Pilot Study on Nucleation-Induced Pelleting Coagulation in Treatment of High-Algae Surface Water: Coagulant Dosage and Hydraulic Loading Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Water
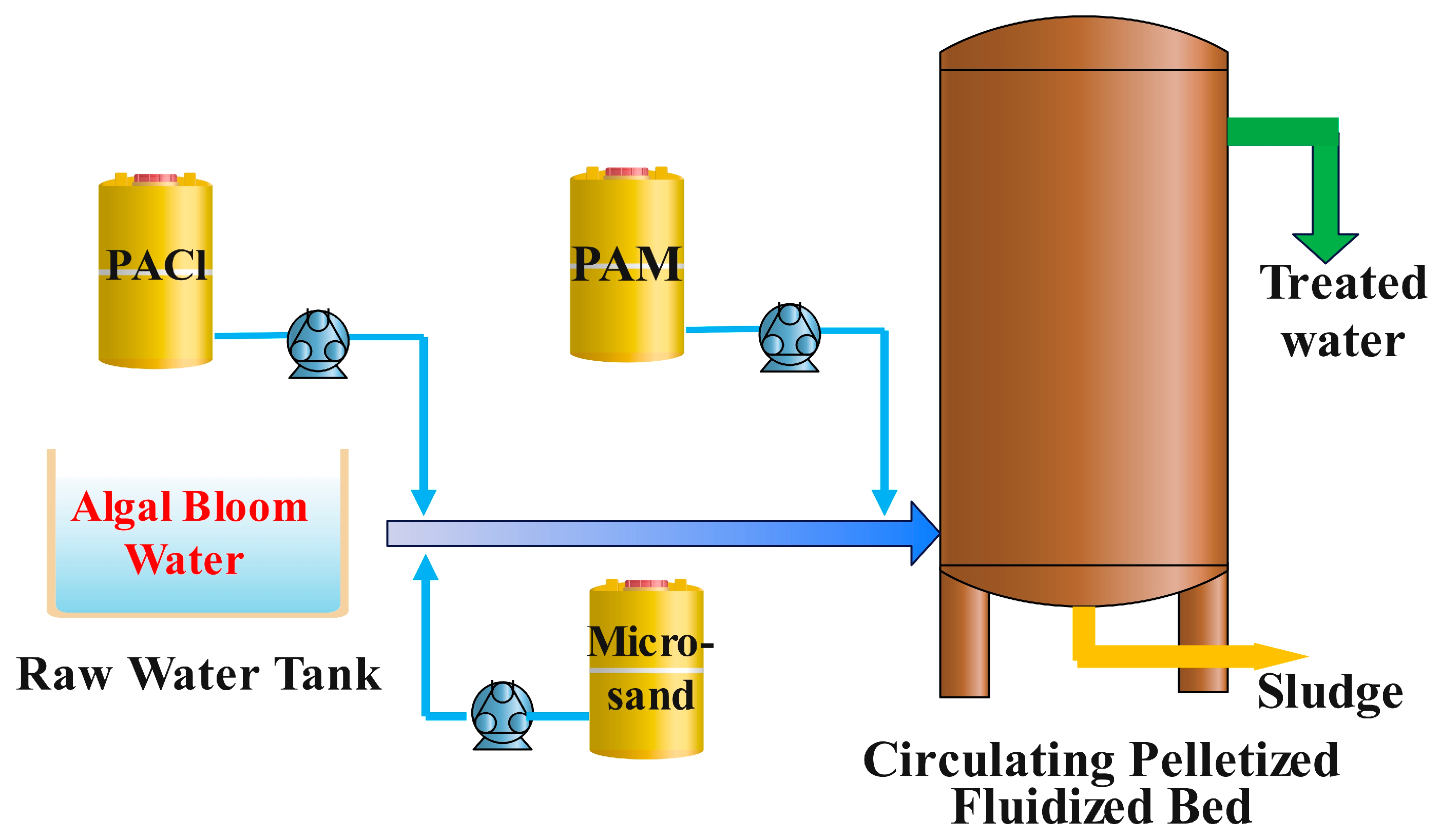
2.2. Experimental Device
2.3. Preparation of Chemicals and Micro-Sand
2.4. Analytical Methods
3. Results and Discussion
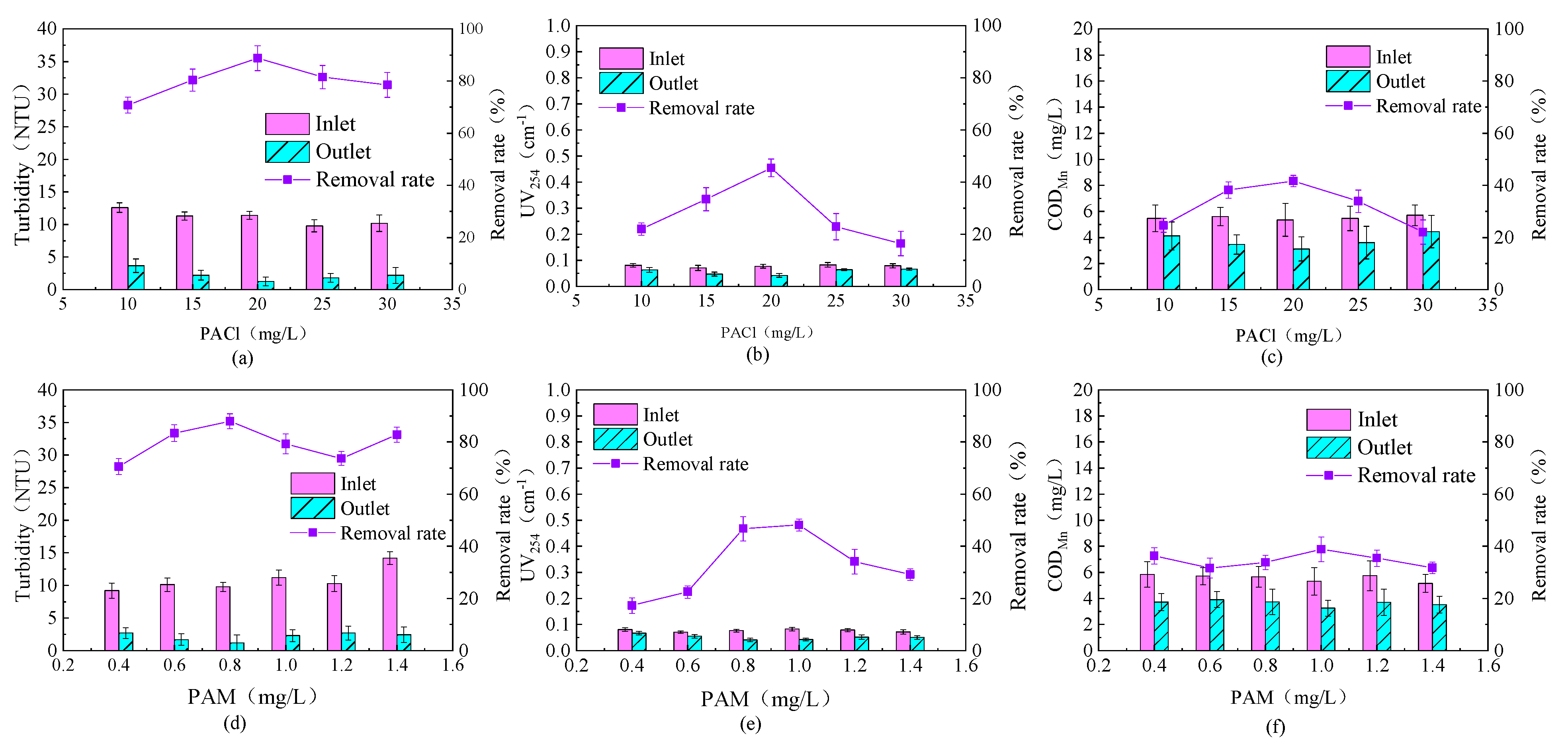
3.1. Determination of the Optimal Dosage of Coagulant
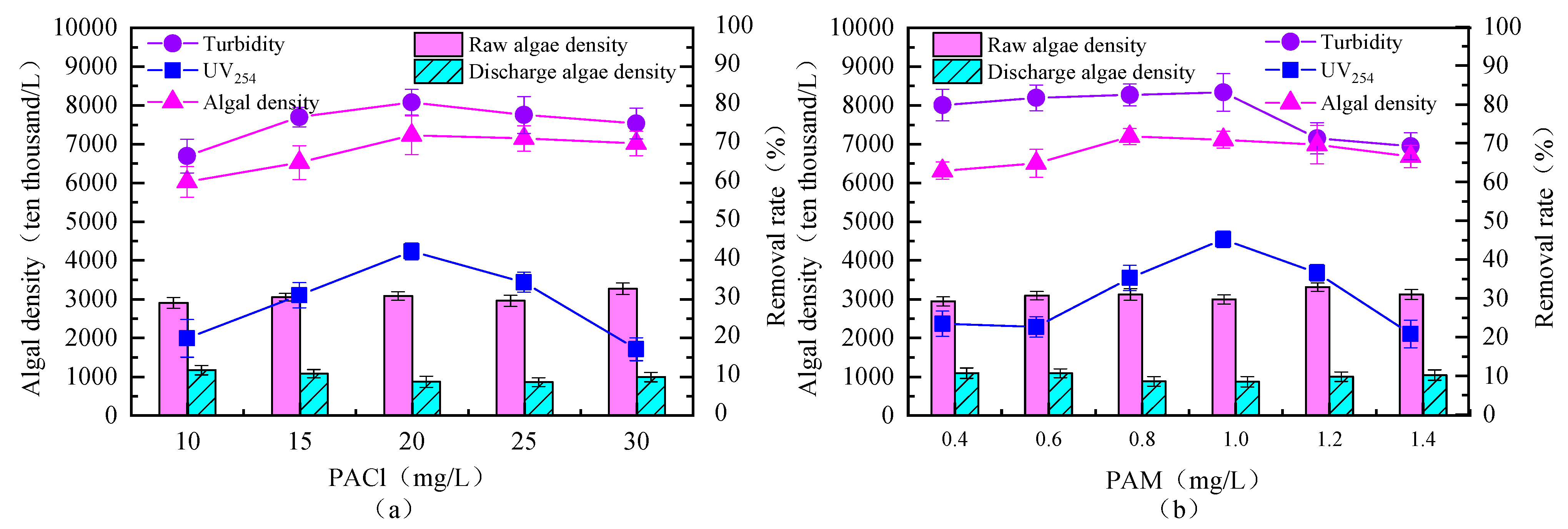
3.1.1. Determination of PACl Dosage
3.1.2. Determination of PAM Dosage
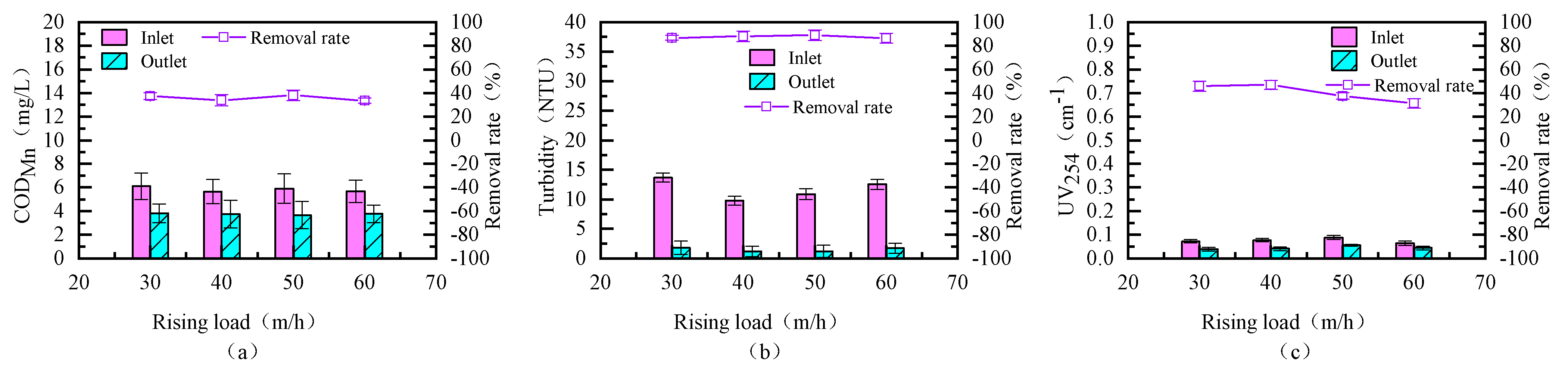
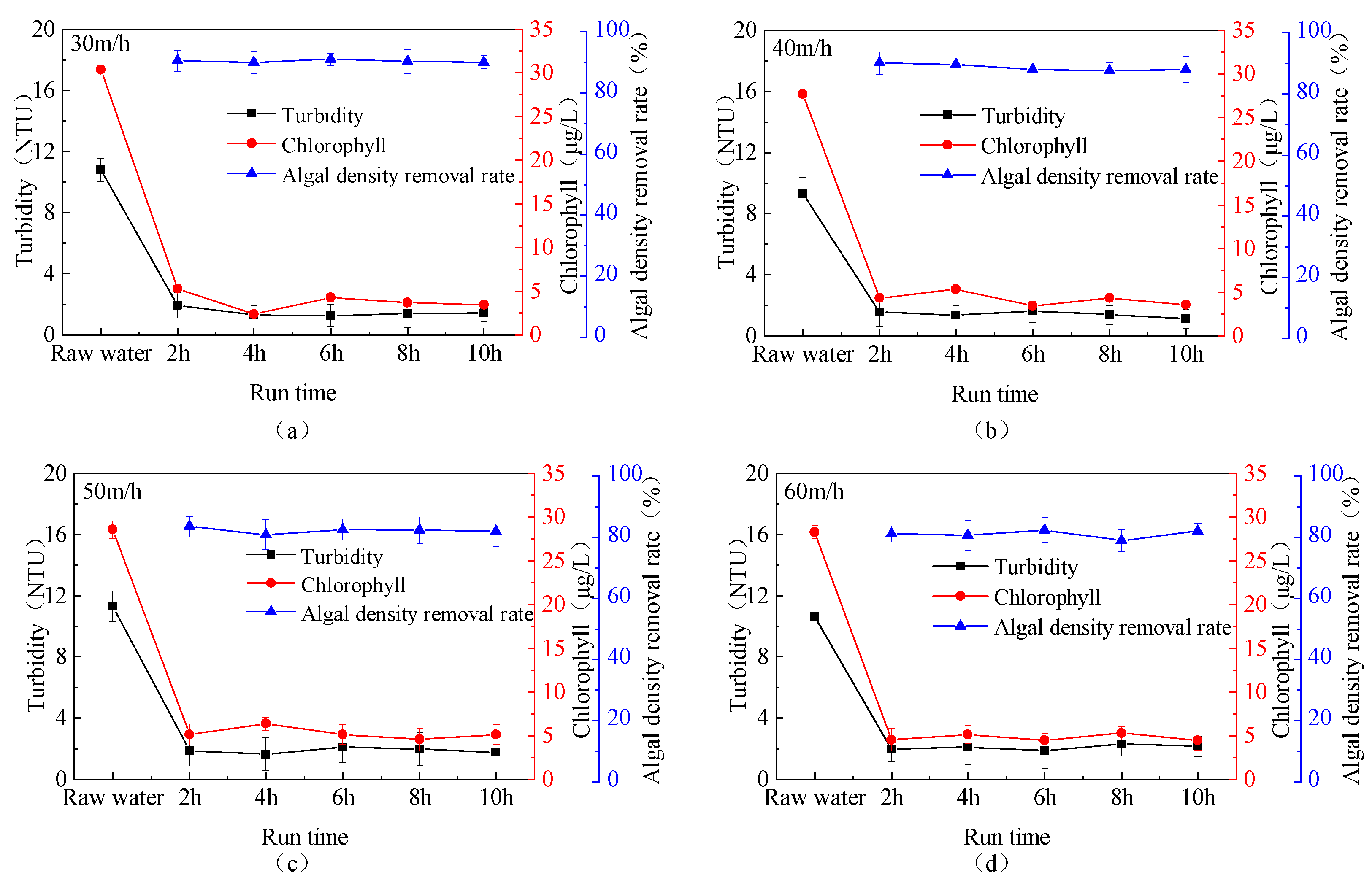
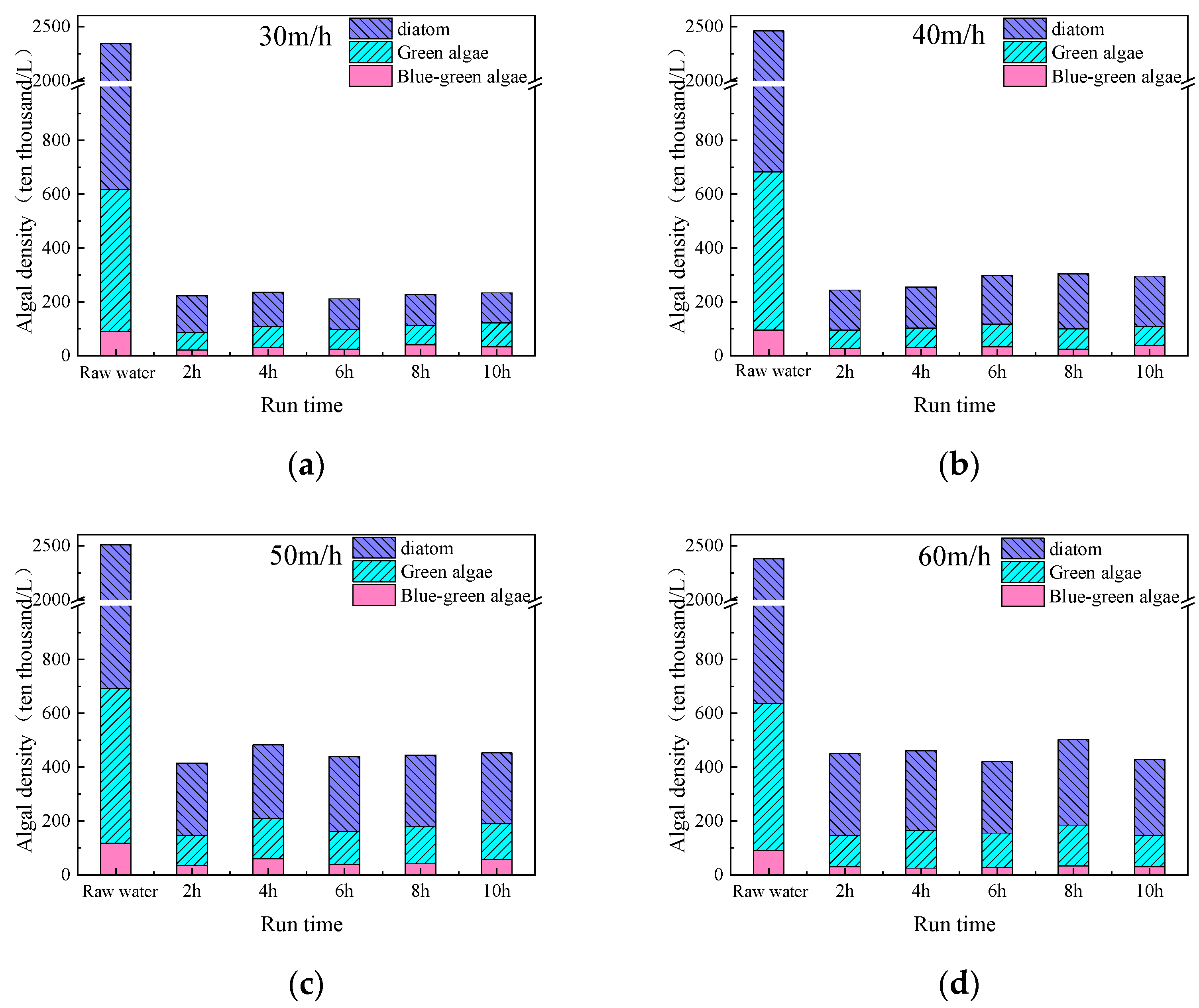
3.2. Determination of Optimal Upflow Velocity
3.3. Water Treatment Efficiency Under Stable NPC Operation
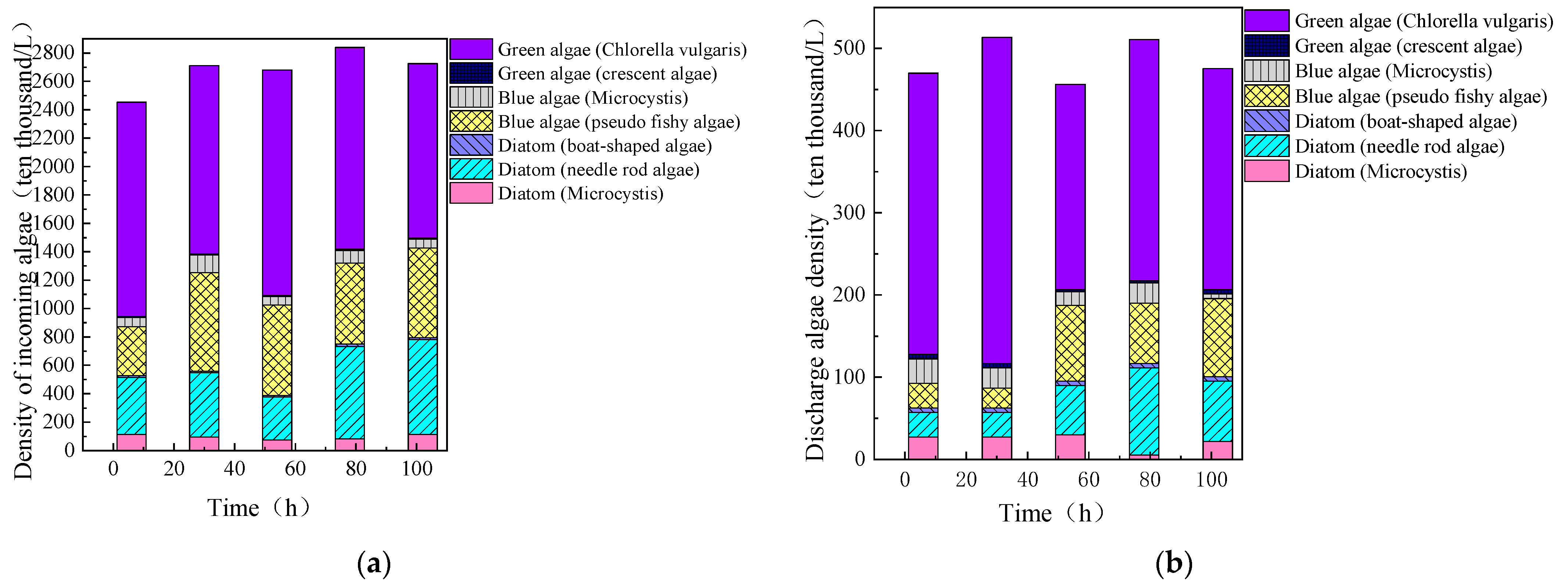
3.3.1. Removal Efficiency of Algae and Organic Matter During Continuous Fluidized Bed Operation
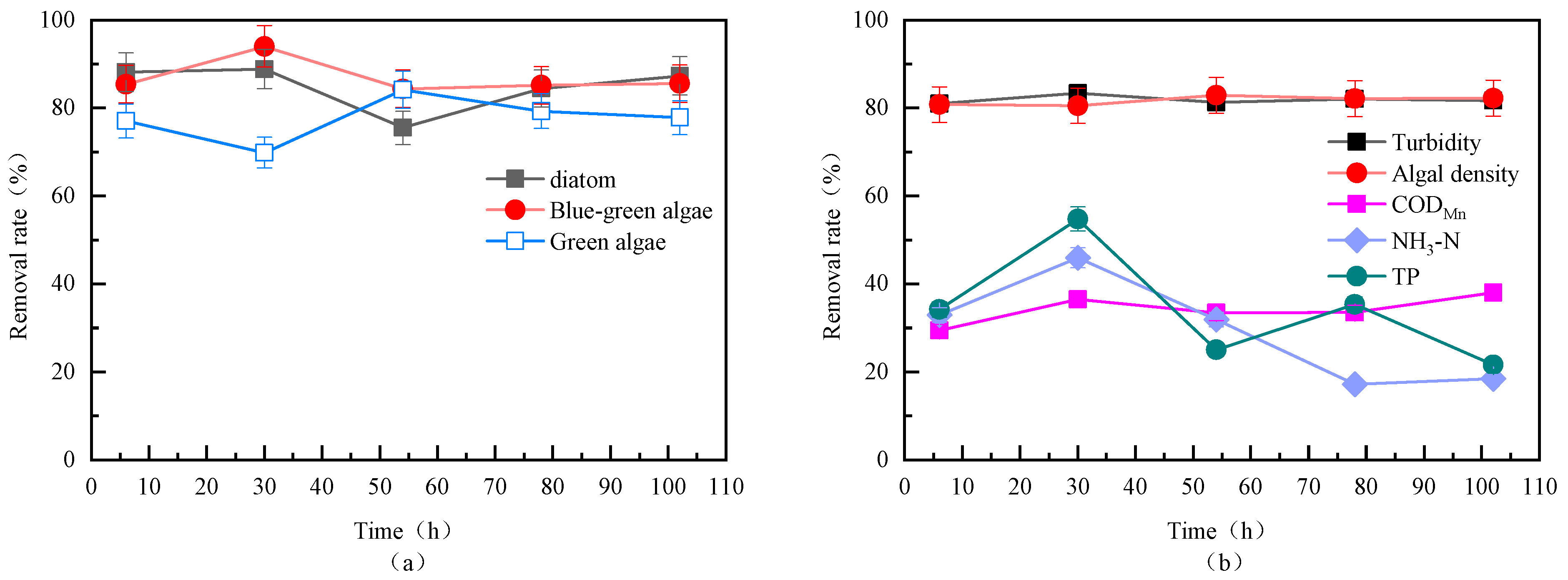
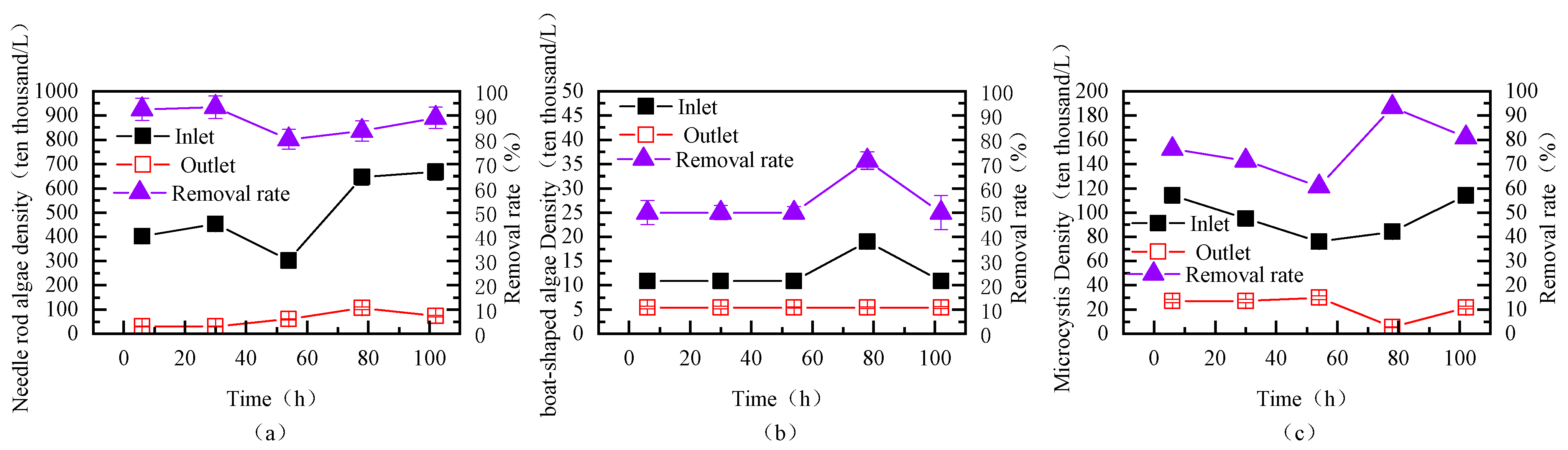
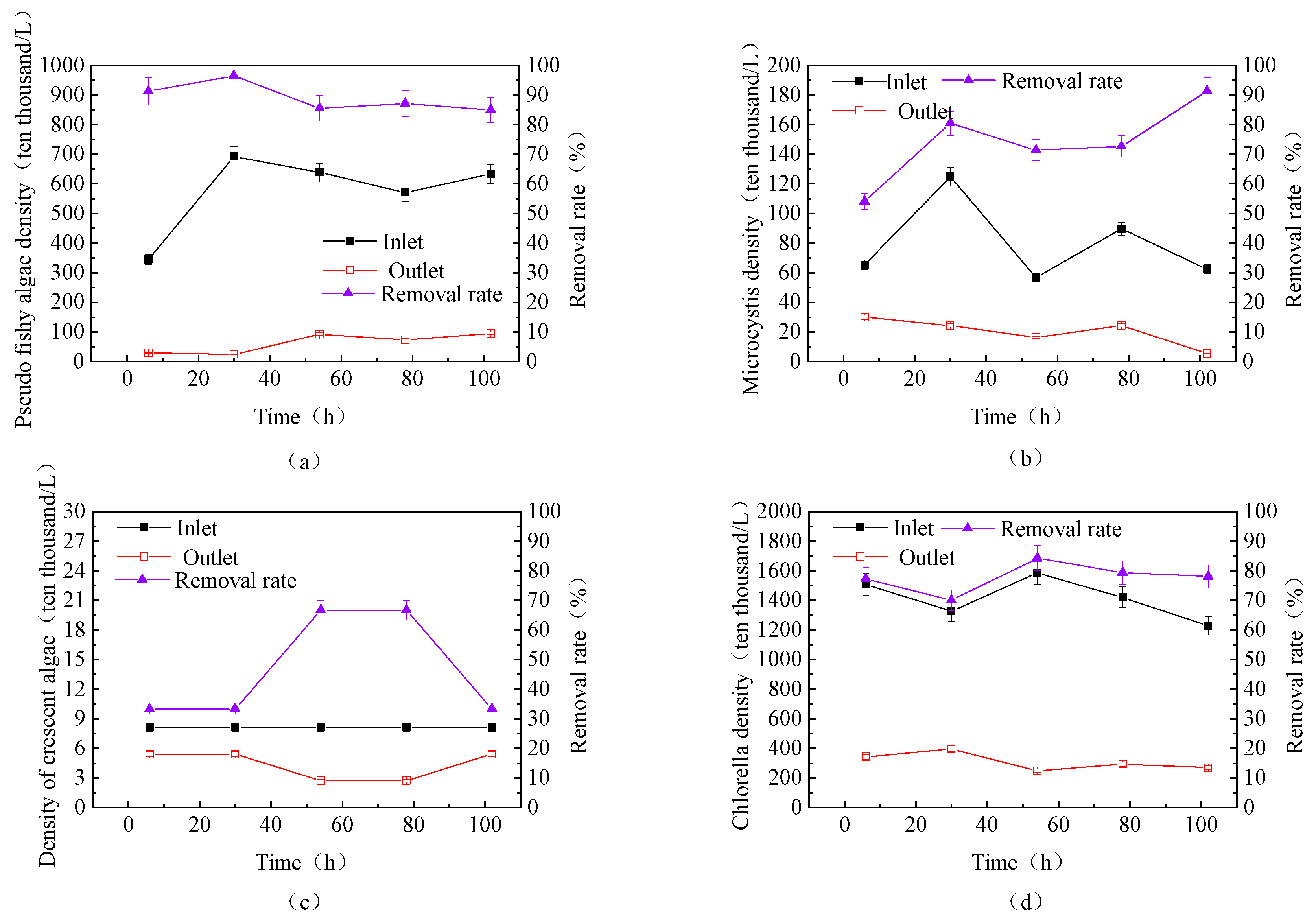
3.3.2. Removal Efficiency of Different Algae During Continuous Fluidized Bed Operation
3.4. Water Quality Safety of NPC-Treated Algae-Rich Water
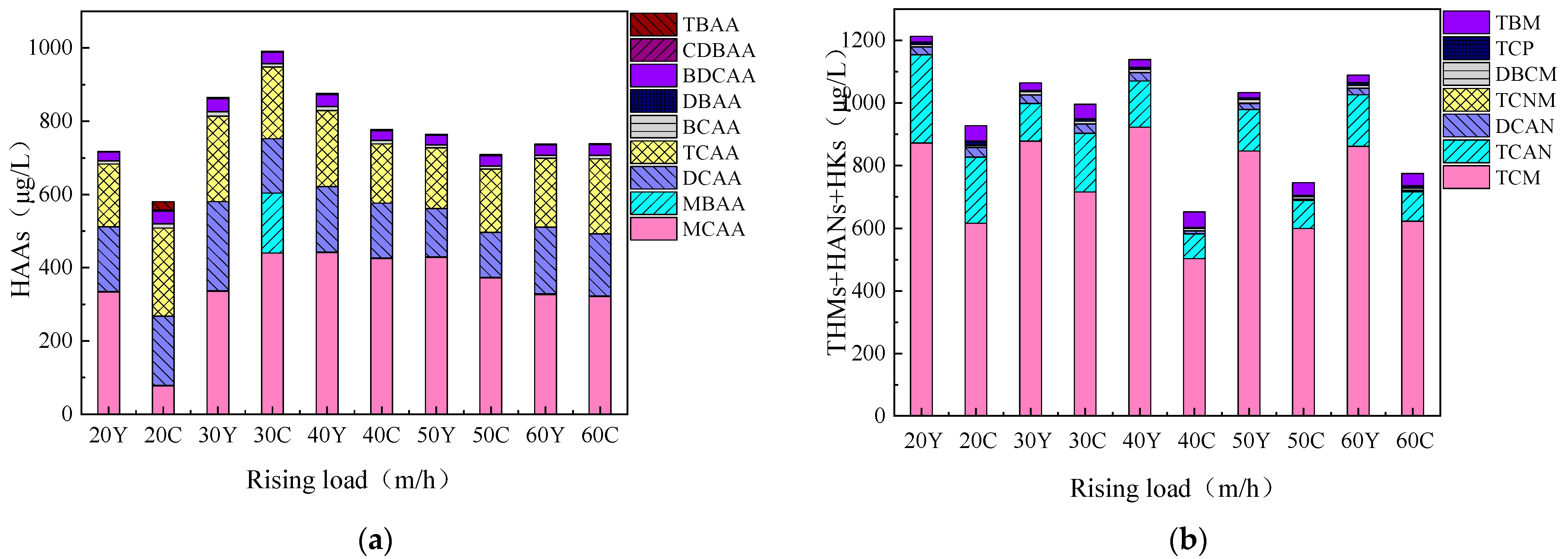
3.4.1. Changes in Disinfection Byproduct Formation Potential Before and After Fluidized Bed Treatment
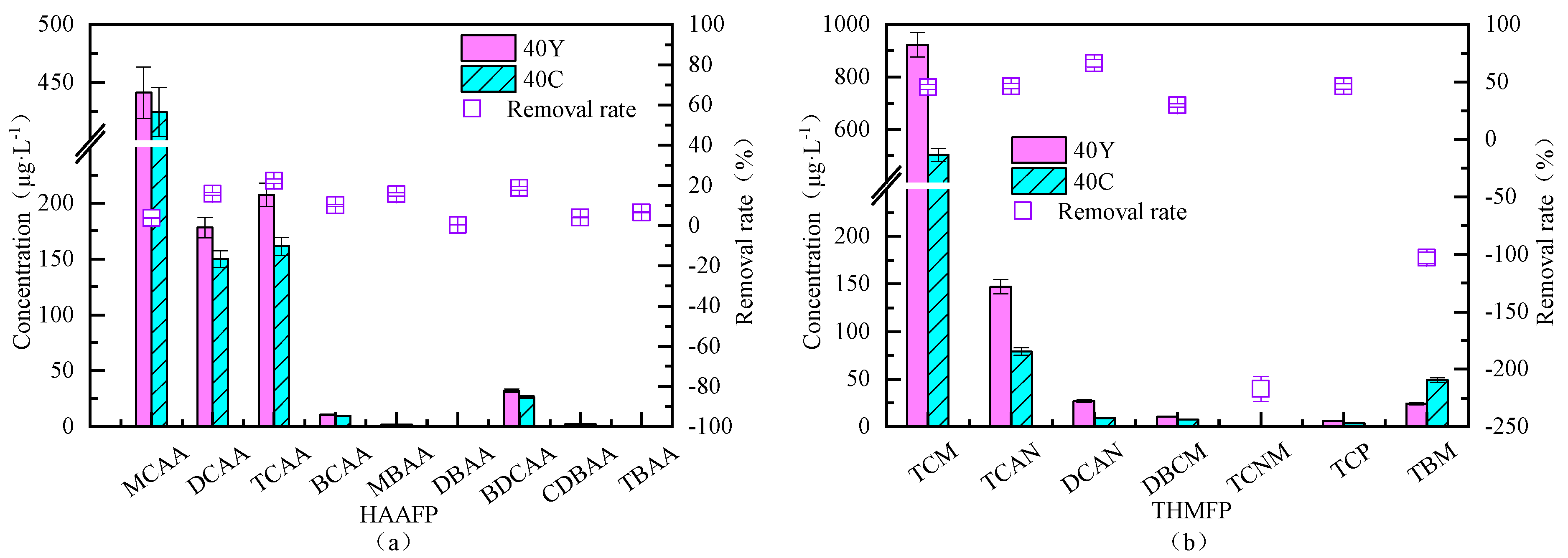
3.4.2. Removal Efficiency of Fluidized Bed for Different Types of Disinfection Byproduct Formation Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, H.; Jia, Y.; Wang, Z. Microcystin pollution in lakes and reservoirs: A nationwide meta-analysis and assessment in China. Environ. Pollut. 2022, 309, 119791. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Ma, R.; Shen, M.; Wu, J.; Hu, M.; Guo, Y.; Cao, Z.; Xiong, J. Horizontal and vertical migration of cyanobacterial blooms in two eutrophic lakes observed from the GOCI satellite. Water Res. 2023, 240, 120099. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Liang, Z.; Xin, P.; Wang, C.; Zhang, Y.; Chen, X. Identification of key water environmental factor contributions and spatiotemporal differential characteristics for eutrophication in Dianchi Lake. Environ. Monit. Assess. 2024, 196, 1217. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Choi, S.Y.; Ahn, S.K.; Kweon, J.H. Disinfection by-product formation potential of algogenic organic matter from Microcystis aeruginosa: Effects of growth phases and powdered activated carbon adsorption. J. Hazard. Mater. 2020, 408, 124864. [Google Scholar] [CrossRef]
- Matsumoto, M.; Murata, Y.; Hirose, N.; Shigeta, Y.; Iso, T.; Hirose, A. Hazard assessment of disinfection by-products, bromo chloroacetic acid and bromo dichloroacetic acid, in drinking water. Toxicol. Lett. 2021, 350, S226. [Google Scholar] [CrossRef]
- Pan, R.; Huang, Y.; Ao, J.; Wu, Y.; Bu, L.; Zhou, S.; Deng, L.; Shi, Z. A molecular-level mechanism analysis of PFS coagulation behaviors: Differences in natural organic matter and algal organic matter. Sep. Purif. Technol. 2023, 314, 123485. [Google Scholar] [CrossRef]
- Bai, Y.; Li, K.; Cao, R.; Xu, H.; Wang, J.; Huang, T.; Wen, G. Changes of characteristics and disinfection by-products formation potential of intracellular organic matter with different molecular weight in metalimnetic oxygen minimum. Chemosphere 2024, 354, 141718. [Google Scholar] [CrossRef]
- Gang, Z.; Huang, T.; Tan, C.; Li, Z.; He, W.; Han, H.; Li, C. Settling behaviour of pellet flocs in pelleting flocculation process: Analysis through operational conditions. Water Sci. Technol. A J. Int. Assoc. Water Pollut. Res. 2010, 62, 1346–1352. [Google Scholar] [CrossRef]
- Han, X.; Huang, T.; Xing, X.; Hu, R.; Li, K.; Sun, S.; Sun, C. Study on Removal of Natural Organic Matter by Micro-charcoal Enhanced Circulating Pelletized Fluidized Bed. China Environ. Sci. 2020, 40, 2513–2520. [Google Scholar]
- Xu, J.Q.; Huang, T.L.; Wen, G.; Xing, X.; Xu, Y. Performance and Enhancement of Circulating Granulation Fluidized Bed in Removing Different Characteristics of Organic Matter. China Water Wastewater 2025, 41, 58–64. [Google Scholar]
- Sun, S.B.; Xie, Y.J.; Huang, T.L.; Xing, X.; Hu, R.Z.; Han, X.L. Experimental study on the treatment of water plant sludge water by circulating granulation fluidized bed. China Water Wastewater 2020, 36, 12–18. [Google Scholar]
- Huang, T.L.; Tai, C.M.; Liu, J.Q. Pilot study on the treatment of low turbidity and high algae water by micro-sand enhanced flocculation technology. Water Wastewater Eng. 2012, 48, 115–119. [Google Scholar]
- Chaitra, H.; Aravind, H.B.; Senapati, A. Removal of Total Suspended Solids and Turbidity by Actiflo Process Using Microsand. Int. J. Res. Eng. Technol. 2017, 6, 138–144. [Google Scholar]
- Jin, X.; Zhang, W.; Ji, Z.; Zhou, L.; Jin, P.; Wang, X.C.; Zhang, Y. Application and mechanism of nucleation-induced pelleting coagulation (NPC) in treatment of fracturing wastewater with high concentration of dissolved organic matter. Chemosphere 2018, 211, 1082–1090. [Google Scholar] [CrossRef]
- Jin, Y.; Li, P.; Xu, B.; Wang, L.; Ma, G.; Chen, S.; Tan, F.; Shao, Y.; Zhang, L.; Yang, Z.; et al. A novel technology using iron in a coupled process of moderate preoxidation–hybrid coagulation to remove cyanobacteria in drinking water treatment plants. J. Clean. Prod. 2022, 342, 130947. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, W.; Zhu, W.; Zhu, L.; Xu, X. Diatomite-enhanced coagulation for algal removal in polluted raw water: Performance optimization and pilot-scale study. Environ. Sci. Pollut. Res. 2021, 28, 50204–50216. [Google Scholar] [CrossRef]
- Wu, X.; Xu, G.; Wang, J. Ultrasound-assisted coagulation for Microcystis aeruginosa removal using Fe3O4-loaded carbon nanotubes. RSC Adv. 2020, 10, 13525–13531. [Google Scholar] [CrossRef]
- Tumsri, K.; Chavalparit, O. Optimizing Electrocoagulation-electroflotation Process for Algae Removal. In Asia-Pacific Chemical, Biological & Environmental Engineering Society (APCBEES), Proceedings of International Conference on Environmental Science and Technology (ICEST 2011), Singapore, 26–28 February 2011; Environmental Engineering Faculty of Engineering Chulalongkorn University: Bangkok, Thailand, 2011; pp. 932–936. [Google Scholar]
- Driscoll, C.T.; Lee, A.; Montesdeoca, M.; Matthews, D.A.; Effler, S.W. Mobilization and toxicity potential of aminum from alum floc deposits in Kensico Reservoir, New York. J. Am. Water Resour. Assoc. 2014, 50, 143–152. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Ibrahem, M.D. Acrylamide-induced hematotoxicity, oxidative stress, and DNA damage in liver, kidney, and brain of catfish (Clarias gariepinus). Environ. Toxicol. 2020, 35, 300–308. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Li, A.; Yang, H. Enhanced coagulation of low-turbidity micro-polluted surface water: Properties and optimization. J. Environ. Manag. 2019, 233, 739–747. [Google Scholar] [CrossRef]
- Khan, Z.; Riaz, S.M.; Qaqzi, A.I. Comparing plain and coagulated horizontal flow roughing filtration for high turbidity water. Water Supply 2013, 13, 413–419. [Google Scholar] [CrossRef]
- Application of gas chromatographic technique for investigation of the formation of by products in drinking water disinfection. Eng. Sanit. Ambiental 2013, 18, 289–294.
- Wang, L.; Al-Dhabi, N.A.; Huang, X.; Luan, Z.; Tang, W.; Xu, Z.; Xu, W. Suitability of inorganic coagulants for algae-laden water treatment: Trade-off between algae removal and cell viability, aggregate properties and coagulant residue. J. Hazard. Mater. 2024, 471, 134314. [Google Scholar] [CrossRef]
- Runkana, V.; Somasundaran, P.; Kapur, P.C. Mathematical modeling of polymer-induced flocculation by charge neutralization. J. Colloid Interface 2004, 270, 347–358. [Google Scholar] [CrossRef]
- Ou, H.S.; Wei, C.H.; Deng, Y.; Gao, N.Y.; Ren, Y.; Hu, Y. Principal component analysis to assess the efficiency and mechanism for enhanced coagulation of natural algae-laden water using a novel dual coagulant system. Environ. Sci. Pollut. Res. Int. 2014, 21, 2122–2131. [Google Scholar] [CrossRef]
- Asharuddin, S.M.; Othman, N.; Al-Maqtari, Q.A.; Al-towayti, W.A.H.; Arifin, S.N.H. The assessment of coagulation and flocculation performance and interpretation of mechanistic behavior of suspended particles aggregation by alum assisted by tapioca peel starch. Environ. Technol. Innov. 2023, 32, 103414. [Google Scholar] [CrossRef]
- Weir, S.; Moody, G. The importance of flocculant choice with consideration to mixing energy to achieve efficient solid/liquid separation. Miner. Eng. 2003, 16, 109–113. [Google Scholar] [CrossRef]
- Huang, T. Study on Granulation Dynamics in Aggregated Pellet Fluidized Bed. Water Supply Drain. 1998, 24, 5. [Google Scholar]
- Macedo, T.Z.; de Souza Dornelles, H.; do Valle Marques, A.L.; PalladinoDelforno, T.; Centurion, V.B.; de Oliveira, V.M.; Silva, E.L.; Varesche, M.B.A. The influence of upflow velocity and hydraulic retention time changes on taxonomic and functional characterization in Fluidized Bed Reactor treating commercial laundry wastewater in co-digestion with domestic sewage. Biodegradation 2020, 31, 73–89. [Google Scholar] [CrossRef]
- Feng, L.; Qiu, T.; Yan, H.; Liu, C.; Chen, Y.; Zhou, X.; Qiu, S. Removal of Ammonia Nitrogen from Aqueous Media with Low-cost Adsorbents: A Review. Water Air Soil Pollut. 2023, 234, 280. [Google Scholar] [CrossRef]
- Zong, J.M.; Wang, X.X.; Zhong, Q.Y.; Xiao, X.M.; Ma, J.; Zhao, B. Increasing Outbreak of Cyanobacterial Blooms in Large Lakes and Reservoirs under Pressures from Climate Change and Anthropogenic Interferences in the Middle–Lower Yangtze River Basin. Remote Sens. 2019, 11, 1754. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, T.L.; Ning, G.; Li, Z.P. Application of Pellet Fluidized Bed Process in Ferric Flocs Sludge Conditioning. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, ICBBE 2009.2009, Beijing, China, 11–13 June 2009. [Google Scholar]
- Rupal, S.; Kumar, A.G.; Sarathi, P.G. A review on Trihalomethanes and Haloacetic acids in drinking water: Global status, health impact, insights of control and removal technologies. J. Environ. Chem. Eng. 2021, 9, 106511. [Google Scholar]
- Song, Q.; Yang, B.; Liu, M.; Song, S.; Graham, N.; Yu, W. Floc aging: Crystallization and improving low molecular weight organic removal in re-coagulation. Water Res. 2023, 243, 120328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-X.; Liu, B.-M.; Lu, M.-F.; Li, Y.-P.; Jiang, Y.-Y.; Zhao, M.-X.; Huang, Z.-X.; Pan, Y.; Miao, H.-F.; Ruan, W.-Q. Characterization of algal organic matter as precursors for carbonaceous and nitrogenous disinfection byproducts formation: Comparison with natural organic matter. J. Environ. Manag. 2021, 282, 111951. [Google Scholar] [CrossRef]
- Wu, Y.; Bu, L.; Duan, X.; Zhou, S.; Crittenden, J.C. Insights into the molecular compositions of CX3R-type disinfection byproduct precursors in algal organic matter from algae-laden water. Chem. Eng. J. 2022, 446, 136921. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Z.; Kong, Y.; Li, Y.; Zhou, Y.; Shi, X.; Shi, X. Effects of ultrasound on Microcystis aeruginosa cell destruction and release of intracellular organic matter. Ultrason. Sonochem. 2020, 63, 104909. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Gao, B.Y.; Yue, Q.; Zhao, Y. The disinfection by-products precursors removal efficiency and the subsequent effects on chlorine decay for humic acid synthetic water treated by coagulation process and coagulation–ultrafiltration process. Chem. Eng. J. 2012, 193–194, 59–67. [Google Scholar] [CrossRef]
| pH | Turbidity/NTU | CODMn/mg·L−1 | Algal Density (×104 cells/L) | Temperature (°C) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 7.73~8.45 | 2~5000 | 4.9~6.6 | 2680~3290 | 9–17 | −22.8~−23.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, X.; Huang, T.; Hu, R.; Li, K. Pilot Study on Nucleation-Induced Pelleting Coagulation in Treatment of High-Algae Surface Water: Coagulant Dosage and Hydraulic Loading Optimization. Toxics 2025, 13, 418. https://doi.org/10.3390/toxics13060418
Xing X, Huang T, Hu R, Li K. Pilot Study on Nucleation-Induced Pelleting Coagulation in Treatment of High-Algae Surface Water: Coagulant Dosage and Hydraulic Loading Optimization. Toxics. 2025; 13(6):418. https://doi.org/10.3390/toxics13060418
Chicago/Turabian StyleXing, Xiangxuan, Tinglin Huang, Ruizhu Hu, and Kai Li. 2025. "Pilot Study on Nucleation-Induced Pelleting Coagulation in Treatment of High-Algae Surface Water: Coagulant Dosage and Hydraulic Loading Optimization" Toxics 13, no. 6: 418. https://doi.org/10.3390/toxics13060418
APA StyleXing, X., Huang, T., Hu, R., & Li, K. (2025). Pilot Study on Nucleation-Induced Pelleting Coagulation in Treatment of High-Algae Surface Water: Coagulant Dosage and Hydraulic Loading Optimization. Toxics, 13(6), 418. https://doi.org/10.3390/toxics13060418









