Analysis of Mercury Concentration in Cosmetic Clays
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Statistical Analysis
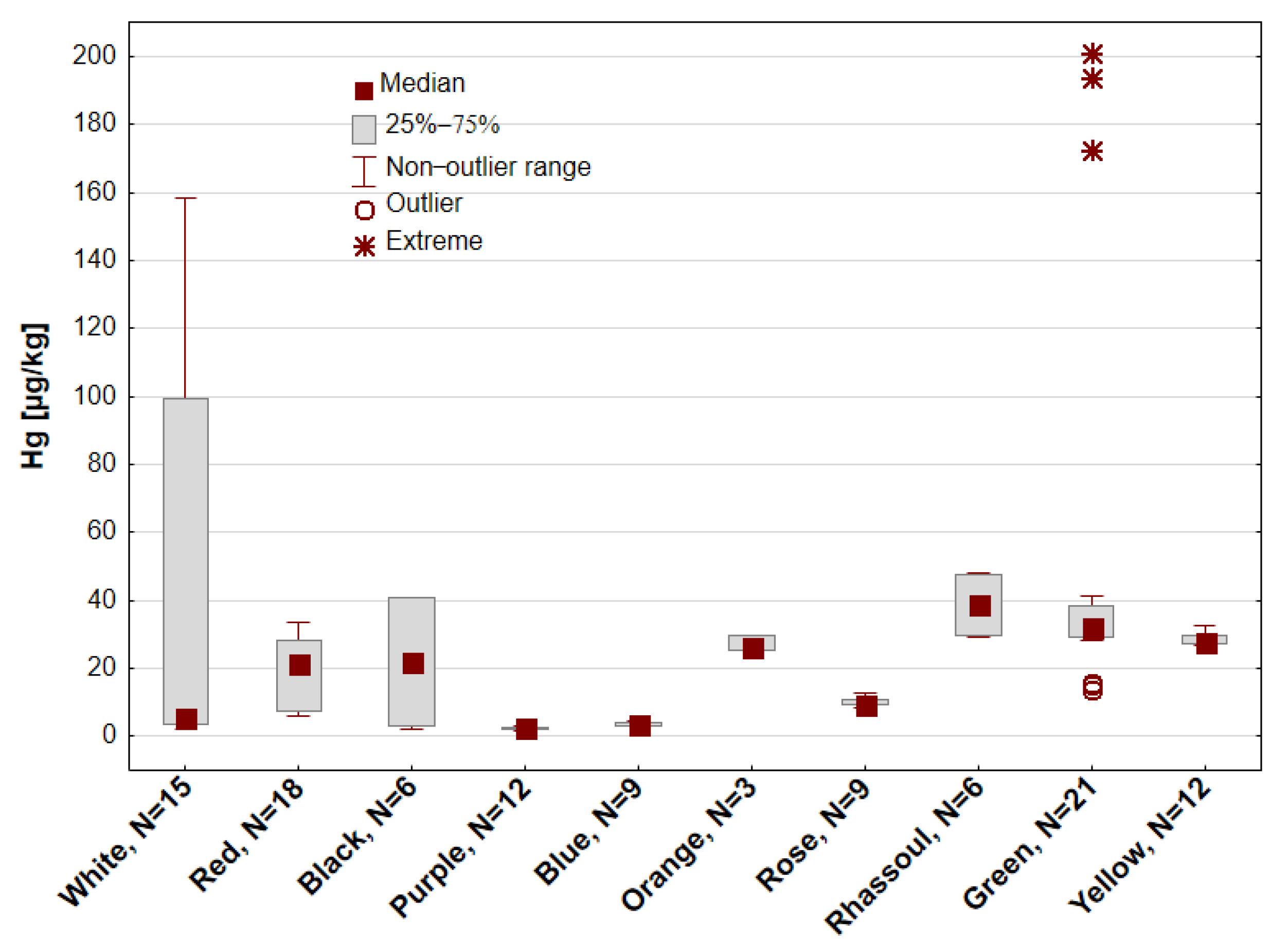
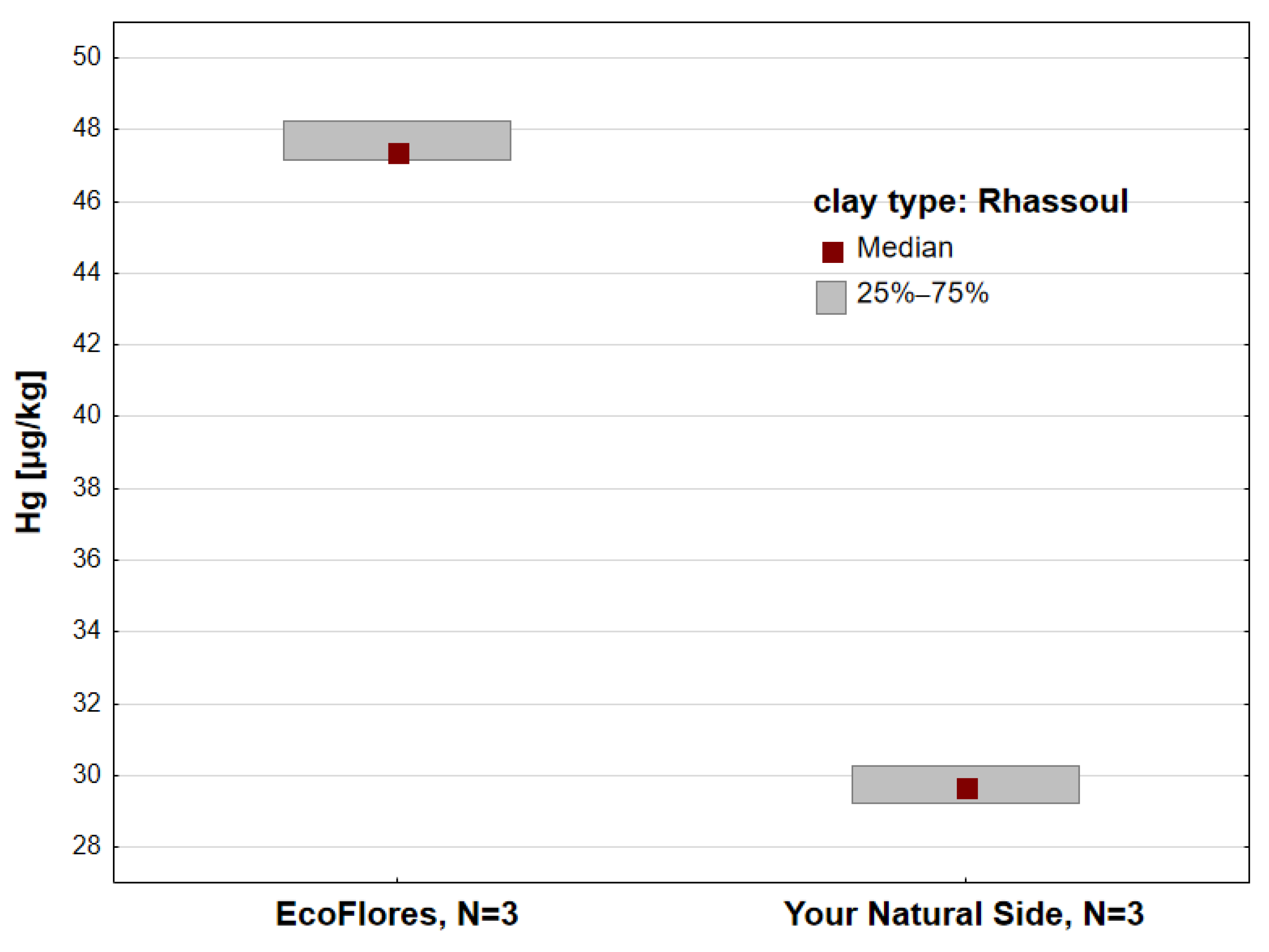
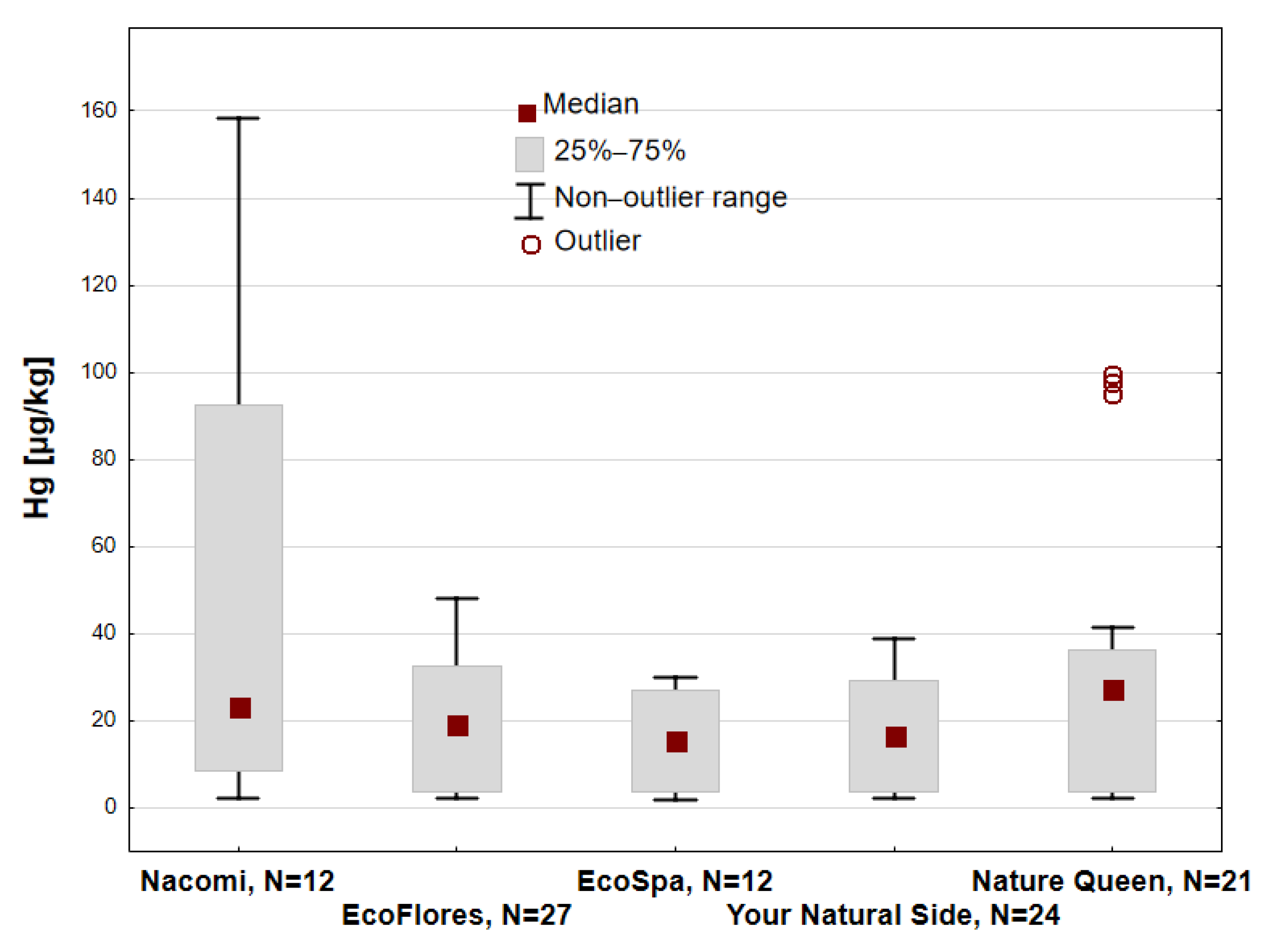
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viseras, C.; Sánchez-Espejo, R.; Palumbo, R.; Liccardi, N.; García-Villén, F.; Borrego-Sánchez, A.; Massaro, M.; Riela, S.; López-Galindo, A. Clays in cosmetics and personal-care products. Clays Clay Min. 2021, 69, 561–575. [Google Scholar] [CrossRef]
- Tokarský, J. Ghassoul–Moroccan clay with excellent adsorption properties. Mater. Today Proc. 2018, 5, 78–87. [Google Scholar] [CrossRef]
- Williams, L.B. Natural antibacterial clays: Historical uses and modern advances. Clays Clay Min. 2019, 67, 724. [Google Scholar] [CrossRef]
- Chrząstek, L.; Dondela, B. Homeopathy. Pt. 1. Chem. Environ. Biotechnol. 2014, 17, 21–42. [Google Scholar]
- Yue, F.; Xiangyu, C.; Rong-Rong, H.; Zhongqiu, L.; Yuri, M.L.; Mingxian, L. The horizons of medical mineralogy: Structure-bioactivity relationship and biomedical applications of halloysite nanoclay. ACS Nano 2024, 18, 20001–20026. [Google Scholar] [CrossRef]
- Centini, M.; Tredici, M.R.; Biondi, N.; Buonocore, A.; Maffei Facino, R.; Anselmi, C. Thermal mud maturation: Organic matter and biological activity. Int. J. Cosmet. Sci. 2015, 37, 3. [Google Scholar] [CrossRef]
- Bernat, M.; Matysek-Nawrocka, M.; Domaniuk, M.; Ślusarczyk, I. Cosmetic application of aluminum and its derivatives. Aesthetic Cosmetol. 2017, 6, 603–608. [Google Scholar]
- Safety Assessment of Clays as Used in Cosmetics. Cosmetic Ingredient Review; Washington, DC, USA, 7–8 March 2022. Available online: https://www.cir-safety.org/sites/default/files/Clays.pdf (accessed on 3 January 2025).
- Piszcz, P.; Dzwigałowska, M.; Głód, B.K. Total antioxidant potential of cosmetic products containing plant extracts. Camera Separatoria 2017, 9, 53–64. [Google Scholar]
- Polish Geological Institute, National Research Institute. Crystal Structure. Available online: https://www.pgi.gov.pl/en/1135-muzeum-pig-pib/kopalnia-wiedzy-1/10151-mineraly.html?start=1 (accessed on 4 January 2025).
- Kumari, N.; Mohan, C. Basics of clay minerals and their characteristic properties. Clay Clay Miner. 2021, 24, 1–29. [Google Scholar] [CrossRef]
- Amakrane, J.; El Hammounti, K.; Azdimousa, A.; El Halim, M.; El Ouahabi, M.; Lamouri, B.; Fagel, N. Mineralogical and physicochemical characterization of the Jbel Rhassoul clay deposit (Moulouya Plain, Morocco). J. Mater. Environ. Sci. 2018, 9, 2549–2557. [Google Scholar]
- Drzymała, J. Fundamentals of Mineralogy; Publishing House of the Wrocław University of Science and Technology: Wrocław, Poland, 2009; ISBN 978-83-7493-467-1. [Google Scholar]
- Molski, M. Beautiful Chemistry, Division of Substances According to Structure and Function, 2nd ed.; PWN: Warszawa, Poland, 2021; ISBN 978-83-01-21573-6. [Google Scholar]
- Sarruf, F.D.; Contreras, V.J.P.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. The scenario of clays and clay minerals use in cosmetics/dermocosmetics. Cosmetics 2024, 11, 7. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Tao, H.; He, X.; Hsu, K.; Wang, W.; Fang, X.; Steel, A. Comprehensive assessment of the efficacy and safety of a clay mask in oily and acne skin. Ski. Res. Technol. 2023, 29, 11. [Google Scholar] [CrossRef]
- Matike, D.M.E.; Ekosse, G.I.E.; Ngole, V.M. Physico-chemical properties of clayey soils used traditionally for Cosmetics in Eastern Cape, South Africa. Int. J. Phys. Sci. 2011, 6, 7557–7566. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay minerals and their beneficial effects upon human health. A review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R. Anestezjologia; Edra Urban & Partner: Wrocław, Poland, 2020; Volume 3, ISBN 978-83-66310-88-9. [Google Scholar]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.; Yang, Q.; Mao, L.; Yang, H. Emerging nanoclay composite for effective hemostasis. Adv. Funct. Mater. 2018, 28, 1704452. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Liu, Q.; Li, S.; Cheng, Y.; Cheng, C.H.; Sun, Z.; Du, Y.; Butch, C.J.; Wei, H. An orally administered CeO2@Montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Adv. Funct. Mater. 2020, 30, 2004692. [Google Scholar] [CrossRef]
- Kang, F.; Ge, Y.; Hu, X.; Goikavi, C.; Waigi, M.G.; Gao, Y.; Ling, W. Understanding the sorption mechanisms of aflatoxin B1 to kaolinite, illite, and smectite clays via a comparative computational study. J. Hazard. Mater. 2016, 15, 80–87. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Zague, V.; Dario, M.F.; Nishikawa, D.O.; Pinto, C.A.; Almeida, M.M.; Trossini, G.H.G.; Coelho, A.C.V.; Baby, A.R. Characterization and short-term clinical study of clay facial mask. Rev. Ciênc Farm. Básica Apl. 2016, 37, 1–6. [Google Scholar]
- Cosmetic Clay Market Overview, Trends Evaluation|Evaluating Trends and Forecasted Outlook for 2024–2031. Available online: https://www.linkedin.com/pulse/cosmetic-clay-market-overview-trends-evaluation-evaluating-gshcf (accessed on 18 December 2024).
- Nkosi, S.M.; Thembane, N. Physical, chemical and biological characteristics of clays from Durban (South Africa) for applications in cosmetics. Anal. Sci. Adv. 2024, 5, 2300062. [Google Scholar] [CrossRef]
- Reeuwijk, N.M.; Klerx, W.N.M.; Kooijman, M.; Hoogenboom, L.A.P.; Rietjens, I.M.C.M.; Martena, M.J. Levels of lead, arsenic, mercury and cadmium in clays for oral use on the Dutch market and estimation of associated risks. Food Addit. Contam. Part A 2013, 30, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Gyamfi, O.; Aboko, J.; Ankapong, E.; Marfo, J.T.; Awuah-Boateng, N.; Sarpong, K.; Dartey, E. A systematic review of heavy metals contamination in cosmetics. Cutan. Ocul. Toxicol. 2023, 43, 5–12. [Google Scholar] [CrossRef]
- International Cooperation on Cosmetics Regulation. Recommendation for Acceptable Trace Mercury Levels in Cosmetic Products. 2016. Available online: https://www.iccr-cosmetics.org/topics-documents/12:traces (accessed on 5 May 2025).
- Ferreira-Rodríguez, N.; Castro, A.J.; Tweedy, B.N.; Quintas-Soriano, C.; Vaughn, C.C. Mercury consumption and human health: Linking pollution and social risk perception in the Southeastern United States. J. Environ. Manag. 2021, 282, 111528. [Google Scholar] [CrossRef]
- Gworek, B.; Dmuchowski, W.; Baczewska-Dąbrowska, A.H. Mercury in the terrestrial environment: A review. Environ. Sci. Eur. 2020, 32, 128. [Google Scholar] [CrossRef]
- Oloruntoba, A.; Omoniyi, A.O.; Shittu, Z.A.; Ajala, R.O.; Kolawole, S.A. Heavy metal contamination in soils, water, and food in Nigeria from 2000–2019: A systematic review on methods, pollution level and policy implications. Water Air Soil. Pollut. 2024, 235, 586. [Google Scholar] [CrossRef]
- Kang, B.; Wang, J.; Guo, S.; Yang, L. Mercury-induced toxicity: Mechanisms, molecular pathways, and gene regulation. Sci. Total Environ. 2024, 15, 173577. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, S.; Nieuwenhuijsen, M.J.; Elliott, P.; Jarup, L. Kidney disease mortality and environmental exposure to mercury. Am. J. Epidemiol. 2007, 1, 16572–16577. [Google Scholar] [CrossRef]
- Rojas-Franco, P.; Franco-Colín, M.; Torres-Manzo, A.P.; Blas-Valdivia, V.; Thompson-Bonilla, M.D.R.; Kandir, S.; Cano-Europa, E. Endoplasmic reticulum stress participates in the pathophysiology of mercury-caused acute kidney injury. Ren. Fail. 2019, 41, 1001–1010. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, B.; Hu, S.; Wang, T.; Zhang, Y.; Wang, J.; Liu, Y.; Zhang, H. The role of endoplasmic reticulum stress in renal damage caused by acute mercury chloride poisoning. J. Toxicol. Sci. 2020, 45, 589–598. [Google Scholar] [CrossRef]
- Hu, X.F.; Lowe, M.; Chan, H.M. Mercury exposure, cardiovascular disease, and mortality: A systematic review and dose-response meta-analysis. Environ. Res. 2021, 193, 110538. [Google Scholar] [CrossRef]
- Houston, M.C. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J. Clin. Hypertens. 2011, 13, 621–762. [Google Scholar] [CrossRef] [PubMed]
- Neisi, A.; Nasab, F.K.; Sepahvand, A.; Falahi, B.; Taherian, M.; Farhadi, A.; Asban, P.; Pour, N.T.; Farhadi, M.; Dargahi, A. Exposure to mercury in the air and its effect on cardiovascular diseases (CVD): A systematic review. Iran. J. Public Health 2024, 53, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wu, N.; Du, X.; Li, H.; Mei, X.; Song, Y. Toxic nephropathy secondary to chronic mercury poisoning: Clinical characteristics and outcomes. Kidney Int. Rep. 2022, 18, 1189–1197. [Google Scholar] [CrossRef]
- Spectro-Lab. Analizator Rtęci AMA 254. Available online: http://www.spectro-lab.pl/produkt/analizator-rteci-ama-254 (accessed on 12 November 2024).
- Kozak, L.; Niedzielski, P. Theoretical basis of atomic absorption spectrometry. LAB Lab. Apar. Badania 2011, 16, 12–14. [Google Scholar]
- Certified Reference Material. Available online: http://www.ichtj.waw.pl/drupal/pliki/certyfikaty/2016/MPH.pdf (accessed on 12 November 2024).
- Transform Your Enterprise with TIBCO Platform: Real-Time Data Solutions. TIBCO. Available online: https://www.tibco.com (accessed on 12 November 2024).
- StatSoft (2006). Elektroniczny Podręcznik Statystyki PL, Krakow, Poland. Version 13.3.0. Available online: http://www.statsoft.pl/textbook/stathome.html (accessed on 2 November 2024).
- Cosmetic Clay-Global Market Share and Ranking, Overall Sales and Demand Forecast 2024–2030. Available online: https://reports.valuates.com/market-reports/QYRE-Auto-20G9914/global-cosmetic-clay (accessed on 12 January 2025).
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Hair care cosmetics: From traditional shampoo to solid clay and herbal shampoo, A Review. Cosmetics 2019, 6, 13. [Google Scholar] [CrossRef]
- Ustymenko, R. Trends and Innovations in Cosmetic Marketing. Econ. Educ. 2023, 8, 12–17. [Google Scholar] [CrossRef]
- Wargala, E.; Sławska, M.; Zalewska, A.; Toporowska, M. Health effects of dyes, minerals, and vitamins used in cosmetics. Women 2021, 1, 223–237. [Google Scholar] [CrossRef]
- Statista. Cosmetics–Worldwide. Available online: https://www.statista.com/outlook/cmo/beauty-personal-care/cosmetics/worldwide (accessed on 18 November 2024).
- Mattioli, M.; Giardini, L.; Roselli, C.; Desideri, D. Mineralogical characterization of commercial clays used in cosmetics and possible risk for health. Appl. Clay Sci. 2016, 119, 449–454. [Google Scholar] [CrossRef]
- Moraes, J.D.D.; Bertolino, S.R.A.; Cuffini, S.L.; Ducart, D.F.; Bretzke, P.E.; Leonardi, G.R. Clay minerals: Properties and applications to dermocosmetic products and perspectives of natural raw materials for therapeutic purposes—A Review. Int. J. Pharm. 2017, 534, 213–219. [Google Scholar] [CrossRef]
- Meena, P.; Jha, V. The unfairness of fairness creams: Unveiling the toxic impact on kidneys of mercury in beauty products. Kidney Int. 2024, 106, 337–340. [Google Scholar] [CrossRef]
- National Archives. Code of Federal Regulation. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-G/part-700/subpart-B/section-700.13 (accessed on 18 November 2024).
- Regulation (EC) No 1223/2009; The European Parliament and of the Council of 30 November 2009 on Cosmetic Products. European Commission: Brussels, Belgium, 2009.
- Nasirudeen, M.; Amaechi, A. Spectrophotometric determination of heavy metals in cosmetics sourced from Kaduna Metropolis, Nigeria. Sci. World J. 2015, 10, 1–5. [Google Scholar]
- Parmar, A.; Patel, A. Review on risk of toxic heavy metals present in cosmetic products. Int. J. Pharm. Sci. 2024, 2, 277–286. [Google Scholar] [CrossRef]
- Saah, S.A.; Boadi, N.O.; Sakyi, P.O.; Darko, G.; Mensah, M.B. Risk of exposure to trace elements through the application of facial makeup powders. J. Chem. 2022, 2022, 9229134. [Google Scholar] [CrossRef]
- Shomar, B.; Rashkeev, S.N. A comprehensive risk assessment of toxic elements in international brands of face foundation powders. Environ. Res. 2021, 192, 110274. [Google Scholar] [CrossRef]
- Akhtar, A.; Kazi, T.G.; Afridi, H.I.; Khan, M. Human exposure to toxic elements through facial cosmetic products: Dermal risk assessment. Regul. Toxicol. Pharmacol. 2022, 131, 105145. [Google Scholar] [CrossRef]
- Mohammed, T.; Rambocas, N.; Basdeo, S.; Kissoon, Y. Evaluation of mercury in skin lightening creams commonly used in Trinidad and Tobago and their associated health risk. Eur. Res. J. 2024, 10, 276–285. [Google Scholar] [CrossRef]
- Moniczewski, A.; Starek, M.; Rutkowska, A. Toxicological aspects of metal impurities in cosmetics. Med. Inter. Rev. 2016, 27, 81–90. Available online: https://interrev.com/mir/index.php/mir/article/view/4 (accessed on 4 May 2025).
- Sin, K.W.; Tsang, H.F. Large-scale mercury exposure due to a cream cosmetic: Community-wide case series. Hong Kong Med. J. 2003, 5, 329–334. [Google Scholar]
- Podgórska, A.; Puścion-Jakubik, A.; Grodzka, A.; Naliwajko, S.K.; Markiewicz-Żukowska, R.; Socha, K. Natural and Conventional Cosmetics —Mercury Exposure Assessment. Molecules 2021, 26, 4088. [Google Scholar] [CrossRef]
- Hepp, N.M.; Mindak, W.R.; Gasper, J.W.; Thompson, C.B.; Barrows, J.N. Survey of cosmetics for arsenic, cadmium, chromium, cobalt, lead, mercury, and nickel content. J. Cosmet. Sci. 2014, 65, 125–145. [Google Scholar]
- Gomes, C.; Rautureau, M.; Poustis, J.; Gomes, J. Benefits and risks of clays and clay minerals to human health from ancestral to current times: A synoptic overview. Clays Clay Miner. 2021, 69, 612–632. [Google Scholar] [CrossRef]
- Adepoju-Bello, A.A.; Oguntibeju, O.O.; Adebisi, R.A.; Okpala, N.; Coker, H.A.B. Evaluation of the concentration of toxic metals in cosmetic products in Nigeria. Afr. J. Biotech. 2012, 11, 16360–16364. Available online: https://academicjournals.org/journal/AJB/article-abstract/1E25B0825009 (accessed on 12 January 2025).
- Amended Safety Assessment of Naturally-Sourced Clays Washington, 2022; 1620 L Street NW, Suite 1200, DC. Available online: https://www.cir-safety.org/sites/default/files/Clays_0.pdf (accessed on 12 January 2025).
- Attard, T.; Attard, E. Heavy Metals in Cosmetics; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Ahmed, M.; Ahmad, M.; Sohail, A.; Sanaullah, M.; Saeed, A.; Qamar, S.; Wani, T.A.; Zargar, S.; Alkahtani, H.M.; Khalid, K. Multivariate statistical analysis of cosmetics due to potentially toxic/heavy metal (loid) contamination: Source identification for sustainability and human health risk assessment. Sustainability 2024, 16, 6127. [Google Scholar] [CrossRef]
- Ricketts, P.; Knight, C.; Gordon, A.; Boischio, A.; Voutchkov, M. Mercury exposure associated with use of skin lightening products in Jamaica. J. Health Pollut. 2020, 4, 200601. [Google Scholar] [CrossRef]
- Habib, U.; Pervaiz, A.; Murtaza, A.; Hussain, A.; Sarwar, H.; Ullah, M. Assessing the cumulative effects of heavy metal exposure from cosmetic products on local consumers. J. Mt. Area Res. 2024, 9, 149–167. [Google Scholar] [CrossRef]
- Guillaume, M.; |Guillemin, L.; Pirnay, S. Rising consumer demand for green cosmetics: The necessity of an ecotoxicological score in the cosmetic industry. Int. J. Cosmet. Sci. 2025, 9, 535–541. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin. Toxicol. 2011, 49, 886–891. [Google Scholar] [CrossRef]

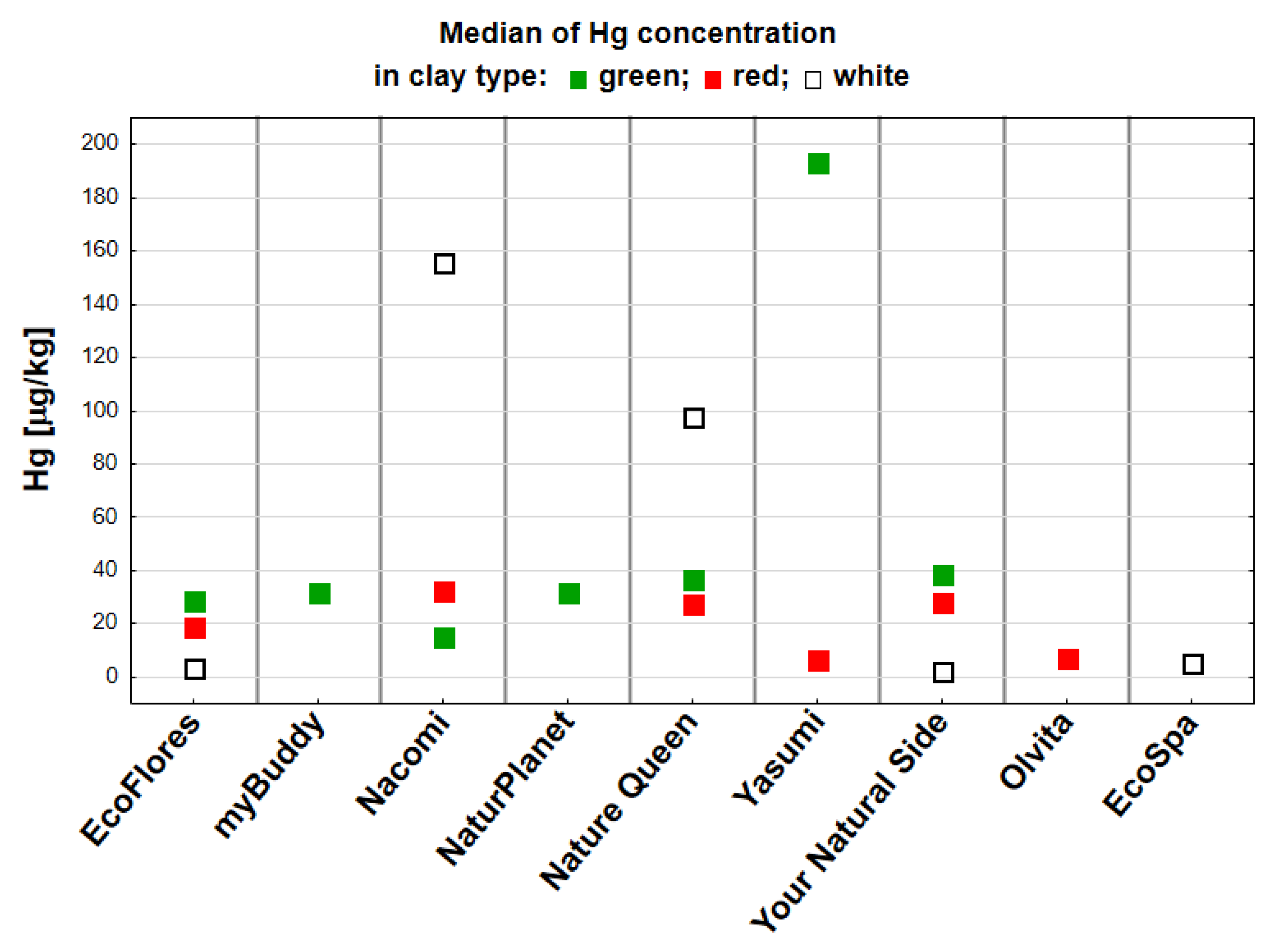
| Manufacturer | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yasumi (Kalisz, Poland | Olvita (Marcinowice, Poland | Nacomi (Wilkowice, Poland) | NaturPlanet (Katowice, Poland) | MyBuddy (Rzeszow, Poland) | EcoFlores (Nowy Targ, Poland) | EcoSpa (Warszawa, Poland) | Your Natural Side (Krakow, Poland) | Nature Queen (Poznan, Poland) | Total N | % | |
| Red | 3 | 3 | 3 | 3 | 3 | 3 | 18 | 16 | |||
| Green | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 21 | 19 | ||
| Purple | 3 | 3 | 3 | 3 | 12 | 11 | |||||
| Blue | 3 | 3 | 3 | 9 | 8 | ||||||
| Rose | 3 | 3 | 3 | 9 | 8 | ||||||
| White | 3 | 3 | 3 | 3 | 3 | 15 | 14 | ||||
| Black | 3 | 3 | 6 | 5 | |||||||
| Yellow | 3 | 3 | 3 | 3 | 12 | 11 | |||||
| Rhassoul | 3 | 3 | 6 | 5 | |||||||
| Orange | 3 | 3 | 3 | ||||||||
| Total | 6 | 3 | 12 | 3 | 3 | 27 | 12 | 24 | 21 | 111 | 100 |
| Clay | N | AM ± SD | Me | Min | Max | Quartile | CV [%] | |
|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | |||||||
| White | 15 | 52.80 ± 64.97 | 5.31 | 2.31 | 158.41 | 3.51 | 99.39 | 123 |
| Red | 18 | 19.71 ± 10.20 | 21.29 | 5.82 | 33.63 | 7.26 | 28.09 | 52 |
| Black | 6 | 21.79 ± 20.75 | 21.88 | 2.38 | 40.98 | 3.02 | 40.62 | 95 |
| Purple | 12 | 2.56 ± 0.46 | 2.54 | 1.87 | 3.31 | 2.24 | 2.88 | 18 |
| Blue | 9 | 3.69 ± 0.52 | 3.52 | 3.12 | 4.61 | 3.32 | 4.08 | 14 |
| Orange | 3 | 27.24 ± 2.31 | 26.52 | 25.38 | 29.83 | 25.38 | 29.83 | 9 |
| Rose | 9 | 10.08 ± 1.38 | 9.56 | 8.36 | 12.63 | 9.37 | 11.03 | 14 |
| Rhassoul | 6 | 38.66 ± 9.80 | 38.72 | 29.23 | 48.26 | 29.67 | 47.38 | 25 |
| Green | 21 | 53.26 ± 57.45 | 32.24 | 13.69 | 200.81 | 29.41 | 38.64 | 108 |
| Yellow | 12 | 28.74 ± 1.96 | 28.03 | 26.73 | 32.58 | 27.14 | 29.64 | 7 |
| All | 111 | 28.91 ± 39.34 | 23.72 | 1.87 | 200.81 | 4.08 | 32.05 | 136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, A.; Brodziak-Dopierała, B.; Jańska, W.; Jeyranyan, L.; Malara, B. Analysis of Mercury Concentration in Cosmetic Clays. Toxics 2025, 13, 507. https://doi.org/10.3390/toxics13060507
Fischer A, Brodziak-Dopierała B, Jańska W, Jeyranyan L, Malara B. Analysis of Mercury Concentration in Cosmetic Clays. Toxics. 2025; 13(6):507. https://doi.org/10.3390/toxics13060507
Chicago/Turabian StyleFischer, Agnieszka, Barbara Brodziak-Dopierała, Wiktoria Jańska, Luiza Jeyranyan, and Beata Malara. 2025. "Analysis of Mercury Concentration in Cosmetic Clays" Toxics 13, no. 6: 507. https://doi.org/10.3390/toxics13060507
APA StyleFischer, A., Brodziak-Dopierała, B., Jańska, W., Jeyranyan, L., & Malara, B. (2025). Analysis of Mercury Concentration in Cosmetic Clays. Toxics, 13(6), 507. https://doi.org/10.3390/toxics13060507









