Metal-Induced Genotoxic Events: Possible Distinction Between Sporadic and Familial ALS
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
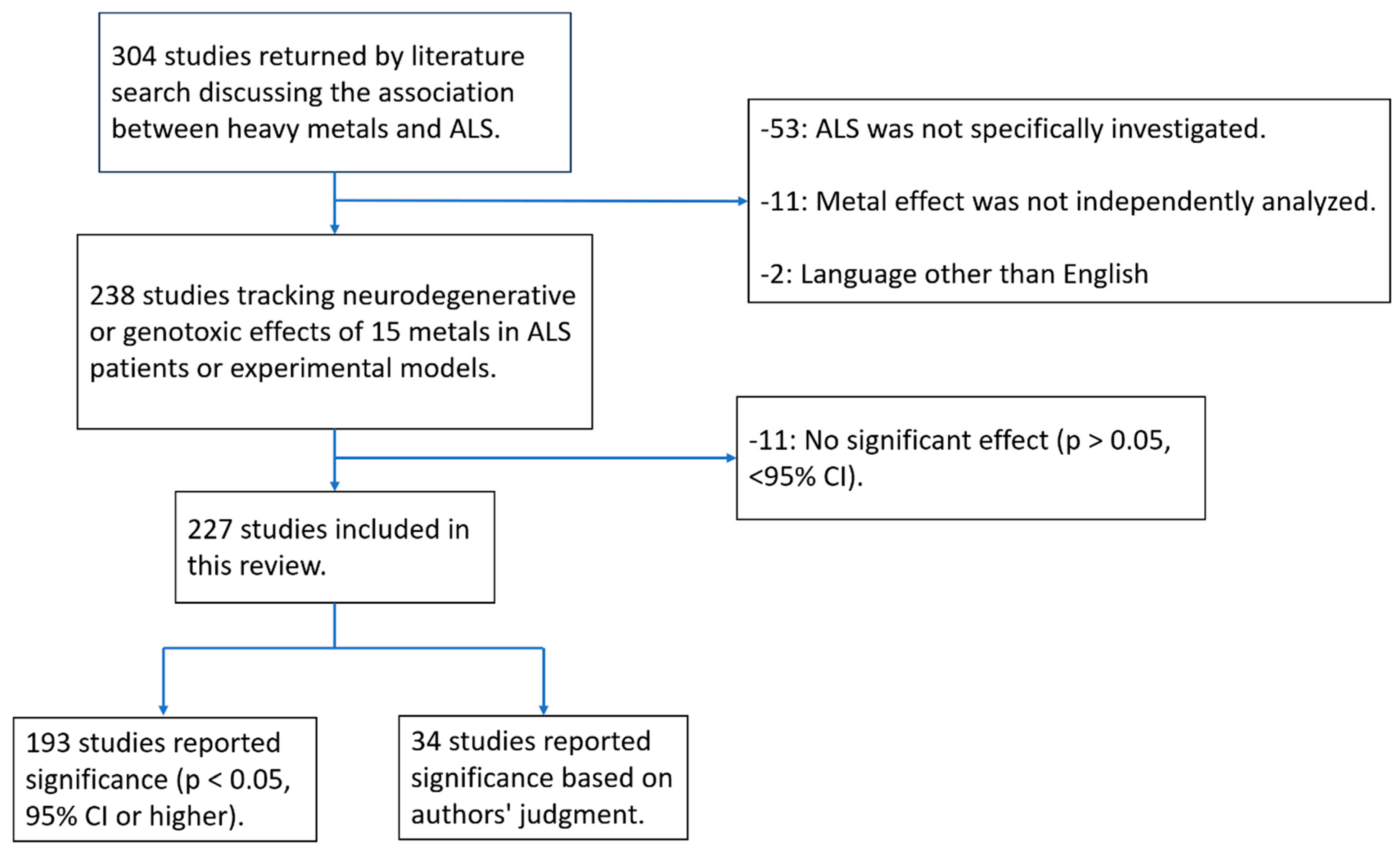
2.2. Study Selection
2.3. Data Extraction
3. Results
3.1. Toxicological Profile Findings
3.2. Summary of Studies
3.3. Metal-Induced Genotoxicity in ALS
3.4. Metal Associations with ALS Mutations
4. Discussion
4.1. Metal Exposure-Induced Genotoxicity in ALS; Unexplored Genotoxic Endpoints
4.2. Genotoxic Biomarkers and Early Diagnosis Potential
4.3. Metal Exposure and ALS-Linked Genetic Mutations
4.4. Divergent Associations of Key Metals in ALS
4.5. A Potential Framework for Distinguishing fALS and sALS
5. Studies Reporting No Statistically Significant Association
Challenges and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Zarei, S.; Carr, K.; Reiley, L.; Diaz, K.; Guerra, O.; Altamirano, P.F.; Pagani, W.; Lodin, D.; Orozco, G.; Chinea, A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015, 6, 171. [Google Scholar] [CrossRef]
- Pugh, K.H.; Zarus, G.M. The burden of environmental disease in the United States. J. Environ. Health 2012, 74, 30–34. [Google Scholar] [PubMed]
- Mehta, P.; Raymond, J.; Punjani, R.; Larson, T.; Han, M.; Bove, F.; Horton, D.K. Incidence of amyotrophic lateral sclerosis in the United States, 2014–2016. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 378–382. [Google Scholar] [CrossRef]
- Mehta, P.; Raymond, J.; Zhang, Y.; Punjani, R.; Han, M.; Larson, T.; Muravov, O.; Lyles, R.H.; Horton, D.K. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph. Lateral Scler. Front. Degener. 2023, 24, 702–708. [Google Scholar] [CrossRef]
- Mehta, P.; Raymond, J.; Nair, T.; Han, M.; Berry, J.; Punjani, R.; Larson, T.; Mohidul, S.; Horton, D.K. Amyotrophic lateral sclerosis estimated prevalence cases from 2022 to 2030, data from the national ALS Registry. Amyotroph. Lateral Scler. Front. Degener. 2025, 26, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ash, P.E.A.; Dhawan, U.; Boudeau, S.; Lei, S.; Carlomagno, Y.; Knobel, M.; Mohanna, L.F.A.A.; Boomhower, S.T.; Newland, M.C.; Sherr, D.H.; et al. Heavy Metal Neurotoxicants Induce ALS-Linked TDP-43 Pathology. Toxicol. Sci. 2019, 167, 105–115. [Google Scholar] [CrossRef]
- Garnier, C.; Devred, F.; Byrne, D.; Puppo, R.; Roman, A.Y.; Malesinski, S.; Golovin, A.V.; Lebrun, R.; Ninkina, N.N.; Tsvetkov, P.O. Zinc binding to RNA recognition motif of TDP-43 induces the formation of amyloid-like aggregates. Sci. Rep. 2017, 7, 6812. [Google Scholar] [CrossRef]
- Talbott, E.O.; Malek, A.M.; Lacomis, D. The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 2016, 138, 225–238. [Google Scholar] [CrossRef]
- Van Daele, S.H.; Moisse, M.; van Vugt, J.J.F.A.; Zwamborn, R.A.J.; van der Spek, R.; van Rheenen, W.; Van Eijk, K.; Kenna, K.; Corcia, P.; Vourc’h, P.; et al. Genetic variability in sporadic amyotrophic lateral sclerosis. Brain A J. Neurol. 2023, 146, 3760–3769. [Google Scholar] [CrossRef]
- Ruf, W.P.; Boros, M.; Freischmidt, A.; Brenner, D.; Grozdanov, V.; de Meirelles, J.; Meyer, T.; Grehl, T.; Petri, S.; Grosskreutz, J.; et al. Spectrum and frequency of genetic variants in sporadic amyotrophic lateral sclerosis. Brain Commun. 2023, 5, fcad152. [Google Scholar] [CrossRef]
- Logan, R.; Dubel-Haag, J.; Schcolnicov, N.; Miller, S.J. Novel Genetic Signatures Associated with Sporadic Amyotrophic Lateral Sclerosis. Front. Genet. 2022, 13, 851496. [Google Scholar] [CrossRef]
- Wang, H.; Guan, L.; Deng, M. Recent progress of the genetics of amyotrophic lateral sclerosis and challenges of gene therapy. Front. Neurosci. 2023, 17, 1170996. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Substance Priority List; U.S. Department of Health and Human Services: Chamblee, GA, USA, 2024. Available online: https://www.atsdr.cdc.gov/spl/ (accessed on 1 May 2024).
- Peters, S.; Broberg, K.; Gallo, V.; Levi, M.; Kippler, M.; Vineis, P.; Veldink, J.; van den Berg, L.; Middleton, L.; Travis, R.C.; et al. Blood Metal Levels and Amyotrophic Lateral Sclerosis Risk: A Prospective Cohort. Ann. Neurol. 2021, 89, 125–133. [Google Scholar] [CrossRef]
- Roelofs-Iverson, R.A.; Mulder, D.W.; Elveback, L.R.; Kurland, L.T.; Molgaard, C.A. ALS and heavy metals: A pilot case-control study. Neurology 1984, 34, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Oliveira, T.; Montezinho, L.; Mendes, C.; Firuzi, O.; Saso, L.; Oliveira, P.J.; Silva, F.S.G. Oxidative Stress in Amyotrophic Lateral Sclerosis: Pathophysiology and Opportunities for Pharmacological Intervention. Oxidative Med. Cell. Longev. 2020, 2020, 5021694. [Google Scholar] [CrossRef] [PubMed]
- Sheykhansari, S.; Kozielski, K.; Bill, J.; Sitti, M.; Gemmati, D.; Zamboni, P.; Singh, V.A. Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: A review. Cell Death Dis. 2018, 9, 348. [Google Scholar] [CrossRef]
- Zakharova, M.N.; Bakulin, I.S.; Abramova, A.A. Toxic damage to motor neurons. Neurochem. J. 2021, 15, 410–421. [Google Scholar] [CrossRef]
- Sirangelo, I.; Iannuzzi, C. The Role of Metal Binding in the Amyotrophic Lateral Sclerosis-Related Aggregation of Copper-Zinc Superoxide Dismutase. Molecules 2017, 22, 1429. [Google Scholar] [CrossRef]
- Kok, J.R.; Palminha, N.M.; Dos Santos Souza, C.; El-Khamisy, S.F.; Ferraiuolo, L. DNA damage as a mechanism of neurodegeneration in ALS and a contributor to astrocyte toxicity. Cell. Mol. Life Sci. CMLS 2021, 78, 5707–5729. [Google Scholar] [CrossRef]
- Sudweeks, S.; Elgethun, K.; Abadin, H.; Zarus, G.; Irvin, E. Applied toxicology at the Agency for Toxic Substances and Disease Registry (ATSDR). Encycl. Toxicol. 2023, 1, 761–767. [Google Scholar]
- Kisby, G.E.; Wilson, D.M., III; Spencer, P.S. Introducing the Role of Genotoxicity in Neurodegenerative Diseases and Neuropsychiatric Disorders. Int. J. Mol. Sci. 2024, 25, 7221. [Google Scholar] [CrossRef]
- Shoeb, M.; Zarus, G.M.; Abadin, H.E. Profiling Metal-Induced Genotoxic Endpoints. J. Environ. Health 2023, 86, 30. [Google Scholar]
- Figueroa-Romero, C.; Mikhail, K.A.; Gennings, C.; Curtin, P.; Bello, G.A.; Botero, T.M.; Goutman, S.A.; Feldman, E.L.; Arora, M.; Austin, C. Early life metal dysregulation in amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2020, 7, 872–882. [Google Scholar] [CrossRef]
- Sirabella, R.; Valsecchi, V.; Anzilotti, S.; Cuomo, O.; Vinciguerra, A.; Cepparulo, P.; Brancaccio, P.; Guida, N.; Blondeau, N.; Canzoniero, L.M.T.; et al. Ionic homeostasis maintenance in ALS: Focus on new therapeutic targets. Front. Neurosci. 2018, 12, 510. [Google Scholar] [CrossRef] [PubMed]
- Andrew, A.S.; Caller, T.A.; Tandan, R.; Duell, E.J.; Henegan, P.L.; Field, N.C.; Bradley, W.G.; Stommel, E.W. Environmental and Occupational Exposures and Amyotrophic Lateral Sclerosis in New England. Neuro-Degener. Dis. 2017, 17, 110–116. [Google Scholar] [CrossRef]
- Yu, B.; Pamphlett, R. Environmental insults: Critical triggers for amyotrophic lateral sclerosis. Transl. Neurodegener. 2017, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Ronnevi, C.; Berntsson, E.; Wärmländer, S.K.T.S.; Roos, P.M. Metals in ALS TDP-43 Pathology. Int. J. Mol. Sci. 2021, 22, 12193. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Calvo, A.; Chio, A.; Colville, S.; Ellis, C.M.; Hardiman, O.; Heverin, M.; Howard, R.S.; Huisman, M.H.B.; Keren, N.; et al. Analysis of amyotrophic lateral sclerosis as a multistep process: A population-based modelling study. Lancet Neurol. 2014, 13, 1108–1113. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profiles; U.S. Department of Health and Human Services: Chamblee, GA, USA, 2014. Available online: https://www.atsdr.cdc.gov/toxicological-profiles/about/index.html (accessed on 11 April 2024).
- Zarus, G.M.; Ruiz, P.; Benedict, R.; Brenner, S.; Carlson, K.; Jeong, L.; Morata, T.C. Which Environmental Pollutants Are Toxic to Our Ears? Evidence of the Ototoxicity of Common Substances. Toxics 2024, 12, 650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trojsi, F.; Monsurr_o, M.; Tedeschi, G. Exposure to environmental toxicants and pathogenesis of amyotrophic lateral sclerosis: State of the art and research perspectives. Int. J. Mol. Sci. 2013, 14, 15286–15311. [Google Scholar] [CrossRef] [PubMed]
- Koski, L.; Berntsson, E.; Vikström, M.; Wärmländer, S.K.T.S.; Roos, P.M. Metal ratios as possible biomarkers for amyotrophic lateral sclerosis. J. Trace Elem. Med. Biol. 2023, 78, 127163. [Google Scholar] [CrossRef] [PubMed]
- Ingre, C.; Roos, P.M.; Piehl, F.; Kamel, F.; Fang, F. Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 2015, 7, 181–193. [Google Scholar]
- Shoeb, M.; Meier, H.C.S.; Antonini, J.M. Telomeres in toxicology: Occupational health. Pharmacol Ther. 2021, 220, 107742. [Google Scholar] [CrossRef]
- Jang, D.G.; Dou, J.F.; Koubek, E.J.; Teener, S.; Zhou, L.; Bakulski, K.M.; Mukherjee, B.; Batterman, S.A.; Feldman, E.L.; Goutman, S.A. Multiple metal exposures associate with higher amyotrophic lateral sclerosis risk and mortality independent of genetic risk and correlate to self-reported exposures: A case-control study. J. Neurol. Neurosurg. Psychiatry 2025, 96, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Berdyński, M.; Miszta, P.; Safranow, K.; Andersen, P.M.; Morita, M.; Filipek, S.; Żekanowski, C.; Kuźma-Kozakiewicz, M. SOD1 mutations associated with amyotrophic lateral sclerosis analysis of variant severity. Sci. Rep. 2022, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Gruzman, A.; Wood, W.L.; Alpert, E.; Prasad, M.D.; Miller, R.G.; Rothstein, J.D.; Bowser, R.; Hamilton, R.; Wood, T.D. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12524–12529. [Google Scholar] [CrossRef]
- Bosco, D.A.; Morfini, G.; Karabacak, N.M.; Song, Y.; Gros-Louis, F.; Pasinelli, P.; Goolsby, H.; Fontaine, B.A.; Lemay, N.; McKenna-Yasek, D.; et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat. Neurosci. 2010, 13, 1396–1403. [Google Scholar] [CrossRef]
- Rotunno, M.S.; Bosco, D.A. An emerging role for misfolded wild-type SOD1 in sporadic ALS pathogenesis. Front. Cell. Neurosci. 2013, 7, 253. [Google Scholar] [CrossRef]
- Tokuda, E.; Takei, Y.I.; Ohara, S.; Fujiwara, N.; Hozumi, I.; Furukawa, Y. Wild-type Cu/Zn-superoxide dismutase is misfolded in cerebrospinal fluid of sporadic amyotrophic lateral sclerosis. Mol. Neurodegener. 2019, 14, 42. [Google Scholar] [CrossRef]
- Peters, T.L.; Beard, J.D.; Umbach, D.M.; Allen, K.; Keller, J.; Mariosa, D.; Sandler, D.P.; Schmidt, S.; Fang, F.; Ye, W.; et al. Blood levels of trace metals and amyotrophic lateral sclerosis. Neurotoxicology 2016, 54, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Noguchi, T.; Ikegami, S.; Sakurai, T.; Kakita, A.; Toyoshima, Y.; Kambe, T.; Yamada, M.; Inden, M.; Hara, H.; et al. Zinc transporters ZnT3 and ZnT6 are downregulated in the spinal cords of patients with sporadic amyotrophic lateral sclerosis. J. Neurosci. Res. 2015, 93, 370–379. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.Y.; Hwang, J.J.; Lee, J.Y.; Shin, J.H.; Gwag, B.J.; Koh, J.Y. Accumulation of labile zinc in neurons and astrocytes in the spinal cords of G93A SOD-1 transgenic mice. Neurobiol. Dis. 2009, 34, 221–229. [Google Scholar] [CrossRef]
- Roos, P.M.; Vesterberg, O.; Syversen, T.; Flaten, T.P.; Nordberg, M. Metal concentrations in cerebrospinal fluid and blood plasma from patients with amyotrophic lateral sclerosis. Biol. Trace Elem. Res. 2013, 151, 159–170. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Wu, P.; Wen, T.; Qin, X.; Zhang, R.; Jia, R.; Jin, J.; Hu, F.; Xie, X.; Dang, J. Association of cerebral spinal fluid copper imbalance in amyotrophic lateral sclerosis. Front. Aging Neurosci. 2022, 14, 970711. [Google Scholar] [CrossRef] [PubMed]
- Kapaki, E.; Zournas, C.; Kanias, G.; Zambelis, T.; Kakami, A.; Papageorgiou, C. Essential trace element alterations in amyotrophic lateral sclerosis. J. Neurol. Sci. 1997, 147, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.B.; Kysenius, K.; Liddell, J.R.; Mercer, S.W.; Paul, B.; Beckman, J.S.; McLean, C.A.; White, A.R.; Donnelly, P.S.; Bush, A.I.; et al. Evidence for disrupted copper availability in human spinal cord supports CuII (atsm) as a treatment option for sporadic cases of ALS. Sci. Rep. 2024, 14, 5929. [Google Scholar] [CrossRef]
- Forte, G.; Bocca, B.; Oggiano, R.; Clemente, S.; Asara, Y.; Sotgiu, M.A.; Farace, C.; Montella, A.; Fois, A.G.; Malaguarnera, M. Essential trace elements in amyotrophic lateral sclerosis (ALS): Results in a population of a risk area of Italy. Neurol. Sci. 2017, 38, 1609–1615. [Google Scholar] [CrossRef]
- Qin, X.; Wu, P.; Wen, T.; Jia, R.; Zhang, R.; Jin, J.; Hu, F.; Chen, Q.Y.; Dang, J. Comparative assessment of blood Metal/metalloid levels, clinical heterogeneity, and disease severity in amyotrophic lateral sclerosis patients. Neurotoxicology 2022, 89, 12–19. [Google Scholar] [CrossRef]
- Patti, F.; Fiore, M.; Chisari, C.G.; D’Amico, E.; Fermo, S.L.; Toscano, S.; Copat, C.; Ferrante, M.; Zappia, M. CSF neurotoxic metals/metalloids levels in amyotrophic lateral sclerosis patients: Comparison between bulbar and spinal onset. Environ. Res. 2020, 188, 109820. [Google Scholar] [CrossRef]
- Enge, T.G.; Ecroyd, H.; Jolley, D.F.; Yerbury, J.J.; Dosseto, A. Longitudinal assessment of metal concentrations and copper isotope ratios in the G93A SOD1 mouse model of amyotrophic lateral sclerosis. Metallomics 2017, 9, 161–174. [Google Scholar] [CrossRef]
- Tokuda, E.; Ono, S.I.; Ishige, K.; Naganuma, A.; Ito, Y.; Suzuki, T. Metallothionein proteins expression, copper and zinc concentrations, and lipid peroxidation level in a rodent model for amyotrophic lateral sclerosis. Toxicology 2007, 229, 33–41. [Google Scholar] [CrossRef]
- Sauzéat, L.; Bernard, E.; Perret-Liaudet, A.; Quadrio, I.; Vighetto, A.; Krolak-Salmon, P.; Broussolle, E.; Leblanc, P.; Balter, V. Isotopic evidence for disrupted copper metabolism in amyotrophic lateral sclerosis. iScience 2018, 6, 264–271. [Google Scholar] [CrossRef]
- Tokuda, E.; Okawa, E.; Watanabe, S.; Ono, S.I. Overexpression of metallothionein-I, a copper-regulating protein, attenuates intracellular copper dyshomeostasis and extends lifespan in a mouse model of amyotrophic lateral sclerosis caused by mutant superoxide dismutase-1. Hum. Mol. Genet. 2014, 23, 1271–1285. [Google Scholar] [CrossRef]
- Enge, T.G.; Ecroyd, H.; Jolley, D.F.; Yerbury, J.J.; Kalmar, B.; Dosseto, A. Assessment of metal concentrations in the SOD1G93A mouse model of amyotrophic lateral sclerosis and its potential role in muscular denervation, with particular focus on muscle tissue. Mol. Cell. Neurosci. 2018, 88, 319–329. [Google Scholar] [CrossRef]
- McAlary, L.; Shephard, V.K.; Wright, G.S.A.; Yerbury, J.J. A copper chaperone–mimetic polytherapy for SOD1-associated amyotrophic lateral sclerosis. J. Biol. Chem. 2022, 298, 101612. [Google Scholar] [CrossRef]
- Roberts, B.R.; Lim, N.K.; McAllum, E.J.; Donnelly, P.S.; Hare, D.J.; Doble, P.A.; Turner, B.J.; Price, K.A.; Lim, S.C.; Pasterson, B.M.; et al. Oral treatment with CuII (atsm) increases mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2014, 34, 8021–8031. [Google Scholar] [CrossRef]
- Soon, C.P.; Donnelly, P.S.; Turner, B.J.; Hung, L.W.; Crouch, P.J.; Sherratt, N.A.; Tan, J.L.; Lim, N.K.-H.; Lam, L.; Bica, L.; et al. Diacetylbis (N(4)-methylthiosemicarbazonato) copper(II) (CuII (atsm)) protects against peroxynitrite-induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J. Biol. Chem. 2011, 286, 44035–44044. [Google Scholar] [CrossRef]
- Wu, F.; Malek, A.M.; Buchanich, J.M.; Arena, V.C.; Rager, J.R.; Sharma, R.K.; Vena, J.E.; Bear, T.; Talbott, E.O. Exposure to ambient air toxicants and the risk of amyotrophic lateral sclerosis (ALS): A matched case control study. Environ. Res. 2024, 242, 117719. [Google Scholar] [CrossRef]
- Moriwaka, F.; Satoh, H.; Ejima, A.; Watanabe, C.; Tashiro, K.; Hamada, T.; Matsumoto, A.; Shima, K.; Yanagihara, T.; Fukazawa, T.; et al. Mercury and selenium contents in amyotrophic lateral sclerosis in Hokkaido, the northernmost island of Japan. J. Neurol. Sci. 1993, 118, 38–42. [Google Scholar] [CrossRef]
- Oggiano, R.; Solinas, G.; Forte, G.; Bocca, B.; Farace, C.; Pisano, A.; Sotgiu, M.A.; Clemente, S.; Malaguarnera, M.; Fois, A.G.; et al. Trace elements in ALS patients and their relationships with clinical severity. Chemosphere 2018, 197, 457–466. [Google Scholar] [CrossRef]
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013, 38, 25–32. [Google Scholar] [CrossRef]
- Mandrioli, J.; Michalke, B.; Solovyev, N.; Grill, P.; Violi, F.; Lunetta, C.; Conte, A.; Sansone, V.A.; Sabatelli, M.; Vinceti, M. Elevated levels of selenium species in cerebrospinal fluid of amyotrophic lateral sclerosis patients with disease-associated gene mutations. Neurodegener. Dis. 2017, 17, 171–180. [Google Scholar] [CrossRef]
- Hadzhieva, M.; Kirches, E.; Wilisch-Neumann, A.; Pachow, D.; Wallesch, M.; Schoenfeld, P.; Paege, I.; Vilehaber, S.; Petri, S.; Keihoff, G.; et al. Dysregulation of iron protein expression in the G93A model of amyotrophic lateral sclerosis. Neuroscience 2013, 230, 94–101. [Google Scholar] [CrossRef]
- Yasui, M.; Ota, K.; Garruto, R.M. Concentrations of zinc and iron in the brains of Guamanian patients with amyotrophic lateral sclerosis and parkinsonism-dementia. Neurotoxicology 1993, 14, 445–450. [Google Scholar]
- Jeong, S.Y.; Rathore, K.I.; Schulz, K.; Ponka, P.; Arosio, P.; David, S. Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2009, 29, 610–619. [Google Scholar] [CrossRef]
- Kwan, J.Y.; Jeong, S.Y.; Van Gelderen, P.; Deng, H.X.; Quezado, M.M.; Danielian, L.E.; Butman, J.A.; Chen, L.; Bayat, E.; Russell, J.; et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: Correlating 7 tesla MRI and pathology. PLoS ONE 2012, 7, e35241. [Google Scholar] [CrossRef]
- Ignjatović, A.; Stević, Z.; Lavrnić, D.; Nikolić-Kokić, A.; Blagojević, D.; Spasić, M.; Spasojević, I. Inappropriately chelated iron in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. 2012, 13, 357–362. [Google Scholar] [CrossRef]
- Goodall, E.F.; Haque, M.S.; Morrison, K.E. Increased serum ferritin levels in amyotrophic lateral sclerosis (ALS) patients. J. Neurol. 2008, 255, 1652–1656. [Google Scholar] [CrossRef]
- Ikeda, K.; Hirayama, T.; Takazawa, T.; Kawabe, K.; Iwasaki, Y. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: A cross-sectional study. Intern. Med. 2012, 51, 1501–1508. [Google Scholar] [CrossRef]
- Wu, A.S.; Kiaei, M.; Aguirre, N.; Crow, J.P.; Calingasan, N.Y.; Browne, S.E.; Beal, M.F. Iron porphyrin treatment extends survival in a transgenic animal model of amyotrophic lateral sclerosis. J. Neurochem. 2003, 85, 142–150. [Google Scholar] [CrossRef]
- Walker, A.K.; Atkin, J.D.; Horne, M.K. Role of DNA damage and repair in motor neuron diseases. Int. J. Mol. Sci. 2021, 22, 2536. [Google Scholar] [CrossRef]
- Kraus, K.L.; Nawreen, N.; Godale, C.M.; Chordia, A.P.; Packard, B.; LaSarge, C.L.; Herman, J.P.; Danzer, S.C. Hippocampal glucocorticoid receptors modulate status epilepticus severity. Neurobiol. Dis. 2023, 178, 106014. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.; He, C.; Yan, F.; Li, J.R.; Xu, H.Z.; Zhuang, J.F.; Zhou, H.; Peng, Y.C.; Fu, X.J.; et al. Selective Ferroptosis Inhibitor Liproxstatin-1 Attenuates Neurological Deficits and Neuroinflammation After Subarachnoid Hemorrhage. Neurosci. Bull. Apr. 2021, 37, 535–549. [Google Scholar] [CrossRef]
- Malhi, G.S.; Tanious, M.; Das, P.; Berk, M. The science and practice of lithium therapy. Aust. N. Z. J. Psychiatry 2013, 47, 675–684. [Google Scholar] [CrossRef]
- Pagano, G.; Guida, M.; Tommasi, F.; Oral, R. Health impacts of exposure to rare earth elements: An overview. Front. Environ. Sci. 2022, 10, 948041. [Google Scholar] [CrossRef]
- Engström, K.; Love, T.M.; Raqib, R.; Vahter, M.; Kippler, M. Early life cadmium exposure and DNA methylation of apoptosis-related genes in Bangladeshi children. Environ. Res. 2020, 180, 108812. [Google Scholar] [CrossRef]
- Kepp, K.P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar] [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar] [CrossRef]
- Zecca, L.; Youdim, M.B.; Riederer, P.; Connor, J.R.; Crichton, R.R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004, 5, 863–873. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Bush, A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Forcella, M.; Lau, P.O.U.I.; Oldani, M.; Melchioretto, P.; Bogni, A.; Gribaldo, L.; Fusi, P.; Urani, C. Neuronal specific and non-specific responses to cadmium possibly involved in neurodegeneration: A toxicogenomics study in a human neuronal cell model. Neurotoxicology 2020, 76, 162–173. [Google Scholar] [CrossRef]
- Urani, C.; Forcella, M.; Pierre, L.; Alessia, B.; Melchioretto, P.; Laura, G.; Fusi, P. Toxicogenomics reveals neuronal specific and non-specific responses to cadmium possibly involved in neurodegeneration. Altex. Ethik 2018, 7, 236. [Google Scholar]
- Polykretis, P.; Cencetti, F.; Donati, C.; Luchinat, E.; Banci, L. Cadmium effects on superoxide dismutase 1 in human cells revealed by NMR. Redox Biol. 2019, 21, 101102. [Google Scholar] [CrossRef]
- Bovio, F.; Sciandrone, B.; Urani, C.; Fusi, P.; Forcella, M.; Regonesi, M.E. Superoxide dismutase 1 (SOD1) and cadmium: A three models approach to the comprehension of its neurotoxic effects. Neurotoxicology 2021, 84, 125–135. [Google Scholar] [CrossRef]
- Freydenzon, A.; Nabais, M.F.; Lin, T.; Williams, K.L.; Wallace, L.; Henders, A.K.; Blair, L.P.; Wray, N.R.; Pamphlett, R.; McRae, A.F. Association between DNA methylation variability and self-reported exposure to heavy metals. Sci. Rep. 2022, 12, 10582. [Google Scholar] [CrossRef]
- Xu, R.; Wu, C.; Zhang, X.; Zhang, Q.; Yang, Y.; Yi, J.; Yang, R.; Tao, Y. Linking hypoxic and oxidative insults to cell death mechanisms in models of ALS. Brain Res. 2011, 1372, 133–144. [Google Scholar] [CrossRef]
- Lee, J.K.; Shin, J.H.; Gwag, B.J.; Choi, E.J. Iron accumulation promotes TACE-mediated TNF-α secretion and neurodegeneration in a mouse model of ALS. Neurobiol. Dis. 2015, 80, 63–69. [Google Scholar] [CrossRef]
- Halon-Golabek, M.; Flis, D.J.; Zischka, H.; Akdogan, B.; Wieckowski, M.R.; Antosiewicz, J.; Ziolkowski, W. Amyotrophic lateral sclerosis associated disturbance of iron metabolism is blunted by swim training-role of AKT signaling pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2024, 1870, 167014. [Google Scholar] [CrossRef]
- Eum, K.D.; Seals, R.M.; Taylor, K.M.; Grespin, M.; Umbach, D.M.; Hu, H.; Sandler, D.P.; Kamel, F.; Weisskopf, M.G. Modification of the association between lead exposure and amyotrophic lateral sclerosis by iron and oxidative stress related gene polymorphisms. Amyotroph. Lateral Scler. Front. Degener. 2015, 16, 72–79. [Google Scholar] [CrossRef]
- Eum, K.D.; Seals, R.; Grespin, M.; Umbach, D.; Sandler, D.; Hu, H.; Kamel, F.; Weisskopf, M.G. Interaction Between HFE Polymorphisms and Cumulative Lead Exposure on the Risk of Amyotrophic Lateral Sclerosis. In ISEE Conference Abstracts 25; The International Society for Environmental Epidemiology: Herndon, VA, USA, 2013; Volume 2013, p. 4638. [Google Scholar]
- Kamel, F.; Umbach, D.M.; Lehman, T.A.; Park, L.P.; Munsat, T.L.; Shefner, J.M.; Sandler, D.P.; Hu, H.; Taylor, J.A. Amyotrophic lateral sclerosis, lead, and genetic susceptibility: Polymorphisms in the delta-aminolevulinic acid dehydratase and vitamin D receptor genes. Environ. Health Perspect. 2003, 111, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Barbeito, A.G.; Martinez-Palma, L.; Vargas, M.R.; Pehar, M.; Mañay, N.; Beckman, J.S.; Barbeito, L.; Cassina, P. Lead exposure stimulates VEGF expression in the spinal cord and extends survival in a mouse model of ALS. Neurobiol. Dis. 2010, 37, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Stamenković, S.; Dučić, T.; Stamenković, V.; Kranz, A.; Andjus, P.R. Imaging of glial cell morphology, SOD1 distribution and elemental composition in the brainstem and hippocampus of the ALS hSOD1G93A rat. Neuroscience 2017, 357, 37–55. [Google Scholar] [CrossRef]
- Maraldi, T.; Riccio, M.; Zambonin, L.; Vinceti, M.; De Pol, A.; Hakim, G. Low levels of selenium compounds are selectively toxic for a human neuron cell line through ROS/RNS increase and apoptotic process activation. Neurotoxicology 2011, 32, 180–187. [Google Scholar] [CrossRef]
- Estevez, A.O.; Morgan, K.L.; Szewczyk, N.J.; Gems, D.; Estevez, M. The neurodegenerative effects of selenium are inhibited by FOXO and PINK1/PTEN regulation of insulin/insulin-like growth factor signaling in Caenorhabditis elegans. Neurotoxicology 2014, 41, 28–43. [Google Scholar] [CrossRef]
- Puttaparthi, K.; Gitomer, W.L.; Krishnan, U.; Son, M.; Rajendran, B.; Elliott, J.L. Disease progression in a transgenic model of familial amyotrophic lateral sclerosis is dependent on both neuronal and non-neuronal zinc binding proteins. J. Neurosci. 2002, 22, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, G.J.; van Weenen, J.D.L.; Van Muiswinkel, F.L.; Veldman, H.; Veldink, J.H.; Wokke, J.H.J.; Bär, P.R.; Van Den Berg, L.H. Zinc amplifies mSOD1-mediated toxicity in a transgenic mouse model of amyotrophic lateral sclerosis. Neurosci. Lett. 2003, 352, 175–178. [Google Scholar] [CrossRef]
- Baziyar, P.; Seyedalipour, B.; Hosseinkhani, S. Zinc binding loop mutations of hSOD1 promote amyloid fibrils under physiological conditions: Implications for initiation of amyotrophic lateral sclerosis. Biochimie 2022, 199, 170–181. [Google Scholar] [CrossRef]
- Nutini, M.; Frazzini, V.; Marini, C.; Spalloni, A.; Sensi, S.L.; Longone, P. Zinc pre-treatment enhances NMDAR-mediated excitotoxicity in cultured cortical neurons from SOD1G93A mouse, a model of amyotrophic lateral sclerosis. Neuropharmacology 2011, 60, 1200–1208. [Google Scholar] [CrossRef]
- Isabel Post, J.; Karl Eibl, J.; Michiel Ross, G. Zinc induces motor neuron death via a selective inhibition of brain-derived neurotrophic factor activity. Amyotroph. Lateral Scler. 2008, 9, 149–155. [Google Scholar] [CrossRef]
- Caragounis, A.; Price, K.A.; Soon, C.P.; Filiz, G.; Masters, C.L.; Li, Q.X.; Crouch, P.J.; White, A.R. Zinc induces depletion and aggregation of endogenous TDP-43. Free Radic. Biol. Med. 2010, 48, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.J.; Liu, H.; Goto, J.J.; Nersissian, A.; Roe, J.A.; Graden, J.A.; Café, C.; Ellerby, L.M.; Bredesen, D.E.; Gralla, E.B.; et al. Mutations in copper-zinc superoxide dismutase that cause amyotrophic lateral sclerosis alter the zinc binding site and the redox behavior of the protein. Proc. Natl. Acad. Sci. USA 1996, 93, 12240–12244. [Google Scholar] [CrossRef]
- Lyons, T.J.; Nersissian, A.; Huang, H.; Yeom, H.; Nishida, C.R.; Graden, J.A.; Gralla, E.B.; Valentine, J.S. The metal binding properties of the zinc site of yeast copper-zinc superoxide dismutase: Implications for amyotrophic lateral sclerosis. JBIC J. Biol. Inorg. Chem. 2000, 5, 189–203. [Google Scholar] [CrossRef]
- Watanabe, S.; Nagano, S.; Duce, J.; Kiaei, M.; Li, Q.X.; Tucker, S.M.; Tiwari, A.; Brown, R.H.; Beal, M.F.; Hayward, L.J.; et al. Increased affinity for copper mediated by cysteine 111 in forms of mutant superoxide dismutase 1 linked to amyotrophic lateral sclerosis. Free Radic. Biol. Med. 2007, 42, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, M.; Bush, A.I.; Morrison, B.M.; Morrison, J.H.; Cherny, R.A.; Volitakis, I.; Beal, M.F.; Gordon, J.W. Genetically decreased spinal cord copper concentration prolongs life in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2004, 24, 7945–7950. [Google Scholar] [CrossRef]
- Carrì, M.T.; Battistoni, A.; Polizio, F.; Desideri, A.; Rotilio, G. Impaired copper binding by the H46R mutant of human Cu, Zn superoxide dismutase, involved in amyotrophic lateral sclerosis. FEBS Lett. 1994, 356, 314–316. [Google Scholar] [CrossRef]
- Corson, L.B.; Strain, J.J.; Culotta, V.C.; Cleveland, D.W. Chaperone-facilitated copper binding is a property common to several classes of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants. Proc. Natl. Acad. Sci. USA 1998, 95, 6361–6366. [Google Scholar] [CrossRef]
- Kreuzer, M.; Stamenković, S.; Chen, S.; Andjus, P.; Dučić, T. Lipids status and copper in a single astrocyte of the rat model for amyotrophic lateral sclerosis: Correlative synchrotron-based X-ray and infrared imaging. J. Biophotonics 2020, 13, e202000069. [Google Scholar] [CrossRef]
- Tokuda, E.; Okawa, E.; Watanabe, S.; Ono, S.I.; Marklund, S.L. Dysregulation of intracellular copper homeostasis is common to transgenic mice expressing human mutant superoxide dismutase-1s regardless of their copper-binding abilities. Neurobiol. Dis. 2013, 54, 308–319. [Google Scholar] [CrossRef]
- Dang, T.N.; Lim, N.K.; Grubman, A.; Li, Q.X.; Volitakis, I.; White, A.R.; Crouch, P.J. Increased metal content in the TDP-43A315T transgenic mouse model of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Front. Aging Neurosci. 2014, 6, 15. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Brown, H.H.; Borchelt, D.R.; Vogt, S.; Miller, L.M. Metal-deficient aggregates and diminished copper found in cells expressing SOD1 mutations that cause ALS. Front. Aging Neurosci. 2014, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.B.; White, A.R.; Crouch, P.J. Endogenous Cu in the central nervous system fails to satiate the elevated requirement for Cu in a mutant SOD1 mouse model of ALS. Metallomics 2016, 8, 1002–1011. [Google Scholar] [CrossRef]
- Hilton, J.B.; Kysenius, K.; White, A.R.; Crouch, P.J. The accumulation of enzymatically inactive cuproenzymes is a CNS-specific phenomenon of the SOD1G37R mouse model of ALS and can be restored by overexpressing the human copper transporter hCTR1. Exp. Neurol. 2018, 307, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Consales, C.; Panatta, M.; Butera, A.; Filomeni, G.; Merla, C.; Carrì, M.T.; Marino, C.; Benassi, B. 50-Hz magnetic field impairs the expression of iron-related genes in the in vitro SOD1G93A model of amyotrophic lateral sclerosis. Int. J. Radiat. Biol. 2019, 95, 368–377. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Chen, S.; Zhang, X.; Zhang, S.; Youdium, M.; Le, W. Prevention of motor neuron degeneration by novel iron chelators in SOD1G93A transgenic mice of amyotrophic lateral sclerosis. Neurodegener. Dis. 2011, 8, 310–321. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Weinreb, O.; Amit, T.; Mandel, S.; Carri, M.T.; Youdim, M.B. Neuroprotective and neuritogenic activities of novel multimodal iron-chelating drugs in motor-neuron-like NSC-34 cells and transgenic mouse model of amyotrophic lateral sclerosis. FASEB J. 2009, 23, 3766–3779. [Google Scholar] [CrossRef]
- Popović-Bijelić, A.; Mojović, M.; Stamenković, S.; Jovanović, M.; Selaković, V.; Andjus, P.; Bačić, G. Iron-sulfur cluster damage by the superoxide radical in neural tissues of the SOD1G93A ALS rat model. Free Radic. Biol. Med. 2016, 96, 313–322. [Google Scholar] [CrossRef]
- Soll, M.; Goldshtein, H.; Rotkopf, R.; Russek-Blum, N.; Gross, Z. A synthetic SOD/Catalase mimic compound for the treatment of ALS. Antioxidants 2021, 10, 827. [Google Scholar] [CrossRef]
- Magnavita, N.; Sabatelli, M.; Scoditti, E.; Chirico, F. Personalized prevention in mercury-induced amyotrophic lateral sclerosis: A case report. Appl. Sci. 2020, 10, 7839. [Google Scholar] [CrossRef]
- Johnson, F.O.; Yuan, Y.; Hajela, R.K.; Chitrakar, A.; Parsell, D.M.; Atchison, W.D. Exposure to an environmental neurotoxicant hastens the onset of amyotrophic lateral sclerosis-like phenotype in human Cu2+/Zn2+ superoxide dismutase 1 G93A mice: Glutamate-mediated excitotoxicity. J. Pharmacol. Exp. Ther. 2011, 338, 518–527. [Google Scholar] [CrossRef]
- Jhan, C.R.; Satange, R.; Wang, S.C.; Zeng, J.Y.; Horng, Y.C.; Jin, P.; Neidle, S.; Hou, M.H. Targeting the ALS/FTD-associated A-DNA kink with anthracene-based metal complex causes DNA backbone straightening and groove contraction. Nucleic Acids Res. 2021, 49, 9526–9538. [Google Scholar] [CrossRef] [PubMed]
- Maraldi, T.; Beretti, F.; Anselmi, L.; Franchin, C.; Arrigoni, G.; Braglia, L.; Mandrioli, J.; Vinceti, M.; Marmiroli, S. Influence of selenium on the emergence of neuro tubule defects in a neuron-like cell line and its implications for amyotrophic lateral sclerosis. Neurotoxicology 2019, 75, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Amporndanai, K.; Rogers, M.; Watanabe, S.; Yamanaka, K.; O’Neill, P.M.; Hasnain, S.S. Novel Selenium-based compounds with therapeutic potential for SOD1-linked amyotrophic lateral sclerosis. eBioMedicine 2020, 59, 102980. [Google Scholar] [CrossRef]
- McAllum, E.J.; Roberts, B.R.; Hickey, J.L.; Dang, T.N.; Grubman, A.; Donnelly, P.S.; Liddell, J.R.; White, A.R.; Crouch, P.J. ZnII (atsm) is protective in amyotrophic lateral sclerosis model mice via a copper delivery mechanism. Neurobiol. Dis. 2015, 81, 20–24. [Google Scholar] [CrossRef]
- Lelie, H.L.; Liba, A.; Bourassa, M.W.; Chattopadhyay, M.; Chan, P.K.; Gralla, E.B.; Miller, L.M.; Borchelt, D.R.; Valentine, J.S.; Whitelegge, J.P. Copper and zinc metallation status of copper-zinc superoxide dismutase from amyotrophic lateral sclerosis transgenic mice. J. Biol. Chem. 2011, 286, 2795–2806. [Google Scholar] [CrossRef]
- Tiwari, A.; Xu, Z.; Hayward, L.J. Aberrantly Increased Hydrophobicity Shared by Mutants of Cu, Zn-Superoxide Dismutase in Familial Amyotrophic Lateral Sclerosis. J. Biol. Chem. 2005, 280, 29771–29779. [Google Scholar] [CrossRef]
- Ermilova, I.P.; Ermilov, V.B.; Levy, M.; Ho, E.; Pereira, C.; Beckman, J.S. Protection by dietary zinc in ALS mutant G93A SOD transgenic mice. Neurosci. Lett. 2005, 379, 42–46. [Google Scholar] [CrossRef]
- Hayward, L.J.; Rodriguez, J.A.; Kim, J.W.; Tiwari, A.; Goto, J.J.; Cabelli, D.E.; Valentine, J.S.; Brown, R.H. Decreased metallation and activity in subsets of mutant superoxide dismutases associated with familial amyotrophic lateral sclerosis* 210. J. Biol. Chem. 2002, 277, 15923–15931. [Google Scholar] [CrossRef]
| Metals | SPL Rank | In Vitro | In Vivo | Epidemiological | Hybrid | Total |
|---|---|---|---|---|---|---|
| Arsenic (As) | 1 | 3 | 3 | |||
| Lead (Pb) | 2 | 32 | 2 | 34 | ||
| Mercury (Hg) | 3 | 8 | 9 | 1 | 18 | |
| Cadmium (Cd) | 7 | 7 | 8 | 15 | ||
| Cobalt (Co) | 51 | 3 | 1 | 1 | 5 | |
| Nickel (Ni) * | 57 | 1 | 1 | 5 | 1 | 8 |
| Zinc (Zn) * | 74 | 8 | 9 | 12 | 2 | 31 |
| Chromium (Cr) | 78 | 2 | 2 | |||
| Uranium (U) | 99 | 1 | 1 | |||
| Copper (Cu) * | 120 | 4 | 14 | 15 | 1 | 34 |
| Manganese (Mn) | 143 | 2 | 15 | 17 | ||
| Selenium (Se) * | 151 | 2 | 1 | 16 | 1 | 20 |
| Aluminum (Al) | 188 | 3 | 6 | 4 | 13 | |
| Vanadium (V) * | 208 | 1 | 1 | 2 | ||
| Iron (Fe) * | 2 | 9 | 11 | 2 | 24 | |
| Total | 31 | 52 | 133 | 11 | 227 |
| Metal | Genotoxic Endpoints | Evidence |
|---|---|---|
| Aluminum (Al) | Not Specified | |
| Arsenic (Ar) | Not Specified | |
| Cadmium (Cd) | Yes | DNA Methylation, DNA Repair Inhibition, DNA Fragmentation |
| Chromium (Cr) | Not Specified | |
| Cobalt (Co) | Inferred | DNA Fragmentation |
| Copper (Cu) * | Inferred | DNA Fragmentation |
| Iron (Fe) * | Inferred | DNA Fragmentation |
| Lead (Pb) | Inferred | DNA Fragmentation |
| Manganese (Mn) | Not Specified | |
| Mercury (Hg) | Inferred | DNA Fragmentation |
| Nickel (Ni) * | Not Specified | |
| Selenium (Se) * | Inferred | DNA Fragmentation |
| Uranium (U) | Not Specified | |
| Vanadium (V) * | Not Specified | |
| Zinc (Zn) * | Inferred | DNA Fragmentation |
| Gene | Mutation | Co | Cu | Fe | Pb | Mn | Hg | Ni | Se | Zn | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C9orf72 | G4C2 | 1 | 1 | ||||||||
| SOD1 | A4V | 4 | 1 | 1 | 6 | ||||||
| SOD1 | D76Y | 1 | 1 | ||||||||
| SOD1 | D83H | 1 | 1 | ||||||||
| SOD1 | D124V | 2 | 2 | ||||||||
| SOD1 | D125H | 1 | 1 | 2 | |||||||
| SOD1 | G37R | 6 | 1 | 3 | 10 | ||||||
| SOD1 | G41D | 1 | 1 | ||||||||
| SOD1 | G85R | 2 | 3 | 3 | 8 | ||||||
| SOD1 | G86R | 1 | 1 | 2 | |||||||
| SOD1 | G93A | 2 | 8 | 6 | 1 | 1 | 1 | 9 | 28 | ||
| SOD1 | G93C | 1 | 1 | ||||||||
| SOD1 | G93R | 1 | 1 | ||||||||
| SOD1 | H46R | 2 | 3 | 3 | 8 | ||||||
| SOD1 | H48Q | 1 | 1 | 2 | |||||||
| SOD1 | H63A | 2 | 1 | 3 | |||||||
| SOD1 | H63E | 2 | 1 | 3 | |||||||
| SOD1 | H80C | 1 | 1 | ||||||||
| SOD1 | H80R | 1 | 1 | ||||||||
| SOD1 | I113T | 1 | 1 | ||||||||
| SOD1 | L67P | 1 | 1 | ||||||||
| SOD1 | L106V | 1 | 1 | 2 | |||||||
| SOD1 | L126S | 1 | 1 | 2 | |||||||
| SOD1 | S134N | 1 | 1 | ||||||||
| TBK1 | deletion in c.1852_1854delGAA: p.E618del | 1 | 1 | ||||||||
| TDP43 | A315T | 1 | 1 | 1 | 3 | ||||||
| TUBA4A | A383T | 1 | 1 | ||||||||
| TUBA4A | R320C | 1 | 1 | ||||||||
| Total | 13 | 34 | 9 | 1 | 1 | 2 | 2 | 3 | 30 | 95 |
| Category | Familial ALS (fALS) | Sporadic ALS (sALS) |
|---|---|---|
| Prevalence | ~5–10% of cases | ~90–95% of cases |
| Age of Onset | Average onset in 40 –50 s | Average onset in 50–60 s |
| Genetic Contribution | Strong; linked to SOD1, FUS, TARDBP, C9orf72 mutations | Multifactorial; less often linked to identifiable mutations |
| Environmental Factors | May act as secondary triggers | Often significant; includes metals and pesticides |
| Family History | Frequently positive; may include FTD | Rarely positive |
| Progression Rate | Variable: some mutations linked to faster decline | Generally slower; varies individually |
| Biomarkers | Detectable via genetic testing | No confirmed biomarkers; clinical diagnosis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.W.; Zarus, G.; Alman, B.; Ruiz, P.; Han, M.; Mehta, P.; Ji, C.; Qureshi, H.; Antonini, J.; Shoeb, M. Metal-Induced Genotoxic Events: Possible Distinction Between Sporadic and Familial ALS. Toxics 2025, 13, 493. https://doi.org/10.3390/toxics13060493
Kim WW, Zarus G, Alman B, Ruiz P, Han M, Mehta P, Ji C, Qureshi H, Antonini J, Shoeb M. Metal-Induced Genotoxic Events: Possible Distinction Between Sporadic and Familial ALS. Toxics. 2025; 13(6):493. https://doi.org/10.3390/toxics13060493
Chicago/Turabian StyleKim, William Wu, Gregory Zarus, Breanna Alman, Patricia Ruiz, Moon Han, Paul Mehta, Chao Ji, Hoormat Qureshi, James Antonini, and Mohammad Shoeb. 2025. "Metal-Induced Genotoxic Events: Possible Distinction Between Sporadic and Familial ALS" Toxics 13, no. 6: 493. https://doi.org/10.3390/toxics13060493
APA StyleKim, W. W., Zarus, G., Alman, B., Ruiz, P., Han, M., Mehta, P., Ji, C., Qureshi, H., Antonini, J., & Shoeb, M. (2025). Metal-Induced Genotoxic Events: Possible Distinction Between Sporadic and Familial ALS. Toxics, 13(6), 493. https://doi.org/10.3390/toxics13060493







