal. Environ. Epidemiol. 2000, 10 Pt 1, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Senofonte, O.; Violante, N.; Caroli, S. Assessment of reference values for elements in human hair of urban schoolboys. J. Trace Elem. Med. Biol. 2000, 14, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Shin, K.O.; Kim, J.S. Assessment of reference values for hair minerals of Korean preschool children. Biol. Trace Elem. Res. 2007, 116, 119–130. [Google Scholar] [CrossRef]
- Skröder, H.; Kippler, M.; Nermell, B.; Tofail, F.; Levi, M.; Rahman, S.M.; Raqib, R.; Vahter, M. Major Limitations in Using Element Concentrations in Hair as Biomarkers of Exposure to Toxic and Essential Trace Elements in Children. Environ. Health Perspect. 2017, 125, 067021. [Google Scholar] [CrossRef]
- Ilmiawati, C.; Yoshida, T.; Itoh, T.; Nakagi, Y.; Saijo, Y.; Sugioka, Y.; Sakamoto, M.; Ikegami, A.; Ogawa, M.; Kayama, F. Biomonitoring of mercury, cadmium, and lead exposure in Japanese children: A cross-sectional study. Environ. Health Prev. Med. 2015, 20, 18–27. [Google Scholar] [CrossRef]
- Tian, W.; Egeland, G.M.; Sobol, I.; Chan, H.M. Mercury hair concentrations and dietary exposure among Inuit preschool children in Nunavut, Canada. Environ. Int. 2011, 37, 42–48. [Google Scholar] [CrossRef]
- Carneiro, M.F.; Moresco, M.B.; Chagas, G.R.; de Oliveira Souza, V.C.; Rhoden, C.R.; Barbosa, F., Jr. Assessment of trace elements in scalp hair of a young urban population in Brazil. Biol. Trace Elem. Res. 2011, 143, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Chmielnicka, J. Relationship of lead and cadmium to essential elements in hair, teeth, and nails of environmentally exposed people. Ecotoxicol. Environ. Saf. 2000, 46, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Varrica, D.; Tamburo, E.; Alaimo, M.G. Levels of trace elements in human hair samples of adolescents living near petrochemical plants. Environ. Geochem. Health 2022, 44, 3779–3797. [Google Scholar] [CrossRef] [PubMed]
- Berglund, M.; Lindberg, A.; Rahman, M.; Yunus, M.; Grandér, M.; Lönnerdal, B.; Vahter, M. Gender and age differences in mixed metal exposure and urinary excretion. Environ. Res. 2011, 111, 1271–1279. [Google Scholar] [CrossRef]
- Tamburo, E.; Varrica, D.; Dongarrà, G. Coverage intervals for trace elements in human scalp hair are site specific. Environ. Toxicol. Pharmacol. 2015, 39, 70–76. [Google Scholar] [CrossRef]
- Lavoie, M.; Risk, D.; Rainham, D. Sociodemographic and Population Exposure to Upstream Oil and Gas Operations in Canada. Int. J. Environ. Res. Public Health 2024, 21, 1692. [Google Scholar] [CrossRef]
- Relić, D.; Sakan, S.; Anđelković, I.; Popović, A.; Đorđević, D. Pollution and Health Risk Assessments of Potentially Toxic Elements in Soil and Sediment Samples in a Petrochemical Industry and Surrounding Area. Molecules 2019, 24, 2139. [Google Scholar] [CrossRef]
- González, N.; Esplugas, R.; Marquès, M.; Domingo, J.L. Concentrations of arsenic and vanadium in environmental and biological samples collected in the neighborhood of petrochemical industries: A review of the scientific literature. Sci. Total Environ. 2021, 771, 145149. [Google Scholar] [CrossRef]
- Xie, W.; Peng, C.; Wang, H.; Chen, W. Health Risk Assessment of Trace Metals in Various Environmental Media, Crops and Human Hair from a Mining Affected Area. Int. J. Environ. Res. Public Health 2017, 14, 1595. [Google Scholar] [CrossRef]
- Nadal, M.; García, F.; Schuhmacher, M.; Domingo, J.L. Metals in biological tissues of the population living near a hazardous waste incinerator in Catalonia, Spain: Two decades of follow-up. Environ. Res. 2019, 176, 108578. [Google Scholar] [CrossRef]
- Chojnacka, K.; Michalak, I.; Zielińska, A.; Górecka, H.; Górecki, H. Inter-relationship between elements in human hair: The effect of gender. Ecotoxicol. Environ. Saf. 2010, 73, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Campo, L.; Bechtold, P.; Borsari, L.; Fustinoni, S. A systematic review on biomonitoring of individuals living near or working at solid waste incinerator plants. Crit. Rev. Toxicol. 2019, 49, 479–519. [Google Scholar] [CrossRef] [PubMed]
- Schaller, J.; Headley, T.; Prigent, S.; Breuer, R. Potential mining of lithium, beryllium and strontium from oilfield wastewater after enrichment in constructed wetlands and ponds. Sci. Total Environ. 2014, 493, 910–913. [Google Scholar] [CrossRef]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Levels of metals, PCBs, PCNs and PAHs in soils of a highly industrialized chemical/petrochemical area: Temporal trend. Chemosphere 2007, 66, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Yusta-García, R.; Orta-Martínez, M.; Mayor, P.; González-Crespo, C.; Rosell-Melé, A. Water contamination from oil extraction activities in Northern Peruvian Amazonian rivers. Environ. Pollut. 2017, 225, 370–380. [Google Scholar] [CrossRef]
- Hussein Were, F.; Njue, W.; Murungi, J.; Wanjau, R. Use of human nails as bio-indicators of heavy metals environmental exposure among school age children in Kenya. Sci. Total Environ. 2008, 393, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Semenova, Y.; Zhunussov, Y.; Pivina, L.; Abisheva, A.; Tinkov, A.; Belikhina, T.; Skalny, A.; Zhanaspayev, M.; Bulegenov, T.; Glushkova, N.; et al. Trace element biomonitoring in hair and blood of occupationally unexposed population residing in polluted areas of East Kazakhstan and Pavlodar regions. J. Trace Elem. Med. Biol. 2019, 56, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, I.; Daponte, A.; Gil, F.; Hernández, A.F.; Godoy, P.; Pla, A.; Ramos, J.L.; DASAHU Group. Urinary levels of arsenic and heavy metals in children and adolescents living in the industrialised area of Ria of Huelva (SW Spain). Environ. Int. 2010, 36, 563–569. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for Aluminum; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2008.
- Bryliński, Ł.; Kostelecka, K.; Woliński, F.; Duda, P.; Góra, J.; Granat, M.; Flieger, J.; Teresiński, G.; Buszewicz, G.; Sitarz, R.; et al. Aluminium in the Human Brain: Routes of Penetration, Toxicity, and Resulting Complications. Int. J. Mol. Sci. 2023, 24, 7228. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum environmental pollution: The silent killer. Environ. Sci. Pollut. Res. Int. 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Niu, Q. Overview of the Relationship Between Aluminum Exposure and Health of Human Being. Adv. Exp. Med. Biol. 2018, 1091, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Corkins, M.R.; COMMITTEE ON NUTRITION. Aluminum Effects in Infants and Children. Pediatrics 2019, 144, e20193148. [Google Scholar] [CrossRef]
- Chuchu, N.; Patel, B.; Sebastian, B.; Exley, C. The aluminium content of infant formulas remains too high. BMC Pediatr. 2013, 13, 162. [Google Scholar] [CrossRef]
- Crisponi, G.; Fanni, D.; Gerosa, C.; Nemolato, S.; Nurchi, V.M.; Crespo-Alonso, M.; Lachowicz, J.I.; Faa, G. The meaning of aluminium exposure on human health and aluminium-related diseases. Biomol. Concepts. 2013, 4, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gleason, G.R.; Sharmanov, T. Anemia prevention and control in four central Asian republics and Kazakhstan. J. Nutr. 2002, 132 (Suppl. S4), 867S–870S. [Google Scholar] [CrossRef] [PubMed]
- Awadh, S.M.; Yaseen, Z.M.; Al-Suwaiyan, M.S. The role of environmental trace element toxicants on autism: A medical biogeochemistry perspective. Ecotoxicol. Environ. Saf. 2023, 251, 114561. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; McCarty, K.M.; Steckling, N.; Lettmeier, B. Mercury exposure and children’s health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef]
- Ronchetti, R.; Zuurbier, M.; Jesenak, M.; Koppe, J.G.; Ahmed, U.F.; Ceccatelli, S.; Villa, M.P. Children’s health and mercury exposure. Acta Paediatr. Suppl. 2006, 95, 36–44. [Google Scholar] [CrossRef]
- Takeuchi, H.; Shiota, Y.; Yaoi, K.; Taki, Y.; Nouchi, R.; Yokoyama, R.; Kotozaki, Y.; Nakagawa, S.; Sekiguchi, A.; Iizuka, K.; et al. Mercury levels in hair are associated with reduced neurobehavioral performance and altered brain structures in young adults. Commun. Biol. 2022, 5, 529. [Google Scholar] [CrossRef]
- Al Osman, M.; Yang, F.; Massey, I.Y. Exposure routes and health effects of heavy metals on children. Biometals 2019, 32, 563–573. [Google Scholar] [CrossRef]
- Kampalath, R.A.; Jay, J.A. Sources of Mercury Exposure to Children in Low- and Middle-Income Countries. J. Health Pollut. 2015, 5, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Fido, A.; Al-Saad, S. Toxic trace elements in the hair of children with autism. Autism 2005, 9, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Priya, M.D.; Geetha, A. Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol. Trace Elem. Res. 2011, 142, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Shen, L.; Khan, N.U.; Zhang, X.; Chen, L.; Zhao, H.; Luo, P. Trace elements in children with autism spectrum disorder: A meta-analysis based on case-control studies. J. Trace Elem. Med. Biol. 2021, 67, 126782. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA); Summary and Conclusions of the 67th Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 940; International Programme on Chemical Safety; World Health Organization: Geneva, Switzerland. 2006. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/9cdcf62e-54b6-4655-af56-dbc4c70bd90a/content (accessed on 30 May 2025).
- National Research Council, NRC. Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK225778/pdf/Bookshelf_NBK225778.pdf (accessed on 30 May 2025).
- Ruggieri, F.; Majorani, C.; Domanico, F.; Alimonti, A. Mercury in Children: Current State on Exposure through Human Biomonitoring Studies. Int. J. Environ. Res. Public Health 2017, 14, 519. [Google Scholar] [CrossRef]
- Myers, G.J.; Thurston, S.W.; Pearson, A.T.; Davidson, P.W.; Cox, C.; Shamlaye, C.F.; Cernichiari, E.; Clarkson, T.W. Postnatal exposure to methyl mercury from fish consumption: A review and new data from the Seychelles Child Development Study. Neurotoxicology 2009, 30, 338–349. [Google Scholar] [CrossRef]
- Wahlberg, K.; Love, T.M.; Pineda, D.; Engström, K.; Watson, G.E.; Thurston, S.W.; Yeates, A.J.; Mulhern, M.S.; McSorley, E.M.; Strain, J.J.; et al. Maternal polymorphisms in glutathione-related genes are associated with maternal mercury concentrations and early child neurodevelopment in a population with a fish-rich diet. Environ. Int. 2018, 115, 142–149. [Google Scholar] [CrossRef]
- Torrente, M.; Colomina, M.T.; Domingo, J.L. Metal concentrations in hair and cognitive assessment in an adolescent population. Biol. Trace Elem. Res. 2005, 104, 215–221. [Google Scholar] [CrossRef]


| Studied Population Characteristics | 1 N | 2 % | 3 Median (Min–Max) |
|---|---|---|---|
| Total | 1595 | 100 | |
| Gender | 1595 | 100 | |
| Boys | 764 | 47.9 | |
| Girls | 831 | 52.1 | |
| Age (years) | 1595 | 100 | 11 (5–18) |
| 5–9 | 619 | 38.8 | |
| 11–18 | 976 | 61.2 | |
| Height, cm | 1595 | 100 | 144 (84–189) |
| Weight, kg | 1595 | 100 | 37 (14–124) |
| Body mass index, kg/m2 | 1595 | 100 | 17.36 (10.77–40.32) |
| Oblasts | 1595 | 100 | |
| Aktobe | 573 | 35.9 | |
| Mangistau | 410 | 25.7 | |
| Atyrau | 612 | 38.4 | |
| Place of residence | 1595 | 100 | |
| Urban | 805 | 50.5 | |
| Rural | 790 | 49.5 | |
| Distance of place of residence to oil and gas fields, km | 1595 | 100 | |
| 0–16 | 524 | 32.9 | |
| 17–110 | 579 | 36.3 | |
| 110 km and more | 492 | 30.8 | |
| Passive smoking | 1595 | 100 | |
| Yes | 729 | 45.7 | |
| No | 866 | 54.3 | |
| Monthly income, tenge | 1595 | 100 | |
| Up to 50 thousand | 119 | 7.5 | |
| 50–100 thousand | 479 | 30.0 | |
| 100–150 thousand | 245 | 15.4 | |
| 150–200 thousand | 240 | 15.0 | |
| 200–250 thousand | 389 | 24.4 | |
| 250 thousand and above | 123 | 7.7 |
| Element | AM | GM | Me | Min | Percentile | Max | |||
|---|---|---|---|---|---|---|---|---|---|
| 2.5th | 25th | 75th | 97.5th | ||||||
| Al | 6.45 | 4.41 | 4.40 | 0.309 | 0.904 | 2.59 | 7.29 | 24.36 | 277.05 |
| As | 0.039 | 0.033 | 0.032 | 0.003 | 0.012 | 0.023 | 0.044 | 0.106 | 0.984 |
| Be | 0.002 | 0.001 | 0.001 | 0.000005 | 0.0002 | 0.0004 | 0.001 | 0.003 | 1.05 |
| Cd | 0.022 | 0.011 | 0.011 | 0.0001 | 0.001 | 0.005 | 0.024 | 0.111 | 0.878 |
| Hg | 0.105 | 0.060 | 0.058 | 0.003 | 0.008 | 0.028 | 0.122 | 0.466 | 2.46 |
| Pb | 0.324 | 0.174 | 0.170 | 0.013 | 0.025 | 0.079 | 0.362 | 1.57 | 13.60 |
| Element | Remoteness < 16 km (n = 524) | Remoteness 16–110 km (n = 579) | Remoteness > 110 km (n = 492) | p K-W | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | GM | Me (q25; q75) | P2.5; P97.5 | AM | GM | Me (q25; q75), | P2.5; P97.5 | AM | GM | Me (q25; q75), | P2.5; P97.5 | ||
| Al | 6.154 | 4.791 | 4.824 c (2.975; 7.771) | (1.250; 19.414) | 7.061 | 4.617 | 4.517 c* (2.656; 7.346) | (1.076; 28.907) | 6.061 | 3.822 | 3.704 a,b* (2.126; 7.009) | (0.540; 25.350) | 0.001 |
| As | 0.033 | 0.030 | 0.030 b,c (0.022; 0.040) | (0.012; 0.077) | 0.042 | 0.034 | 0.032 a (0.022; 0.040) | (0.013; 0.132) | 0.041 | 0.034 | 0.033 a (0.024; 0.047) | (0.013; 0.116) | <0.001 |
| Be | 0.0006 | 0.0010 | 0.0005 b,c (0.0003; 0.0008) | (0.0001; 0.0020) | 0.003 | 0.001 | 0.0010 a,c (0.0005; 0.0016) | (0.0001; 0.0030) | 0.0008 | 0.0007 | 0.0007 a,b (0.0004; 0.0012) | (0.0001; 0.0022) | <0.001 |
| Cd | 0.020 | 0.010 | 0.010 b,c** (0.005; 0.020) | (0.001; 0.124) | 0.023 | 0.010 | 0.013 a (0.005; 0.020) | (0.001; 0.119) | 0.022 | 0.012 | 0.012 a** (0.005; 0.026) | (0.001; 0.100) | <0.001 |
| Hg | 0.137 | 0.090 | 0.096 b,c (0.047; 0.173) | (0.013; 0.474) | 0.105 | 0.053 | 0.051 a,c*** (0.024; 0.111) | (0.008; 0.609) | 0.071 | 0.044 | 0.043 a,b*** (0.025; 0.077) | (0.005; 0.355) | <0.001 |
| Pb | 0.260 | 0.132 | 0.122 b,c (0.063; 0.269) | (0.021; 0.904) | 0.325 | 0.178 | 0.174 a,c (0.082; 0.337) | (0.029; 1.811) | 0.392 | 0.226 | 0.229 a,b (0.108; 0.467) | (0.033; 1.921) | <0.001 |
| Element | Model 0 | 95% CI | p | Model A | 95% CI | p | Model B | 95% CI | p | Model C | 95% CI | p | Model D | 95% CI | p | Model E | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | −0.062 | (−0.103; −0.022) | 0.003 | −0.052 | (−0.088; −0.015) | 0.006 | −0.044 | (−0.080; −0.008) | 0.017 | −0.044 | (−0.080; −0.008) | 0.017 | −0.072 | (−0.108; −0.036) | <0.001 | −0.072 | (−0.109; −0.036) | <0.001 |
| As | 0.066 | (0.040;0.092) | <0.001 | 0.071 | (0.045; 0.096) | <0.001 | 0.080 | (0.055; 0.104) | <0.001 | 0.079 | (0.055; 0.103) | <0.001 | 0.064 | (0.040; 0.089) | <0.001 | 0.064 | (0.039; 0.089) | <0.001 |
| Be | 0.038 | (−0.003; 0.078) | 0.071 | 0.040 | (−0.001; 0.081) | 0.055 | 0.040 | (−0.001; 0.081) | 0.054 | 0.039 | (−0.002; 0.080) | 0.060 | 0.031 | (−0.011; 0.073) | 0.144 | 0.030 | (−0.011; 0.072) | 0.155 |
| Cd | 0.073 | (0.018;0.128) | 0.009 | 0.089 | (0.041; 0.137) | <0.001 | 0.102 | (0.055; 0.149) | <0.001 | 0.101 | (0.055; 0.148) | <0.001 | 0.092 | (0.044; 0.140) | <0.001 | 0.093 | (0.045; 0.141) | <0.001 |
| Hg | −0.245 | (−0.294; −0.195) | <0.001 | −0.244 | (−0.293; −0.194) | <0.001 | −0.249 | (−0.299; −0.200) | <0.001 | −0.250 | (−0.299; −0.200) | <0.001 | −0.294 | (−0.344; −0.244) | <0.001 | −0.293 | (−0.343; −0.243) | <0.001 |
| Pb | 0.220 | (0.169; 0.271) | <0.001 | 0.235 | (0.191; 0.279) | <0.001 | 0.253 | (0.212; 0.295) | <0.001 | 0.252 | (0.211; −0.293) | <0.001 | 0.244 | (0.202; −0.288) | <0.001 | 0.244 | (0.202; 0.287) | <0.001 |
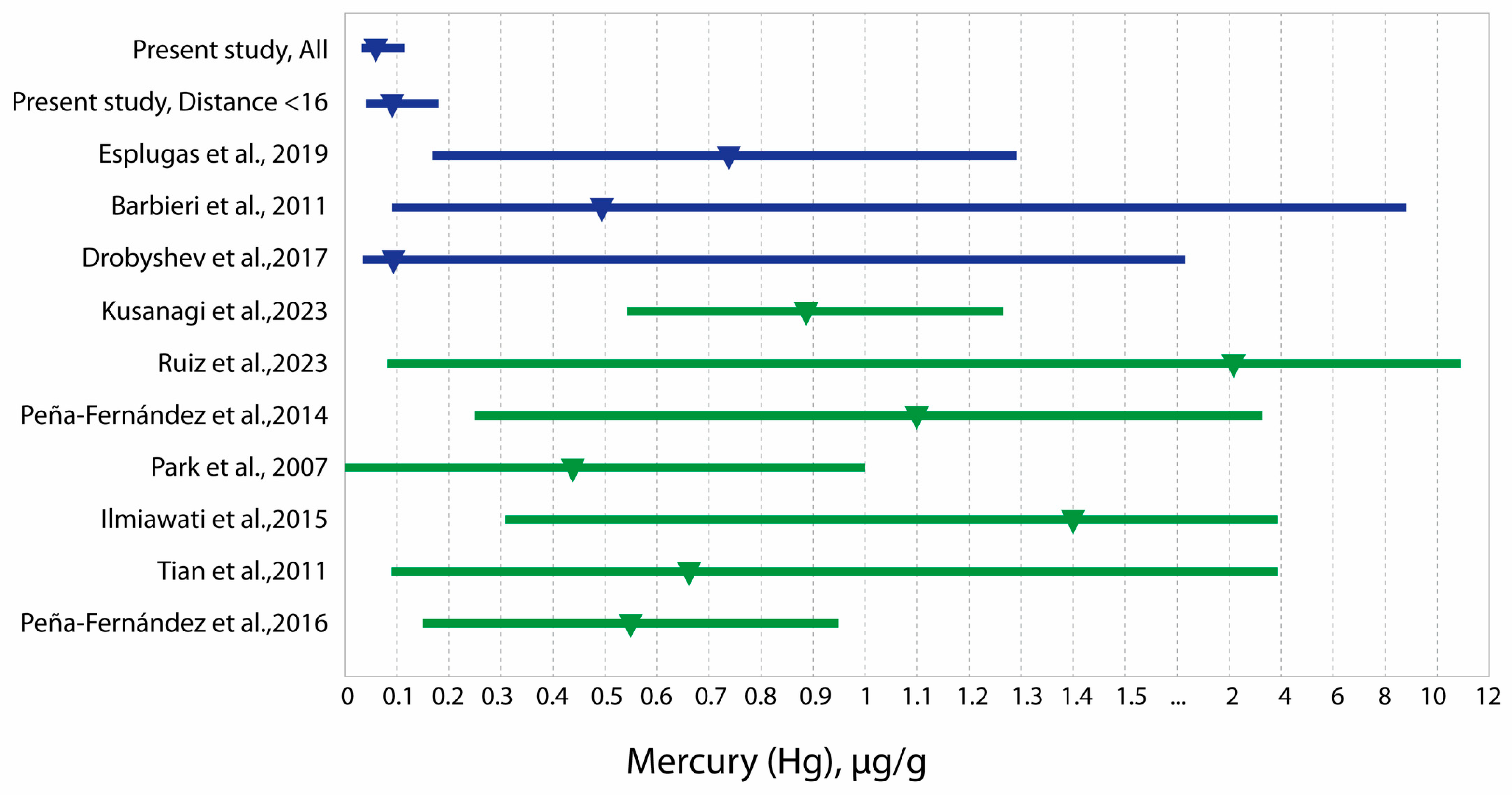
| Sample Type & Location | Age (Years) | n | Al Median (Range) | As Median (Range) | Be Median (Range) | Cd Median (Range) | Hg Median (Range) | Pb Median (Range) | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Present study, total sample | 5–18 | 1595 | Median P25; P75 | 4.40 (2.59; 7.29) | 0.032 (0.023; 0.044) | 0.001 (0.0004; 0.001) | 0.011 (0.005; 0.024) | 0.058 (0.028; 0.122) | 0.170 (0.079; 0.362) | |
| Present study, living at a distance of 0–16 km from oil fields | 5–18 | 524 | Median P25; P75 | 4.824 (2.975; 7.771) | 0.030 (0.022; 0.040) | 0.0005 (0.0003; 0.0008) | 0.010 (0.005; 0.020) | 0.096 (0.047; 0.173) | 0.122 (0.063; 0.269 | |
| West Kazakhstan | adults | 850 | Median P25; P75 | 4.080 (1.007; 33.586) | 0.030 (0.004; 0.116) | 0.0004 (0.0001; 0.0026) | 0.010 (0.001; 0.141) | 0.145 (0.019; 1.035) | 0.181 (0.032; 2.651) | Batyrova et al., 2022 [36] |
| Spain, Catalonia | 10–13 | 94 | Mean ± SD | - | - | - | 0.04 ±0.05 | 0.73 ± 0.56 | 1.44 ± 1.89 | Esplugas et al., 2019 [27] |
| Russia, Karabash | 14.7 ± 1.1 years | 46 | Median P25; P75 | - | 2.038 (0.975–3.699) | - | 0.118 (0.055–0.210) | - | 5.44 (3.57–13.98) | Skalny et al., 2018 [26] |
| Bolivia (mining area) | 7–9 | 60 | GM P5–P95 | - | 0.79 0.10–3.30 | - | 0.07 0.00–2.03 | 0.49 0.09–8.44 | 14.08 3.24–65.4 | Barbieri et al., 2011 [42] |
| Russia | 7.6 ± 1.3 | 82 | Median Min–max | 19.5 5.1–71.7 | 0.020 <0.003–0.330 | 0.11 0.01–1.01 | 0.09 <0.03–2.23 | 2.48 0.33–28.0 | Drobyshev et al., 2017 [43] | |
| Italy, Sicily, Industrial area | 11–13 | 60 | Median P10; P90 | 4.1 (2.0; 8.3) | 0.038 (0.002; 0.119) | - | 0.077 (0.001; 0.148) | - | 1.58 (0.59; 3.29) | Dongarrà et al., 2012 [44] |
| Italy, the active volcanic area of Mt. Etna | 11–13 | 376 | Median P10–P90 | 4.90 2.08; 8.38 | 0.03 0.004; 0.06 | - | 0.01 0.002; 0.04 | - | 0.58 0.14; 1.89 | Varrica et al., 2014 [20] |
| Poland | 8–15 | 158 | Me (Min; Max) | - | - | - | 0.83 (0.08;3.73) | - | 6.74 (0.57;36.14) | Chłopicka et al., 1998 [45] |
| Sicily | 11–14 | 963 | Median P25; P75 | 5.0 (0.01; 10.6) | 0.03 (0.0003; 0.17) | - | 0.01 (0.0003; 0.18) | - | 0.63 (0.03; 4.0) | Tamburo et al., 2016 [46] |
| Non-polluted area | ||||||||||
| Italy, Sicily, Rural area | 11–13 | 47 | Median P10; P90 | 4.2 (2.5; 8.1) | 0.04 (0.02; 0.07) | - | 0.037 (0.008; 0.057) | - | 0.70 (0.42; 1.78) | Dongarrà et al., 2012 [44] |
| Japan | 3–6 | 118 | Median P25; P75 | 8.17 (5.41; 10.78) | - | - | 0.001 (0.0007; 0.0011) | 0.88 (0.54; 1.26) | 0.96 (0.62; 1.49) | Kusanagi et al., 2023 [34] |
| Spain | 3–12 | 419 | Median P5; P95 | 5.98 (0.00; 24.78) | 0.020 (0.002; 0.068) | 0.000 (0.000; 0.004) | 0.025 (0.002; 0.146) | 2.09 (0.08; 10.98) | 1.17 (0.08; 7.39) | Ruiz et al., 2023 [14] |
| Africa, Ethiopia | 0–18 | 81 | GM P25; P75 mg kg−1 | 1 (<DL; 42) | 0.04 (<DL; 0.09) | 0.008 (0.004; 0.027) | 0.10 (0.06; 0.21) | 0.056 (0.026; 0.111) | 3.1 (1.7; 5.4) | Astolfi et al., 2020 [7] |
| Germany | 6–14 | 711 | GM P10; P90 | 6.57 (2.4; 18.3) | - | - | 0.048 (0.01; 0.19) | - | 1.02 (0.3; 3.5) | Seifert et al., 2000 [47] |
| Italy, Rome | 3–14 | 412 | Median P5; P95 | 8.45 (2.4; 20.0) | 0.06 (0.14; 0.24) | - | 0.14 (0.04; 0.61) | - | 5.60 (1.0; 19.8) | Senofonte et al., 2000 [48] |
| Spain, Madrid | 0–18 | 648 | Mean P5; P95 | 23.8 4.9; 63.2 | 0.12 <0.05; 0.26 | - | 0.029 0.004; 0.079 | - | 1.23 0.17; 4.28 | Llorente Ballesteros et al., 2017 [25] |
| Spain, Alcalá de Henares | 6–9 | 117 | AM P5; P95 | 9.05 1.71; 22.76 | ND | ND | 0.52 0.17; 0.93 | 1.10 0.25; 3.35 | 1.48 0.25; 3.47 | Peña-Fernández et al., 2014 [23] |
| South Korea | 3–6 | 655 | Median P5; P95 | 8.08 (3; 16) | 0.11 (0.05–0.20) | - | 0.07 (0.01–0.20) | 0.43 (0–1) | 1.43 (<3) | Park et al., 2007 [49] |
| Bolivia | 7–9 | 71 | GM P5–P95 | - | 0.39 0.11–1.24 | - | 0.08 0.03–0.19 | 0.15 0.05–0.50 | 2.32 0.33–10.19 | Barbieri et al., 2011 [42] |
| Bangladesh | 9–10 | 207 | Median P5; P95 | - | 0.53 (0.14–2.9) | - | 29 (0.76–150) | - | 1.6 (0.50–6.4) | Skröder et al., 2017 [50] |
| Japan | 9–10 | 229 | Median Min–Max | -- | -- | - | - | 1.40 0.31–3.96 | - | Ilmiawati et al., 2015 [51] |
| Canada | 3–5 | 361 | GM P10–P90 | - | - | - | - | 0.66 0.09; 3.96 | - | Tian et al., 2011 [52] |
| Italy, Sicily | 11–13 | 131 | Median P10; P90 | 6.09 (0.01; 10.49) | 0.0003 (0.0003; 0.014) | - | 0.03 (0.01; 0.11) | - | 0.78 (0.37; 1.84) | Dongarrà et al., 2012 [44] |
| Spain | 13–16 | 96 | M ± S.D | 5.34 ± 2.96 | ND | ND | 0.11 ± 0.14 | 0.55 ± 0.40 | 0.70 ± 0.52 | Peña-Fernández et al., 2016 [1] |
| Southern Brazil | 12–18 | 126 | Median P25; P75 | - | 0.006 (0.001; 0.02) | - | 0.003 (0.000; 0.02) | - | 0.1 (0.009; 0.4) | Carneiro et al., 2011 [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batyrova, G.; Kononets, V.; Umarova, G.; Taskozhina, G.; Umarov, Y.; Issanguzhina, Z.; Kudabayeva, K.; Batyrov, R. Trace Metal and Metalloid Profiles in Hair Samples from Children in the Oil-Producing Region of Kazakhstan. Toxics 2025, 13, 522. https://doi.org/10.3390/toxics13070522
Batyrova G, Kononets V, Umarova G, Taskozhina G, Umarov Y, Issanguzhina Z, Kudabayeva K, Batyrov R. Trace Metal and Metalloid Profiles in Hair Samples from Children in the Oil-Producing Region of Kazakhstan. Toxics. 2025; 13(7):522. https://doi.org/10.3390/toxics13070522
Chicago/Turabian StyleBatyrova, Gulnara, Victoria Kononets, Gulmira Umarova, Gulaim Taskozhina, Yeskendir Umarov, Zhamilya Issanguzhina, Khatimya Kudabayeva, and Rabbil Batyrov. 2025. "Trace Metal and Metalloid Profiles in Hair Samples from Children in the Oil-Producing Region of Kazakhstan" Toxics 13, no. 7: 522. https://doi.org/10.3390/toxics13070522
APA StyleBatyrova, G., Kononets, V., Umarova, G., Taskozhina, G., Umarov, Y., Issanguzhina, Z., Kudabayeva, K., & Batyrov, R. (2025). Trace Metal and Metalloid Profiles in Hair Samples from Children in the Oil-Producing Region of Kazakhstan. Toxics, 13(7), 522. https://doi.org/10.3390/toxics13070522







