Associations Between Individual Health Risk Perceptions and Biomarkers of PAH Exposure Before and After PM2.5 Pollution in the Suburbs of Chiang Mai Province
Abstract
1. Introduction
2. Materials and Methods
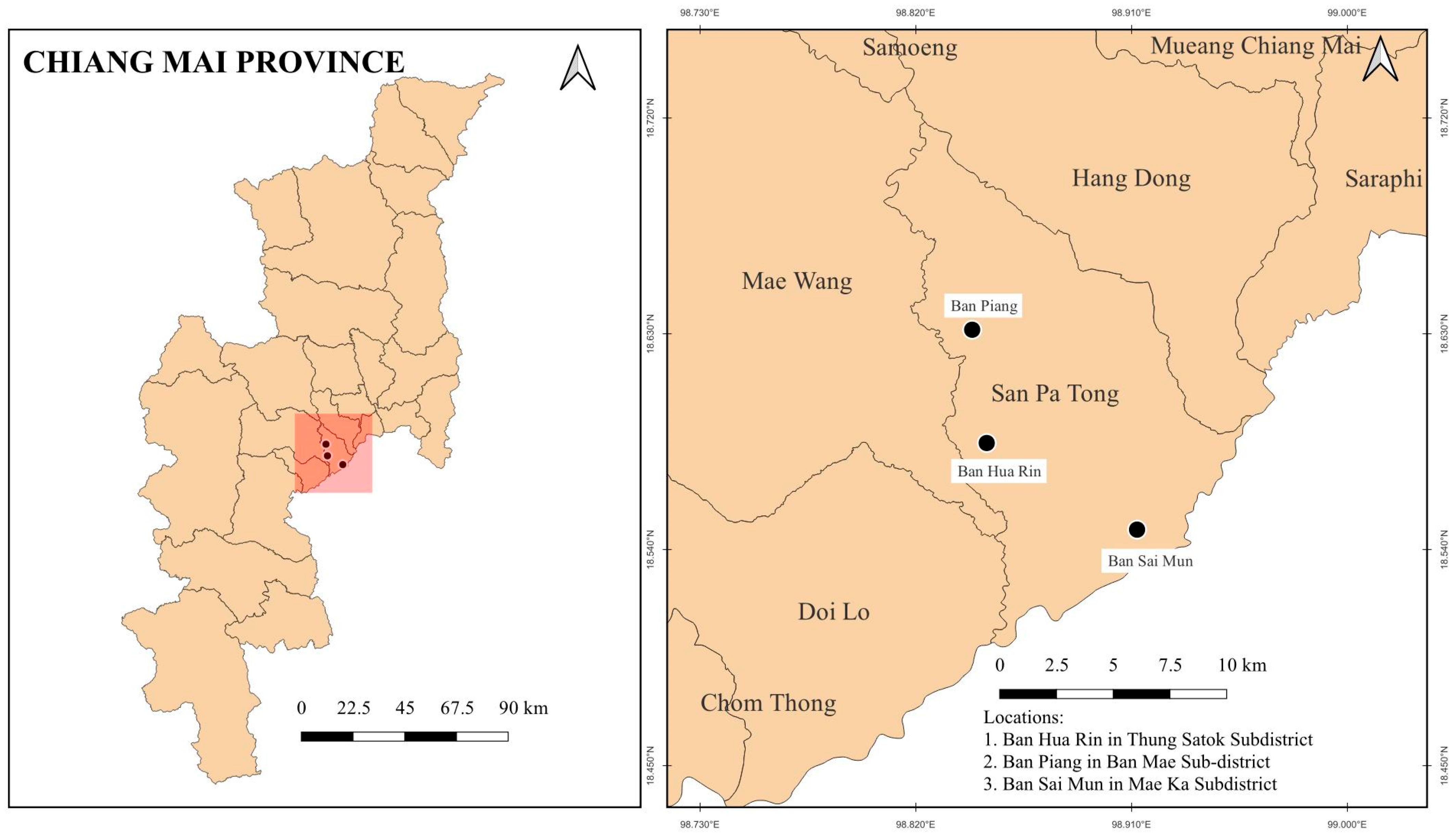
2.1. Study Design
2.2. Ethics Approval and Consent to Participate
2.3. Survey Instrument
2.4. Monitoring of PM2.5 Concentrations
2.5. Biological Sample Collection
2.6. Urinary 1-Hydroxypyrene (1-OHP) Metabolite Analysis
2.6.1. Urine Sample Collection
2.6.2. Standard Chemicals and Reagents
2.6.3. Analysis of Urinary 1-Hydroxypyrene (1-OHP) Metabolites by High-Performance Liquid Chromatography with Fluorescence Detection (HPLC-FLD)
2.6.4. Urinary Creatinine Analysis
2.7. BPDE-DNA Measurement
2.8. Statistical Analysis
3. Results
3.1. The Socioeconomic and Health-Related Characteristics of the Population
3.2. Seasonal Variation in PM2.5 Levels Across Subdistricts
3.3. Comparison of 1-Hydroxypyrene (1-OHP) and BPDE Levels Across Occupations During Low (Visit 1)- and High (Visit 2)-PM2.5 Seasons
3.4. Impact of Pollution Levels on Risk Knowledge and Preventive Behaviors
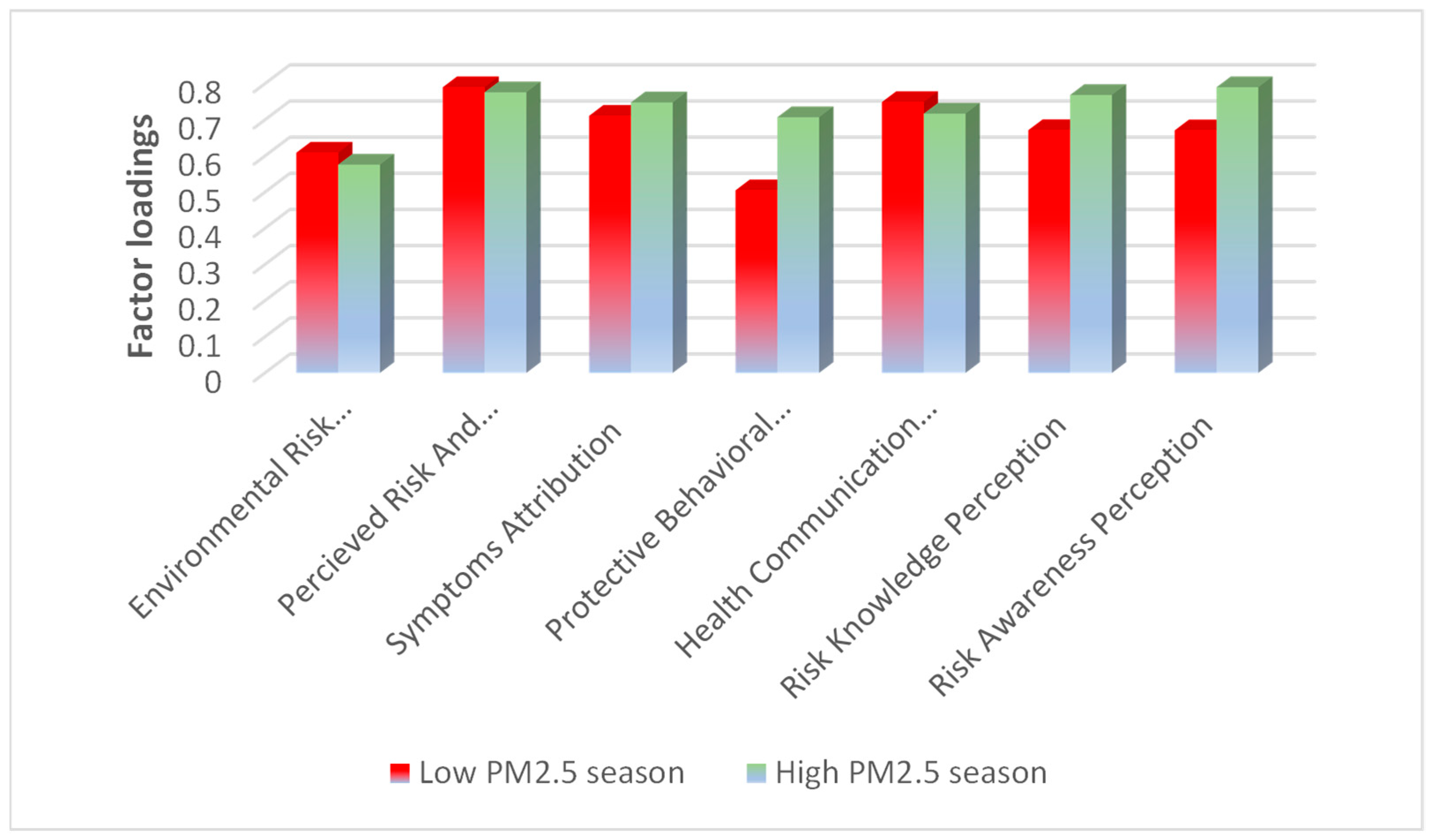
3.5. Impact of Pollution Levels on Public Awareness, Health Perception, and Preventive Behaviors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM2.5 | Particulate matter with a diameter less than 2.5 |
| PAHs | Polycyclic aromatic hydrocarbons |
| BPDA | Benzo[a]pyrene diol epoxide |
| FEV1/FVC | Forced expiratory volume in the first second (FEV1) to forced vital capacity (FVC) |
| 1-OHP | 1-Hydroxypyrene |
| NTAQHI | Northern Thailand Air Quality Health Index |
References
- Abulikemu, A.; Zhang, X.; Su, X.; Meng, T.; Su, W.; Shi, Q.; Yu, T.; Niu, Y.; Yu, H.; Yuan, H. Particulate matter, polycyclic aromatic hydrocarbons and metals, platelet parameters and blood pressure alteration: Multi-pollutants study among population. Sci. Total Environ. 2024, 941, 173657. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F., Jr.; Rocha, B.A.; Souza, M.C.; Bocato, M.Z.; Azevedo, L.F.; Adeyemi, J.A.; Santana, A.; Campiglia, A.D. Polycyclic aromatic hydrocarbons (PAHs): Updated aspects of their determination, kinetics in the human body, and toxicity. J. Toxicol. Environ. Health Part B 2023, 26, 28–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Reduction strategies for polycyclic aromatic hydrocarbons in processed foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1598–1626. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Zhang, H.; Xing, W.; Wang, Y.; Bai, P.; Zhang, L.; Hayakawa, K.; Toriba, A.; Wei, Y. Assessing approaches of human inhalation exposure to polycyclic aromatic hydrocarbons: A review. Int. J. Environ. Res. Public Health 2021, 18, 3124. [Google Scholar] [CrossRef]
- Farooqi, H.M.; Kim, K.H.; Kausar, F.; Muhammad, J.; Bukhari, H.; Choi, K.H. Frequency and Molecular Characterization of Staphylococcus aureus from Placenta of Mothers with Term and Preterm Deliveries. Life 2022, 12, 257. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, Z.; Wang, L.; Qiang, M. Correlation of internal exposure levels of polycyclic aromatic hydrocarbons to methylation of imprinting genes of sperm DNA. Int. J. Environ. Res. Public Health 2019, 16, 2606. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, H.M.; Sammantasinghar, A.; Kausar, F.; Farooqi, M.A.; Chethikkattuveli Salih, A.R.; Hyun, K.; Lim, J.H.; Khalil, A.A.; Mumtaz, A.S.; Choi, K.H. Study of the Anticancer Potential of Plant Extracts Using Liver Tumor Microphysiological System. Life 2022, 12, 135. [Google Scholar] [CrossRef]
- Chuesaard, T.; Chetiyanukornkul, T.; Kameda, T.; Hayakawa, K.; Toriba, A. Influence of biomass burning on the levels of atmospheric polycyclic aromatic hydrocarbons and their nitro derivatives in Chiang Mai, Thailand. Aerosol Air Qual. Res. 2014, 14, 1247–1257. [Google Scholar] [CrossRef]
- Kongpran, J.; Kliengchuay, W.; Niampradit, S.; Sahanavin, N.; Siriratruengsuk, W.; Tantrakarnapa, K. The health risks of airborne polycyclic aromatic hydrocarbons (PAHs): Upper North Thailand. GeoHealth 2021, 5, e2020GH000352. [Google Scholar] [CrossRef]
- Kausar, S.; Tongchai, P.; Yadoung, S.; Sabir, S.; Pata, S.; Khamduang, W.; Chawansuntati, K.; Yodkeeree, S.; Wongta, A.; Hongsibsong, S. Impact of fine particulate matter (PM2.5) on ocular health among people living in Chiang Mai, Thailand. Sci. Rep. 2024, 14, 26479. [Google Scholar] [CrossRef]
- Duc, H.N.; Bang, H.Q.; Quan, N.H.; Quang, N.X. Impact of Biomass Burnings in Southeast Asia on Air Quality and Pollutant Transport during the End of the 2019 Dry Season. Environ. Monit. Assess. 2021, 193, 565. [Google Scholar] [CrossRef]
- Minhas, L.A.; Kaleem, M.; Farooqi, H.M.; Kausar, F.; Waqar, R.; Bhatti, T.; Aziz, S.; Jung, D.W.; Mumtaz, A.S. Algae-Derived Bioactive Compounds as Potential Pharmaceuticals for Cancer Therapy: A Comprehensive Review. Algal Res. 2024, 78, 103396. [Google Scholar] [CrossRef]
- Ciarrocca, M.; Rosati, M.V.; Tomei, F.; Capozzella, A.; Andreozzi, G.; Tomei, G.; Bacaloni, A.; Casale, T.; Andre, J.C.; Fioravanti, M. Is urinary 1-hydroxypyrene a valid biomarker for exposure to air pollution in outdoor workers? A meta-analysis. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Strickland, P.; Kang, D.; Sithisarankul, P. Polycyclic aromatic hydrocarbon metabolites in urine as biomarkers of exposure and effect. Environ. Health Perspect. 1996, 104, 927–932. [Google Scholar]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo [a] pyrene—Environmental occurrence, human exposure, and mechanisms of toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jin, H.; Lei, Y.; Li, Q.; Zhang, Y.; Lu, Q. The dual effects of Benzo (a) pyrene/Benzo (a) pyrene-7, 8-dihydrodiol-9, 10-epoxide on DNA Methylation. Sci. Total Environ. 2024, 950, 175042. [Google Scholar] [CrossRef]
- Moubarz, G.; Saad-Hussein, A.; Shahy, E.M.; Mahdy-Abdallah, H.; Mohammed, A.M.; Saleh, I.A.; Abo-Zeid, M.A.; Abo-Elfadl, M.T. Lung cancer risk in workers occupationally exposed to polycyclic aromatic hydrocarbons with emphasis on the role of DNA repair gene. Int. Arch. Occup. Environ. Health 2023, 96, 313–329. [Google Scholar] [CrossRef]
- He, B.; Xu, H.-M.; Liu, H.-W.; Zhang, Y.-F. Unique regulatory roles of ncRNAs changed by PM2.5 in human diseases. Ecotoxicol. Environ. Saf. 2023, 255, 114812. [Google Scholar] [CrossRef]
- Holme, J.A.; Vondráček, J.; Machala, M.; Lagadic-Gossmann, D.; Vogel, C.F.; Le Ferrec, E.; Sparfel, L.; Øvrevik, J. Lung cancer associated with combustion particles and fine particulate matter (PM2.5)-The roles of polycyclic aromatic hydrocarbons (PAHs) and the aryl hydrocarbon receptor (AhR). Biochem. Pharmacol. 2023, 216, 115801. [Google Scholar] [CrossRef]
- Dobkin, F.; Kerr, G. Demographic disparities in United States Clean Air Act PM2.5 attainment counties: Assessing population living in nonattainment conditions. J. Environ. Stud. Sci. 2024, 15, 298–309. [Google Scholar] [CrossRef]
- Casey, J.A.; Kioumourtzoglou, M.-A.; Padula, A.; González, D.J.; Elser, H.; Aguilera, R.; Northrop, A.J.; Tartof, S.Y.; Mayeda, E.R.; Braun, D. Measuring long-term exposure to wildfire PM2.5 in California: Time-varying inequities in environmental burden. Proc. Natl. Acad. Sci. USA 2024, 121, e2306729121. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Wang, H.; Kang, H.; Hwang, M.-H.; Lee, D.G. Influences of PM2.5 pollution on the public’s negative emotions, risk perceptions, and coping behaviors: A cross-national study in China and Korea. J. Risk Res. 2023, 26, 367–379. [Google Scholar] [CrossRef]
- NTAQHI Air Quality Data for Area Point 50240392. Available online: https://www2.ntaqhi.info/?area-point=50240392 (accessed on 8 June 2025).
- Jongeneelen, F.J. Benchmark guideline for urinary 1-hydroxypyrene as biomarker of occupational exposure to polycyclic aromatic hydrocarbons. Ann. Occup. Hyg. 2001, 45, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pollution Control Department. PM2.5 Exposure Limit Update. Available online: https://www.pcd.go.th/ (accessed on 8 June 2025).
- WHO. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Amnuaylojaroen, T.; Kaewkanchanawong, P.; Panpeng, P. Distribution and meteorological control of PM2.5 and its effect on visibility in Northern Thailand. Atmosphere 2023, 14, 538. [Google Scholar] [CrossRef]
- Othman, M.; Latif, M.T.; Hamid, H.H.A.; Uning, R.; Khumsaeng, T.; Phairuang, W.; Daud, Z.; Idris, J.; Sofwan, N.M.; Lung, S.-C.C. Spatial–temporal variability and health impact of particulate matter during a 2019–2020 biomass burning event in Southeast Asia. Sci. Rep. 2022, 12, 7630. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xing, J.; Ji, Q.; Li, Z.; Wang, Y.; Zhao, H.; Wang, Q.; Wang, T.; Yu, L.; Zhang, X. Declining pulmonary function in populations with long-term exposure to polycyclic aromatic hydrocarbons-enriched PM2.5. Environ. Sci. Technol. 2018, 52, 6610–6616. [Google Scholar] [CrossRef]
- Darcey, E.; Carey, R.N.; Reid, A.; Driscoll, T.; Glass, D.C.; Benke, G.P.; Peters, S.; Fritschi, L. Prevalence of exposure to occupational carcinogens among farmers. Rural Remote Health 2018, 18, 4348. [Google Scholar] [CrossRef] [PubMed]
- Ciarrocca, M.; Rosati, M.V.; Tomei, F.; Capozzella, A.; Andreozzi, G.; Tomei, G.; Bacaloni, A.; Casale, T.; Andrè, J.C.; Fioravanti, M.; et al. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Urinary 1-Hydroxypyrene Levels: A Meta-Analysis. Int. J. Environ. Res. Public Health 2013, 10, 5419–5437. [Google Scholar]
- Kang, D.; Rothman, N.; Cho, S.H.; Lim, H.S.; Kwon, H.J.; Kim, S.M.; Schwartz, B.; Strickland, P.T. Association of Exposure to Polycyclic Aromatic Hydrocarbons (Estimated from Job Category) with Concentration of 1-Hydroxypyrene Glucuronide in Urine from Workers at a Steel Plant. Int. Arch. Occup. Environ. Health 1995, 67, 233–239. [Google Scholar] [CrossRef]
- Driscoll, T.R.; Carey, R.N.; Peters, S.; Glass, D.C.; Benke, G.; Reid, A.; Fritschi, L. The Australian Work Exposures Study: Occupational Exposure to Polycyclic Aromatic Hydrocarbons. Ann. Occup. Hyg. 2016, 60, 124–131. [Google Scholar] [CrossRef]
- Stowers, S.J.; Anderson, M.W. Formation and Persistence of Benzo(a)pyrene Metabolite-DNA Adducts. Environ. Health Perspect. 1985, 62, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.R.C.; Farooqi, H.M.U.; Kim, Y.S.; Lee, S.H.; Choi, K.H. Impact of serum concentration in cell culture media on tight junction proteins within a multiorgan microphysiological system. Microelectron. Eng. 2020, 232, 111405. [Google Scholar] [CrossRef]
- Alexandrov, A.; Martzen, M.R.; Phizicky, E.M. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA 2002, 8, 1253–1266. [Google Scholar] [CrossRef]
- Johnson, T.; Mol, A.P.; Zhang, L.; Yang, S. Living under the dome: Individual strategies against air pollution in Beijing. Habitat Int. 2017, 59, 110–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Mao, Y.; Huo, X.; Wu, L. Exploring Community Resilience: The Joint Roles of Environmental Knowledge and Risk Perception in Pro-Environmental Behavior. Buildings 2025, 15, 169. [Google Scholar] [CrossRef]
- Dwivedi, R.; Yaqoob, M.; Khan, A.; Priyadarshi, P.; Ahmad, S.; Attri, S.; Mutharaju, R. In Search of a “Social-AQI”. Democratic Deficits in the Air Pollution Data Regime in Delhi. South Asia Multidiscip. Acad. J. 2023. [Google Scholar] [CrossRef]
- Maung, T.Z.; Bishop, J.E.; Holt, E.; Turner, A.M.; Pfrang, C. Indoor air pollution and the health of vulnerable groups: A systematic review focused on particulate matter (PM), volatile organic compounds (VOCs) and their effects on children and people with pre-existing lung disease. Int. J. Environ. Res. Public Health 2022, 19, 8752. [Google Scholar] [CrossRef] [PubMed]
- Orakij, W.; Chetiyanukornkul, T.; Kasahara, C.; Boongla, Y.; Chuesaard, T.; Furuuchi, M.; Hata, M.; Tang, N.; Hayakawa, K.; Toriba, A. Polycyclic aromatic hydrocarbons and their nitro derivatives from indoor biomass-fueled cooking in two rural areas of Thailand: A case study. Air Qual. Atmos. Health 2017, 10, 747–761. [Google Scholar] [CrossRef]
- Boldeanu, L.; Văduva, C.-C.; Caragea, D.C.; Novac, M.B.; Manasia, M.; Siloși, I.; Manolea, M.M.; Boldeanu, M.V.; Dijmărescu, A.L. Association between serum 8-iso-prostaglandin F2α as an oxidative stress marker and immunological markers in a cohort of preeclampsia patients. Life 2023, 13, 2242. [Google Scholar] [CrossRef]

| Characteristic | n | % | Characteristic | n | % |
|---|---|---|---|---|---|
| Age group | Gender identity | ||||
| ≤49 years | 21 | 14.0 | Female | 115 | 76.7 |
| 50–59 years | 47 | 31.3 | Male | 35 | 23.3 |
| ≥60 years | 82 | 54.7 | |||
| Occupation | Education level | ||||
| Employees and workers | 80 | 53.3 | Primary education | 83 | 55.3 |
| Farmers | 41 | 27.3 | Secondary education | 56 | 37.3 |
| Private business/others | 29 | 19.3 | Bachelor’s or higher | 11 | 7.3 |
| Monthly income (THB) | Smoking status | ||||
| <5000 | 74 | 49.3 | Non-smoker | 128 | 85.3 |
| 5001–10,000 | 65 | 43.3 | Current/former smoker | 22 | 14.7 |
| >10,000 | 11 | 7.4 | |||
| Alcohol consumption | Family history | ||||
| Never | 96 | 64.0 | Diabetes | 46 | 30.9 |
| Occasionally (≤4×/month) | 41 | 27.3 | Cardiovascular disease | 45 | 30.0 |
| Frequently (≥2×/week) | 13 | 8.7 | Cancer | 26 | 17.3 |
| Exercise frequency | Chronic diseases | ||||
| No exercise | 41 | 27.3 | Any chronic disease | 91 | 60.7 |
| 1–4 times/week | 83 | 55.3 | Diabetes | 46 | 49.5 |
| Daily | 26 | 17.3 | High blood pressure | 35 | 38.5 |
| High blood fat | 40 | 44.0 | |||
| Asthma | 9 | 9.9 | |||
| Other (thyroid, gout, etc.) | 12 | 13.2 |
| Occupation | 1-OHP (μmol/mol Cre) | BPDE (ng/mL) | ||||
|---|---|---|---|---|---|---|
| Low-PM2.5 Season Mean ± S.D. | High-PM2.5 Season Mean ± S.D. | p-Value | Low-PM2.5 Season Mean ± S.D. | High-PM2.5 Season Mean ± S.D. | p-Value | |
| Private workers and others (n = 32) | 0.15 ± 0.27 | 0.59 ± 0.79 | <0.001 | 1.4 ± 2.16 | 1.02 ± 1.18 | 0.23 |
| Employees and laborers (n = 77) | 0.30 ± 0.71 | 0.90 ± 1.30 | <0.001 | 1.69 ± 2.42 | 0.94 ± 1.53 | 0.02 |
| Farmers (n = 41) | 0.13 ± 0.10 | 1.06 ± 1.53 | <0.001 | 1.35 ± 1.04 | 1.01 ± 1.40 | 0.018 |
| All participants (n = 150) | 0.22 ± 0.52 | 0.89 ± 1.27 | <0.001 | 1.55 ± 2.06 | 0.98 ± 1.42 | 0.0001 |
| Dimensions | Questions | Factor Loading 1 | Factor Loading 2 | |
|---|---|---|---|---|
| Environmental Risk Knowledge | The general perception of burning as a cause of pollution | Q1.1 The cause of the smog problem in Chiang Mai is all kinds of burning. | 0.436 | 0.756 |
| Q1.2 Open burning is one of the causes of dirty air because there are contaminants in the air such as smoke, dust. | 0.762 | 0.615 | ||
| Q1.3 Chiang Mai City is located in a lowland basin, so the air is not well-ventilated, causing dust to cover the city. | 0.578 | 0.611 | ||
| Q1.4 During winter, the air is still and low, causing more pollution than at other times. | 0.672 | 0.283 | ||
| Q1.5 Dust from construction or factories must be removed before being released into the atmosphere. | 0.648 | 0.254 | ||
| Perceived health impacts | Q1.6 Fine particles from various types of burning can damage the respiratory system of people. | 0.864 | / | |
| Q1.7 People in areas with polluted air are more likely to suffer from lung diseases than people in other areas. | 0.772 | 0.843 | ||
| Environmental and economic impacts | Q1.8 Air pollution causes soil and water quality to deteriorate. | 0.334 | 0.224 | |
| Q1.9 Smog causes traffic accidents. | 0.557 | 0.23 | ||
| Q1.10 Smog causes poor visibility in flights, preventing planes from taking off or landing. | 0.333 | 0.634 | ||
| Q1.11 Chiang Mai loses a lot of economic income from air pollution. | 0.729 | 0.639 | ||
| Government response and regulations | Q1.12 People lose a lot of health care expenses from air pollution. | 0.39 | 0.914 | |
| Q1.13 Local officials have the power to order a ban on open burning. Those who fail to comply with the order may be subject to punishment. | 0.842 | 0.91 | ||
| Perceived Risk and Responsibility | Environmental awareness | Q2.1 Burning pollutes the air in the area where it is burned and spreads the pollution to other areas. | 0.431 | 0.998 |
| Q2.2 All types of burning make the air in Mueang Chiang Mai hotter. | 1.063 | 0.999 | ||
| Waste management | Q2.3 Reducing the amount of garbage in your home reduces air pollution. | 0.495 | 0.7 | |
| Q2.4 Repairing and reusing damaged items reduces air pollution. | 0.974 | 0.386 | ||
| Q2.5 Sorting garbage before throwing it away can solve the problem of air pollution. | 1.084 | 1.17 | ||
| Community and personal responsibility | Q2.6 Everyone should avoid burning. | / | 1.103 | |
| Q2.7 Everyone should help plant trees. | 0.694 | 0.403 | ||
| Q2.8 Everyone should help monitor garbage burning in the community. | / | / | ||
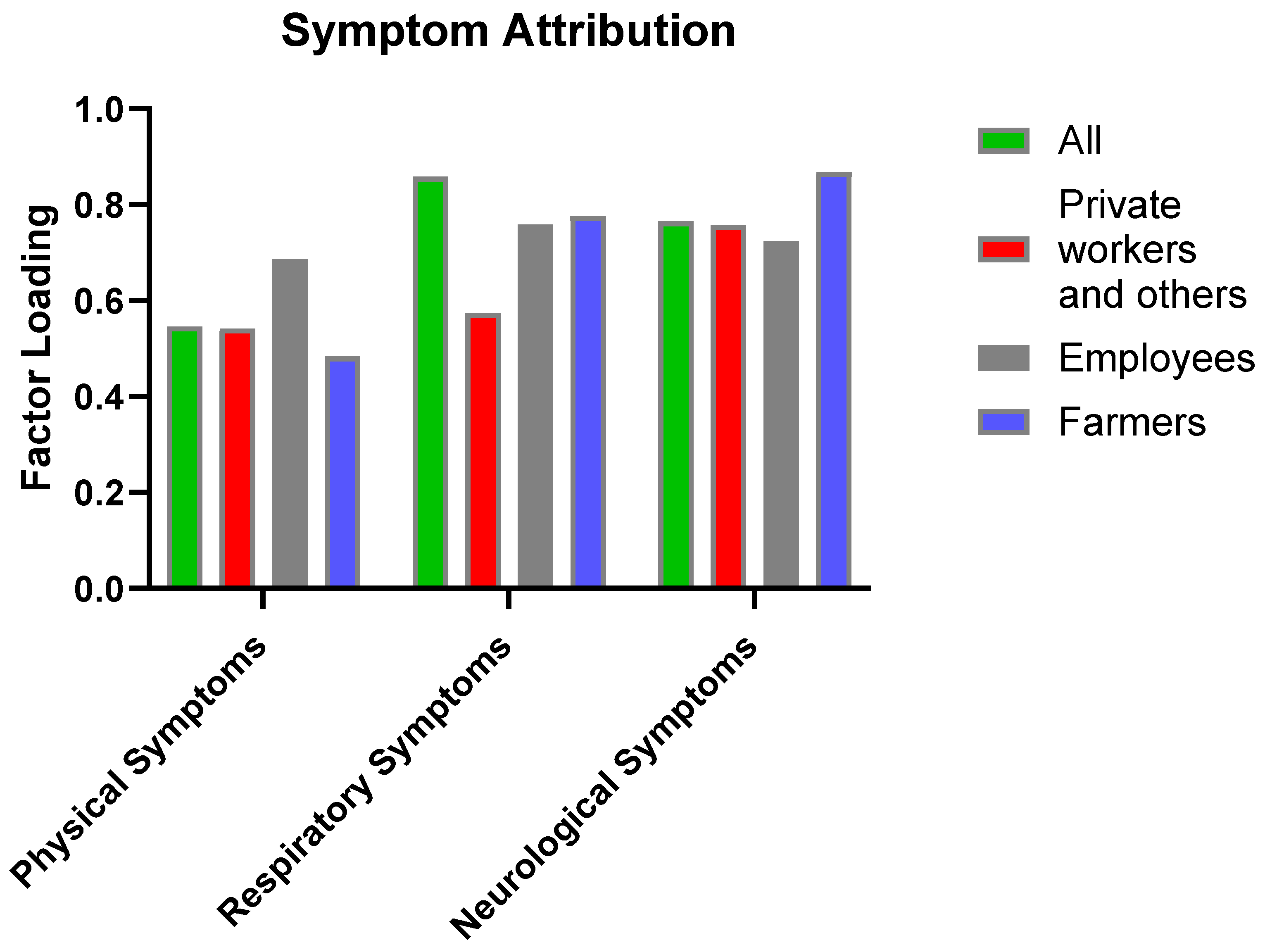
| Symptom Attribution | Physical symptoms | Q3.1 Eye irritation. | 0.792 | 0.312 |
| Q3.2 Skin irritation. | 0.488 | 0.78 | ||
| Respiratory symptoms | Q3.3 Respiratory irritation such as coughing, sneezing, chest tightness. | 0.851 | 0.833 | |
| Q3.4 Feeling short of breath. | 0.442 | 0.673 | ||
| Q3.5 Reported pneumonia diagnosis . | 0.673 | 1.07 | ||
| Neurological symptoms | Q3.6 Feeling Faint/Lightheaded. | 0.876 | 0.834 | |
| Q3.7 Reported loss of consciousness. | 0.956 | 1.049 | ||
| Q3.8 Feeling dizzy. | 0.821 | 0.572 | ||
| Q3.9 Having poor visibility while driving. | 0.496 | 0.605 | ||
| Protective Behavioral Intentions | Preventive actions | Q4.1 Avoid outdoor exercise or stay in open areas. | 0.31 | / |
| Q4.2 Avoid burning of any kind. | 1.007 | 0.228 | ||
| Q4.3 Reduce the amount of household waste. | 0.187 | / | ||
| Q4.4 Close the doors and windows of the house to prevent dust. | 0.455 | 0.575 | ||
| Environmental improvements | Q4.5 Use water to wash around the house to reduce the amount of dust. | 0.468 | 1.246 | |
| Q4.6 Help plant trees. | 0.599 | 0.33 | ||
| Safety and health impacts | Q4.7 Having an accident caused by smog on the roadside. | / | 0.52 | |
| Q4.8 If driving a vehicle, turn off the engine when parked. | 0.871 | 0.905 | ||
| Q4.9 Have an annual health check to monitor health. | 0.142 | 1.148 | ||
| Health Communication and Comprehension | Knowledge and understanding | Q5.1 I can read knowledge, diagrams, or specific terms about air pollution, such as PM2.5, Air Quality Index (AQI), etc., with understanding. | 0.357 | 0.937 |
| Q5.2 I can easily understand and know the explanations about PM2.5 dust from various media. | 0.987 | 0.407 | ||
| Q5.3 I understand the explanations about how to reduce the health impacts of PM2.5 dust that are published in various places. | 0.704 | 1.136 | ||
| Q5.4 I understand the causes and health impacts of the problem of fine dust in the air. | 0.592 | 0.527 | ||
| Application and Personal Information | Q5.5 I know and understand enough about PM2.5 dust to be able to use it to protect my health and that of others. | 0.873 | 0.341 | |
| Communication and Advocacy | Q5.6 I can explain to others about the level of PM2.5 dust that affects health. | 0.954 | 0.671 | |
| Q5.7 I am open to advice on preventing and reducing the impacts of fine dust and can explain it to others. | 0.776 | 1.003 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, S.; Cao, X.; Yadoung, S.; Wongta, A.; Zhou, K.; Kosashunhanan, N.; Hongsibsong, S. Associations Between Individual Health Risk Perceptions and Biomarkers of PAH Exposure Before and After PM2.5 Pollution in the Suburbs of Chiang Mai Province. Toxics 2025, 13, 491. https://doi.org/10.3390/toxics13060491
Kausar S, Cao X, Yadoung S, Wongta A, Zhou K, Kosashunhanan N, Hongsibsong S. Associations Between Individual Health Risk Perceptions and Biomarkers of PAH Exposure Before and After PM2.5 Pollution in the Suburbs of Chiang Mai Province. Toxics. 2025; 13(6):491. https://doi.org/10.3390/toxics13060491
Chicago/Turabian StyleKausar, Sobia, Xianfeng Cao, Sumed Yadoung, Anurak Wongta, Kai Zhou, Natthapol Kosashunhanan, and Surat Hongsibsong. 2025. "Associations Between Individual Health Risk Perceptions and Biomarkers of PAH Exposure Before and After PM2.5 Pollution in the Suburbs of Chiang Mai Province" Toxics 13, no. 6: 491. https://doi.org/10.3390/toxics13060491
APA StyleKausar, S., Cao, X., Yadoung, S., Wongta, A., Zhou, K., Kosashunhanan, N., & Hongsibsong, S. (2025). Associations Between Individual Health Risk Perceptions and Biomarkers of PAH Exposure Before and After PM2.5 Pollution in the Suburbs of Chiang Mai Province. Toxics, 13(6), 491. https://doi.org/10.3390/toxics13060491








