Maternal Exposure to Ambient Ozone and Fetal Critical Congenital Heart Disease in China: A Large Multicenter Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Ascertainment and Classification of CCHD
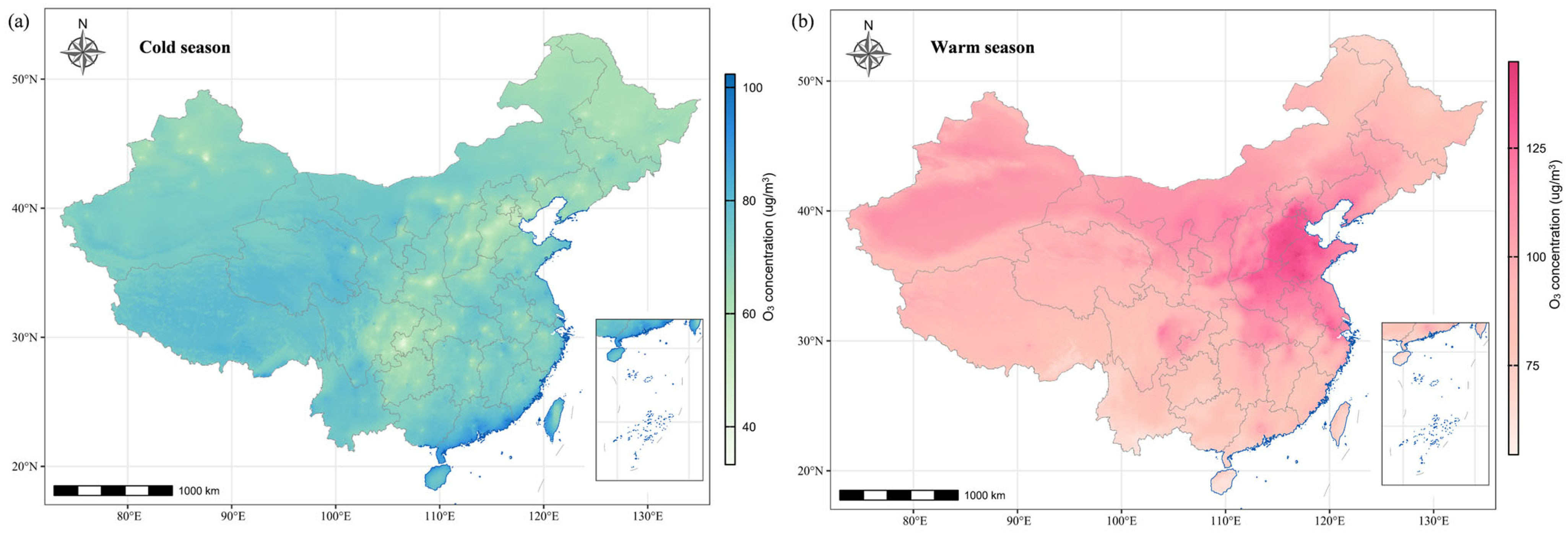
2.3. Exposure Assessment
2.4. Covariate Assessment
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Exposure–Response Analysis
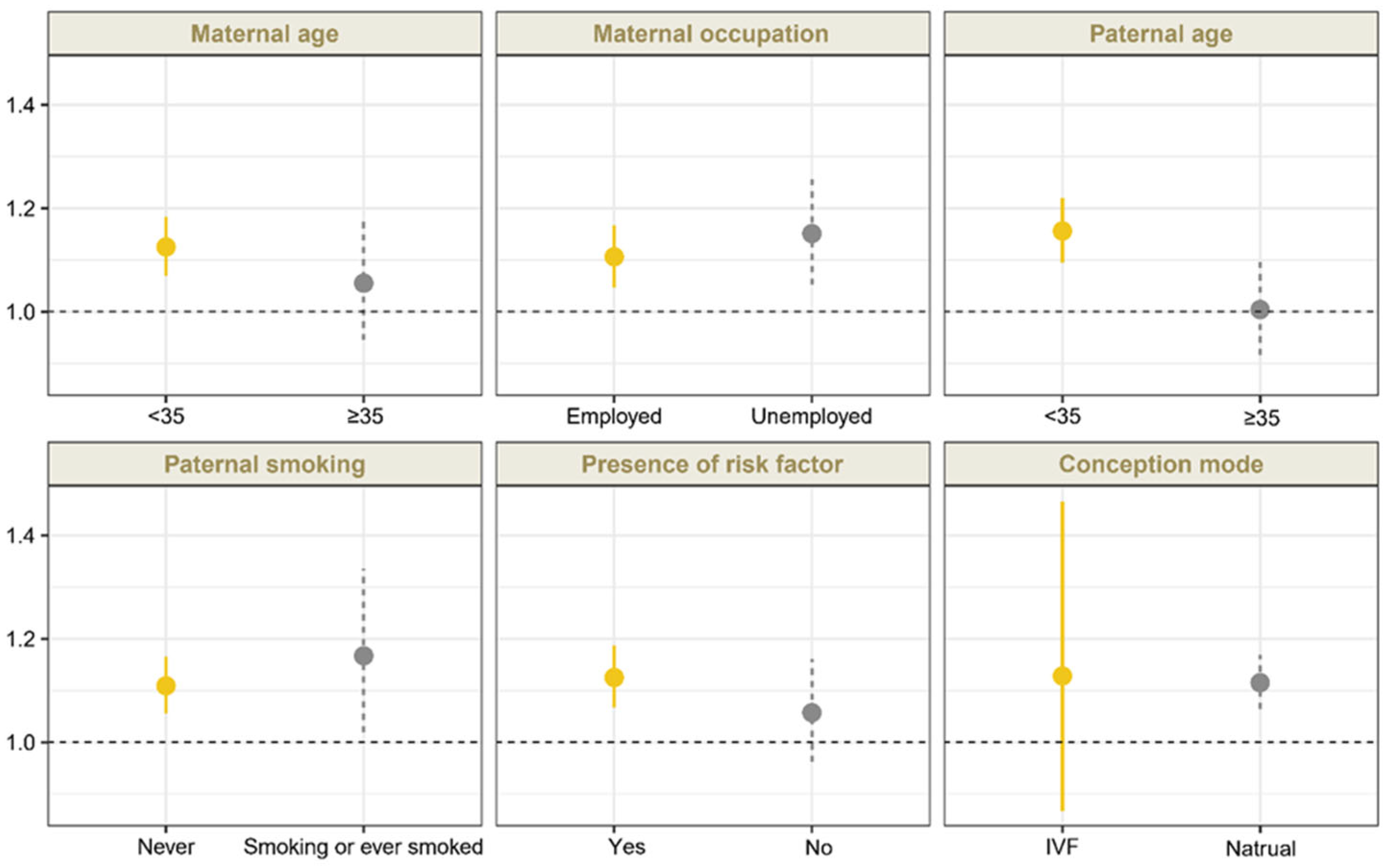
3.3. Stratified and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Child Adolesc. Health 2020, 4, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Ossa Galvis, M.M.; Bhakta, R.T.; Tarmahomed, A.; Mendez, M.D. Cyanotic Heart Disease. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lee, K.; Khoshnood, B.; Chen, L.; Wall, S.N.; Cromie, W.J.; Mittendorf, R.L. Infant mortality from congenital malformations in the United States, 1970–1997. Obstet. Gynecol. 2001, 98, 620–627. [Google Scholar] [CrossRef]
- Oster, M.E.; Lee, K.A.; Honein, M.A.; Riehle-Colarusso, T.; Shin, M.; Correa, A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics 2013, 131, e1502-8. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Zou, Z.Y.; Hay, S.I.; Liu, Y.W.; Li, S.J.; Chen, H.W.; Mohsen, N.; Zimmerman, M.S.; Martin, G.R.; Wilner, L.B.; et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: An age-period-cohort analysis for the Global Burden of Disease 2019 study. EClinicalMedicine 2022, 43, 101249. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Liu, F.; Wu, L.; Ma, X.J.; Niu, C.; Huang, G.Y. Prevalence of congenital heart disease at live birth in China. J. Pediatr. 2019, 204, 53–58. [Google Scholar] [CrossRef]
- Martin, G.R.; Beekman, R.H., III; Mikula, E.B.; Fasules, J.; Garg, L.F.; Kemper, A.R.; Morrow, W.R.; Pearson, G.D.; Mahle, W.T. Implementing recommended screening for critical congenital heart disease. Pediatrics 2013, 132, e185–e192. [Google Scholar] [CrossRef]
- American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of fetal echocardiography. J. Ultrasound Med. 2013, 32, 1067–1082. [Google Scholar] [CrossRef]
- Carvalho, J.S.; Allan, L.D.; Chaoui, R.; Copel, J.A.; DeVore, G.R.; Hecher, K.; Lee, W.; Munoz, H.; Paladini, D.; Tutschek, B.; et al. ISUOG Practice Guidelines (updated): Sonographic screening examination of the fetal heart. Ultrasound Obstet. Gynecol. 2013, 41, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Tometzki, A.J.; Suda, K.; Kohl, T.; Kovalchin, J.P.; Silverman, N.H. Accuracy of prenatal echocardiographic diagnosis and prognosis of fetuses with conotruncal anomalies. J. Am. Coll. Cardiol. 1999, 33, 1696–1701. [Google Scholar] [CrossRef]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Liberman, R.F.; Getz, K.D.; Lin, A.E.; Higgins, C.A.; Sekhavat, S.; Markenson, G.R.; Anderka, M. Delayed diagnosis of critical congenital heart defects: Trends and associated factors. Pediatrics 2014, 134, e373–e381. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Wei, S.X.; Wang, Y.Q.; Jiang, J.; Lian, X.Y.; Zou, Z.Y.; Li, J. The association between maternal air pollution exposure and the incidence of congenital heart diseases in children: A systematic review and meta-analysis. Sci. Total Environ. 2023, 892, 164431. [Google Scholar] [CrossRef]
- Sun, H.Z.; Zhao, J.; Liu, X.; Qiu, M.; Shen, H.; Guillas, S.; Giorio, C.; Staniaszek, Z.; Yu, P.; Wan, M.W.L.; et al. Antagonism between ambient ozone increase and urbanization-oriented population migration on Chinese cardiopulmonary mortality. Innovation 2023, 4, 100517. [Google Scholar] [CrossRef]
- Arjomandi, M.; Wong, H.; Donde, A.; Frelinger, J.; Dalton, S.; Ching, W.; Power, K.; Balmes, J.R. Exposure to medium and high ambient levels of ozone causes adverse systemic inflammatory and cardiac autonomic effects. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H1499–H1509. [Google Scholar] [CrossRef]
- Day, D.B.; Xiang, J.; Mo, J.; Li, F.; Chung, M.; Gong, J.; Weschler, C.J.; Ohman-Strickland, P.A.; Sundell, J.; Weng, W. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern. Med. 2017, 177, 1344–1353. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Carraway, M.S.; Dhingra, R.; Tong, H.; Neas, L.; Diaz-Sanchez, D.; Cascio, W.; Case, M.; Crooks, J.; Hauser, E.R. Ozone exposure is associated with acute changes in inflammation, fibrinolysis, and endothelial cell function in coronary artery disease patients. Environ. Health 2017, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Agay-Shay, K.; Friger, M.; Linn, S.; Peled, A.; Amitai, Y.; Peretz, C. Air pollution and congenital heart defects. Environ. Res. 2013, 124, 28–34. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Z.; Ni, B.; Xie, W.; Zhou, H.; Li, X. Independent and interactive effects of air pollutants and ambient heat exposure on congenital heart defects. Reprod. Toxicol. 2021, 104, 106–113. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.M.; Liang, Y.; Ruan, Z.L.; Acharya, B.K.; Zhang, S.Y.; Qian, Z.M.; McMillin, S.E.; Hinyard, L.; Sun, J.; et al. Maternal air pollution exposure associated with risk of congenital heart defect in pre-pregnancy overweighted women. Sci. Total Environ. 2020, 712, 136470. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, J.Z.; Yang, R.; Qian, Z.M.; Liang, S.W.; Bassig, B.A.; Zhang, Y.M.; Hu, K.; Xu, S.Q.; Dong, G.H.; et al. Ozone and other air pollutants and the risk of congenital heart defects. Sci. Rep. 2016, 6, 34852. [Google Scholar] [CrossRef] [PubMed]
- Dadvand, P.; Rankin, J.; Rushton, S.; Pless-Mulloli, T. Ambient air pollution and congenital heart disease: A register-based study. Environ. Res. 2011, 111, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Farhi, A.; Boyko, V.; Almagor, J.; Benenson, I.; Segre, E.; Rudich, Y.; Stern, E.; Lerner-Geva, L. The possible association between exposure to air pollution and the risk for congenital malformations. Environ. Res. 2014, 135, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Lima, I.; Hatzopoulou, M.; Ryswyk, K.V.; Decou, M.L.; Luo, W.; Donkelaar, A.V.; Martin, R.V.; Chen, H.; Stieb, D.M.; et al. Spatial variations in ambient ultrafine particle concentrations and risk of congenital heart defects. Environ. Int. 2019, 130, 104953. [Google Scholar] [CrossRef]
- Vinikoor-Imler, L.C.; Stewart, T.G.; Luben, T.J.; Davis, J.A.; Langlois, P.H. An exploratory analysis of the relationship between ambient ozone and particulate matter concentrations during early pregnancy and selected birth defects in Texas. Environ. Pollut. 2015, 202, 1–6. [Google Scholar] [CrossRef]
- Vrijheid, M.; Martinez, D.; Manzanares, S.; Dadvand, P.; Schembari, A.; Rankin, J.; Nieuwenhuijsen, M. Ambient air pollution and risk of congenital anomalies: A systematic review and meta-analysis. Environ. Health Perspect. 2011, 119, 598–606. [Google Scholar] [CrossRef]
- Yuan, X.L.; Liang, F.C.; Zhu, J.; Huang, K.Y.; Dai, L.; Li, X.H.; Wang, Y.P.; Li, Q.; Lu, X.F.; Huang, J.F.; et al. Maternal Exposure to PM2.5 and the risk of congenital heart defects in 1.4 Million Births: A nationwide surveillance-based study. Circulation 2023, 147, 565–574. [Google Scholar] [CrossRef]
- Louis, G.M.; Cooney, M.A.; Lynch, C.D.; Handal, A. Periconception window: Advising the pregnancy-planning couple. Fertil. Steril. 2008, 89, e119–e121. [Google Scholar] [CrossRef]
- Luderer, U.; Lim, J.W.; Ortiz, L.; Nguyen, J.D.; Shin, J.H.; Allen, B.D.; Liao, L.S.; Malott, K.; Perraud, V.; Wingen, L.M.; et al. Exposure to environmentally relevant concentrations of ambient fine particulate matter (PM2.5) depletes the ovarian follicle reserve and causes sex-dependent cardiovascular changes in apolipoprotein E null mice. Part. Fibre Toxicol. 2022, 19, 5. [Google Scholar] [CrossRef]
- Wu, S.S.; Zhang, Y.S.; Wu, X.Q.; Hao, G.M.; Ren, H.Q.; Qiu, J.H.; Zhang, Y.F.; Bi, X.Y.; Yang, A.M.; Bai, L.N.; et al. Association between exposure to ambient air pollutants and the outcomes of in vitro fertilization treatment: A multicenter retrospective study. Environ. Int. 2021, 153, 106544. [Google Scholar] [CrossRef]
- Luoto, R.; Mottola, M.F.; Hilakivi-Clarke, L. Pregnancy and lifestyle: Short- and long-term effects on mother’s and her children’s health. J. Pregnancy 2013, 2013, 537526. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Han, J.C.; Yu, S.M.; Guo, Y.; Ruan, Y.P.; Fu, Y.W.; Hao, X.Y.; Wang, X.; Wang, S.Y.; Zhou, X.X.; et al. Comparison of fetal echocardiogram with fetal cardiac autopsy findings in fetuses with congenital heart disease. J. Matern. Fetal Neonatal Med. 2021, 34, 3844–3850. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Mai, C.T.; Riehle-Colarusso, T.; O’Halloran, A.; Cragan, J.D.; Olney, R.S.; Lin, A.; Feldkamp, M.; Botto, L.D.; Rickard, R.; Anderka, M.; et al. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2005–2009: Featuring critical congenital heart defects targeted for pulse oximetry screening. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 970–983. [Google Scholar] [CrossRef]
- Oster, M.E.; Aucott, S.W.; Glidewell, J.; Hackell, J.; Kochilas, L.; Martin, G.R.; Phillippi, J.L.; Pinto, N.M.; Saarinen, A.; Sontag, M.; et al. Lessons learned from newborn screening for critical congenital heart defects. Pediatrics 2016, 137, e20154573. [Google Scholar] [CrossRef]
- Xiao, Q.; Geng, G.; Xue, T.; Liu, S.; Cai, C.; He, K.; Zhang, Q. Tracking PM2.5 and O3 pollution and the related health burden in China 2013–2020. Environ. Sci. Technol. 2022, 56, 6922–6932. [Google Scholar] [CrossRef]
- Hu, C.Y.; Huang, K.; Fang, Y.; Yang, X.J.; Ding, K.; Jiang, W.; Hua, X.G.; Huang, D.Y.; Jiang, Z.X.; Zhang, X.J. Maternal air pollution exposure and congenital heart defects in offspring: A systematic review and meta-analysis. Chemosphere 2020, 253, 126668. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.Q.; Cribb, M.; Huang, W.; Xue, W.H.; Sun, L.; Guo, J.P.; Peng, Y.R.; Li, J.; Lyapustin, A.; et al. Improved 1km resolution PM2.5 estimates across China using enhanced space–time extremely randomized trees. Atmos. Chem. Phys. 2020, 20, 3273–3289. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Lyapustin, A.; Sun, L.; Peng, Y.; Xue, W.; Su, T.N.; Cribb, M. Reconstructing 1-km-resolution high-quality PM2.5 data records from 2000 to 2018 in China: Spatiotemporal variations and policy implications. Remote Sens. Environ. 2021, 252, 112136. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Wang, J.; Li, C.; Gupta, P.; Cribb, M. Ground-level gaseous pollutants (NO2, SO2, and CO) in China: Daily seamless mapping and spatiotemporal variations. Atmos. Chem. Phys. 2023, 23, 1511–1532. [Google Scholar] [CrossRef]
- Xin, Y.; Yang, Y.; Chen, X.; Yue, X.; Liu, Y.; Yin, C. Evaluation of IMERG and ERA5 precipitation products over the Mongolian Plateau. Sci. Rep. 2022, 12, 21776. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2023. Available online: https://www.r-project.org/ (accessed on 31 May 2024).
- Huang, C.C.; Chen, B.Y.; Pan, S.C.; Ho, Y.L.; Guo, Y.L. Prenatal exposure to PM2.5 and Congenital Heart Diseases in Taiwan. Sci. Total Environ. 2019, 655, 880–886. [Google Scholar] [CrossRef]
- Girguis, M.S.; Strickland, M.J.; Hu, X.; Liu, Y.; Bartell, S.M.; Vieira, V.M. Maternal exposure to traffic-related air pollution and birth defects in Massachusetts. Environ. Res. 2016, 146, 1–9. [Google Scholar] [CrossRef]
- Schembari, A.; Nieuwenhuijsen, M.J.; Salvador, J.; De Nazelle, A.; Cirach, M.; Dadvand, P.; Beelen, R.; Hoek, G.; Basagaña, X.; Vrijheid, M. Traffic-related air pollution and congenital anomalies in Barcelona. Environ. Health Perspect. 2014, 122, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, Z.; Pinker, R.T.; Wang, J.; Sun, L.; Xue, W.; Li, R.; Cribb, M. Himawari-8-derived diurnal variations in ground-level PM2.5 pollution across China using the fast space-time Light Gradient Boosting Machine (LightGBM). Atmos. Chem. Phys. 2021, 21, 7863–7880. [Google Scholar] [CrossRef]
- Ogliari, K.S.; Lichtenfels, A.J.; de Marchi, M.R.; Ferreira, A.T.; Dolhnikoff, M.; Saldiva, P.H. Intrauterine exposure to diesel exhaust diminishes adult ovarian reserve. Fertil. Steril. 2013, 99, 1681–1688. [Google Scholar] [CrossRef]
- Perin, P.M.; Maluf, M.; Januário, D.N.; Saldiva, P.H. Effects of short-term exposure of female mice to diesel exhaust particles on in vitro fertilization and embryo development. Fertil. Steril. 2008, 90, S206. [Google Scholar] [CrossRef]
- Klepac, P.; Locatelli, I.; Korošec, S.; Künzli, N.; Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef]
- Proietti, E.; Röösli, M.; Frey, U.; Latzin, P. Air pollution during pregnancy and neonatal outcome: A review. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 9–23. [Google Scholar] [CrossRef]
- Januário, D.A.; Perin, P.M.; Maluf, M.; Lichtenfels, A.J.; Nascimento, S.P.H. Biological effects and dose-response assessment of diesel exhaust particles on in vitro early embryo development in mice. Toxicol. Sci. 2010, 117, 200–208. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Song, X.; Lazar, L.; Li, Z.; Zhao, J. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 2019, 169, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yauk, C.; Polyzos, A.; Rowan-Carroll, A.; Somers, C.M.; Godschalk, R.W.; Van Schooten, F.J.; Berndt, M.L.; Pogribny, I.P.; Koturbash, I.; Williams, A.; et al. Germ-line mutations, DNA damage, and global hypermethylation in mice exposed to particulate air pollution in an urban/industrial location. Proc. Natl. Acad. Sci. USA 2008, 105, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Blanc, N.; Liao, J.; Gilliland, F.; Zhang, J.J.; Berhane, K.; Huang, G.; Yan, W.; Chen, Z. A systematic review of evidence for maternal preconception exposure to outdoor air pollution on Children’s health. Environ. Pollut. 2023, 318, 120850. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (N = 24,516) | CCHD (N = 1541) | No CHD (N = 22,975) | p Value |
|---|---|---|---|---|
| Maternal factors | ||||
| Maternal age, years | 31.0 (4.2) | 30.5 (4.6) | 31.1 (4.2) | <0.001 |
| Maternal occupation status, n (%) | <0.001 | |||
| Employed | 19,633 (80.1) | 1074 (69.7) | 18,559 (80.8) | |
| Unemployed | 4055 (16.5) | 430 (27.9) | 3625 (15.8) | |
| Unknown | 828 (3.4) | 37 (2.4) | 791 (3.4) | |
| Paternal factors | ||||
| Paternal age, years | 32.6 (5.1) | 31.7 (5.2) | 32.7 (5.0) | <0.001 |
| Smoking status, n (%) | <0.001 | |||
| Smoking or ever smoked | 2139 (8.7) | 205 (13.3) | 1934 (8.4) | |
| Never | 22,377 (91.3) | 1336 (86.7) | 21,041 (91.6) | |
| Alcohol consumption, n (%) | <0.001 | |||
| Drinking or ever drank | 989 (4.0) | 112 (7.3) | 877 (3.8) | |
| Never | 23,527 (96.0) | 1429 (92.7) | 22,098 (96.2) | |
| Clinical factors | ||||
| Gestational week at time of fetal echocardiography a, n (%) | <0.001 | |||
| 15–28 | 21,205 (86.5) | 1281 (83.1) | 19,924 (86.7) | |
| 29–40 | 3311 (13.5) | 260 (16.9) | 3051 (13.3) | |
| Conception method, n (%) | 0.615 | |||
| Natural | 23,591 (96.2) | 1487 (96.5) | 22,104 (96.2) | |
| IVF | 925 (3.8) | 54 (3.5) | 871 (3.8) | |
| Fetus number, n (%) | 0.005 | |||
| Singleton | 24,027 (98.0) | 1495 (97.0) | 22,532 (98.1) | |
| Multiple | 489 (2.0) | 46 (3.0) | 443 (1.9) | |
| Presence of risk factors b, n (%) | <0.001 | |||
| Yes | 16,681 (68.0) | 1135 (73.7) | 15,546 (67.7) | |
| No | 7835 (32.0) | 406 (26.3) | 7429 (32.3) | |
| Conception season c, n (%) | 0.996 | |||
| Warm | 12,018 (49.0) | 756 (49.1) | 11,262 (49.0) | |
| Cold | 12,498 (51.0) | 785 (50.9) | 11,713 (51.0) | |
| O3 exposure, μg/m3 | ||||
| Periconceptional period | 93.9 (29.9) | 92.3 (27.7) | 94.0 (30.0) | 0.036 |
| Embryonic period | 95.6 (43.3) | 93.9 (40.1) | 95.7 (43.5) | 0.138 |
| The first trimester | 95.9 (40.2) | 94.2 (37.1) | 96.0 (40.4) | 0.090 |
| Preconception period | 92.1 (39.4) | 90.7 (35.9) | 92.2 (39.7) | 0.140 |
| O3 Exposure | ORs (95% CIs) b | ||
|---|---|---|---|
| Single c | Adjusted for PM2.5 d | Adjusted for NO2 e | |
| Per 10 ug/m3 | 1.260 (1.189, 1.335) | 1.372 (1.289, 1.461) | 1.255 (1.168, 1.348) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 1.381 (1.170, 1.629) | 1.338 (1.085, 1.650) | 1.096 (0.874, 1.374) |
| Quartile 3 | 1.511 (1.151, 1.983) | 1.824 (1.304, 2.552) | 1.406 (0.979, 2.018) |
| Quartile 4 | 1.803 (1.293, 2.514) | 1.685 (1.115, 2.546) | 1.267 (0.818, 1.965) |
| p for linear trend f | <0.001 | <0.05 | 0.330 |
| O3 Exposure | ORs (95% CIs) a | ||
|---|---|---|---|
| Embryonic Period b | The First Trimester c | Preconception Period d | |
| Per 10 ug/m3 | 1.136 (1.090, 1.184) | 1.181 (1.128, 1.237) | 1.117 (1.058, 1.181) |
| Quartile 1 | Ref | Ref | Ref |
| Quartile 2 | 1.332 (1.123, 1.579) | 1.456 (1.220, 1.737) | 1.297 (1.096, 1.536) |
| Quartile 3 | 2.472 (1.826, 3.347) | 2.947 (2.185, 3.975) | 1.278 (1.063, 1.536) |
| Quartile 4 | 2.210 (1.492, 3.273) | 2.879 (1.933, 4.289) | 1.077 (0.861, 1.347) |
| p for linear trend e | <0.001 | <0.001 | 0.804 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Y.; Wang, Y.; Zou, Z.; Li, J.; He, Y. Maternal Exposure to Ambient Ozone and Fetal Critical Congenital Heart Disease in China: A Large Multicenter Retrospective Cohort Study. Toxics 2025, 13, 463. https://doi.org/10.3390/toxics13060463
Ruan Y, Wang Y, Zou Z, Li J, He Y. Maternal Exposure to Ambient Ozone and Fetal Critical Congenital Heart Disease in China: A Large Multicenter Retrospective Cohort Study. Toxics. 2025; 13(6):463. https://doi.org/10.3390/toxics13060463
Chicago/Turabian StyleRuan, Yanping, Yaqi Wang, Zhiyong Zou, Jing Li, and Yihua He. 2025. "Maternal Exposure to Ambient Ozone and Fetal Critical Congenital Heart Disease in China: A Large Multicenter Retrospective Cohort Study" Toxics 13, no. 6: 463. https://doi.org/10.3390/toxics13060463
APA StyleRuan, Y., Wang, Y., Zou, Z., Li, J., & He, Y. (2025). Maternal Exposure to Ambient Ozone and Fetal Critical Congenital Heart Disease in China: A Large Multicenter Retrospective Cohort Study. Toxics, 13(6), 463. https://doi.org/10.3390/toxics13060463








