Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area and Sample Collection
2.2. Heavy Metal Analysis
2.3. Quality Control and Assurance
2.4. The Environmental Assessment Indices
2.4.1. Heavy Metal Evaluation Index (HEI)
2.4.2. Contamination Factor (CF)
2.4.3. Pollution Load Index (PLI)
2.4.4. Contamination Degree (CD)
2.4.5. Enrichment Factor (EF)
2.4.6. Geoaccumulation Index (Igeo)
2.5. Statistical Analysis
3. Results
3.1. Physicochemical Parameters of Surface Sediments
3.2. Heavy Metal Concentrations in Surface Sediments and Water of Pattani Bay
3.3. Assessment of Heavy Metal Pollution in Surface Sediments and Water
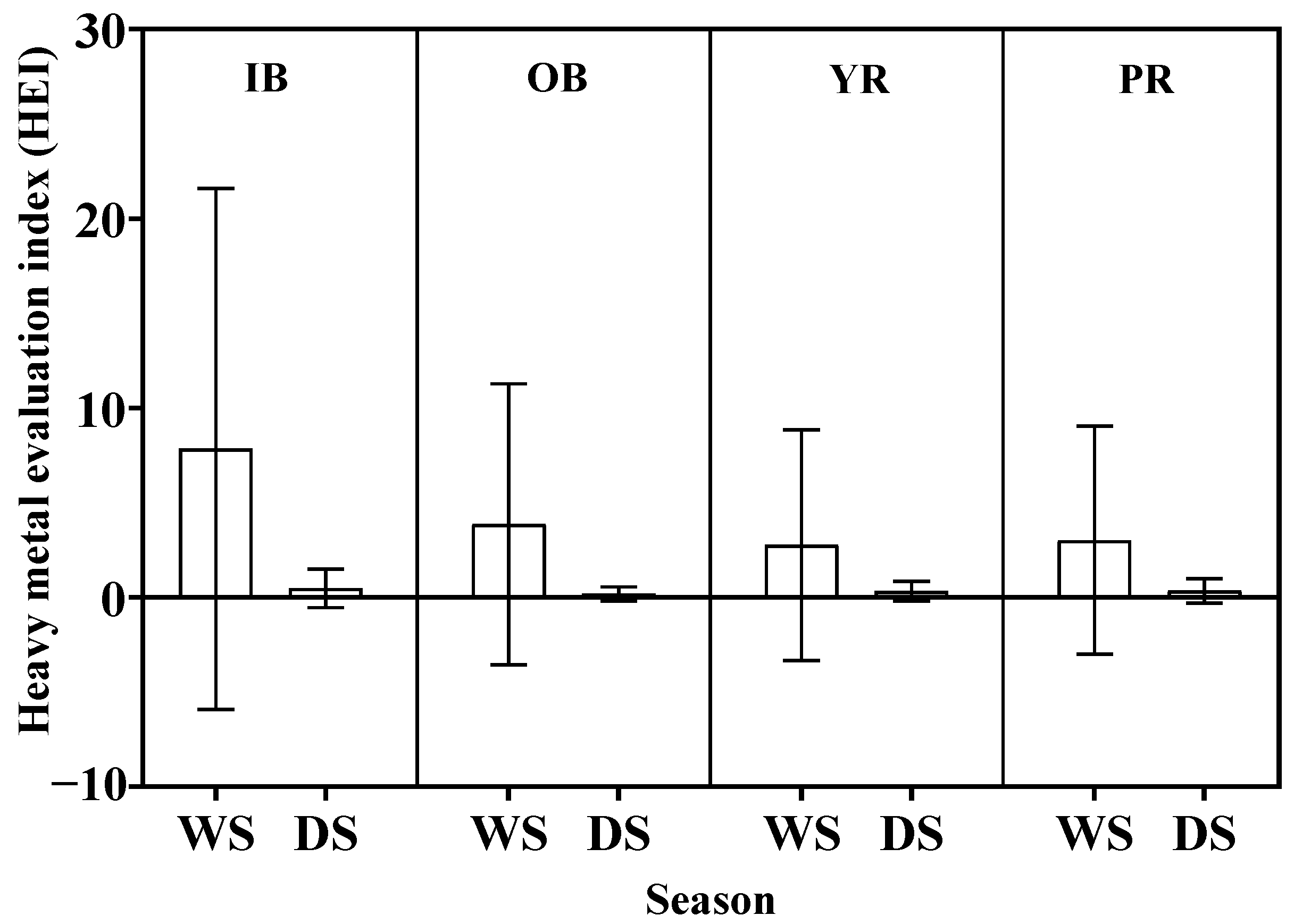
3.3.1. Heavy Metal Evaluation Index (HEI)
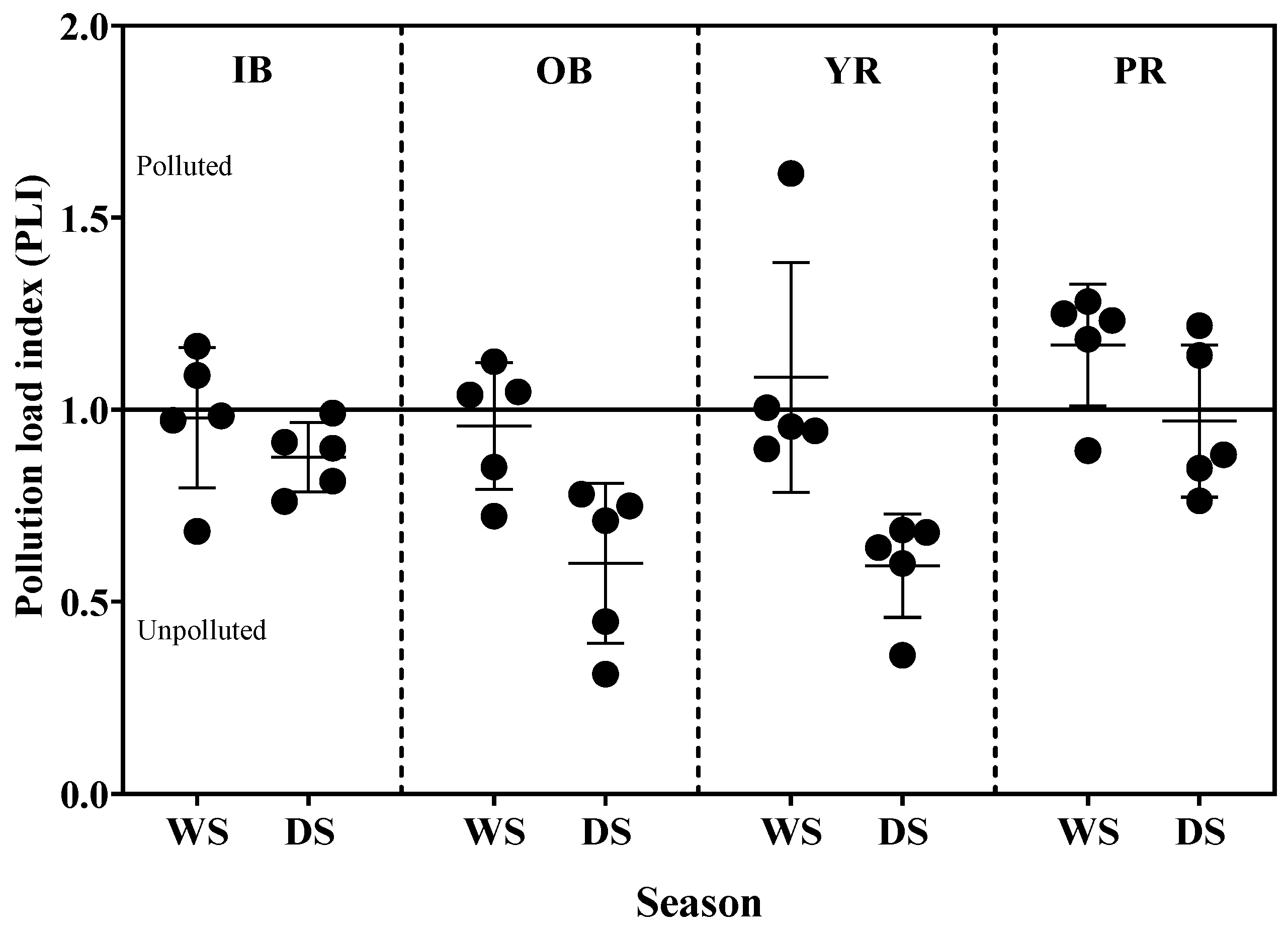
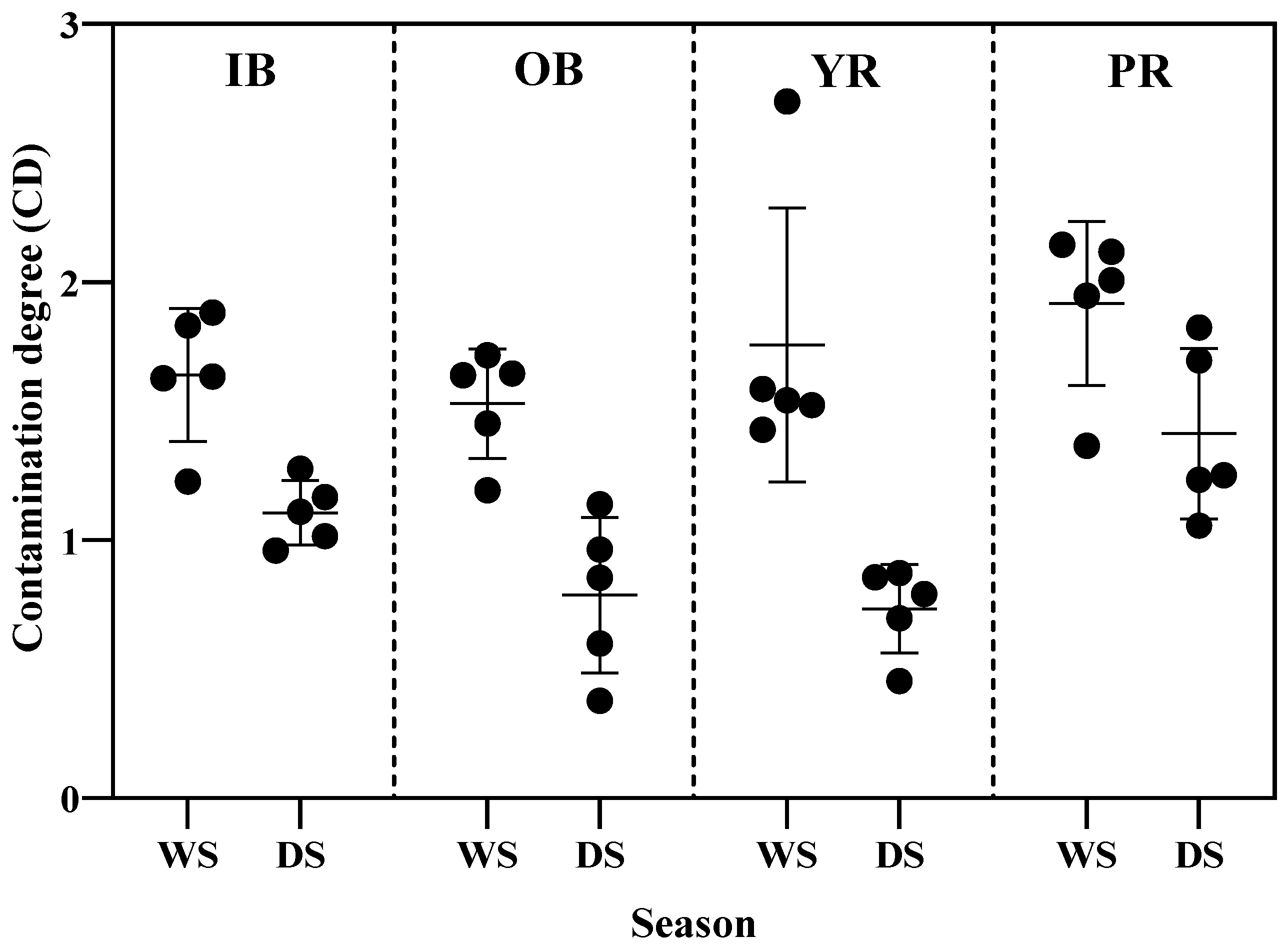
3.3.2. Contamination Factor (CF) and Pollution Load Index (PLI)
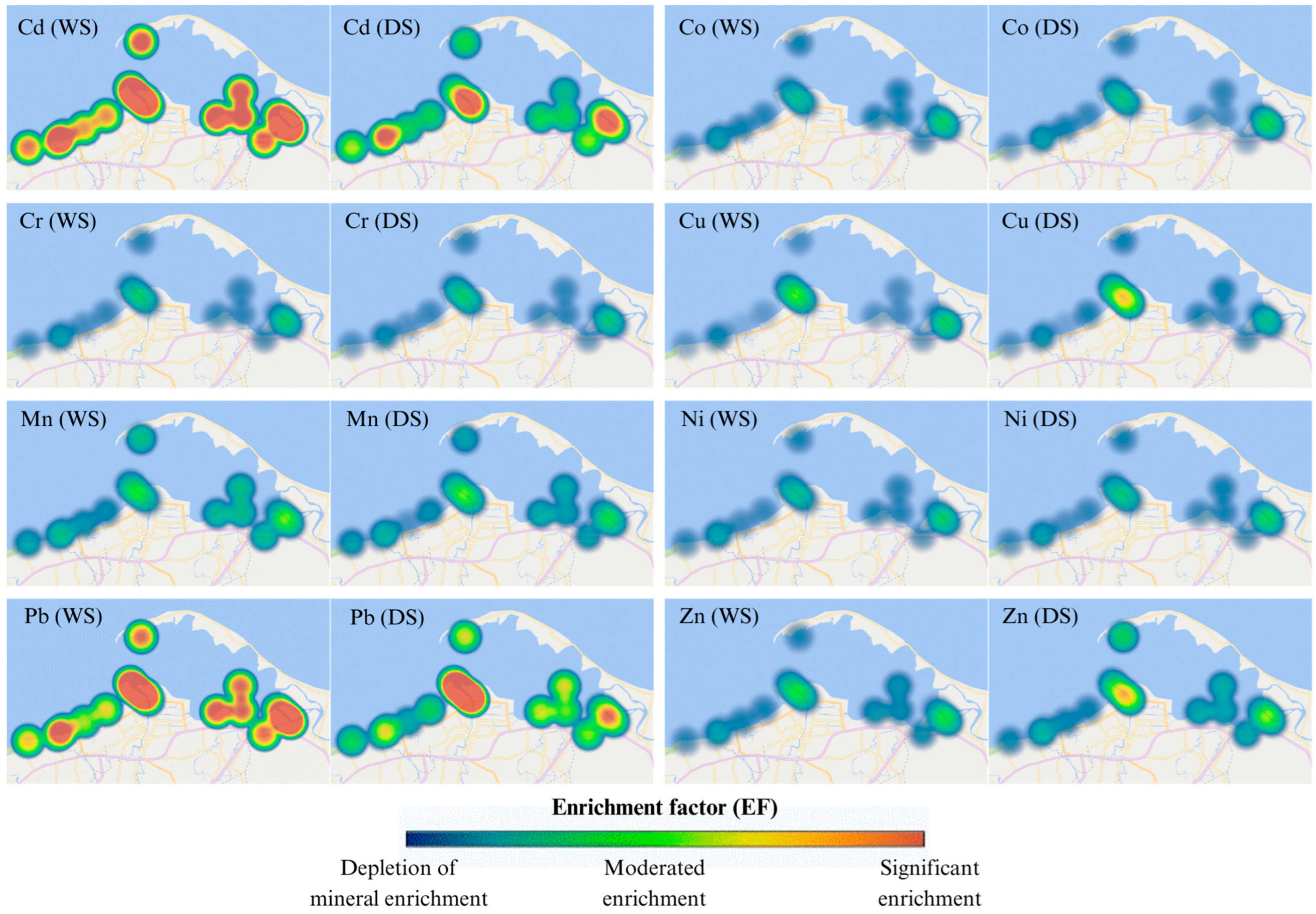
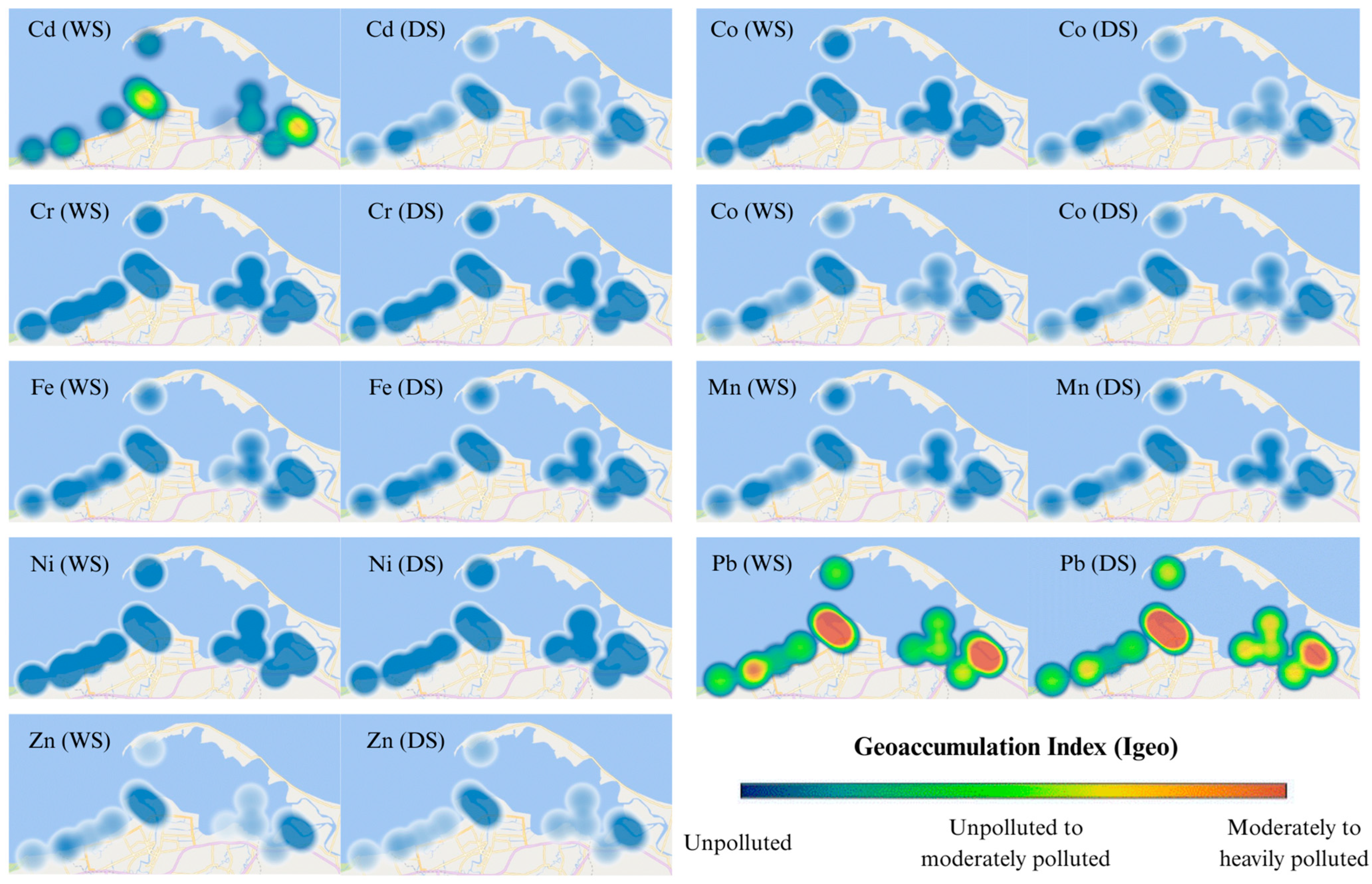
3.3.3. Enrichment Factor (EF) and Geoaccumulation Index (Igeo)
3.4. Principal Component Analysis and Source Identification
4. Discussion
| Country | Location | Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Zn | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Thailand | In-Pattani Bay | 0.43 | 7.82 | 21.71 | 11.68 | 25,196.00 | 746.77 | 14.16 | 78.42 | 42.00 | This study |
| Thailand | Off-Pattani bay | 0.42 | 8.01 | 19.24 | 8.49 | 25,075.89 | 448.14 | 13.32 | 54.63 | 40.09 | This study |
| Thailand | Yaring River | 0.43 | 8.59 | 20.31 | 11.40 | 28,165.17 | 417.08 | 14.22 | 60.94 | 49.68 | This study |
| Thailand | Pattani River | 0.45 | 7.41 | 23.88 | 24.30 | 26,743.70 | 465.45 | 12.92 | 120.12 | 69.81 | This study |
| Thailand | Western Gulf of Thailand | Na | 10.44 | 50.78 | 12.25 | Na | Na | 25.86 | 21.35 | 46.17 | Liu, Shi [73] |
| Thailand | Chao Phraya River | 0.58 | Na | Na | 214 | 16,636 | 419 | Na | 62.6 | 240 | Wijaya, Ohde [74] |
| China | DYB | 0.07 | 59.03 | 16.46 | 37.01 | 87.81 | Zhao, Ye [75] | ||||
| China | Daya Bay | 0.052 | 12.7 | Na | 20.8 | Na | 837 | 31.2 | 45.7 | 113 | Gao, Arthur Chen [76] |
| China | Pearl River Estuary | Na | 106 | 45.7 | 57.9 | 176.8 | Yu, Yan [77] | ||||
| China | Beibu Gulf | 0.01–0.153 | Na | 3.07–85.07 | 1.06–25.23 | Na | Na | Na | 9.42–43.05 | 4.59–112.6 | Yi, Song [78] |
| Sediment quality standard | Proposed SQG-Thailand | 0.17 | - | 72 | 33 | - | - | 27.5 | 35.8 | 95 | PCD [79] |
| TEL | 0.6 | - | 37.3 | 35.7 | - | - | 18 | 35 | 123 | MacDonald, Dipinto [80] | |
| ERL | 5 | - | 70 | 70 | - | - | 30 | 35 | 120 | MacDonald, Dipinto [80] | |
| LEL | 0.6 | - | 16 | 16 | - | - | 16 | 31 | 120 | MacDonald, Dipinto [80] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, R.; Wang, Y.; Zhang, X.; Zhu, L.; Luo, M.; Liu, B.; Yuan, X. Sources and factors controlling the distribution of heavy metals in coastal sediments of Haiyang, China. Mar. Pollut. Bull. 2022, 175, 113152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, Z.; Qiu, X.; Yu, Q.; Bu, J.; Sun, Z.; Long, R.; Brandis, K.J.; He, J.; Feng, Q.; et al. Heavy metal habitat: A novel framework for mapping heavy metal contamination over large-scale catchment with a species distribution model. Water Res. 2022, 226, 119310. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, J.; Xu, M.; Zhao, L.; Wang, Z. Spatial distribution, source apportionment and ecological risk assessment of heavy metals in the sediments of Haizhou Bay national ocean park, China. Mar. Pollut. Bull. 2019, 149, 110651. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Sun, X.; Hu, Z.; Fan, D. Accumulation and transformation of heavy metals in surface sediments from the Yangtze River estuary to the East China Sea shelf. Environ. Pollut. 2019, 245, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Luo, C.; Lou, L.; Li, X.; Shen, Z. Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. and their potential use for contamination indicators and in wastewater treatment. Sci. Total. Environ. 2008, 392, 22–29. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environ. Int. 2007, 33, 576–582. [Google Scholar] [CrossRef]
- Cajaraville, M.P.; Bebianno, M.J.; Blasco, J.; Porte, C.; Sarasquete, C.; Viarengo, A. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. Sci. Total Environ. 2000, 247, 295–311. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Liu, L. Source Identification and Potential Ecological Risk Assessment of Heavy Metals in the Topsoil of the Weining Plain (Northwest China). Expo. Health 2022, 14, 281–294. [Google Scholar] [CrossRef]
- He, Z.; Li, F.; Dominech, S.; Wen, X.; Yang, S. Heavy metals of surface sediments in the Changjiang (Yangtze River) Estuary: Distribution, speciation and environmental risks. J. Geochem. Explor. 2019, 198, 18–28. [Google Scholar] [CrossRef]
- Sun, C.; Gong, W.; Pan, G.; Mortimer, R.J.G.; Yao, E.; Wen, S.; Chen, M.; Zhong, J. Comprehensive effects of sediment dredging on environmental risk and bioavailability of heavy metals from the sediment of Lake Taihu, China. J. Hazard. Mater. 2025, 484, 136789. [Google Scholar] [CrossRef] [PubMed]
- Sowana, A.; Shrestha, R.P.; Parkpian, P.; Pongquan, S. Influence of Coastal Land Use on Soil Heavy-Metal Contamination in Pattani Bay, Thailand. J. Coast. Res. 2011, 27, 252–262. [Google Scholar] [CrossRef]
- Simachaya, W.; Nivickaphum, S.; Leelapanang, P. Lead contamination control plan and environmental risk assessment in the Pattani River Basin and the upper Maeklong River Basin in Thailand. In Proceedings of the 2nd Asian Pacific International Conference on Pollutants Analysis and Control, Ho Chi Minh City, Vietnam, 1–3 December 2003. [Google Scholar]
- Kaewtubtim, P.; Meeinkuirt, W.; Seepom, S.; Pichtel, J. Occurrence of heavy metals and radionuclides in sediments and seawater in mangrove ecosystems in Pattani Bay, Thailand. Environ. Sci. Pollut. Res. 2017, 24, 7630–7639. [Google Scholar] [CrossRef]
- Varathorn, P. Heavy Metals in Pattani River Basin; Bureau of Environmental Health, Department of Health, Ministry of Public Health: Bangkok, Thailand, 1997; 3p. [Google Scholar]
- Tanhan, P.; Lansubsakul, N.; Phaochoosak, N.; Sirinupong, P.; Yeesin, P.; Imsilp, K. Human Health Risk Assessment of Heavy Metal Concentration in Seafood Collected from Pattani Bay, Thailand. Toxics 2023, 11, 18. [Google Scholar]
- Imsilp, K.; Lansubsakul, N.; Thong-asa, W.; Sirinupong, P.; Yeesin, P.; Phaochoosak, N.; Tanhan, P. Potential non-carcinogenic and carcinogenic health risks of elements of health concern bioaccumulated in seafood from local fish rafts in Trang province, Thailand. J. Agric. Food Res. 2024, 18, 101272. [Google Scholar] [CrossRef]
- Imsilp, K.; Tanhan, P.; Lansubsakul, N.; Thong-asa, W. Oxidative response to cadmium and lead accumulations in the tissues of blue swimming crabs Portunus pelagicus from the Trang Province coastline, Southern Thailand. J. Aquat. Anim. Health 2024, 36, 265–274. [Google Scholar] [CrossRef]
- Tanhan, P.; Imsilp, K.; Lansubsakul, N.; Tantiwisawaruji, S.; Thong-asa, W. Oxidative response to accumulation of trace metals in tissue of two bivalves, the Asian green mussel Perna viridis and the blood cockle Tegillarca granosa, living in Pattani Bay, Thailand. J. Aquat. Anim. Health 2024, 36, 32–44. [Google Scholar] [CrossRef]
- Burada, A.; Topa, C.M.; Georgescu, L.P.; Teodorof, L.; Nastase, C.; Seceleanu-Odor, D.; Iticescu, C. Heavy Metals Environment Accumulation in Somova. Rev. Chim. 2015, 66, 48–54. [Google Scholar]
- Hasimuna, O.J.; Chibesa, M.; Ellender, B.R.; Maulu, S. Variability of selected heavy metals in surface sediments and ecological risks in the Solwezi and Kifubwa Rivers, Northwestern province, Zambia. Sci. Afr. 2021, 12, e00822. [Google Scholar] [CrossRef]
- Enuneku, A.; Omoruyi, O.; Tongo, I.; Ogbomida, E.; Ogbeide, O.; Ezemonye, L. Evaluating the potential health risks of heavy metal pollution in sediment and selected benthic fauna of Benin River, Southern Nigeria. Appl. Water Sci. 2018, 8, 224. [Google Scholar] [CrossRef]
- M’kandawire, E.; Choongo, K.; Yabe, J.; Mwase, M.; Saasa, N.; Nakayama, S.M.M.; Bortey-Sam, N.; Blindauer, C.A. Sediment Metal Contamination in the Kafue River of Zambia and Ecological Risk Assessment. Bull. Environ. Contam. Toxicol. 2017, 99, 108–116. [Google Scholar] [CrossRef]
- Kim, I.-G.; Kim, Y.-B.; Kim, R.-H.; Hyon, T.-S. Spatial distribution, origin and contamination assessment of heavy metals in surface sediments from Jangsong tidal flat, Kangryong river estuary, DPR Korea. Mar. Pollut. Bull. 2021, 168, 112414. [Google Scholar] [CrossRef]
- Rajkumar, H.; Naik, P.K.; Rishi, M.S. A new indexing approach for evaluating heavy metal contamination in groundwater. Chemosphere 2020, 245, 125598. [Google Scholar] [CrossRef]
- Rahman, M.S.; Ahmed, Z.; Seefat, S.M.; Alam, R.; Islam, A.R.M.T.; Choudhury, T.R.; Begum, B.A.; Idris, A.M. Assessment of heavy metal contamination in sediment at the newly established tannery industrial Estate in Bangladesh: A case study. Environ. Chem. Ecotoxicol. 2022, 4, 1–12. [Google Scholar] [CrossRef]
- Kaewtubtim, P.; Meeinkuirt, W.; Seepom, S.; Pichtel, J. Phytomanagement of radionuclides and heavy metals in mangrove sediments of Pattani Bay, Thailand using Avicennia marina and Pluchea indica. Mar. Pollut. Bull. 2018, 127, 320–333. [Google Scholar] [CrossRef]
- APHA, AWWA, and WEF. Standard Methods for Examination of Water and Wastewater; APHA, AWWA, and WEF: Washington, DC, USA, 2011. [Google Scholar]
- de Groot, A.J.; Zschuppel, K.H.; Salomons, W. Standardization of methods of analysis for heavy metals in sediments. Hydrobiologia 1982, 91, 689–695. [Google Scholar] [CrossRef]
- Mohamed, N.; Driss, N.; Nadia, B.; Roberto, P.; Abdeljaouad, L.; Nor-dine, R. Characterization of the New Status of Nador Lagoon (Morocco) after the Implementation of the Management Plan. J. Mar. Sci. Eng. 2017, 5, 7. [Google Scholar]
- Lin, J.-G.; Chen, S.-Y. The relationship between adsorption of heavy metal and organic matter in river sediments. Environ. Int. 1998, 24, 345–352. [Google Scholar] [CrossRef]
- Gaudette, H.E.; Flight, W.R.; Toner, L.; Folger, D.W. An inexpensive titration method for the determination of organic carbon in recent sediments. J. Sediment. Res. 1974, 44, 249–253. [Google Scholar] [CrossRef]
- Tamasi, G.; Cini, R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci. Total Environ. 2004, 327, 41–51. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Edet, A.E.; Offiong, O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 2002, 57, 295–304. [Google Scholar] [CrossRef]
- Hakanson, L. An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Basaham, A.S. Distribution and behavior of some heavy metals in the surface sediments of Al-Arbaeen lagoon, Jeddah, Red Sea coast. Earth Sci. 1998, 10, 59–71. [Google Scholar]
- Ao, L.; Chang, R.; Tang, Y.; Zhang, S. Ecological risk assessment and source tracing of heavy metals in surface sediments of a hilly riverine reservoir in Chongqing, China. Environ. Sci. Eur. 2024, 36, 69. [Google Scholar] [CrossRef]
- Cheng, Q.; Lou, G.; Huang, W.; Li, X. Assessment and potential sources of metals in the surface sediments of the Yellow River Delta, Eastern China. Environ. Sci. Pollut. Res. 2017, 24, 17446–17454. [Google Scholar] [CrossRef]
- Taylor, S.R. Abundance of chemical elements in the continental crust: A new table. Geochim. Cosmochim. Acta 1964, 28, 1273–1285. [Google Scholar] [CrossRef]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Mashiatullah, A.; Chaudhary, M.Z.; Ahmad, N.; Javed, T.; Ghaffar, A. Metal pollution and ecological risk assessment in marine sediments of Karachi Coast, Pakistan. Environ. Monit. Assess. 2013, 185, 1555–1565. [Google Scholar] [CrossRef]
- Abrahim, G.M.S.; Parker, R.J. Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ. Monit. Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Wu, K.; Cui, J.; Zhu, A.; Zhang, Y.; Mohamed, C.A.R.; Shi, X. Distribution and assessment of heavy metal contents in surface sediments of the western Sunda Shelf. Mar. Pollut. Bull. 2021, 168, 112433. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Borah, S.S.; Kalamdhad, A.S. Heavy metal pollution and potential ecological risk assessment for surficial sediments of Deepor Beel, India. Ecol. Indic. 2021, 122, 107265. [Google Scholar] [CrossRef]
- Bantan, R.A.; Al-Dubai, T.A.; Al-Zubieri, A.G. Geo-environmental assessment of heavy metals in the bottom sediments of the Southern Corniche of Jeddah, Saudi Arabia. Mar. Pollut. Bull. 2020, 161, 111721. [Google Scholar] [CrossRef]
- Reimann, C.; de Caritat, P. Distinguishing between natural and anthropogenic sources for elements in the environment: Regional geochemical surveys versus enrichment factors. Sci. Total Environ. 2005, 337, 91–107. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosystems 2001, 2, 2000GC000109. [Google Scholar] [CrossRef]
- Mueller, G.; Yahya, A.; Gentner, P. Heavy Metal Contamination of the Sediments of the River Neckar and Its Tributaries. Stocktaking 1990 and Comparison with Previous Investigations; Heidelberg University: Heidelberg, Germany, 1993; Volume 69, Available online: https://inis.iaea.org/records/80h5h-cza28 (accessed on 12 February 2019).
- WHO. Evaluation of Joint FAO/WHO Expert Committee on Food Additives (JECFA). Available online: www.apps.who.int/food-additives-contaminants-jecfa-database (accessed on 12 February 2019).
- EPA. Updates: Water Quality Standards Handbook (Online Version); EPA Office of Water, Office of Science and Technology: Washington, DC, USA, 2014. Available online: https://www.epa.gov/wqs-tech/water-quality-standards-handbook (accessed on 12 April 2025).
- EU. Commission Regulation (EU) No. 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union. 2023. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj/eng (accessed on 12 April 2025).
- Xu, P.; Zhang, Q.; Qian, H.; Li, M.; Hou, K. Characterization of geothermal water in the piedmont region of Qinling Mountains and Lantian-Bahe Group in Guanzhong Basin, China. Environ. Earth Sci. 2019, 78, 442. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Qian, H.; Yang, F. Hydrogeochemistry and fluoride contamination in Jiaokou Irrigation District, Central China: Assessment based on multivariate statistical approach and human health risk. Sci. Total Environ. 2020, 741, 140460. [Google Scholar] [CrossRef]
- Iordache, A.M.; Nechita, C.; Pluhacek, T.; Iordache, M.; Zgavarogea, R.; Ionete, R.E. Past and present anthropic environmental stress reflect high susceptibility of natural freshwater ecosystems in Romania. Environ. Pollut. 2020, 267, 115505. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; He, M.; Christakos, G. Beyond mere pollution source identification: Determination of land covers emitting soil heavy metals by combining PCA/APCS, GeoDetector and GIS analysis. Catena 2020, 185, 104297. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Wong, Y.S. Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ. Pollut. 2000, 110, 195–205. [Google Scholar] [CrossRef]
- Sany, S.B.T.; Salleh, A.; Sulaiman, A.H.; Sasekumar, A.; Rezayi, M.; Tehrani, G.M. Heavy metal contamination in water and sediment of the Port Klang coastal area, Selangor, Malaysia. Environ. Earth Sci. 2013, 69, 2013–2025. [Google Scholar] [CrossRef]
- Nduka, J.K.; Orisakwe, O.E. Water-quality issues in the Niger Delta of Nigeria: A look at heavy metal levels and some physicochemical properties. Environ. Sci. Pollut. Res. 2011, 18, 237–246. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Díaz-Fierros, F.; Barral, M.T. Assessment of enrichment factors and grain size influence on the metal distribution in riverbed sediments (Anllóns River, NW Spain). Environ. Monit. Assess. 2011, 179, 371–388. [Google Scholar] [CrossRef]
- Kang, X.; Song, J.; Yuan, H.; Duan, L.; Li, X.; Li, N.; Liang, X.; Qu, B. Speciation of heavy metals in different grain sizes of Jiaozhou Bay sediments: Bioavailability, ecological risk assessment and source analysis on a centennial timescale. Ecotoxicol. Environ. Saf. 2017, 143, 296–306. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, B.; Lin, M.; Wu, S.; Hou, H.; Zhang, J.; Qian, G.; Huang, X.; Zhou, J. Evaluation of heavy metal mobilization in creek sediment: Influence of RAC values and ambient environmental factors. Sci. Total Environ. 2017, 607–608, 1339–1347. [Google Scholar] [CrossRef]
- Sun, X.-L.; Wang, Y.; Xiong, H.-Q.; Wu, F.; Lv, T.-X.; Fang, Y.-C.; Xiang, H. The Role of Surface Functional Groups of Iron Oxide, Organic Matter, and Clay Mineral Complexes in Sediments on the Adsorption of Copper Ions. Sustainability 2023, 15, 6711. [Google Scholar] [CrossRef]
- Duncan, A.E.; de Vries, N.; Nyarko, K.B. Assessment of Heavy Metal Pollution in the Sediments of the River Pra and Its Tributaries. Water Air Soil Pollut. 2018, 229, 272. [Google Scholar] [CrossRef]
- Li, J.; He, M.; Sun, S.; Han, W.; Zhang, Y.; Mao, X.; Gu, Y. Effect of the behavior and availability of heavy metals on the characteristics of the coastal soils developed from alluvial deposits. Environ. Monit. Assess. 2009, 156, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.I.; Jung, H.S.; Choi, J.Y.; Yang, S.; Ahn, K.S. Geochemical compositions of river and shelf sediments in the Yellow Sea: Grain-size normalization and sediment provenance. Cont. Shelf Res. 2006, 26, 15–24. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, B.; Xiao, R.; Zhao, H. Heavy metals in water, soils and plants in riparian wetlands in the Pearl River Estuary, South China. Proc. Environ. Sci. 2010, 2, 1344–1354. [Google Scholar] [CrossRef]
- Aydin Onen, S.; Kucuksezgin, F.; Kocak, F. Temporal and spatial biomonitoring of heavy metals in eastern Aegean coastal waters using Amphibalanus amphitrite. Mar. Pollut. Bull. 2011, 62, 2548–2556. [Google Scholar] [CrossRef]
- Armah, F.A.; Obiri, S.; Yawson, D.O.; Onumah, E.E.; Yengoh, G.T.; Afrifa, E.K.A.; Odoi, J.O. Anthropogenic sources and environmentally relevant concentrations of heavy metals in surface water of a mining district in Ghana: A multivariate statistical approach. J. Environ. Sci. Health Part A 2010, 45, 1804–1813. [Google Scholar] [CrossRef]
- Sehrin, S.; Palash, M.A.I.; Ahmed, M.S.; Quraishi, S.B.; Baki, M.A.; Amin, M.N.; Begum, A. Public health risk assessment and possible contamination with heavy metals in Saint Martin’s Island, Bay of Bengal. J. Hazard. Mater. Adv. 2025, 17, 100568. [Google Scholar] [CrossRef]
- Liu, S.; Shi, X.; Yang, G.; Khokiattiwong, S.; Kornkanitnan, N. Distribution of major and trace elements in surface sediments of the western Gulf of Thailand: Implications to modern sedimentation. Cont. Shelf Res. 2016, 117, 81–91. [Google Scholar] [CrossRef]
- Wijaya, A.R.; Ohde, S.; Shinjo, R.; Ganmanee, M.; Cohen, M.D. Geochemical fractions and modeling adsorption of heavy metals into contaminated river sediments in Japan and Thailand determined by sequential leaching technique using ICP-MS. Arab. J. Chem. 2019, 12, 780–799. [Google Scholar] [CrossRef]
- Zhao, G.; Ye, S.; Yuan, H.; Ding, X.; Wang, J. Distribution and contamination of heavy metals in surface sediments of the Daya Bay and adjacent shelf, China. Mar. Pollut. Bull. 2016, 112, 420–426. [Google Scholar] [CrossRef]
- Gao, X.; Arthur Chen, C.-T.; Wang, G.; Xue, Q.; Tang, C.; Chen, S. Environmental status of Daya Bay surface sediments inferred from a sequential extraction technique. Estuar. Coast. Shelf Sci. 2010, 86, 369–378. [Google Scholar] [CrossRef]
- Yu, X.; Yan, Y.; Wang, W.-X. The distribution and speciation of trace metals in surface sediments from the Pearl River Estuary and the Daya Bay, Southern China. Mar. Pollut. Bull. 2010, 60, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Song, Z.; Lin, J.; Liu, W.; Li, B. Distribution, sources and influencing factors of heavy metals in the Ledong Sea, South China Sea. Mar. Pollut. Bull. 2024, 202, 116396. [Google Scholar] [CrossRef]
- PCD. Proposed Marine and Coastal Sediment Quality Guidelines. Final Report. UNEP GEF Project “Reversing Environmental Degradation Trends in the South China Sea and Gulf of Thailand”; Pollution Control Department: Bangkok, Thailand, 2006; p. 16. [Google Scholar]
- MacDonald, D.D.; Dipinto, L.M.; Field, J.; Ingersoll, C.G.; Lvong, E.R.; Swartz, R.C. Development and evaluation of consensus-based sediment effect concentrations for polychlorinated biphenyls. Environ. Toxicol. Chem. 2000, 19, 1403–1413. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Jolly, Y.N.; Rahman, A.; Sattar, M.A.; Hai, M.A. Spatial distribution and contamination assessment of six heavy metals in soils and their transfer into mature tobacco plants in Kushtia District, Bangladesh. Environ. Sci. Pollut. Res. 2016, 23, 3414–3426. [Google Scholar] [CrossRef]
- Brady, J.P.; Ayoko, G.A.; Martens, W.N.; Goonetilleke, A. Development of a hybrid pollution index for heavy metals in marine and estuarine sediments. Environ. Monit. Assess. 2015, 187, 306. [Google Scholar] [CrossRef]
- Rahman, M.; Saima, J.; Rima, S.A.; Hossain, M.I.S.; Das, D.K.; Bakar, M.A.; Siddique, M.A.M. Ecological risks of heavy metals on surficial sediment of Nijhum Dweep (Island), an important biodiversity area of Bangladesh. Mar. Pollut. Bull. 2022, 179, 113688. [Google Scholar] [CrossRef]
- Sowrav, S.F.F.; Rahman, S.M.M.; Hossain, M.A.; Hafiz, K.B.; Hossain, N.I.; Nur-E Alam, M.; Rahman, M.S.; Choudhury, T.R. Multi-dimensional approach for an environmental health assessment of a deltaic mangrove ecosystem, Sundarbans. Environ. Adv. 2023, 12, 100377. [Google Scholar] [CrossRef]
- Islam, A.R.M.T.; Uddin, M.N.; Joy, M.F.R.; Proshad, R.; Kormoker, T.; Anik, A.H.; Rahman, M.S.; Siddique, M.A.B.; Alshehri, M.A. Tracing sources-oriented ecological risks of metal(loid)s in sediments of anthropogenically-affected coastal ecosystem from northeast bay of Bengal. Mar. Pollut. Bull. 2025, 211, 117354. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.; Jin, H.; Feng, H.; Shen, G.; Cao, Y.; Yu, C.; Lu, Z.; Zhang, Q. Spatiotemporal variation and potential risks of seven heavy metals in seawater, sediment, and seafood in Xiangshan Bay, China (2011–2016). Chemosphere 2018, 212, 1163–1171. [Google Scholar] [CrossRef]
- Barakat, A.; El Baghdadi, M.; Rais, J.; Nadem, S. Assessment of Heavy Metal in Surface Sediments of Day River at Beni-Mellal Region, Morocco. Res. J. Environ. Earth Sci. 2012, 4, 797–806. [Google Scholar]
- Suresh, G.; Ramasamy, V.; Meenakshisundaram, V.; Venkatachalapathy, R.; Ponnusamy, V. Influence of mineralogical and heavy metal composition on natural radionuclide concentrations in the river sediments. Appl. Radiat. Isot. 2011, 69, 1466–1474. [Google Scholar] [CrossRef]


| Areas | Sand (%) | Clay (%) | Silt (%) | Texture | pH | EC (dS/m) | OM (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WS | DS | WS | DS | WS | DS | WS | DS | WS | DS | WS | DS | WS | DS | |
| IB | 1.78 | 0.20 | 26.64 | 34.72 | 71.58 | 64.18 | Silty clay loam | 7.52 | 7.42 | 11.96 | 14.39 | 13.00 | 10.07 | |
| OB | 6.64 | 7.04 | 23.38 | 23.98 | 69.98 | 69.78 | Silty clay loam | 6.78 | 6.75 | 7.12 | 10.58 | 10.38 | 7.44 | |
| PR | 0.83 | 1.28 | 31.46 | 28.60 | 67.71 | 70.12 | Silty clay loam | 5.44 | 5.59 | 4.71 | 2.65 | 12.49 | 9.82 | |
| YR | 0.06 | 0.58 | 25.40 | 19.46 | 75.54 | 79.96 | Silty clay loam | 4.17 | 4.13 | 4.36 | 6.52 | 11.51 | 12.41 | |
| Heavy Metal | Average Heavy Metal Concentration (µg/L) | Water Quality Standards (mg/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IB | OB | YR | PR | |||||||||
| WS | DS | WS | DS | WS | DS | WS | DS | WHO a | EPA b | EU c | ||
| Cd | Mean | 3.41 * | 0.45 | 26.69 | 1.06 | 5.88 * | 1.10 | 4.56 * | 1.90 | 0.003 | 0.003 | 0.003 |
| Min | 0.20 | 0.30 | 21.00 | 0.30 | 4.20 | 0.30 | 2.40 | 0.60 | ||||
| Max | 7.90 | 0.60 | 39.3 | 1.70 | 8.10 | 2.90 | 6.10 | 3.40 | ||||
| Co | Mean | 7.15 | 14.23 * | 136.89 * | 13.65 | 5.90 | 16.08 * | 4.49 | 16.74 * | - | - | - |
| Min | 2.00 | 2.00 | 98.10 | 4.00 | 2.00 | 2.00 | 1.00 | 3.90 | ||||
| Max | 12.30 | 23.90 | 239.00 | 23.40 | 13.70 | 31.90 | 11.70 | 31.90 | ||||
| Cr | Mean | 16.38 | 94.40 * | 102.55 | Nd | 6.87 * | Nd | 5.94 | 40.30 * | 0.05 | 0.05 | 0.05 |
| Min | 2.50 | 48.80 | 78.60 | Nd | 2.50 | Nd | 2.40 | 40.30 | ||||
| Max | 40.50 | 175.80 | 147.00 | Nd | 14.60 | Nd | 9.80 | 40.30 | ||||
| Cu | Mean | 9.16 | 7.77 | 37.53 * | 4.39 | 5.28 | 5.76 | 3.75 | 10.85 * | 2.00 | 2.00 | 2.00 |
| Min | 4.50 | 3.70 | 30.40 | 1.20 | 1.70 | 1.20 | 2.40 | 2.50 | ||||
| Max | 13.50 | 11.00 | 49.00 | 7.40 | 7.90 | 14.70 | 9.20 | 17.10 | ||||
| Fe | Mean | 3.30 | 6.08 * | 132.05 * | 9.13 | 6.23 | 6.72 | 3.40 | 17.38 * | 0.30 | 0.20 | - |
| Min | 1.10 | Nd | 5.71 | 2.20 | 2.20 | 1.10 | 0.50 | 4.30 | ||||
| Max | 4.40 | 13.00 | 70.75 | 19.80 | 12.10 | 19.60 | 8.80 | 26.10 | ||||
| Mn | Mean | Nd | 2.50 | 8.55 | 3.21 | 2.30 | 3.28 | 0.75 | 4.31 * | 0.40 | 0.50 | - |
| Min | Nd | 0.50 | 3.00 | 0.50 | 1.50 | 0.20 | 0.50 | 0.50 | ||||
| Max | Nd | 10.00 | 14.10 | 8.00 | 3.40 | 10.90 | 1.00 | 16.60 | ||||
| Ni | Mean | 13.73 | 9.16 | 117.43 * | 10.73 | 9.08 | 17.97 * | 10.75 | 17.70 * | 0.07 | 0.02 | 0.02 |
| Min | 4.40 | 2.30 | 86.3 | Nd | Nd | 7.00 | 5.90 | 1.20 | ||||
| Max | 31.70 | 23.40 | 188.4 | 25.80 | 30.30 | 39.80 | 19.50 | 32.80 | ||||
| Pb | Mean | 184.25 * | 8.58 | 386.55 * | 35.82 | 151.87 * | 16.98 | 170.23 * | 15.64 | 0.01 | 0.01 | 0.01 |
| Min | 127.00 | 4.90 | 293.00 | 9.80 | 87.90 | 2.50 | 131.80 | 2.50 | ||||
| Max | 251.50 | 19.60 | 542.00 | 58.60 | 212.40 | 44.20 | 297.50 | 29.50 | ||||
| Zn | Mean | Nd | 12.66 | 3.77 | 15.87 * | 8.98 | 11.63 * | 9.73 | 10.19 | 5.00 | - | - |
| Min | Nd | 9.90 | 0.70 | 2.40 | 7.30 | 7.90 | 5.60 | 5.00 | ||||
| Max | Nd | 17.80 | 5.70 | 31.70 | 10.70 | 19.10 | 13.20 | 17.10 | ||||
| Heavy Metal | Average Heavy Metal Concentration (mg/kg) | Background (Upper Crust) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IB | OB | YR | PR | |||||||
| WS | DS | WS | DS | WS | DS | WS | DS | |||
| Cd | Mean | 0.58 | Nd | 0.61 | Nd | 0.67 | 0.75 | 0.67 * | 0.28 | 0.098 |
| Min | 0.41 | Nd | 0.41 | Nd | 0.47 | 0.07 | 0.47 | 0.02 | ||
| Max | 0.69 | Nd | 0.75 | Nd | 1.00 | 0.07 | 0.83 | 0.47 | ||
| Co | Mean | 9.36 * | 6.67 | 8.23 | 7.41 | 9.52 * | 7.66 | 8.03 * | 6.80 | 17 |
| Min | 7.61 | 3.51 | 5.64 | 5.82 | 9.12 | 5.45 | 4.74 | 5.33 | ||
| Max | 10.93 | 8.39 | 9.77 | 8.52 | 10.23 | 10.13 | 8.99 | 8.88 | ||
| Cr | Mean | 21.38 * | 17.10 | 18.61 | 24.80 * | 22.49 * | 18.12 | 24.48 | 23.27 | 83 |
| Min | 14.72 | 5.54 | 11.92 | 19.06 | 19.99 | 10.69 | 21.02 | 18.68 | ||
| Max | 25.80 | 23.52 | 24.19 | 28.86 | 27.82 | 21.93 | 30.73 | 27.21 | ||
| Cu | Mean | 8.49 | 8.49 | 9.31 | 14.04 * | 14.69 | 8.11 | 21.47 | 27.14 | 25 |
| Min | 3.45 | 4.08 | 5.70 | 10.67 | 6.98 | 4.10 | 8.64 | 18.71 | ||
| Max | 11.50 | 13.61 | 12.34 | 17.18 | 41.50 | 9.72 | 28.67 | 39.89 | ||
| Fe | Mean | 28,904.25 * | 21,247.53 | 23,642.00 | 26,750.01 * | 32,982.84 * | 23,347.50 | 30,734.40 * | 22,753.00 | 35,200 |
| Min | 21,767.87 | 11,679.66 | 15,576.53 | 20,692.12 | 30,842.11 | 14,979.10 | 25,482.41 | 18,408.15 | ||
| Max | 35,792.95 | 26,382.29 | 28,565.03 | 32,266.44 | 35,886.52 | 26,733.37 | 34,360.85 | 26,740.99 | ||
| Mn | Mean | 555.09 * | 341.19 | 845.53 * | 648.02 | 534.07 * | 300.13 | 506.00 | 424.89 | 600 |
| Min | 412.17 | 155.69 | 510.68 | 476.33 | 396.18 | 137.28 | 374.09 | 340.10 | ||
| Max | 716.13 | 555.66 | 1005.49 | 871.16 | 696.77 | 495.82 | 666.67 | 593.27 | ||
| Ni | Mean | 17.17 * | 9.48 | 14.82 | 13.49 | 17.64 * | 10.82 | 15.46 * | 10.39 | 44 |
| Min | 12.56 | 4.52 | 9.77 | 9.36 | 16.30 | 7.65 | 13.55 | 7.38 | ||
| Max | 19.96 | 12.35 | 17.70 | 15.91 | 18.95 | 12.97 | 16.92 | 12.06 | ||
| Pb | Mean | 80.45 * | 28.81 | 95.13 * | 61.72 | 98.22 * | 23.66 | 127.06 | 113.18 | 17 |
| Min | 56.14 | 10.95 | 71.68 | 51.41 | 66.68 | 8.26 | 68.99 | 80.12 | ||
| Max | 98.86 | 45.40 | 114.78 | 69.40 | 182.83 | 30.08 | 173.85 | 138.44 | ||
| Zn | Mean | 42.92 * | 37.25 | 36.52 | 47.49 * | 53.65 | 45.72 | 59.63 | 79.99 * | 71 |
| Min | 31.78 | 21.49 | 25.58 | 41.84 | 38.87 | 30.44 | 50.13 | 55.62 | ||
| Max | 51.56 | 45.79 | 46.70 | 53.63 | 113.12 | 52.58 | 69.06 | 140.12 | ||
| OM | pH | EC | Cd | Co | Cr | Cu | Fe | Mn | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OM | 1 | |||||||||||
| pH | −0.157 | 1 | ||||||||||
| EC | 0.277 | 0.659 ** | 1 | |||||||||
| Cd | 0.096 | 0.193 | 0.223 | 1 | ||||||||
| Co | 0.122 | −0.126 | 0.048 | 0.323 * | 1 | |||||||
| Cr | 0.055 | 0.117 | 0.254 | 0.500 ** | 0.867 ** | 1 | ||||||
| Cu | 0.091 | 0.433 ** | 0.319 * | 0.698 * | 0.541 ** | 0.784 ** | 1 | |||||
| Fe | 0.099 | −0.223 | 0.031 | 0.288 | 0.900 ** | 0.863 ** | 0.494 ** | 1 | ||||
| Mn | 0.298 | 0.448 ** | 0.251 | 0.396 * | 0.069 | 0.189 | 0.570 ** | −0.127 | 1 | |||
| Ni | −0.042 | 0.045 | 0.162 | 0.470 ** | 0.847 ** | 0.937 ** | 0.687 ** | 0.874 ** | 0.35 | 1 | ||
| Pb | 0.175 | 0.494 ** | 0.279 | 0.606 ** | 0.318 * | 0.533 ** | 0.887 ** | 0.181 | 0.825 ** | 0.378 * | 1 | |
| Zn | 0.098 | 0.034 | 0.159 | 0.325 * | 0.912 ** | 0.849 ** | 0.587 ** | 0.812 ** | 0.029 | 0.819 ** | 0.361 * | 1 |
| Heavy Metal | Contamination Factor (CF) * | |||||||
|---|---|---|---|---|---|---|---|---|
| IB | OB | YR | PR | |||||
| WS | DS | WS | DS | WS | DS | WS | DS | |
| Cd | 4.68 | 1.89 | 4.49 | 1.94 | 5.12 | 1.45 | 5.19 | 1.76 |
| Co | 1.48 | 1.33 | 1.68 | 1.20 | 1.71 | 1.37 | 1.44 | 1.22 |
| Cr | 0.24 | 0.32 | 0.27 | 0.22 | 0.29 | 0.23 | 0.31 | 0.30 |
| Cu | 0.37 | 0.56 | 0.34 | 0.34 | 0.59 | 0.32 | 0.86 | 1.09 |
| Fe | 0.68 | 0.76 | 0.83 | 0.61 | 0.94 | 0.67 | 0.65 | 0.79 |
| Mn | 1.31 | 1.01 | 0.86 | 0.53 | 0.83 | 0.47 | 0.79 | 0.66 |
| Ni | 0.50 | 0.46 | 0.58 | 0.32 | 0.60 | 0.37 | 0.53 | 0.35 |
| Pb | 4.02 | 2.61 | 3.40 | 1.22 | 4.15 | 1.00 | 5.37 | 4.78 |
| Zn | 0.52 | 0.68 | 0.61 | 0.53 | 0.77 | 0.65 | 0.85 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imsilp, K.; Sirinupong, P.; Yeesin, P.; Thong-asa, W.; Tanhan, P. Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand. Toxics 2025, 13, 477. https://doi.org/10.3390/toxics13060477
Imsilp K, Sirinupong P, Yeesin P, Thong-asa W, Tanhan P. Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand. Toxics. 2025; 13(6):477. https://doi.org/10.3390/toxics13060477
Chicago/Turabian StyleImsilp, Kanjana, Pattanasuda Sirinupong, Pun Yeesin, Wachiryah Thong-asa, and Phanwimol Tanhan. 2025. "Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand" Toxics 13, no. 6: 477. https://doi.org/10.3390/toxics13060477
APA StyleImsilp, K., Sirinupong, P., Yeesin, P., Thong-asa, W., & Tanhan, P. (2025). Potential Risks and Spatial Variation of Heavy Metals in Water and Surface Sediment of Pattani Bay, Thailand. Toxics, 13(6), 477. https://doi.org/10.3390/toxics13060477







