Removal of Zn(II) and Ag(I) by Staphylococcus epidermidis CECT 4183 and Biosynthesis of ZnO and Ag/AgCl Nanoparticles for Biocidal Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Preparation
2.2. Biosorption Test
2.3. Biomass Characterization
2.4. Synthesis and Characterization of Nanoparticles
2.5. Study of the Biocidal Capacity of Nanoparticles: Planktonic Cells vs. Biofilms
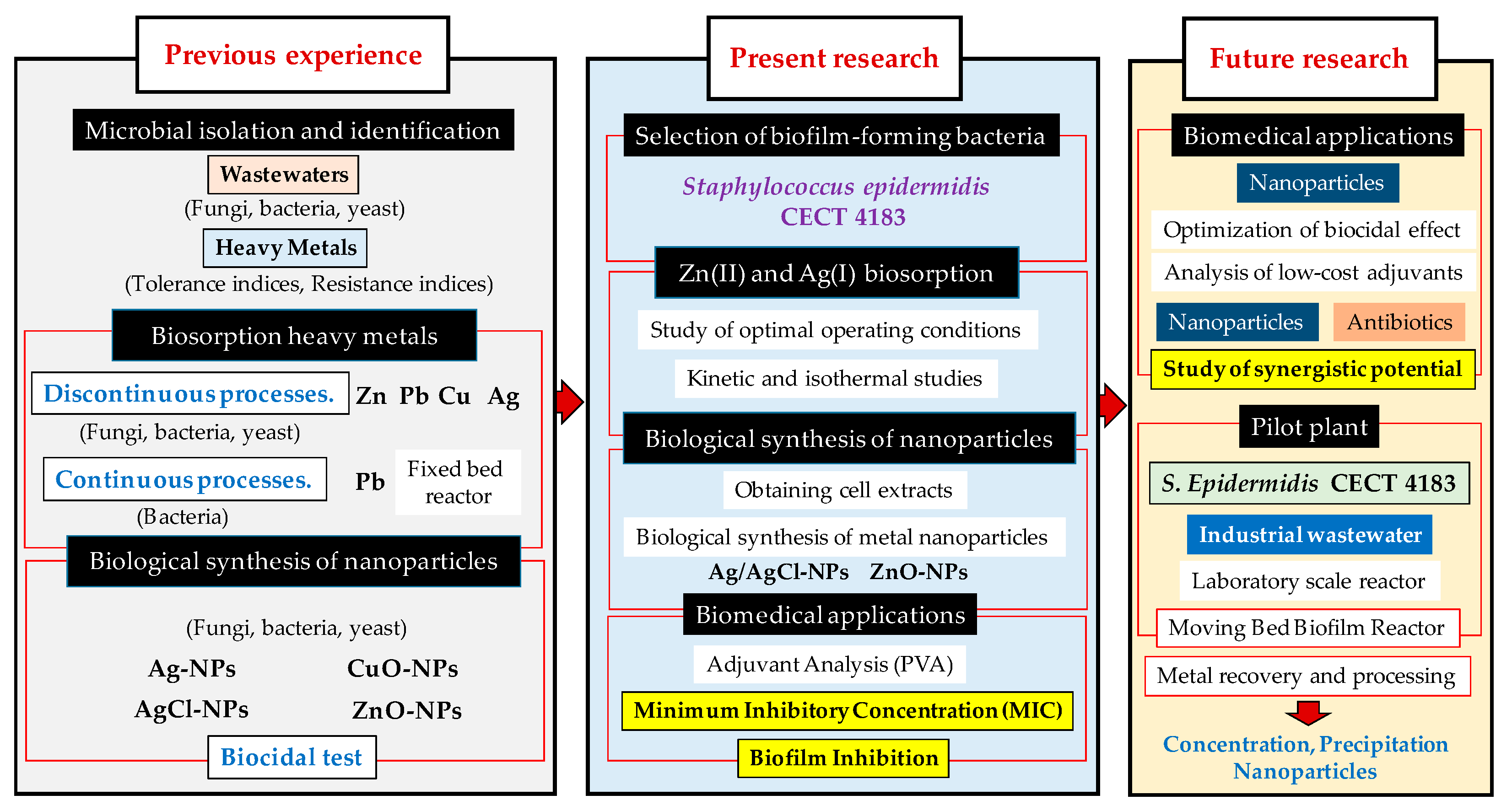
3. Results and Discussion
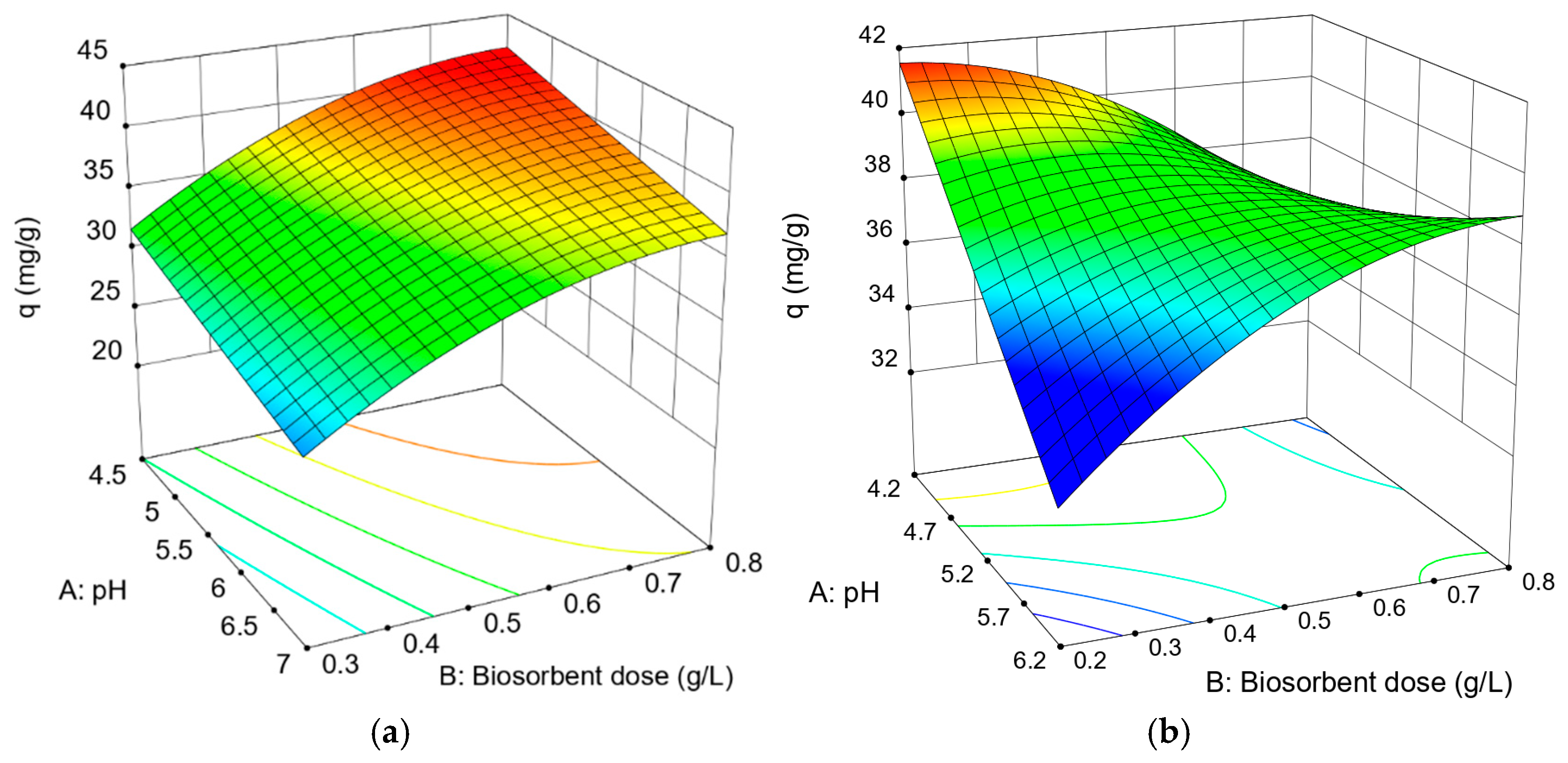
3.1. Experimental Design
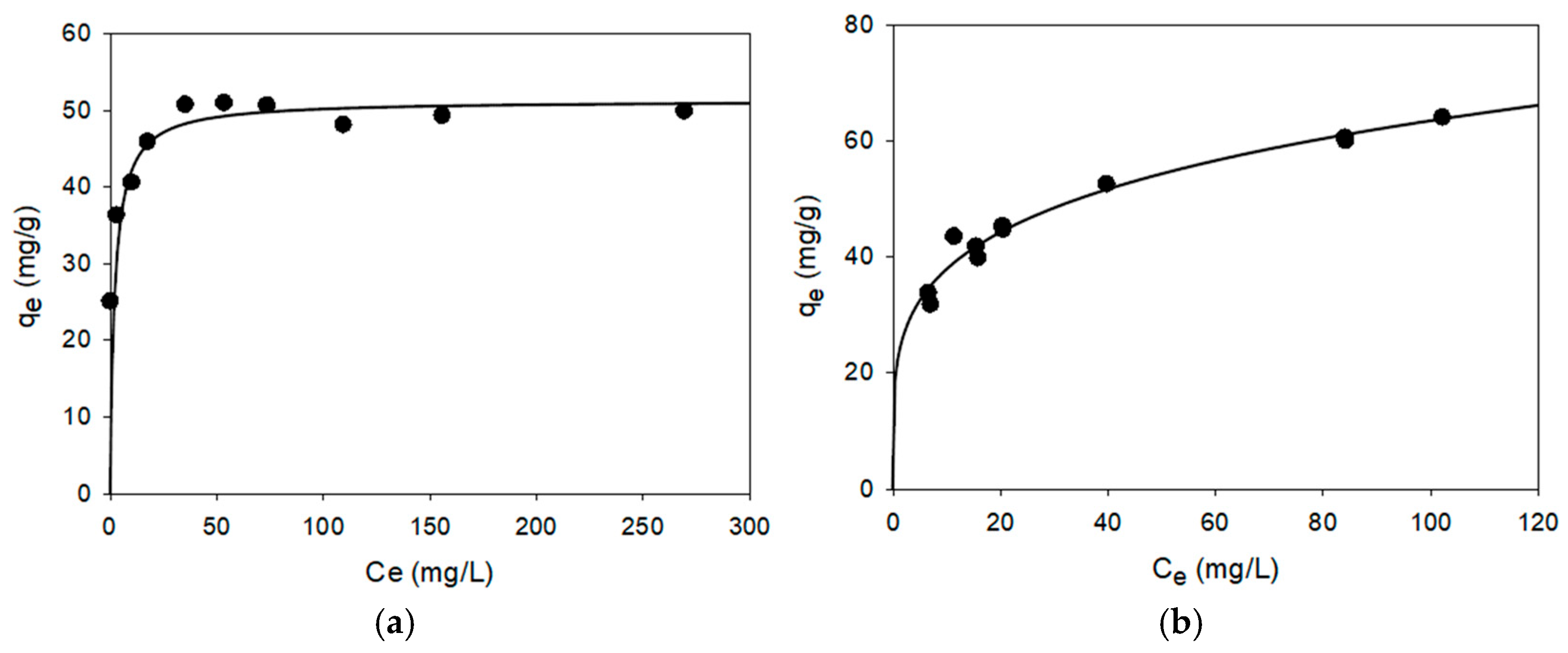
3.2. Kinetic and Equilibrium Studies: Biosorption Isotherms
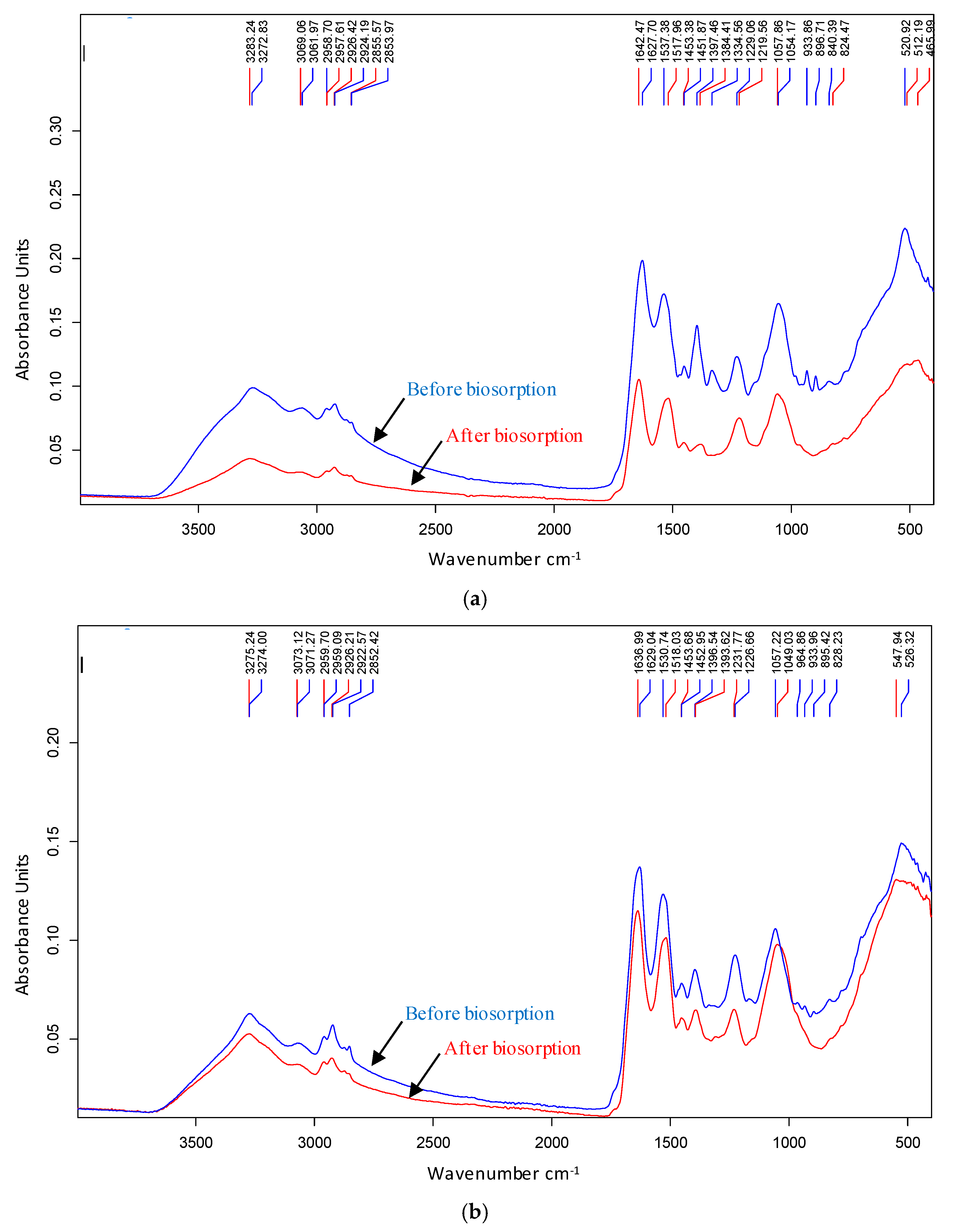
3.3. Biosorption Mechanisms
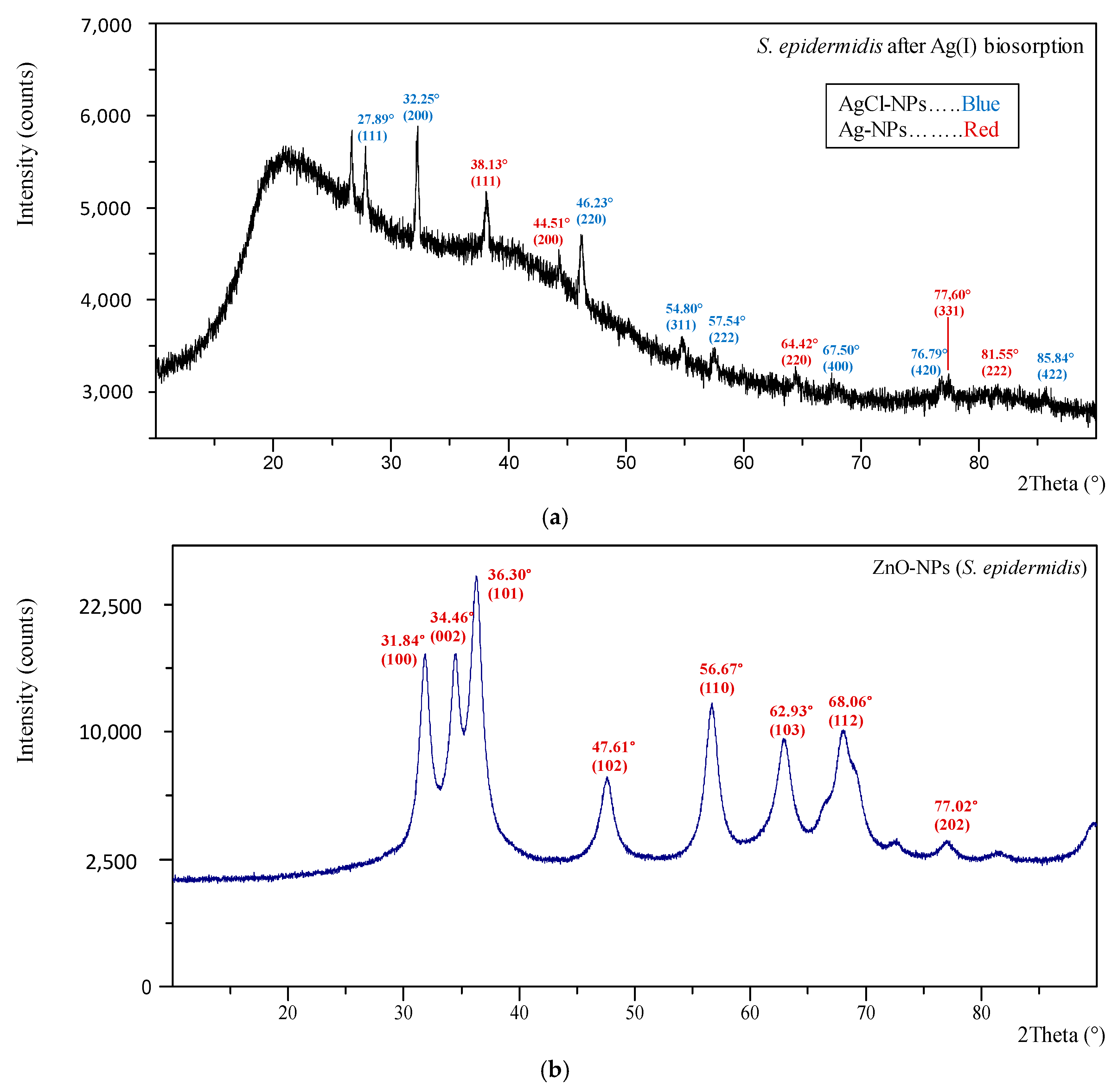
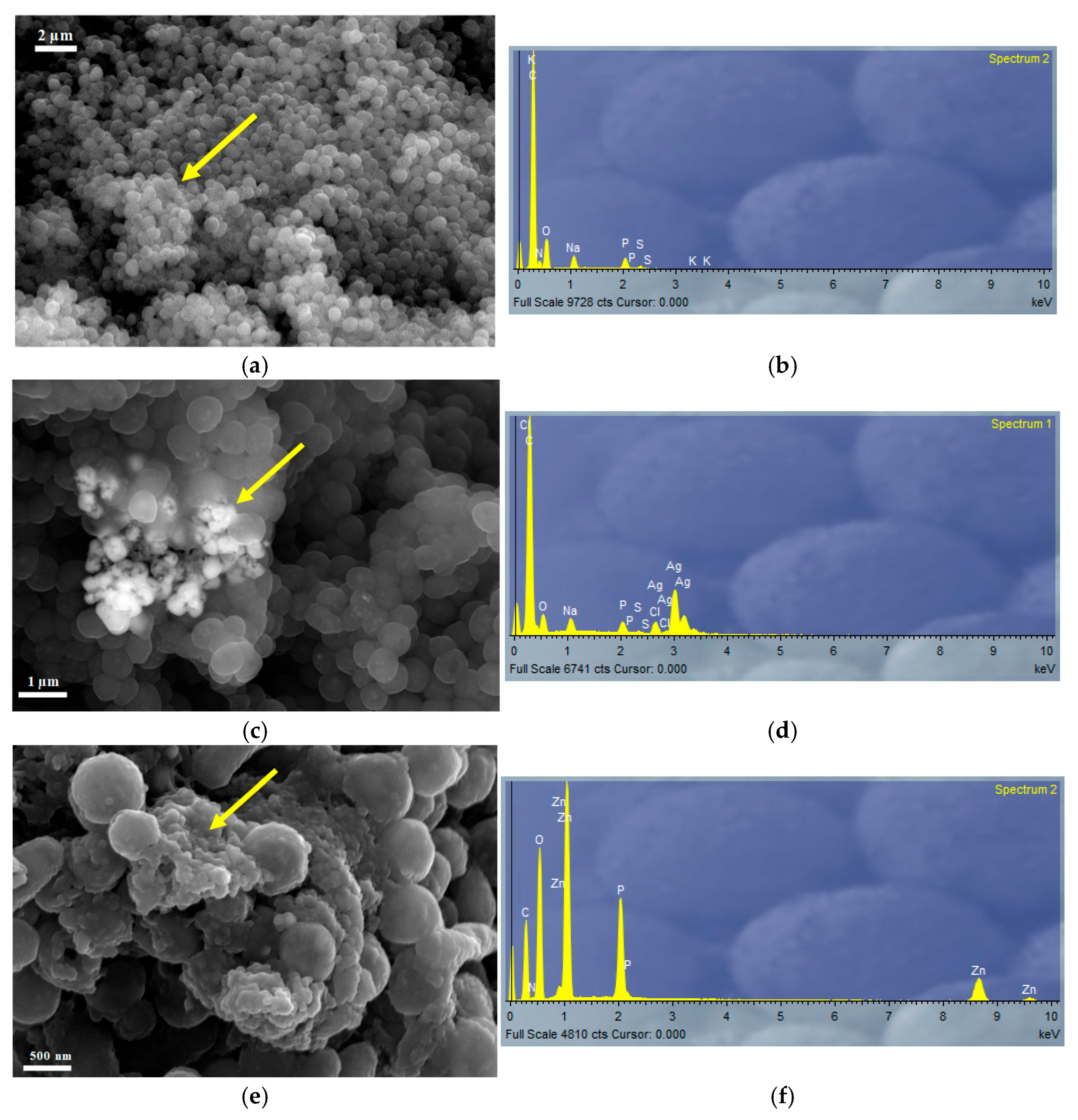
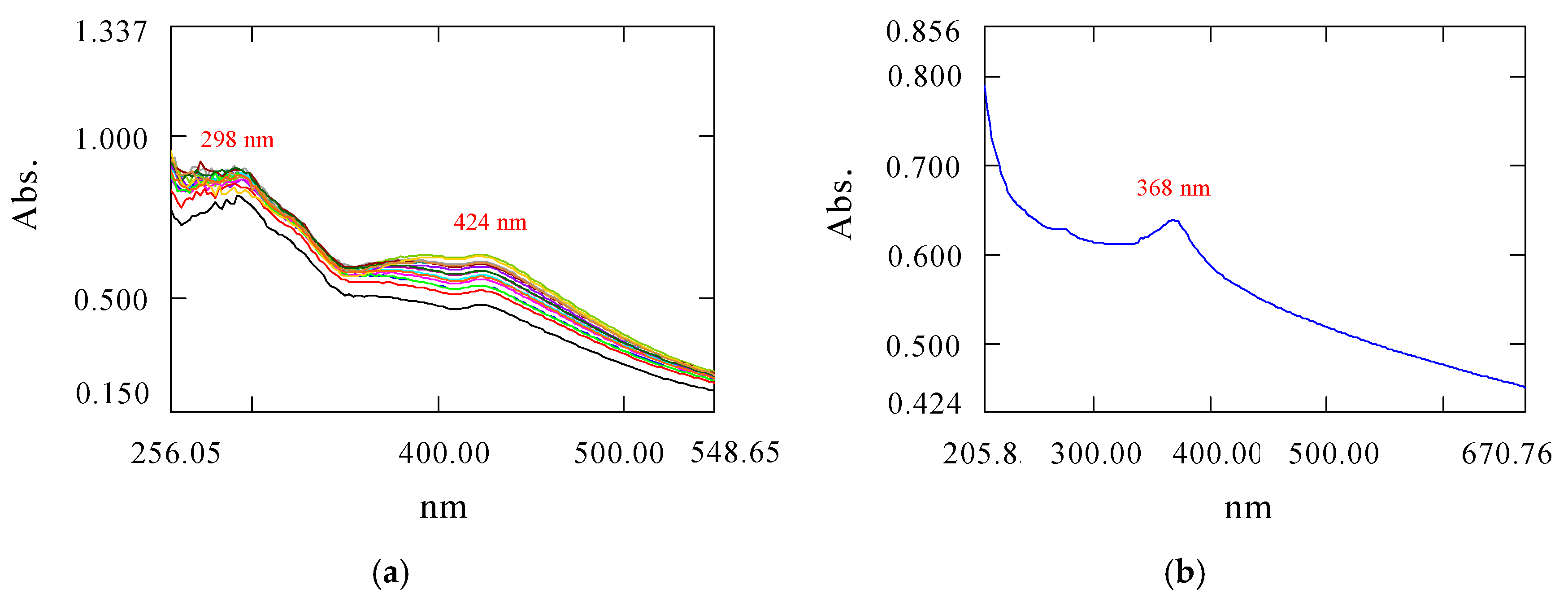
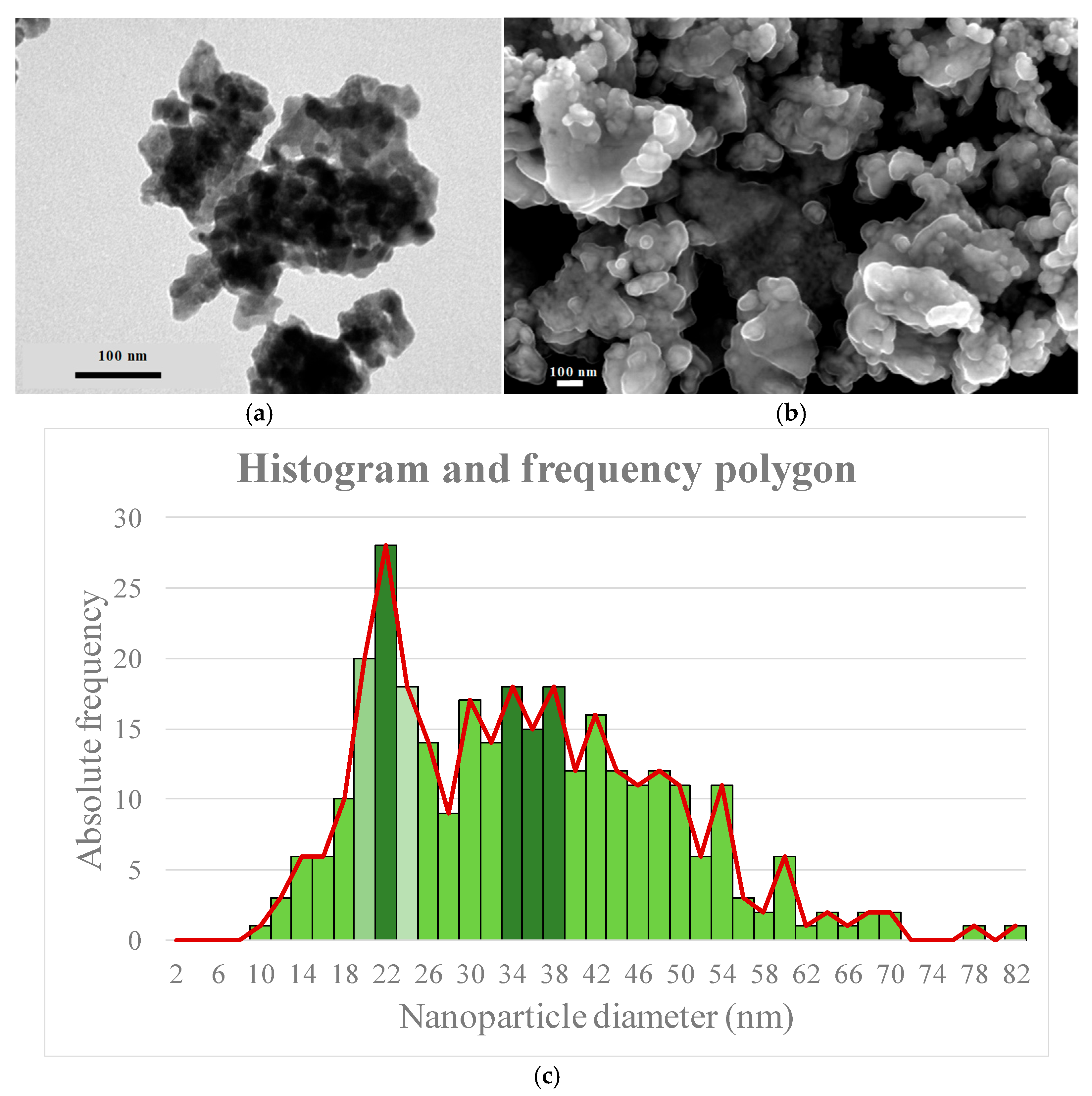
3.4. Characterization of Nanoparticles
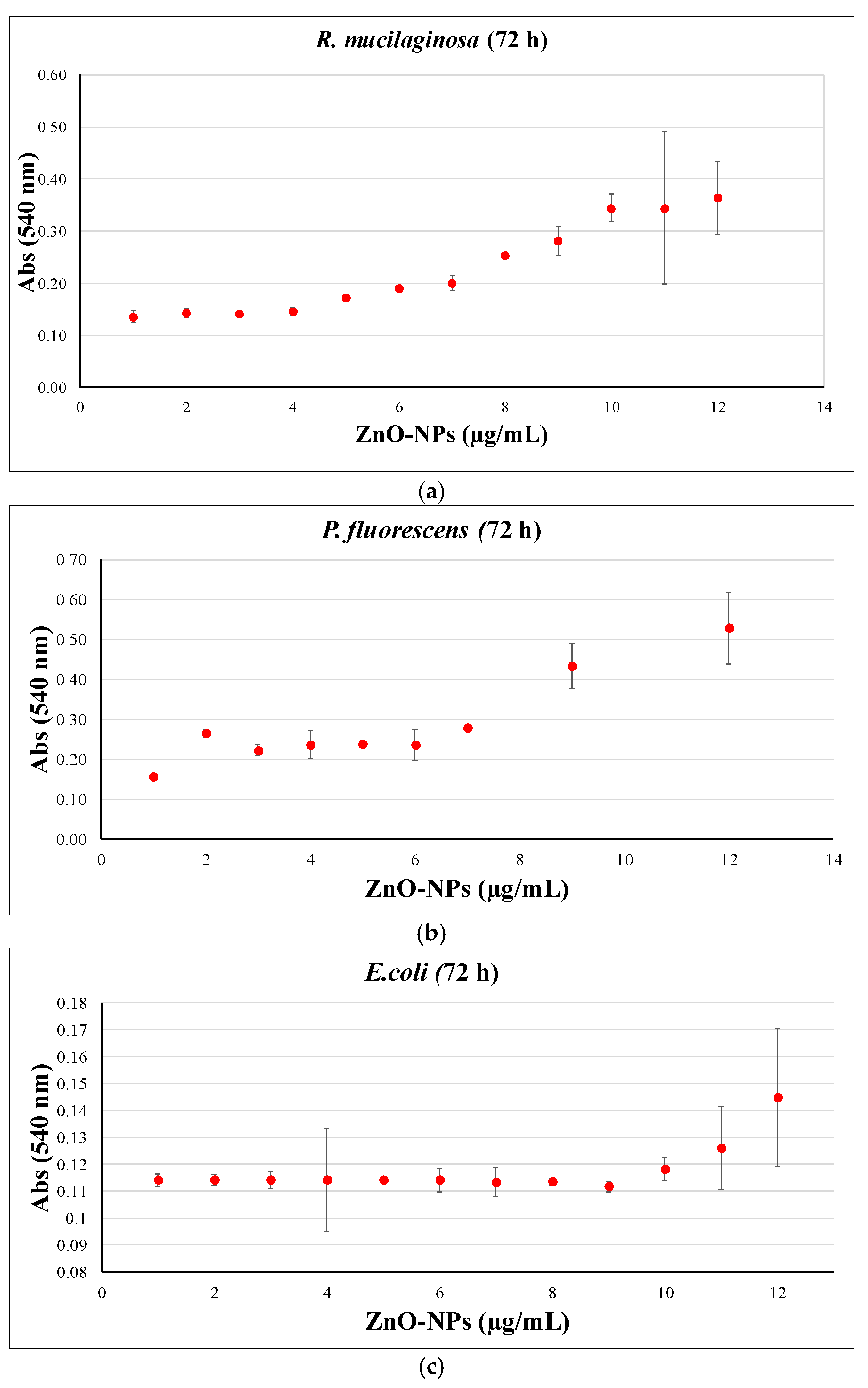
3.5. Biocidal Tests: Determination of MIC and MBIC
4. Conclusions
- (1)
- Staphylococcus epidermidis CECT 4183 exhibited excellent characteristics for use as a biosorbent for Ag(I) and Zn(II) ions, with qm values of 47.43 and 65.08 mg/g, respectively. These values are among the best reported in scientific literature.
- (2)
- These values were obtained under optimal operating conditions: pH 4.5 and a biomass dose of 0.8 g/L for Ag(I), and a biomass dose of 0.2 g/L and pH 4.2 for Zn(II).
- (3)
- The cellular extract of the bacteria demonstrated good characteristics for use as a catalyst in the synthesis of Ag and ZnO nanoparticles, though its performance was significantly better when working with Zn(II) ions.
- (4)
- High-purity ZnO-NPs were obtained, which acted as effective biocidal agents against both planktonic cells and microbial biofilms of the studied microorganisms.
- (5)
- MIC values ranged from 62.5 to 250 µg/mL, while biofilm formation inhibitions of over 70% were achieved with exposures at low doses, as low as 125 µg/mL.
- (6)
- In the case of E. coli, complete inhibition was observed with only 15.63 µg/mL, showcasing the biocidal potential of ZnO-NPs synthesized from S. epidermidis CECT 4183.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehdizadeh, P.; Jamdar, M.; Mahdi, M.A.; Abdulsahib, W.K.; Jasim, L.S.; Yousefi, S.R.; Salavati-Niasari, M. Rapid microwave fabrication of new nanocomposites based on Tb-Co-O nanostructures and their application as photocatalysts under UV/Visible light for removal of organic pollutants in water. Arab. J. Chem. 2023, 16, 104579. [Google Scholar] [CrossRef]
- Guarino, F.; Improta, G.; Triassi, M.; Cicatelli, A.; Castiglione, S. Effects of zinc pollution and compost amendment on the root microbiome of a metal tolerant poplar clone. Front. Microbiol. 2020, 11, 1677. [Google Scholar] [CrossRef] [PubMed]
- Arisekar, U.; Shakila, R.J.; Shalini, R.; Jeyasekaran, G.; Keerthana, M.; Arumugam, N.; Almansour, A.I.; Perumal, K. Distribution and ecological risk assessment of heavy metals using geochemical normalization factors in the aquatic sediments. Chemosphere 2022, 294, 133708. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Shin, K.; Lee, H.; Choi, S.; Kim, G.; Depuydt, S.; De Saeger, J.; Heynderickx, P.; Wu, D.; Asselman, J.; et al. Evaluating ecotoxicological assays for comprehensive risk assessment of toxic metals present in industrial wastewaters in the Republic of Korea. Sci. Total Environ. 2023, 867, 161536. [Google Scholar] [CrossRef]
- Biasi, A.M.; Messina, G.A.; Gómez, N.N. Determinación de Zinc en muestras de agua de ríos y red de la provincia de San Luis y aguas envasadas. Diaeta 2021, 39, 38–48. [Google Scholar]
- Ren, Z.; Zhang, C.; Chen, J.; Zhang, H.; Meng, J.; Han, X.; Liang, J. Highly efficient recovery of Zn (II) from zinc-containing wastewater by tourmaline tailings geopolymers to in-situ construct nanoscale ZnS for the photodegradation of tetracycline hydrochloride. Environ. Res. 2024, 259, 119504. [Google Scholar] [CrossRef]
- Dehkordi, M.M.; Nodeh, Z.P.; Dehkordi, K.S.; Salmanvandi, H.; Khorjestan, R.R.; Ghaffarzadeh, M. Soil, air, and water pollution from mining and industrial activities: Sources of pollution, environmental impacts, and prevention and control methods. Results Eng. 2024, 23, 102729. [Google Scholar] [CrossRef]
- Deycard, V.N.; Schäfer, J.; Petit, J.C.; Coynel, A.; Lanceleur, L.; Dutruch, L.; Bossy, C.; Ventura, A.; Blanc, G. Inputs, dynamics and potential impacts of silver (Ag) from urban wastewater to a highly turbid estuary (SW France). Chemosphere 2017, 167, 501–511. [Google Scholar] [CrossRef]
- Padhye, L.P.; Jasemizad, T.; Bolan, S.; Tsyusko, O.V.; Unrine, J.M.; Kumar Biswal, B.; Balasubramanian, R.; Zhang, Y.; Zhang, T.; Zhao, J.; et al. Silver contamination and its toxicity and risk management in terrestrial and aquatic ecosystems. Sci. Total Environ. 2023, 871, 161926. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Zhang, C.; Wang, R.; Liu, S.; Du, R.; Sun, W. Advances in treatment technologies for silver-containing wastewater. J. Chem. Eng. 2024, 496, 153689. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Dura, A.; Breslin, C.B. Electrocoagulation using aluminium anodes activated with Mg, In and Zn alloying elements. J. Hazard. Mater. 2019, 366, 39–45. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Palanivelu, J.; Hemavathy, R.V. Sustainable approaches for removing toxic heavy metal from contaminated water: A comprehensive review of bioremediation and biosorption techniques. Chemosphere 2024, 357, 141933. [Google Scholar] [CrossRef] [PubMed]
- Bordin, E.R.; Ramsdorf, W.A.; Domingos, L.M.L.; de Souza Miranda, L.P.; Mattoso Filho, N.P.; Cestari, M.M. Ecotoxicological effects of zinc oxide nanoparticles (ZnO-NPs) on aquatic organisms: Current research and emerging trends. J. Environ. Manag. 2024, 349, 119396. [Google Scholar] [CrossRef] [PubMed]
- Natalia, T.; Tatiana, M.; Kolesnikov, S.; Minkina, T. Chapter thirteen-Pollution of silver and silver nanoparticles in the ecosystems and their interactions with plants and soil microbiota. In Emerging Contaminants, Sustainable Agriculture and the Environment; Kumari, A., Rajput, V.D., Mandzhieva, S.S., Minkina, T., Hullebusch, E., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2024; pp. 267–290. [Google Scholar] [CrossRef]
- Hachem, Z.; Kashmar, R.; Abdallah, A.M.; Awad, R.; Khalil, M.I. Characterization, antioxidant, antibacterial, and antibiofilm properties of biosynthesized Ag/AgCl nanoparticles using Origanum ehrenbergii Boiss. Results Mater. 2024, 21, 100550. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E. Removal of heavy metals by Klebsiella sp. 3S1. Kinetics, equilibrium and interaction mechanisms of Zn(II) biosorption. J. Chem. Technol. Biotechno. 2018, 93, 1370–1380. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H. Biosorption of heavy metals from aqueous solution by UV-mutant Bacillus subtilis. Environ. Sci. Pollut. Res. 2013, 20, 7450–7463. [Google Scholar] [CrossRef]
- Kılıç, Z.; Atakol, O.; Aras, S.; Cansaran-Duman, D.; Emregul, E. Biosorption properties of zinc(II) from aqueous solutions by Pseudevernia furfuracea (L.) Zopf. J. Air Waste Manag. Assoc. 2014, 64, 1112–1121. [Google Scholar] [CrossRef]
- Langmuir, I. Adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Adsorption in solutions. Phy. Chem. 1906, 57, 384–410. [Google Scholar]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–549. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024–1039. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Castro, E. Biosorption mechanisms of Ag(I) and the synthesis of nanoparticles by the biomass from Botryosphaeria rhodina MAMB-05. J. Hazar. Mater. 2021, 420, 126598. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espinola, F.; Moya, M.; Martín, C.; Ruiz, E. 2024, Cu(II) Biosorption and Synthesis of CuO Nanoparticles by Staphylococcus epidermidis CECT 4183: Evaluation of the Biocidal Effect. Appl. Sci. 2024, 14, 7623. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Espinola, F.; Ruiz, E.; Moya, M.; Castro, E. Ag(I) Biosorption and Green Synthesis of Silver/Silver Chloride Nanoparticles by Rhodotorula mucilaginosa 1S1. Nanomaterials 2023, 13, 295. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Ruiz, E.; Abriouel, H.; Gálvez, A.; Ezzouhri, L.; Lairini, K.; Espínola, F. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. J. Chem. Eng. 2012, 210, 325–332. [Google Scholar] [CrossRef]
- Cruz, C.D.; Shah, S.; Tammela, P. Defining conditions for biofilm inhibition and eradication assays for Gram-positive clinical reference strains. BMC Microbiol. 2018, 18, 173. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro. Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Li, L.; Hu, Q.; Zeng, J.; Qi, H.; Zhuang, G. Resistence and biosorption mechanism of silver ions by Bacillus cereus biomass. J. Environ. Sci. 2011, 23, 108–111. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Sun, J.; Li, J.; Su, Y.; Ji, Y.; Gong, C. Selective reinforced competitive biosorption of Ag (I) and Cu (II) on Magnetospirillum gryphiswaldense. Desalination 2011, 270, 258–263. [Google Scholar] [CrossRef]
- Das, D.; Das, N.; Mathew, L. Kinetics, equilibrium and thermodynamic studies on biosorption of Ag(I) from aqueous solution by macrofungus Pleurotus platypus. J. Hazard. Mater. 2010, 184, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ahmady-Asbchin, S.; Safari, M.; Tabaraki, R. Biosorption of Zn (II) by Pseudomonas aeruginosa isolated from a site contaminated with petroleum. Desalin. Water Treat. 2015, 54, 3372–3379. [Google Scholar] [CrossRef]
- Tabaraki, R.; Ahmady-Asbchin, S.; Abdi, O. Biosorption of Zn(II) from aqueous solutions by Acinetobacter sp. isolated from petroleum spilled soil. J. Environ. Chem. Eng. 2013, 1, 604–608. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Guan, W.; Chang, J.; Xu, L.; Guo, J.; Wei, G. Biosorption of Zn(II) by live and dead cells of Streptomyces ciscaucasicus strain CCNWHX 72-14. J. Hazard. Mater. 2010, 179, 151–159. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Effects of culture pH on cell surface properties and biosorption of Pb (II), Cd (II), Zn (II) of green alga Neochloris oleoabundans. J. Chem. Eng. 2023, 468, 143579. [Google Scholar] [CrossRef]
- Qu, Y.; Meng, Q.; Zhao, Q.; Ye, Z. Biosorption of Pb(II) and Zn(II) from aqueous solutions by living B350 biomass. Desalin. Water Treat. 2015, 55, 1832–1839. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P. Synthesis of Biogenic Silver Nanoparticles (AgCl-NPs) Using a Pulicaria vulgaris Gaertn. Aerial Part Extract and Their Application as Antibacterial, Antifungal and Antioxidant Agents. Nanomaterials 2020, 10, 638. [Google Scholar] [CrossRef]
- Lin, Z.; Zhou, Z.; Wu, J.; Zhou, J.; Wang, L. A further insight into the mechanism of Ag+ biosorption by Lactobacillus sp. strain A09. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 1195–1200. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.; Xue, F.; Xia, B.; Lu, Y.; Ying, R.; Hu, Z. Adsorption of Zinc(II) Ion by Spent and Raw Agaricus bisporus in Aqueous Solution. Processes 2024, 12, 717. [Google Scholar] [CrossRef]
- Chen, C.; Wen, D.; Wang, J. Cellular surface characteristics of Saccharomyces cerevisiae before and after Ag(I) biosorption. Bioresour. Technol. 2014, 156, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.A.; Uchida, T.; Mizuki, T.; Nakajima, Y.; Katsube, Y.; Hanajiri, T.; Maekawa, T. Synthesis of nanoparticles composed of silver and silver chloride for a plasmonic photocatalyst using an extract from a weed Solidago altissima (goldenrod). Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015002. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Valdez-Salas, B.; Beltran-Partida, E.; Mendez-Trujillo, V.; González-Mendoza, D.; Tzintzun-Camacho, O.; Roumia, A.F. Biosynthesis of Zinc Oxide Nanoparticles Using Garlic Peel Extract and Their Antibacterial Potential. Microbiol. Res. 2024, 15, 1655–1669. [Google Scholar] [CrossRef]
- Subba, B.; Rai, G.B.; Bhandary, R.; Parajuli, P.; Thapa, N.; Kandel, D.K.; Mulmi, S.; Shrestha, S.; Malla, S. Antifungal activity of zinc oxide nanoparticles (ZnO NPs) on Fusarium equiseti phytopathogen isolated from tomato plant in Nepal. Heliyon 2024, 10, e40198. [Google Scholar] [CrossRef]
- Janani, M.; Viswanathan, D.; Pandiaraj, S.; Govindasamy, R.; Gomathi, T.; Vijayakumar, S. Review on phyto-extract methodologies for procuring ZnO NPs and its pharmacological functionalities. Process Biochem. 2024, 147, 186–212. [Google Scholar] [CrossRef]
- Adamu, T.B.; Mengesha, A.M.; Kebede, M.A.; Bogale, B.L.; Kassa, T.W. Facile biosynthesis of zinc oxide nanoparticles (ZnO NPs) using Lupinus albus L (Gibto) seed extract for antibacterial and photocatalytic applications. Results Chem. 2024, 10, 101724. [Google Scholar] [CrossRef]
- Elankathirselvan, K.; Fathima, H.A.; Praveen, K.; Al-Ansari, M.M. Synthesis and characterization of Pyrus communis fruit extract synthesized ZnO NPs and assessed their anti-diabetic and anti-microbial potential. Environ. Res. 2024, 258, 119450. [Google Scholar] [CrossRef]
- Ma, W.; Zhu, G.; Zhang, Y.; Guo, J. Green synthesis of ZnO NPs with long-lasting and ultra-high antimicrobial activity. Surf. Interfaces 2024, 50, 104506. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO Nanomaterials: Current Advancements in Antibacterial Mechanisms and Applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Marqués, G.N.; Moreira, A.J.; Nóbrega, E.T.D.; Braga, S.; Argentín, M.N.; Camargo, I.L.B.C.; Azevedo, E.; Pereira, E.C.; Bernardi, M.I.B.; Mascaro, L.H. Selective inhibitory activity of multidrug-resistant bacteria by zinc oxide nanoparticles. J. Environ. Chem. Eng. 2024, 12, 111870. [Google Scholar] [CrossRef]
- Ebadi, M.; Zolfaghari, M.R.; Aghaei, S.S.; Zargar, M.; Shafiei, M.; Zahiri, H.S.; Noghabi, K.A. A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract of cyanobacterium Nostoc sp. EA03: From biological function to toxicity evaluation. RSC Adv. 2019, 9, 23508–23525. [Google Scholar] [CrossRef] [PubMed]
- Asif, N.; Fatima, S.; Siddiqui, T.; Fatma, T. Investigation of morphological and biochemical changes of zinc oxide nanoparticles induced toxicity against multi drug resistance bacteria. J. Trace Elem. Med. Biol. 2022, 74, 127069. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, R.C.A.; Rajendran, M.; Nagaiah, H.P. Re-Potentiation of-Lactam Antibiotic by Synergistic Combination with Biogenic Copper Oxide Nanocubes against Biofilm Forming Multidrug-Resistant Bacteria. Molecules 2019, 24, 3055. [Google Scholar] [CrossRef] [PubMed]
- Pammi, S.V.N.; Alipour, A.M.; Matangi, R.; Gurugubelli, T.R.; Perumalveeramalai, C.; Ruddaraju, L.K. Unlocking the synergistic potential of green metallic nanoparticles and antibiotics for antibacterial and wound healing activities. iScience 2025, 28, 112518. [Google Scholar] [CrossRef]
- Fadwa, A.O.; Alkoblan, D.K.; Mateen, A.; Albarag, A.M. Synergistic effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J. Biol. Sci. 2021, 28, 928–935. [Google Scholar] [CrossRef]
- Scolari, I.R.; Páez, P.L.; Granero, G.E. Synergistic bactericidal combinations between gentamicin and chitosan capped ZnO nanoparticles: A promising strategy for repositioning this first-line antibiotic. Heliyon 2024, 10, e25604. [Google Scholar] [CrossRef]
| Model | Equation | Parameter | Ag | Zn |
|---|---|---|---|---|
| Langmuir [20] | qm | 47.43 | 65.08 | |
| b | 8.142 | 0.1306 | ||
| R2 | 0.728 | 0.929 | ||
| (R2)adj | 0.694 | 0.921 | ||
| Σ(q − qcal)2 | 175.73 | 83.53 | ||
| Freundlich [21] | KF | 34.051 | 22.524 | |
| n | 12.099 | 4.451 | ||
| R2 | 0.858 | 0.967 | ||
| (R2)adj | 0.840 | 0.963 | ||
| Σ(q − qcal)2 | 91.61 | 39.42 | ||
| Sips [22] | Ks | 81.786 | 22.355 | |
| as | 1.418 | 0.1969 | ||
| n | 3.120 | 2.507 | ||
| R2 | 0.930 | 0.970 | ||
| (R2)adj | 0.910 | 0.962 | ||
| Σ(q − qcal)2 | 45.16 | 35.76 | ||
| Redlich–Peterson [23] | KRP | 820.122 | 31.073 | |
| aRP | 21.839 | 1.0902 | ||
| β | 0.940 | 0.824 | ||
| R2 | 0.902 | 0.971 | ||
| (R2)adj | 0.873 | 0.963 | ||
| Σ(q − qcal)2 | 63.55 | 34.75 |
| Microorganism | Type | Metal | Biosorption Capacity * | Reference |
|---|---|---|---|---|
| Klebsiella sp. 3S1 | Bacteria | Zn(II) | 48.4 | [17] |
| Pseudevernia furfuracea | Fungi | Zn(II) | 8.93 | [19] |
| Botryosphaeria rhodina MAMB-05 | Fungi | Ag(I) | 34.67 39.23 | [24] |
| Rhodotorula mucilaginosa 1S1 | Yeast | Ag(I) | 60.44 58.65 60.53 | [26] |
| Bacillus cereus | Bacteria | Ag(I) | 91.7 | [30] |
| Magnetospirillum gryphiswaldense | Bacteria | Ag(I) | 13.43 | [31] |
| Pleurotus platypus | Macrofungus | Ag(I) | 45.45 43.29 39.48 | [32] |
| Pseudomonas aeruginosa | Bacteria | Zn(II) | 46.1 | [33] |
| Acinetobacter sp. | Bacteria | Zn(II) | 36 | [34] |
| Streptomyces ciscaucasicus (live) Streptomyces ciscaucasicus (death) | Bacteria | Zn(II) | 42.75 54 | [35] |
| S. epidermidis CECT 4183 | Ag(I) Zn(II) | 65.08 47.43 | This work |
| Ag(I) | Zn(II) | |||||
|---|---|---|---|---|---|---|
| Before | After | Shift | Before | After | Shift | Assignment |
| 3273 | 3283 | 10 | - | - | - | Estretching vibrations of amino (-NH) and hydroxyl (-OH) group |
| 3062 | 3069 | 7 | - | - | - | Estretching C-H groups |
| 2852 | - | - | Symmetric C-H estretching vibrations (-CH3) | |||
| 1628 | 1642 | 14 | 1629 | 1637 | 8 | C=C/C=C/C-N Stretching and N-H bending vibrations (Amida I) |
| 1537 | 1518 | 19 | 1531 | 1518 | 13 | -NO asymmetric stretching |
| 1397 | 1384 | 13 | C-H bending (-CH2) | |||
| - | 1335 | 1337 | 1309 | 29 | C-H symmetrical bending vibrations (-CH3)/O-H bending vibrations | |
| 1220 | 1229 | 9 | 1227 | 1232 | - | C-O stretching, |
| 1058 | 1054 | 4 | 1057 | 1049 | 8 | C-O and P-O stretches |
| - | - | - | 965 | - | - | C=C flexion and P-O stretching |
| - | 934 | - | 934 | - | - | C=C flexion and P-O stretching |
| - | 897 | - | 895 | - | - | C=C flexion and P-O stretching |
| 824 | 840 | 16 | 828 | - | - | C=C bending |
| 877 | - | - | - | - | - | C=C bending |
| - | - | - | 777 | - | - | C-H bending |
| - | - | - | 695 | - | - | C-H bending |
| 512 | 521 | 9 | 526 | 548 | 22 | Possible involvement of COO, nitro and disulfide groups |
| 466 | - | - | - | - | - | Possible involvement of COO, nitro and disulfide groups |
| Bacteria | ZnO-NPs | ZnO-NPs + PVA(10%) * |
|---|---|---|
| B. cereus | 1000–2000 | 125–250 |
| S. epidermidis | 62.5–125 | 62.5–125 |
| E. coli | 250–500 | 62.5–125 |
| P. fluorescens | 1000–2000 | 125–250 |
| R. mucilaginosa 1S1 | - | 62.5–125 |
| Rhodotorula mucilaginosa 1S1 | ||||||||||
| ZnO-NPs (µg/mL) | 4000 | 2000 | 1000 | 500 | 250 | 125 | 62.50 | 31.25 | 15.63 | 7.80 |
| Inhibition (%) | 97.1 | 97.5 | 95.6 | 84.3 | 76.3 | 71.7 | 48.1 | 36.3 | 8.7 | 8.6 |
| Pseudomonas fluorescens | ||||||||||
| ZnO-NPs (µg/mL) | 4000 | 2000 | 1000 | 500 | 250 | 125 | 62.50 | 31.25 | 15.63 | 7.80 |
| Inhibition (%) | 71.0 | 82.2 | 78.4 | 77.9 | 78.8 | 67.3 | - | 25.3 | - | - |
| Escherichia coli | ||||||||||
| ZnO-NPs (µg/mL) | 4000 | 2000 | 1000 | 500 | 250 | 125 | 62.50 | 31.25 | 15.63 | 7.80 |
| Inhibition (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 98.9 | 96.8 |
| Precursor of Synthesis | Type | Nanopartícles | Activity | Reference |
|---|---|---|---|---|
| Origanum ehrenbergii | Plant | Ag/AgCl | Antibacterial Antibiofilm Antioxidant | [16] |
| Solidago altissima | Plant | Ag/AgCl | Antibacterial Photocatalytic Activity | [43] |
| Garlic peel | Plant residue | ZnO | Antibacterial | [44] |
| Camellia sinensis | Plant | ZnO | Antifungal | [45] |
| Various | Plant Fruit Vegetable residue | ZnO | Antibacterial Antifungal Antioxidant Anti-inflammatory Antidiabetic Anticancer | [46] |
| Lupinus albus | Plant | ZnO | Antibacterial Photocatalytic Activity | [47] |
| Various | Plant Microorganisms | ZnO | Photocatalytic Activity CO2 Conversion | [48] |
| Magnolia officinalis Goldthread Lonicera japónica | Plant | ZnO | Antibacterial Antifungal Fruit preservative | [49] |
| Chemical synthesis | Various reagents | ZnO | Antibacterial Photocatalytic Activity | [51] |
| Nostoc sp. | Bacteria | ZnO | Antibacterial Antibiofilm | [52] |
| Gleocapsa gelatinosa | Cyanobacteria | ZnO | Antibacterial Antibiofilm | [53] |
| Commercial Nanoparticles | - | ZnO | Antibacterial Synergistic effect | [56] |
| Chemical synthesis | Zinc acetate | ZnO | Antibacterial Synergistic effect | [57] |
| S. epidermidis CECT 4183 | Bacteria | Ag/AgCl ZnO | Antibacterial Antibiofilm | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, A.J.; Martín, C.; Espínola, F.; Moya, M.; Ruiz, E. Removal of Zn(II) and Ag(I) by Staphylococcus epidermidis CECT 4183 and Biosynthesis of ZnO and Ag/AgCl Nanoparticles for Biocidal Applications. Toxics 2025, 13, 478. https://doi.org/10.3390/toxics13060478
Muñoz AJ, Martín C, Espínola F, Moya M, Ruiz E. Removal of Zn(II) and Ag(I) by Staphylococcus epidermidis CECT 4183 and Biosynthesis of ZnO and Ag/AgCl Nanoparticles for Biocidal Applications. Toxics. 2025; 13(6):478. https://doi.org/10.3390/toxics13060478
Chicago/Turabian StyleMuñoz, Antonio Jesús, Celia Martín, Francisco Espínola, Manuel Moya, and Encarnación Ruiz. 2025. "Removal of Zn(II) and Ag(I) by Staphylococcus epidermidis CECT 4183 and Biosynthesis of ZnO and Ag/AgCl Nanoparticles for Biocidal Applications" Toxics 13, no. 6: 478. https://doi.org/10.3390/toxics13060478
APA StyleMuñoz, A. J., Martín, C., Espínola, F., Moya, M., & Ruiz, E. (2025). Removal of Zn(II) and Ag(I) by Staphylococcus epidermidis CECT 4183 and Biosynthesis of ZnO and Ag/AgCl Nanoparticles for Biocidal Applications. Toxics, 13(6), 478. https://doi.org/10.3390/toxics13060478









