Distribution, Diversity, and Ecological Risks of Microplastics in Mangrove Ecosystems of a Southeastern Chinese Estuary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Laboratory Procedure
2.3. Quality Assessment and Control
2.4. Data Analysis
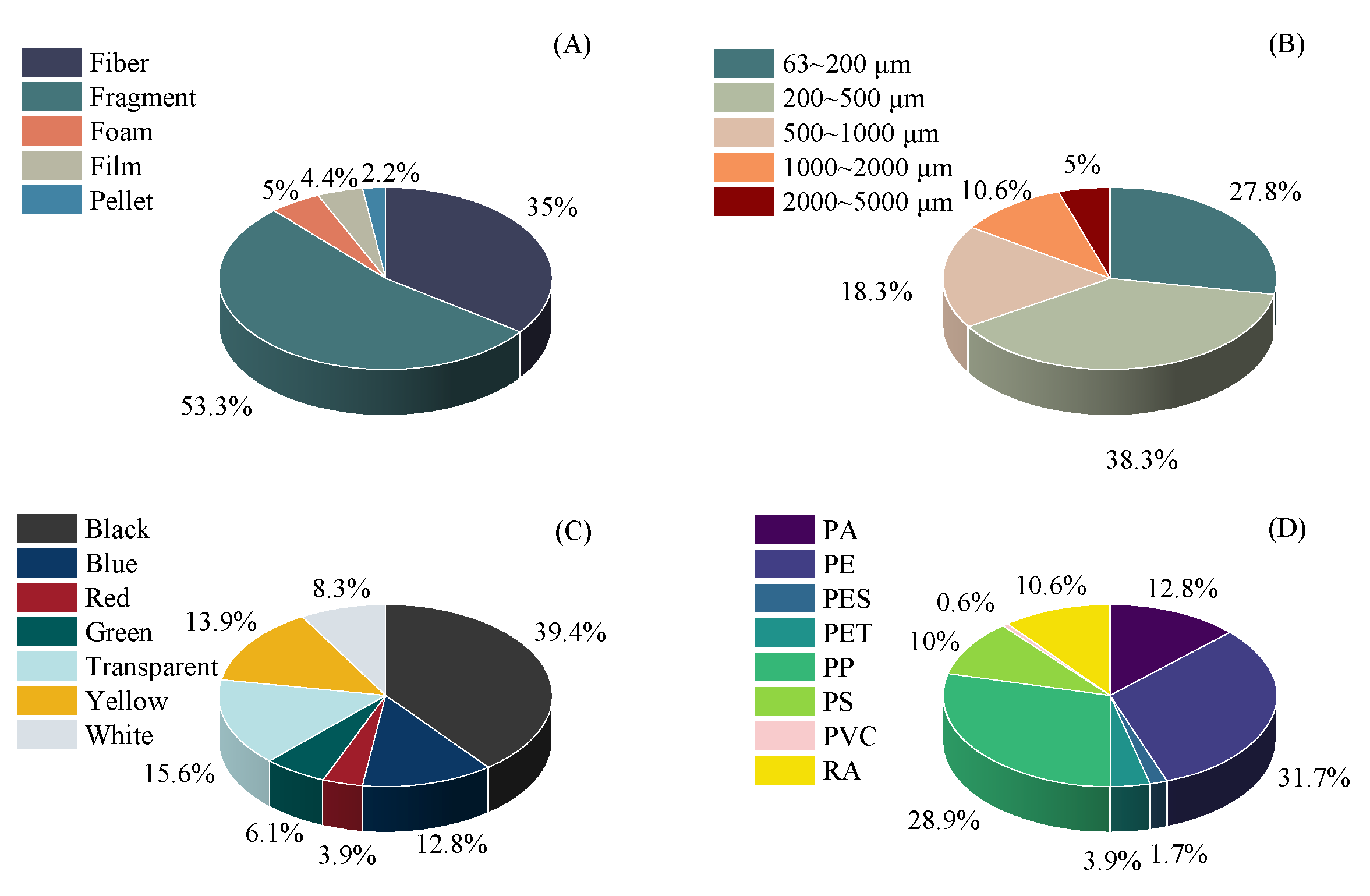
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminants in the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Saito, H.; Fletcher, R.; Yogi, T.; Kayo, M.; Miyagi, S.; Ogido, M.; Fujikura, K. Human footprint in the abyss: 30-year records of deep-sea plastic debris. Mar. Policy. 2018, 96, 204–212. [Google Scholar] [CrossRef]
- Rochman, C.M.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and its analogs in muscle and liver of fish from the North East Atlantic Ocean in relation to microplastic contamination: Exposure and risk to human consumers. J. Hazard. Mater. 2020, 393, 122419. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Lemos, B.; Ren, H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017, 7, 46687. [Google Scholar] [CrossRef]
- Horvatits, T.; Tamminga, M.; Liu, B.; Sebode, M.; Carambia, A.; Fischer, L.; Püschel, K.; Huber, S.; Fischer, E.K. Microplastics detected in cirrhotic liver tissue. EBioMedicine 2022, 82, 104147. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- Asari, N.; Suratman, M.N.; Mohd Ayob, N.A.; Abdul Hamid, N.H. Mangrove as a Natural Barrier to Environmental Risks and Coastal Protection; Springer: Singapore, 2021; pp. 305–322. [Google Scholar] [CrossRef]
- Deng, H.; He, J.; Feng, D.; Zhao, Y.; Sun, W.; Yu, H.; Ge, C. Microplastics pollution in mangrove ecosystems: A critical review of current knowledge and future directions. Sci. Total Environ. 2021, 753, 142041. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Chen, L.; Wang, X. Ecological interception effect of mangroves on microplastics. J. Hazard. Mater. 2022, 423, 127231. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, L.; Lin, B.; Liu, B.; Guan, T.; Guo, S.; Li, Q.; Wei, C. Differences in the uptake and translocation of differentially charged microplastics by the taproot and lateral root of mangroves. Sci. Total Environ. 2024, 945, 174113. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Lykaki, M.; Alrajoula, M.T.; Markiewicz, M.; Kraas, C.; Kolbe, S.; Klinkhammer, K.; Rabe, M.; Klauer, R.; Bendt, E.; et al. Microplastics from textile origin—Emission and reduction measures. Green Chem. 2021, 23, 5247–5271. [Google Scholar] [CrossRef]
- Dimassi, S.N.; Hahladakis, J.N.; Yahia, M.N.D.; Ahmad, M.I.; Sayadi, S.; Al-Ghouti, M.A. Degradation-fragmentation of marine plastic waste and their environmental implications: A critical review. Arab. J. Chem. 2022, 15, 104262. [Google Scholar] [CrossRef]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.; Ragas, A. Wear and tear of tyres: A stealthy source of microplastics in the environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Archodoulaki, V.-M.; Pomakhina, E.; Pukánszky, B.; Zinöcker, E.; Gahleitner, M. Environmental degradation and formation of secondary microplastics from packaging material: A polypropylene film case study. Polym. Degrad. Stabil. 2022, 195, 109794. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Ji, B.; Qiang, Z.; Karanfil, T.; Liu, C. UV Aging of microplastic polymers promotes their chemical transformation and byproduct formation upon chlorination. Sci. Total Environ. 2023, 858, 159842. [Google Scholar] [CrossRef]
- Tang, Y.; Xing, Y.; Wang, X.; Ya, H.; Zhang, T.; Lv, M.; Wang, J.; Zhang, H.; Dai, W.; Zhang, D.; et al. PET microplastics influenced microbial community and heavy metal speciation in heavy-metal contaminated soils. Appl. Soil Ecol. 2024, 201, 105488. [Google Scholar] [CrossRef]
- Zhou, Q.; Tu, C.; Fu, C.; Li, Y.; Zhang, H.; Xiong, K.; Zhao, X.; Li, L.; Waniek, J.J.; Luo, Y. Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci. Total Environ. 2020, 703, 134807. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, X.; Wen, L.; Li, Z.; Peng, M.; Wu, H.; Xie, L. Linking human activity to spatial accumulation of microplastics along mangrove coasts. Sci. Total Environ. 2022, 825, 154014. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Li, Y.; Wang, J.; Yin, X.; Ye, X.; Wang, A.; Wang, L. Sedimentary records of human activity and natural environmental evolution in sensitive ecosystems: A case study of a coral nature reserve in Dongshan Bay and a mangrove forest nature reserve in Zhangjiang River estuary, Southeast China. Org. Geochem. 2018, 121, 22–35. [Google Scholar] [CrossRef]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Li, X.; Wu, F.; Zhang, C.; Wang, T. The effects of rainfall events on the composition and diversity of microplastics on beaches in Xiamen City on a short-term scale. Toxics 2024, 12, 375. [Google Scholar] [CrossRef]
- Yang, D.; Shi, H.; Li, L.; Li, J.; Jabeen, K.; Kolandhasamy, P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015, 49, 13622–13627. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, H.; Wu, Y.; Chen, T.; Deng, L.; Zhang, L.; Feng, G.; Liang, H.; Li, H. Evaluation of soil fauna biodiversity in restored farmland for protection of wetland ecology by planting different crops. Ocean Coast. Manag. 2024, 247, 106945. [Google Scholar] [CrossRef]
- Shekoohiyan, S.; Akbarzadeh, A. The abundance of microplastic pollution along the Jajroud river of Tehran: Estimating the water quality index and the ecological risk. Ecol. Indic. 2022, 145, 109629. [Google Scholar] [CrossRef]
- Yang, T.; Zeng, Y.; Kang, Z.; Cai, M.; Chen, K.; Zhao, Q.; Lin, J.; Liu, R.; Xu, G. Enrichment and ecological risks of microplastics in mangroves of southern Hainan Island, China. Sci. Total Environ. 2023, 889, 164160. [Google Scholar] [CrossRef] [PubMed]
- Lithner, D.; Larsson, Å.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Han, J.; Cheung, S.G.; Chong, R.K.Y.; Lo, C.-M.; Lee, F.W.-F.; Xu, S.J.-L.; Yang, Y.; Tam, N.F.; Zhou, H.-C. How mangrove plants affect microplastic distribution in sediments of coastal wetlands: Case study in Shenzhen Bay, South China. Sci. Total Environ. 2021, 767, 144695. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Sun, Y.; Li, H.; Hu, Y.; Lin, L.; Peng, J.; Xu, X. Microplastics in mangrove sediments of the Pearl River Estuary, South China: Correlation with halogenated flame retardants’ levels. Sci. Total Environ. 2020, 725, 138344. [Google Scholar] [CrossRef]
- Nabizadeh, R.; Sajadi, M.; Rastkari, N.; Yaghmaeian, K. Microplastic pollution on the Persian Gulf shoreline: A case study of Bandar Abbas City, Hormozgan Province, Iran. Mar. Pollut. Bull. 2019, 145, 536–546. [Google Scholar] [CrossRef]
- Aguirre-Sanchez, A.; Purca, S.; Cole, M.; Indacochea, A.G.; Lindeque, P.K. Prevalence of microplastics in Peruvian mangrove sediments and edible mangrove species. Mar. Pollut. Bull. 2024, 200, 116075. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Jian, Q.; Zhang, P.; Lu, Y.; Liu, H. Tidal variation shaped microplastic enrichment patterns in mangrove blue carbon ecosystem of northern Beibu Gulf, China. Front. Mar. Sci. 2022, 9, 927884. [Google Scholar] [CrossRef]
- Cordova, M.R.; Ulumuddin, Y.I.; Lubis, A.A.; Kaisupy, M.T.; Wibowo, S.P.A.; Subandi, R.; Yogaswara, D.; Purbonegoro, T.; Renyaan, J.; Nurdiansah, D.; et al. Microplastics leaving a trace in mangrove sediments ever since they were first manufactured: A study from Indonesia mangroves. Mar. Pollut. Bull. 2023, 195, 115517. [Google Scholar] [CrossRef]
- Amrutha, K.; Warrier, A.K. The first report on the source-to-sink characterization of microplastic pollution from a riverine environment in tropical India. Sci. Total Environ. 2020, 739, 140377. [Google Scholar] [CrossRef]
- Zhang, P.; Tang, X.; Qin, N.; Shuai, Y.; Wang, J.; Wang, H.; Ouyang, Z.; Jia, H. Advanced understanding of the natural forces accelerating aging and release of black microplastics (tire wear particles) based on mechanism and toxicity analysis. Water Res. 2024, 266, 122409. [Google Scholar] [CrossRef]
- Ramakrishnan, D.; Sathiyamoorthy, M. Seasonal distribution, source apportionment and risk exposure of microplastic contaminants along the Muttukadu backwater estuary, Tamil Nadu, India. Results Eng. 2024, 23, 102776. [Google Scholar] [CrossRef]
- Alam, J.M.; Shammi, M.; Tareq, S.M. Distribution of microplastics in shoreline water and sediment of the Ganges River Basin to Meghna Estuary in Bangladesh. Ecotox. Environ. Safe 2023, 266, 115537. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Rao, Q.; Liu, J.; Chen, J.; Xie, P. Occurrence and characteristics of microplastics across the watershed of the world’s third-largest river. J. Hazard. Mater. 2024, 480, 135998. [Google Scholar] [CrossRef] [PubMed]
- Jiao, M.; Ren, L.; Wang, Y.; Ding, C.; Li, T.; Cao, S.; Li, R.; Wang, Y. Mangrove forest: An important coastal ecosystem to intercept river microplastics. Environ. Res. 2022, 210, 112939. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Wu, L.; Yang, Y.; Yu, X.; Liu, Q.; Liu, X.; Li, Y.; Wang, X. Vertical distribution and river-sea transport of microplastics with tidal fluctuation in a subtropical estuary, China. Sci. Total Environ. 2022, 822, 153603. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, Q.; Du, R.; Li, W.; Lin, H. Reinforced human intervention drives microplastic pollution in estuarine beaches and nearshore sediments of Dongshan Bay, China. Gondwana Res. 2023, 119, 153–163. [Google Scholar] [CrossRef]
- Martin, C.; Almahasheer, H.; Duarte, C.M. Mangrove forests as traps for marine litter. Environ. Pollut. 2019, 247, 499–508. [Google Scholar] [CrossRef]
- Zamprogno, G.C.; Caniçali, F.B.; Cozer, C.D.R.; Otegui, M.B.P.; Graceli, J.B.; Costa, M.B.D. Spatial distribution of microplastics in the superficial sediment of a mangrove in Southeast Brazil: A comparison between fringe and basin. Sci. Total Environ. 2021, 784, 146963. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, M.; Li, T.; Li, R.; Liu, B. Role of mangrove forest in interception of microplastics (MPs): Challenges, progress, and prospects. J. Hazard. Mater. 2023, 445, 130636. [Google Scholar] [CrossRef]
- Gonalves, G.R.; Koomson, A.; Aggrey-Fynn, J.; Nyarko, B.K.; Narayanaswamy, B.E. Invisible Peril: Assessing microplastic pollution in Ghanaian mangroves. Mar. Pollut. Bull. 2025, 211, 117361. [Google Scholar] [CrossRef]
- Liang, X.; Liu, C.; Wang, H.; Li, H.; Luo, J.; Luo, G.; Hu, W.; Lan, W.; Wu, L.; Fang, S.; et al. Spatial retention, absorption, transport, and enrichment of microplastics in mangrove sediment complex system. Environ. Pollut. 2025, 375, 126354. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Shin, Y.; Song, I.; Lim, J.; Ok, Y.S.; Weon, S. Atmospheric microplastics: Challenges in site- and target-specific measurements. TrAC Trends Anal. Chem. 2024, 178, 117859. [Google Scholar] [CrossRef]
- Wu, F.; Li, X.; Zhang, C.; Wang, T. Spatiotemporal distribution and diversity of microplastics in the sediment of beaches in Xiamen City, China. J. Oceanol. Limnol. 2025, 43, 396–405. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, Z.; An, C.; Yang, X.; Wang, Z. Tide-induced infiltration and resuspension of microplastics in shorelines: Insights from tidal tank experiments. Water Res. 2023, 236, 119970. [Google Scholar] [CrossRef]
- Lin, J.; Zheng, J.-Y.; Zhan, Z.-G.; Zhao, Y.-M.; Zhou, Q.-Z.; Peng, J.; Li, Y.; Xiao, X.; Wang, J.-H. Abundant small microplastics hidden in water columns of the Yellow Sea and East China Sea: Distribution, transportation and potential risk. J. Hazard. Mater. 2024, 478, 135531. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Guo, J.; Yu, K.; Wang, Y.; Li, R. Dynamic distribution of microplastics in mangrove sediments in Beibu Gulf, South China: Implications of tidal current velocity and tidal range. J. Hazard. Mater. 2020, 399, 122849. [Google Scholar] [CrossRef]
- Prarat, P.; Hongsawat, P.; Chouychai, B. Microplastic occurrence in surface sediments from coastal mangroves in Eastern Thailand: Abundance, characteristics, and ecological risk implications. Reg. Stud. Mar. Sci. 2024, 71, 103389. [Google Scholar] [CrossRef]
- Li, R.; Yu, L.; Chai, M.; Wu, H.; Zhu, X. The distribution, characteristics and ecological risks of microplastics in the mangroves of Southern China. Sci. Total Environ. 2020, 708, 135025. [Google Scholar] [CrossRef]
- Bakir, A.; Rowland, S.J.; Thompson, R.C. Enhanced desorption of persistent organic pollutants from microplastics under simulated physiological conditions. Environ. Pollut. 2014, 185, 16–23. [Google Scholar] [CrossRef]
- Donkers, J.M.; Höppener, E.M.; Grigoriev, I.; Will, L.; Melgert, B.N.; Van Der Zaan, B.; Van De Steeg, E.; Kooter, I.M. Advanced epithelial lung and gut barrier models demonstrate passage of microplastic particles. Microplast. Nanoplast. 2022, 2, 6. [Google Scholar] [CrossRef]
- Zhu, L.; Xie, C.; Chen, L.; Dai, X.; Zhou, Y.; Pan, H.; Tian, K. Transport of microplastics in the body and interaction with biological barriers, and controlling of microplastics pollution. Ecotox. Environ. Safe 2023, 255, 114818. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Craig, N.; Su, L. A hidden pathway for human exposure to micro- and nanoplastics—The mechanical fragmentation of plastic products during daily use. Toxics 2023, 11, 774. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Lee, J.-Y.; Redwan, M. Animal exposure to microplastics and health effects: A review. Emerg. Contam. 2024, 10, 100369. [Google Scholar] [CrossRef]
- Choi, D.; Hwang, J.; Bang, J.; Han, S.; Kim, T.; Oh, Y.; Hwang, Y.; Choi, J.; Hong, J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Sci. Total Environ. 2021, 752, 142242. [Google Scholar] [CrossRef] [PubMed]




| Risk Category | Site | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | |
| PLI | 2.38 | 2.58 | 2.16 | 2.12 | 1.91 | 1.87 | 1.26 |
| Hazard level | I | I | I | I | I | I | I |
| PHI | 468.76 | 16.77 | 16.28 | 12.95 | 14.00 | 16.00 | 15.50 |
| Hazard level | IV | III | III | III | III | III | III |
| PERI | 9.38 | 0.34 | 0.33 | 0.26 | 0.28 | 0.32 | 0.31 |
| Hazard level | I | I | I | I | I | I | I |
| Risk Category | Habitats | |
|---|---|---|
| Interior | Edge | |
| PLI | 2.66 | 1.72 |
| Hazard level | I | I |
| PHI | 506.18 | 54.08 |
| Hazard level | IV | III |
| PERI | 10.12 | 1.08 |
| Hazard level | I | I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Zhang, C.; Li, X.; Liu, S.; Wang, J.; Huang, W. Distribution, Diversity, and Ecological Risks of Microplastics in Mangrove Ecosystems of a Southeastern Chinese Estuary. Toxics 2025, 13, 494. https://doi.org/10.3390/toxics13060494
Wu F, Zhang C, Li X, Liu S, Wang J, Huang W. Distribution, Diversity, and Ecological Risks of Microplastics in Mangrove Ecosystems of a Southeastern Chinese Estuary. Toxics. 2025; 13(6):494. https://doi.org/10.3390/toxics13060494
Chicago/Turabian StyleWu, Fengrun, Chengyi Zhang, Xueyan Li, Sha Liu, Jinpu Wang, and Weiqi Huang. 2025. "Distribution, Diversity, and Ecological Risks of Microplastics in Mangrove Ecosystems of a Southeastern Chinese Estuary" Toxics 13, no. 6: 494. https://doi.org/10.3390/toxics13060494
APA StyleWu, F., Zhang, C., Li, X., Liu, S., Wang, J., & Huang, W. (2025). Distribution, Diversity, and Ecological Risks of Microplastics in Mangrove Ecosystems of a Southeastern Chinese Estuary. Toxics, 13(6), 494. https://doi.org/10.3390/toxics13060494






