Progress of Ship Exhaust Emissions in China’s Lijiang River: Current Status and Aftertreatment Technologies
Abstract
1. Introduction
2. Lijiang River Ship Emission Status and Regulation
2.1. Lijiang River Emission Status
2.2. Lijiang River Emission Regulation
2.2.1. Promoting New Energy Ships
2.2.2. Adjusting the Ship Structure
2.2.3. Construction of Ship Exhaust Monitoring System
3. Ships’ Exhaust Emissions
3.1. Sources and Hazards of SOX
3.2. Sources and Hazards of NOX
3.3. Sources and Hazards of PM
4. Ship Flue Gas Terminal Treatment Technology
4.1. Desulfurization Technology of Exhaust Gases
4.1.1. Dry Exhaust Gas Desulfurization Technology
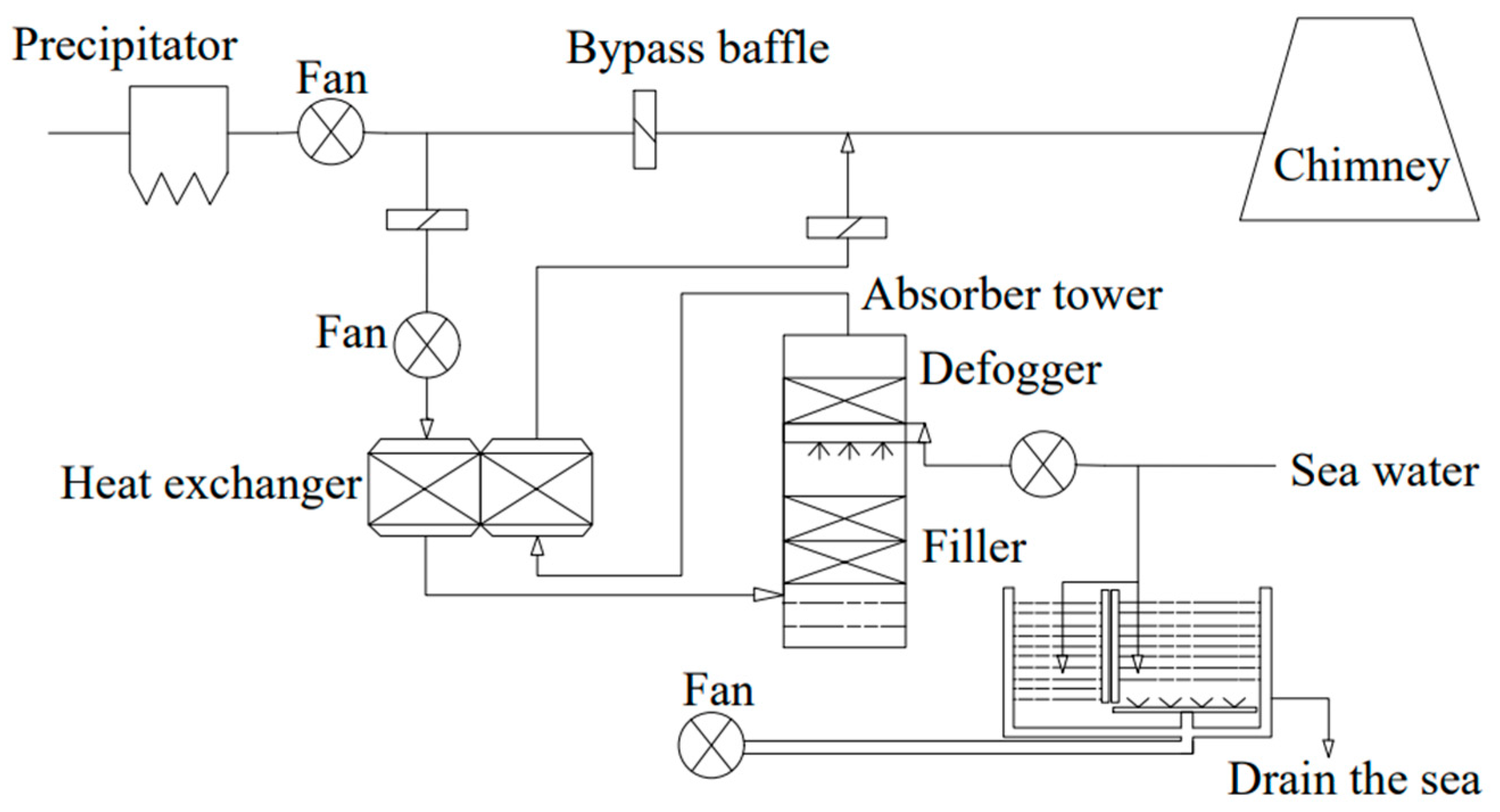
4.1.2. Wet Exhaust Gas Desulfurization Technology
- (1)
- Seawater exhaust gas desulfurization
- (2)
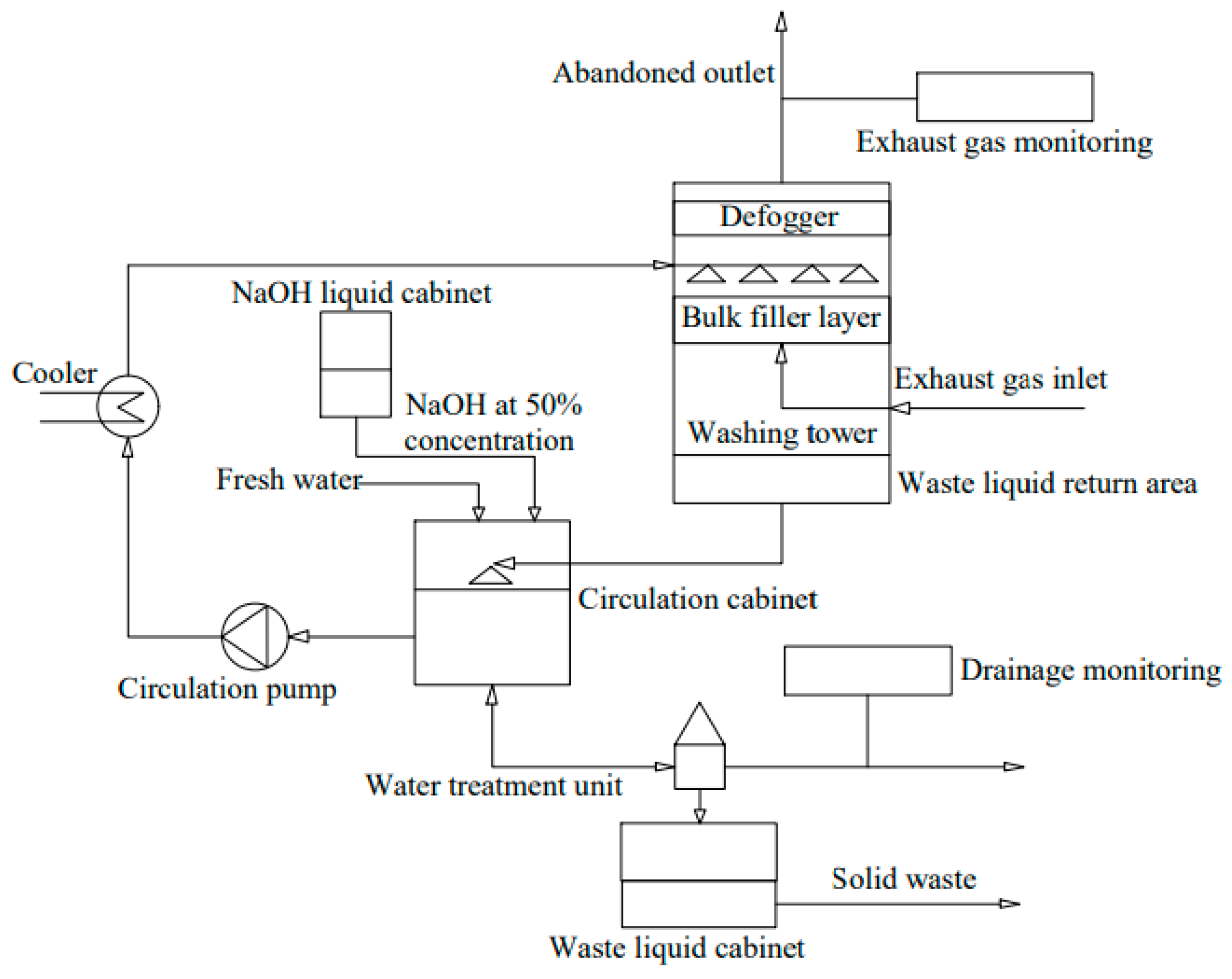
- Sodium alkali exhaust gas desulfurization
- (3)
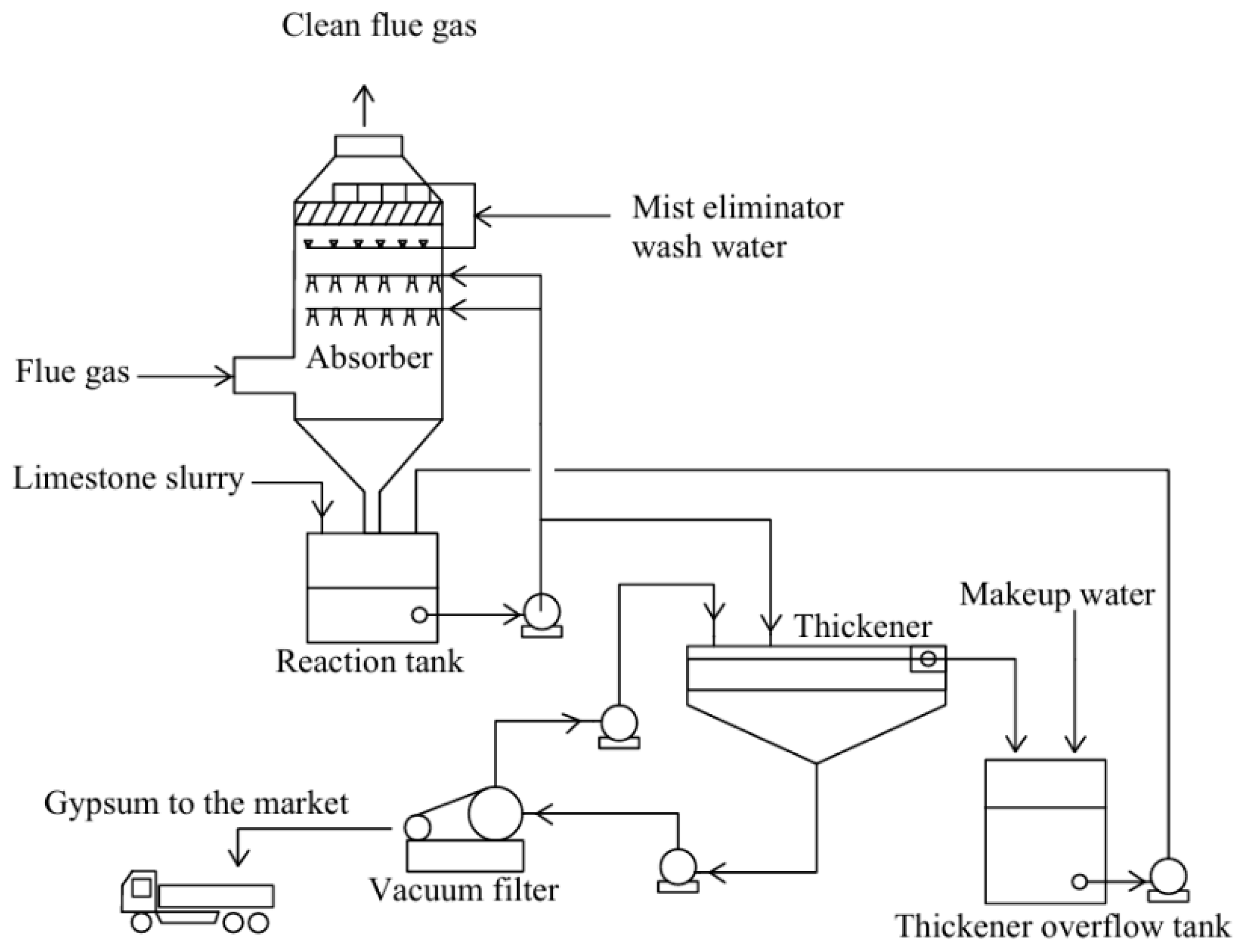
- Limestone–gypsum exhaust gas desulfurization
- (4)
- Ammonia exhaust gas desulfurization
4.2. Denitrification Technology of Exhaust Gas
4.2.1. Selective Catalytic Reduction (SCR) Exhaust Denitrification Technology
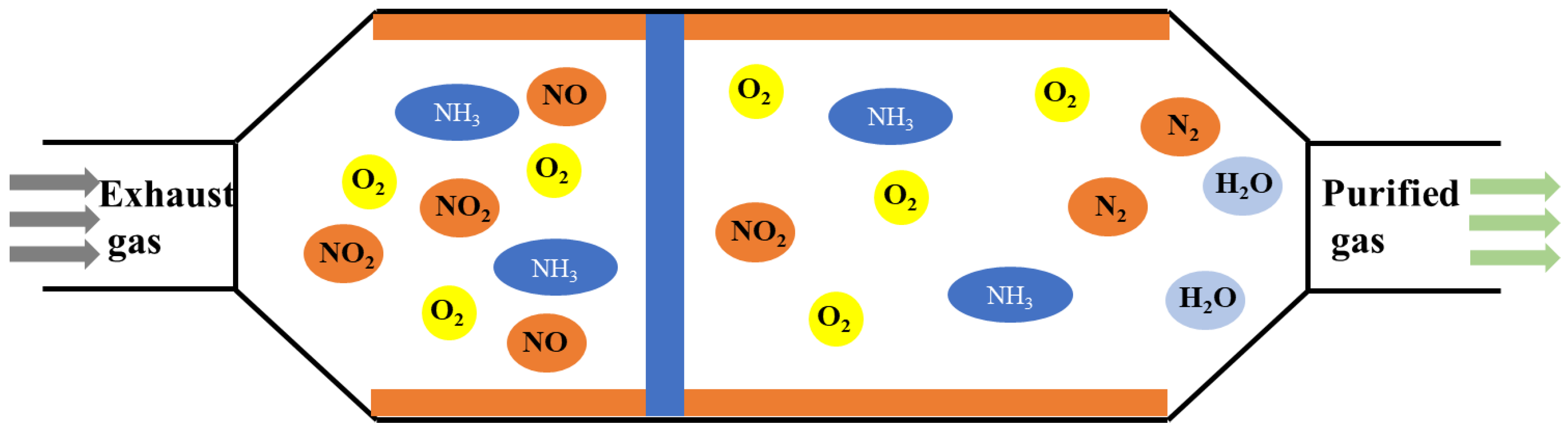
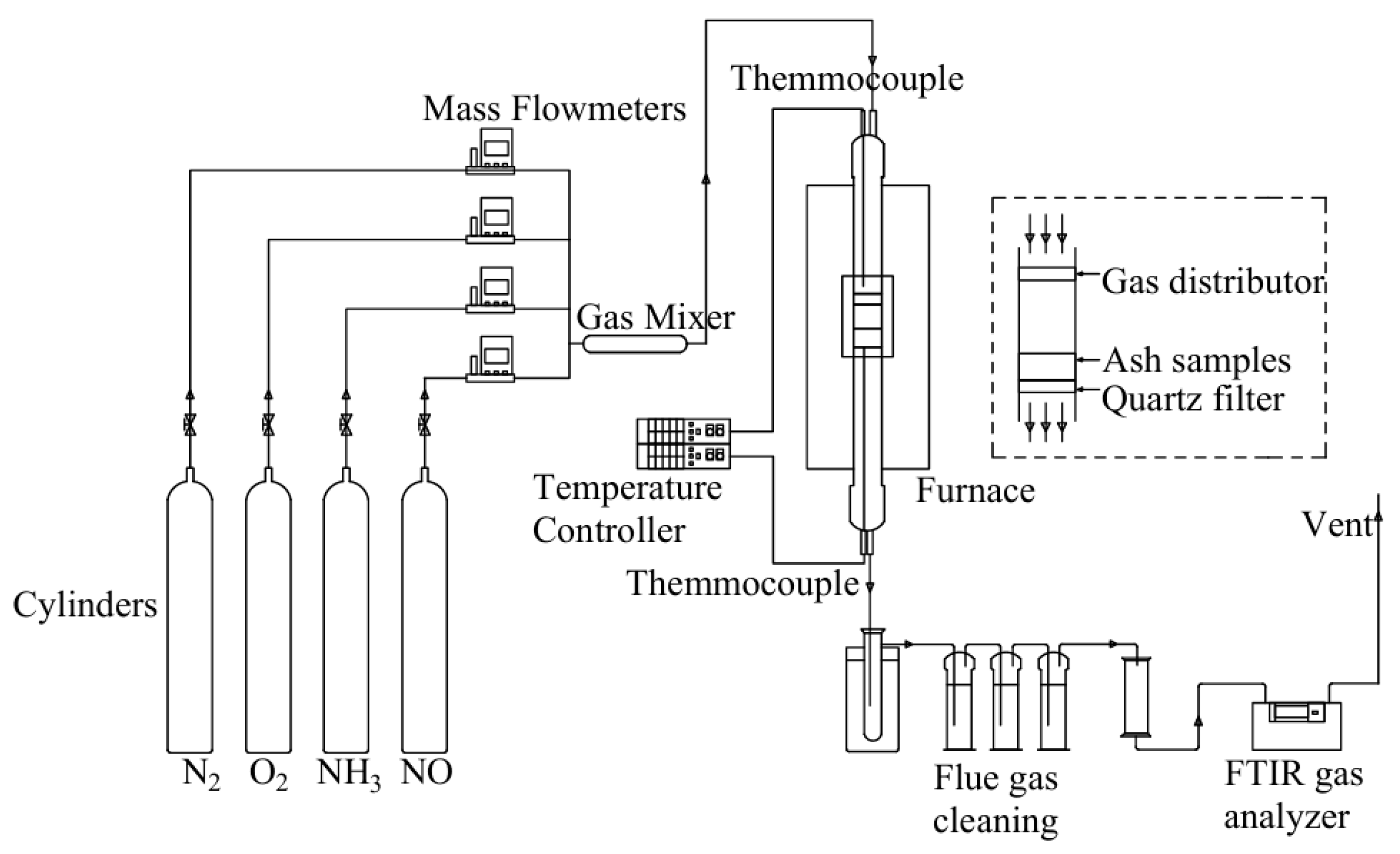
4.2.2. Selective Non-Catalytic Reduction Denitrification Technology
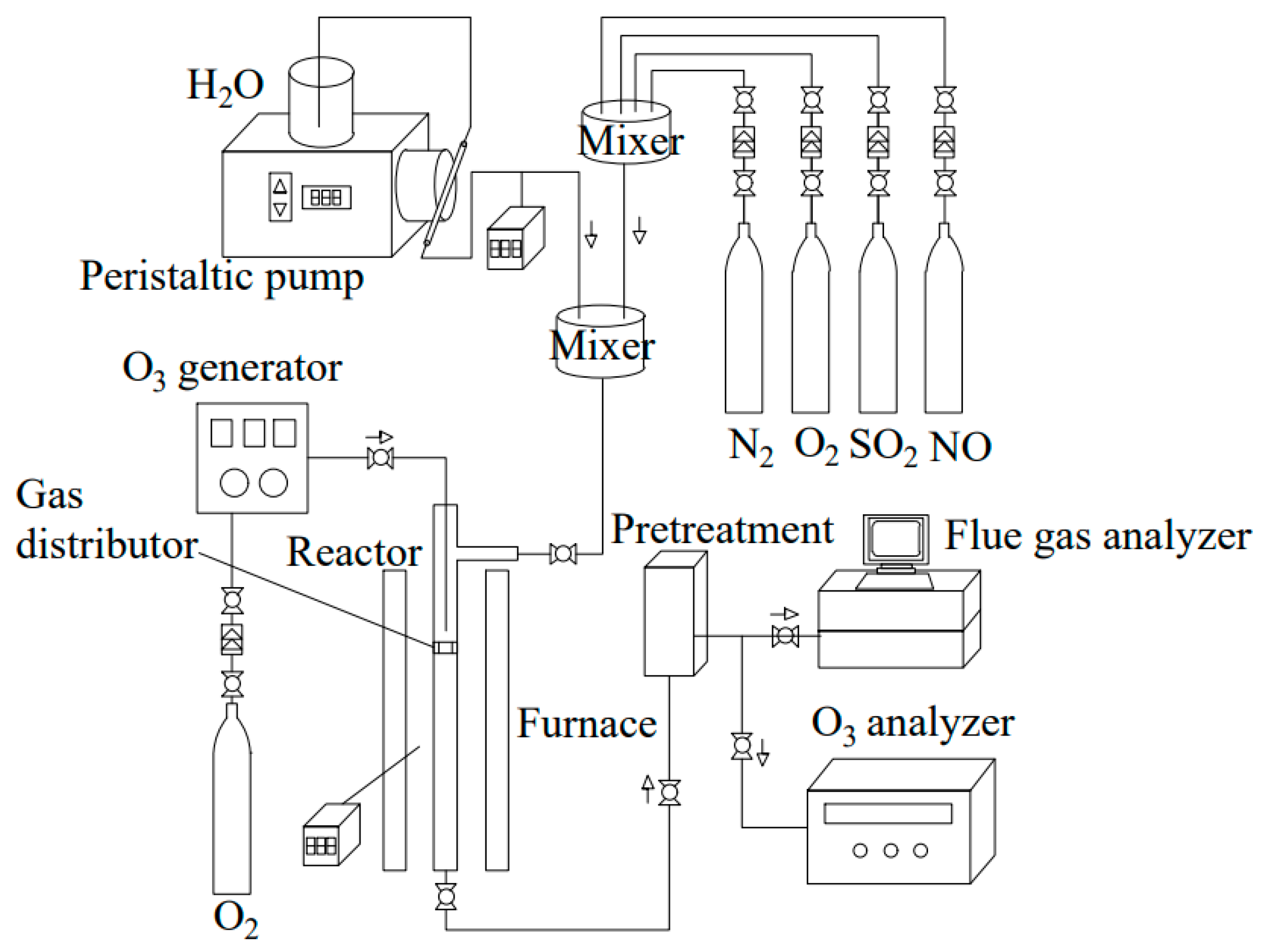
4.2.3. Ozone Oxidation Denitrification Technology
4.3. Integrated Exhaust Gas Desulfurization and Denitrification Technology
4.3.1. Electrostatic Spray Technology
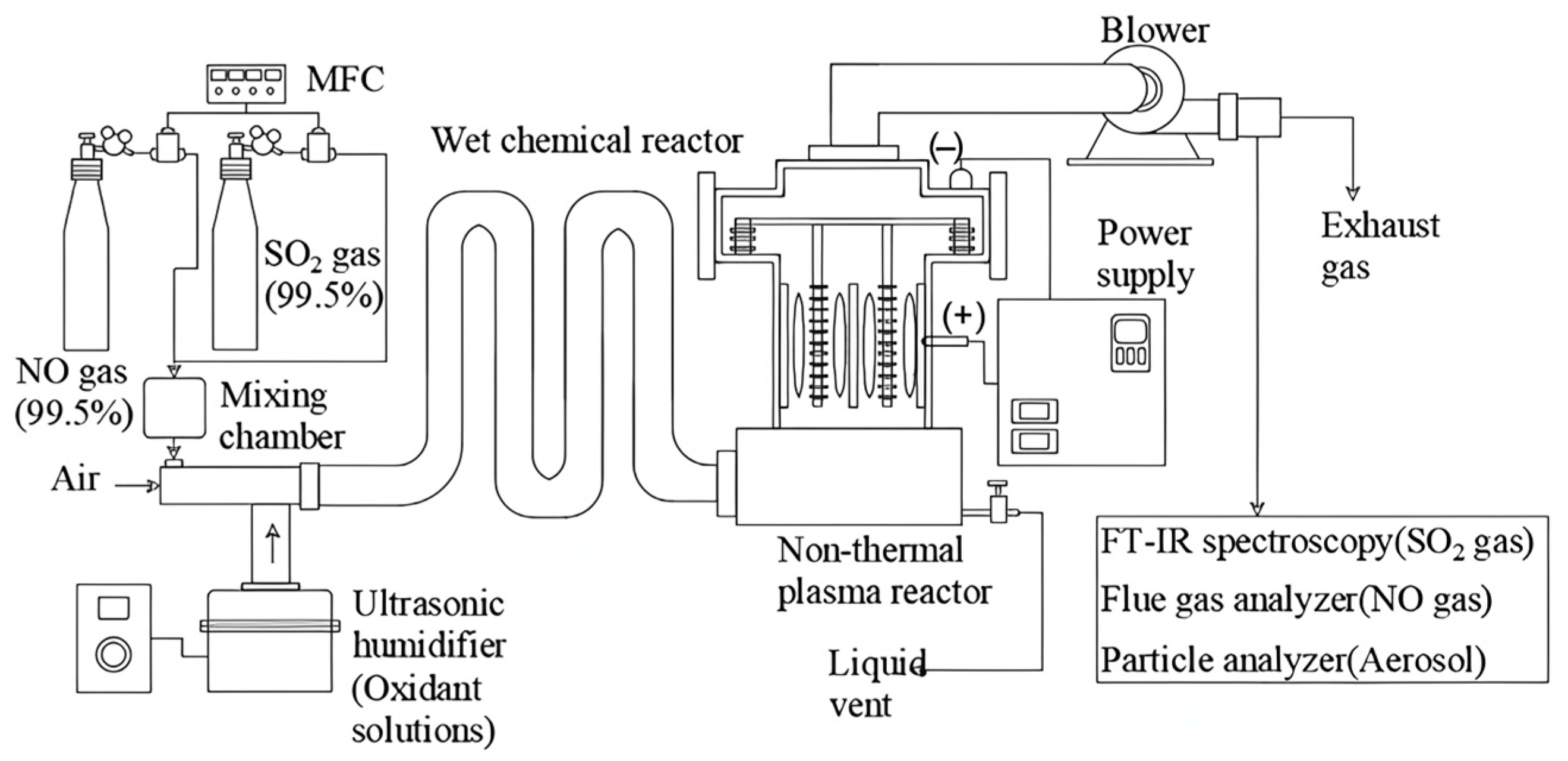
4.3.2. Plasma Oxidation Process
4.3.3. Oxidation–Reduction–Absorption Technology
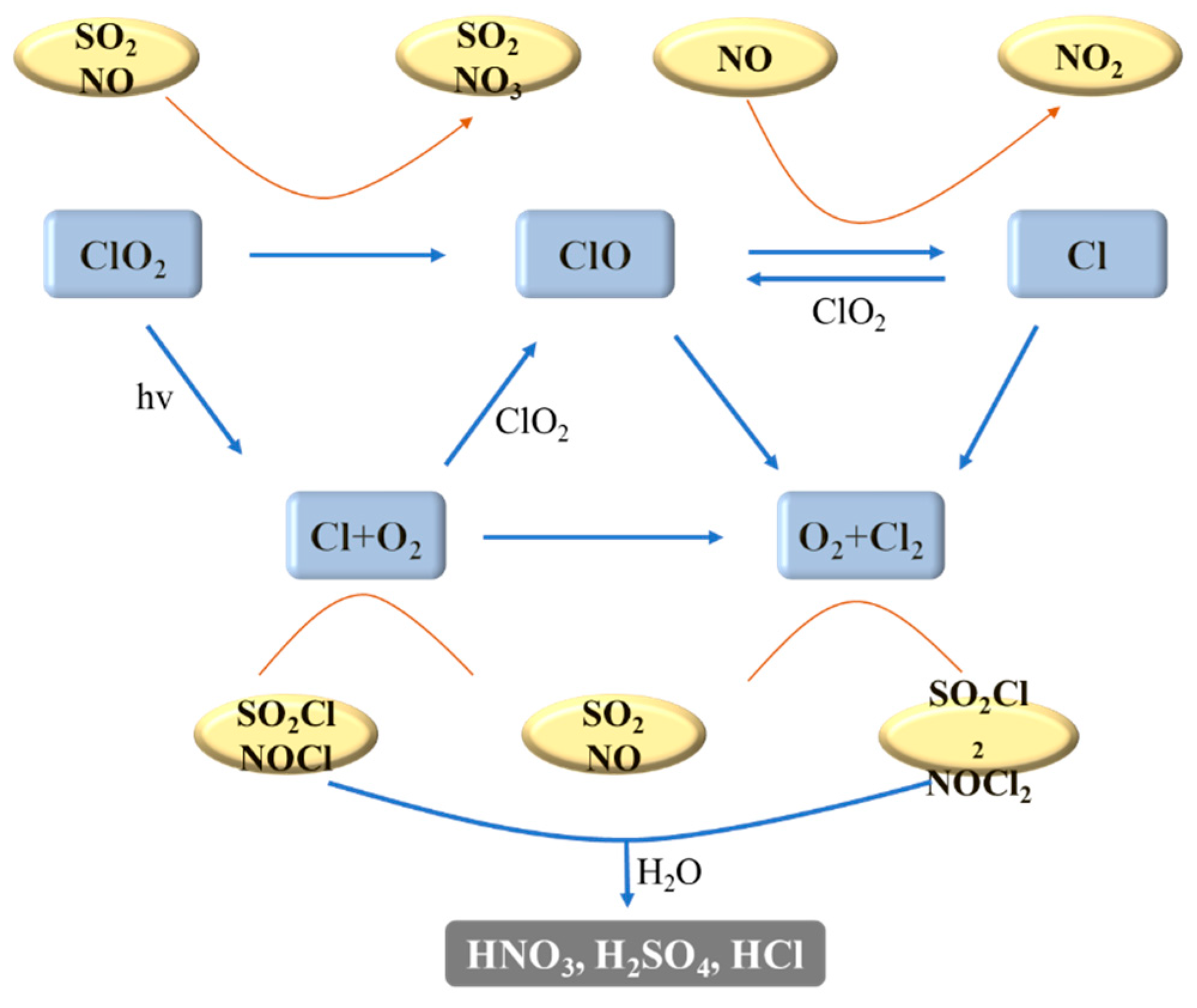
4.3.4. UV/Chlorine Advanced Oxidation Technology
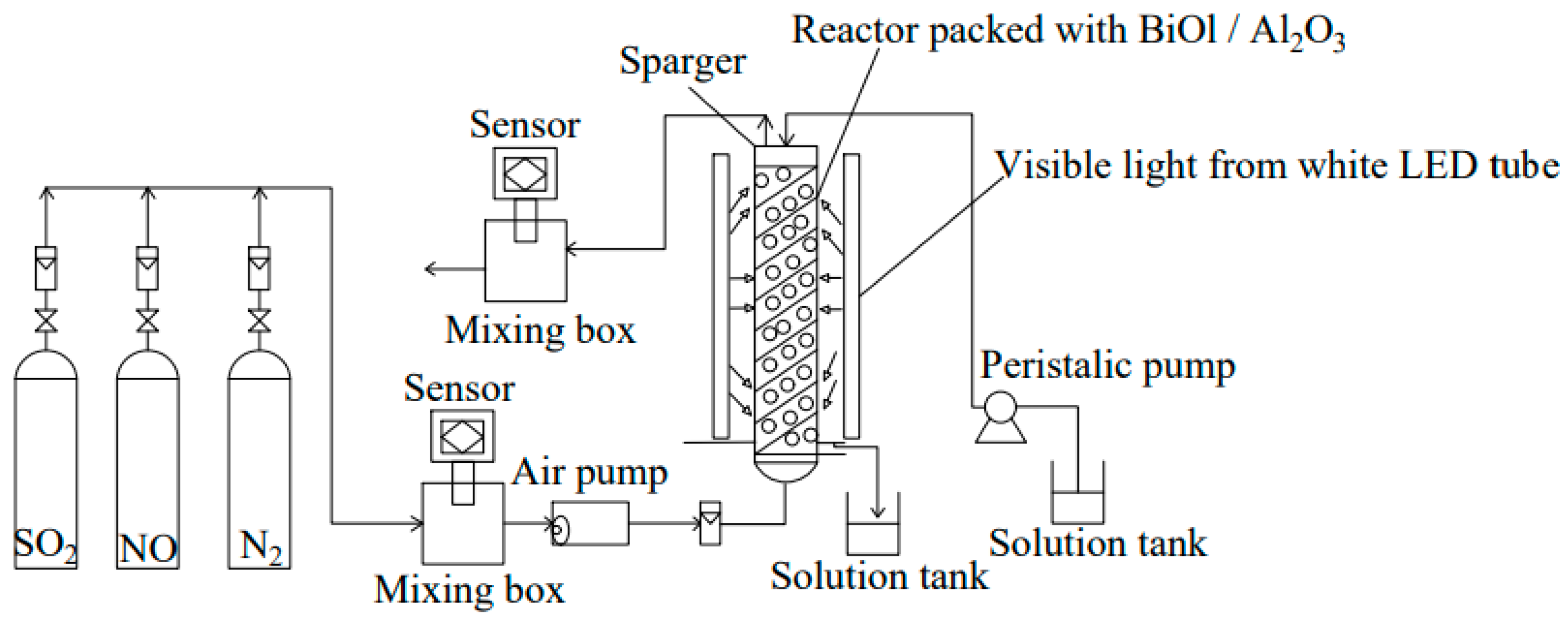
4.3.5. Photocatalysis
5. Conclusions and Recommendations
- (1)
- The Lijiang River has a rich tourist landscape endowed by nature. A large number of tourists have caused a large amount of polluted gas emissions while on the cruise on the Lijiang River. Most ships in the Lijiang River use traditional low-speed diesel engines, with a large number of old ships, and the gas treatment of ships started late, and the equipment was backward. In order to improve air quality, the Guilin Maritime Safety Administration has actively taken measures to solve the problem of ship exhaust pollution in the Lijiang River. In addition to formulating strict emission regulations, the adjustment of ship structure, the promotion of new energy and the construction of a monitoring system are all important measures;
- (2)
- SO2 and PM emissions are largely dependent on the sulfur content of the fuels used by ships. NOX is mainly related to the combustion of nitrogen in the air when the engine is operated at high temperatures. Ships have become a major pollution source in certain inland rivers where shipping lanes are densely populated and ship traffic is high. NOX, SOX, and PM emissions from ships can negatively impact the natural environment and human health along the coastline;
- (3)
- Installing an exhaust aftertreatment system can effectively reduce NOX and SOX emissions, thereby meeting the Tier III standards. The wet flue gas desulfurization system can effectively capture SO2 from flue gas, concentrating it in gypsum and solution. The wet flue gas desulfurization system is applied to large two-stroke marine diesel engines operated with high-sulfur fuel. SCR is the most mature and effective exhaust treatment method for controlling marine diesel engine NOX emission. Most of the exhaust aftertreatment techniques are mature, but they need to be used with appropriate integration and combination to achieve co-reduction in all pollutants and cost-effectiveness. Integrated desulfurization and denitrification treatment technology has its own advantages and disadvantages. The market has not yet found the most ideal solution and still needs to select the appropriate program in accordance with the specific needs of the ship exhaust treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lonati, G.; Cernuschi, S.; Sidi, S. Air quality impact assessment of at-berth ship emissions: Case study for the project of a new freight port. Sci. Total Environ. 2010, 409, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Peng, L.; Wei, J.L.; Liao, Y.Z. Ammonia coverage ratio control of a novel two-cell SCR-DeNOX system configuration based on IA-ISMC. Chem. Eng. Sci. 2024, 295, 120148–120158. [Google Scholar] [CrossRef]
- IMO. Third IMO Greenhouse Gas Study; International Maritime Organization: London, UK, 2014. [Google Scholar]
- Eyring, V.; Köhler, H.; Lauer, A.; Lemper, B. Emissions from international shipping: 2. Impact of future technologies on scenarios until 2050. J. Geophys. Res. 2005, 110, D17. [Google Scholar] [CrossRef]
- Tavella, R.A.; das Neves, D.F.; Silveira, G.d.O.; Vieira de Azevedo, G.M.G.; Brum, R.d.L.; Bonifácio, A.d.S.; Machado, R.A.; Brum, L.W.; Buffarini, R.; Adamatti, D.F.; et al. The relationship between surface meteorological variables and air pollutants in simulated temperature increase scenarios in a medium-sized industrial city. Atmosphere 2025, 16, 363. [Google Scholar] [CrossRef]
- Tavella, R.A.; Penteado, J.O.; Brum, B.D.L.; Bonifácio, A.D.S.; Martin, M.C.S.; Elizabet, S.S.; Brum, A.N.; Buffarini, R.; Filho, W.L.F.C.; Adamatti, D.F.; et al. An exploratory study on the association between air pollution and health problems (ICD-10) with an emphasis on respiratory diseases. Atmos. Pollut. Res. 2025, 16, 102377. [Google Scholar] [CrossRef]
- Tavella, R.A.; Júnior, F.M.R.D.S.; Santos, M.A.; Miraglia, S.G.E.K.; Filho, R.D.P. A review of air pollution from petroleum refining and petrochemical industrial complexes: Sources, key pollutants, health impacts, and challenges. ChemEngineering 2025, 9, 13. [Google Scholar] [CrossRef]
- Jeevahan, J.; Mageshwaran, G.; Joseph, G.B.; Raj, R.B.D.; Kannan, R.T. Various strategies for reducing NOx emissions of biodiesel fuel used in conventional diesel engines: A review. Chem. Eng. Commun. 2017, 204, 1202–1223. [Google Scholar] [CrossRef]
- Long, Y.X.; Li, G.S.; Zhang, Z.H.; Liang, J.J. Application of reformed exhaust gas recirculation on marine LNG engines for NOx emission control. Fuel 2021, 291, 120114. [Google Scholar] [CrossRef]
- Mao, X.Y.; Meng, J.J.; Wang, Q. Modeling the effects of tourism and land regulation on land-use change in tourist regions: A case study of the Lijiang River Basin in Guilin, China. Land Use Policy 2014, 41, 368–377. [Google Scholar] [CrossRef]
- Lin, C.-Y. Strategies for promoting biodiesel use in marine vessels. Mar. Policy 2013, 40, 84–90. [Google Scholar] [CrossRef]
- Deng, J.J.; Wang, X.C.; Wei, Z.L.; Wang, L.; Wang, C.Y.; Chen, Z.B. A review of NOx and SOx emission reduction technologies for marine diesel engines and the potential evaluation of liquefied natural gas fueled vessels. Sci. Total Environ. 2020, 766, 144319–144324. [Google Scholar] [CrossRef]
- Pham, H.T.; Nguyen, T.M. Solution to reduce air environmental pollution from ships. TransNav Int. J. Mar. Navig. Saf. Sea Transp. 2015, 9, 257–261. [Google Scholar]
- Nunes, R.A.O.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Sousa, S.I.V. The activity- based methodology to assess ship emissions—A review. Environ. Pollut. 2017, 231, 87–103. [Google Scholar] [CrossRef]
- Merico, E.; Donateo, A.; Gambaro, A.; Cesari, D.; Gregoris, E.; Barbaro, E.; Dinoi, A.; Giovanelli, G.; Masieri, S.; Contini, D. Influence of in-port ships emissions to gaseous atmospheric pollutants and to particulate matter of different sizes in a Mediterranean harbour in Italy. Atmos. Environ. 2016, 139, 1–10. [Google Scholar] [CrossRef]
- Geng, P.; Tan, Q.M.; Zhang, C.H.; Wei, L.J.; He, X.Z.; Cao, E.M.; Jiang, K. Experimental investigation on NOx and greenhouse gas emissions from a marine auxiliary diesel engine using ultralow sulfur light fuel. Sci. Total Environ. 2016, 572, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, Y.; Zhou, H.Y. Li River Tourism Receives 1.97 Million Tourists in Summer. Guilin Daily, 1 September 2024. [Google Scholar]
- Li, C.L. Considerring of Water Traffie Safety Managementin Li River Travel Sail District; Dalian Maritime University: Dalian, China, 2012. [Google Scholar]
- China New Network. It Is Recommended to Speed Up the Upgrading of the New Energy Tourism Raft Industry. Available online: https://baijiahao.baidu.com/s?id=1792954343862247546&wfr=spider&for=pc (accessed on 1 May 2025).
- Tang, J. Thoughts on ship pollution prevention in Lijiang River Scenic Area in Guilin. Marit. Saf. 2022, 3, 33–36. [Google Scholar]
- DieselNet. IMO Marine Engine Regulations. Available online: https://www.dieselnet.com/standards/inter/imo.php#other (accessed on 17 May 2019).
- Ministry of Transport. Announcement of the Ministry of Transport on Strengthening the Administration of Domestic Waterway Transportation for Imported Ships from Outside China and China-registered International Sailing Ships. Available online: https://www.gov.cn/xinwen/2018-07/04/content_5303394.htm (accessed on 3 May 2025).
- DieselNet. China: Marine Engines. Available online: https://www.dieselnet.com/standards/cn/marine.php (accessed on 1 August 2019).
- Ni, P.Y.; Wang, X.L.; Li, H. A review on regulations, current status, effects and reduction strategies of emissions for marine diesel engines. Fuel 2020, 279, 118477. [Google Scholar] [CrossRef]
- Li, S.J. New Cruise Ship, New Bamboo Raft, New Power, Lijiang River Tour Stepping Into a New Era of Low-Carbon Travel. Guilin Daily, 25 April 2023. [Google Scholar]
- CCTV.com. Lijiang River Launches Pure Electric Power Sightseeing Raft in Guilin. Available online: https://news.cctv.com/2023/04/20/ARTIAvZFPywRADhXTrpYoHsg230420.shtml (accessed on 1 May 2025).
- Guangxi News Network. Guilin Power Supply Bureau Builds a Demonstration Model of Power Supply Service in World-Class Tourist Cities. Available online: https://www.gxnews.com.cn/staticpages/20220425/newgx6266674a-20727577.shtml (accessed on 1 May 2025).
- Mi, J.G.; Huang, Y.T. Keep in mind that Guilin Maritime Bureau should be the “Erlang God” to protect the Lijiang River. China Marit. Aff. 2022, 75–76. [Google Scholar] [CrossRef]
- GB2552-2018; Discharge Standard for Water Pollutants from Ships. China Environmental Science Press: Beijing, China, 2018.
- Guilin Government Network. Notice of Guilin Maritime Safety Administration on Printing and Distributing the Three-year Action Implementation Plan for Pollution Prevention of Ships in Lijiang River. Available online: https://www.guilin.gov.cn/zfxxgk/fdzdgknr/jcxxgk/bmwj/glhsj/202110/t20211014_2141776.shtml (accessed on 3 May 2025).
- Qi, Z.Y.; Peng, S.T.; Hu, J.B.; Deng, M.T.; Zhao, H.X.; Zhu, G.X.; Yu, X.; Su, N. Surveillance practice and automatic data algorithm of sniffing telemetry for SO2 emissions from ship exhaust in Tianjin Port. J. Clean. Prod. 2023, 409, 137225. [Google Scholar] [CrossRef]
- He, L.; Wang, J.J.; Liu, Y.M.; Zhang, Y.; He, C.; Yu, Q.; Ma, W.C. Selection of onshore sites based on monitoring possibility evaluation of exhausts from individual ships for Yantian Port, China. Atmos. Environ. 2021, 247, 118187. [Google Scholar] [CrossRef]
- Peng, X.; Li, T.W.; Wu, L.C.; Huang, L.; Wen, Y.Q.; Zhou, C.H.; Zhang, F.; Han, T.X.; Li, J. Optimal site selection for the remote-monitoring sulfur content of ship fuels in ports. Ocean Coast. Manag. 2022, 225, 106211. [Google Scholar] [CrossRef]
- Paik, B.-G.; Cho, S.-R.; Park, B.-J.; Lee, D.; Bae, B.-D. Development of real-time monitoring system using wired and wireless networks in a full-scale ship. Int. J. Nav. Archit. Ocean Eng. 2010, 2, 132–138. [Google Scholar]
- Sina Finance. Guilin Maritime Safety Administration Introduced UAV Telemetry Equipment for the First Time to Monitor the Tail Gas of Lijiang River Ships. Available online: https://finance.sina.com.cn/jjxw/2021-12-10/doc-ikyamrmy8022940.shtml (accessed on 2 May 2025).
- Zhao, J.; Wei, Q.; Wang, S.; Ren, X.L. Progress of ship exhaust gas control technology. Sci. Total Environ. 2021, 799, 149437–149461. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Wang, X.; Yin, C.Q.; Xu, W.W.; Shi, W.; Qian, G.R.; Xun, Z.M. Inland Vessels Emission Inventory and the emission characteristics of the Beijing-Hangzhou Grand Canal in Jiangsu province. Process Saf. Environ. Prot. 2018, 113, 498–506. [Google Scholar] [CrossRef]
- Russo, M.A.; Leitão, J.; Gama, C.; Ferreira, J.; Monteiro, A. Shipping emissions over Europe: A state-of-the-art and comparative analysis. Atmos. Environ. 2018, 177, 187–194. [Google Scholar] [CrossRef]
- Han, Z.; Zou, T.; Wang, J.; Dong, J.M.; Deng, Y.B.; Pan, X.X. A novel method for simultaneous removal of NO and SO2 from marine exhaust gas via in-site combination of ozone oxidation and wet scrubbing absorption. J. Mar. Sci. Eng. 2020, 8, 943. [Google Scholar] [CrossRef]
- Lu, Q.; Zheng, J.; Ye, S.; Shen, X.Q.; Yuan, Z.B.; Yin, S.S. Emission trends and source characteristics of SO2, NOx, PM10 and VOCs in the Pearl River Delta region from 2000 to 2009. Atmos. Environ. 2013, 76, 11–20. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, Y.; Ma, W.; Ma, H.X.; Feng, J.L.; Yu, Q.; Yang, X.; Simon, K.W.N.; Fu, Q.Y.; Chen, L.M. Spatial and seasonal dynamics of ship emissions over the Yangtze River Delta and east China sea and their potential environmental influence. Environ. Sci. Technol. 2016, 50, 1322–1329. [Google Scholar] [CrossRef]
- Song, Y.N. Research of Emission Inventory and Emission Character of Inland and Offshore Ships; Beijing Institute of Technology: Beijing, China, 2015. [Google Scholar]
- Gan, L.X.; Che, W.Y.; Zhou, M.G.; Zhou, C.H.; Zheng, Y.Z.; Zhang, L.; Rangel-Buitrago, N.; Song, L. Ship exhaust emission estimation and analysis using automatic identification system data: The west area of Shenzhen port, China, as a case study. Ocean Coast. Manag. 2022, 226, 106245. [Google Scholar] [CrossRef]
- Shu, Y.Q.; Hu, A.Y.; Zheng, Y.Z.; Gan, L.X.; Xiao, G.N.; Zhou, C.H.; Song, L. Evaluation of ship emission intensity and the inaccuracy of exhaust emission estimation model. Ocean. Eng. 2023, 287, 115723. [Google Scholar] [CrossRef]
- Ke, S.Z.; Zhou, Z.J. On the Existing Conditions and Problems with the Prevention and Control of Air Pollution from Ships in China; China Maritime Safety: Beijing, China, 2019; pp. 18–21.
- Fu, Q.Y.; Shen, Y.; Zhang, J. On the ship pollutant emission inventory in Shanghai port. J. Saf. Environ. 2012, 12, 57–64. [Google Scholar]
- Schroeder, C.; Schroeder, W.; Yang, S.; Shi, H.T.; Nystrom, A.; Subramanian, S.; Li, S.J.; Gundersen, M.A.; Cronin, S.B. Plasma-enhanced SO2 remediation in a humidified gas matrix: A potential strategy for the continued burning of high sulfur bunker fuel. Fuel 2020, 274, 117810–117813. [Google Scholar] [CrossRef]
- Wang, X.N.; Shen, Y.; Lin, Y.F.; Pan, J.; Zhang, Y.; Louie, P.K.K.; Li, M.; Fu, Q.Y. Atmospheric pollution from ships and its impact on local air quality at a port site in Shanghai. Atmos. Chem. Phys. 2019, 19, 6315–6330. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Ma, Q.X.; Zhang, P.; Chen, T.Z.; Wang, Y.H.; Chu, B.W.; Li, H.; Li, J.K.; Chen, C.C.; Zhao, J.C.; et al. A light -driven acidic positive feedback mechanism of sulfate formation. Atmos. Environ. 2024, 331, 120606–120613. [Google Scholar] [CrossRef]
- Brynolf, S.; Magnusson, M.; Fridell, E.; Andersson, K. Compliance possibilities for the future ECA regulations through the use of abatement technologies or change of fuels. Transp. Res. Part D Transp. Environ. 2014, 28, 6–18. [Google Scholar] [CrossRef]
- Chen, P. Effect of wet scrubbing oxidation denitration technology combined with ultraviolet online irradiation on the efficiency of desulfurization and denitrification of ship exhaust gas. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 012101–012110. [Google Scholar] [CrossRef]
- Chen, J.; Fu, X.; Wang, X.F.; Dong, S.W.; Chen, T.S.; Xue, L.K.; Zhou, Y.; Sheng, L.F.; Wang, W.X. Unveiling the overlooked direct emissions of particulate organic nitrates from ship. Environ. Int. 2024, 185, 108487–108493. [Google Scholar] [CrossRef] [PubMed]
- Saxe, H.; Larsen, T. Air pollution from ships in three danish ports. Atmos. Environ. 2004, 38, 4057–4067. [Google Scholar] [CrossRef]
- Liu, M.W.; Zhang, H.; Li, J.; Qi, X.C.; Wang, Y.F.; Pang, J.D. Recent progress and future prospects of metal-organic frameworks for adsorption, separation and catalytic removal of NOx and N2O. ChemCatChem 2024, 6, 922–934. [Google Scholar] [CrossRef]
- Chin, T.; Tam, I.C.K.; Yin, C.-Y. Comparison of various chemical compounds for the removal of SO2 and NOx with wet scrubbing for marine diesel engines. Environ. Sci. Pollut. Res. 2022, 29, 8873–8891. [Google Scholar] [CrossRef]
- Lee, Y.W.; Sung, J.H.; Han, B.W.; Kim, Y.J.; Kim, H.J. Minimizing the consumption of reducing agents for NOx removal in a wet scrubber without H2S formation. Sep. Purif. Technol. 2022, 282, 120101–120112. [Google Scholar] [CrossRef]
- Zetterdahl, M.; Moldanová, J.; Pei, X.Y.; Pathak, R.K.; Demirdjian, B. Impact of the 0.1% fuel sulfur content limit in SECA on particle and gaseous emissions from marine vessels. Atmos. Environ. 2016, 145, 338–345. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Wan, Q.Q.; Hou, Q.C.; Feng, Y.M.; Yu, J.; Shi, J.; Xia, C. Analysis of diffusion characteristics and influencing factors of particulate matter in ship exhaust plume in arctic environment based on CFD. Atmosphere 2024, 15, 580. [Google Scholar] [CrossRef]
- Zhang, Y.; Eastham, S.D.; Lau, A.K.; Fung, J.C.H.; Selin, N.E. Global air quality and health impacts of domestic and international shipping. Environ. Res. Lett. 2021, 16, 84055–84069. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.; Koponen, P.; Roslund, P.; Laurikko, J.; Nylund, N.-O.N.-O.; Karjalainen, P.; Rönkkö, T.; Timonen, H. Comprehensive emission characterisation of exhaust from alternative fueled cars. Atmos. Environ. 2020, 236, 117643–117644. [Google Scholar] [CrossRef]
- Corbett, J.; Winebrake, E.; Green, P.; Kasibhatla, P.; Eyring, V.; Lauer, A. Mortality from ship emissions: A global assessment. Environ. Sci. Technol. 2007, 41, 8512–8518. [Google Scholar] [CrossRef]
- Mao, X. Present situation and trends of desulfurization and denitrification of flue gas. Shandong Ind. Technol. 2015, 19, 3–6. [Google Scholar]
- Liu, X.W. Progress of desulfurization and denitration technology of flue gas in China. IOP Conf. Ser. Earth Environ. Sci. 2019, 242, 42010–42017. [Google Scholar]
- Zhao, X.; Gao, J.; Wu, S.H.; Qin, Y.K. Development of dry and semi-dry (CalciumBase) flue gas desulfurization technology. Chem. Eng. 2003, 31, 64–67. [Google Scholar]
- Osaka, Y.G.; Kito, T.; Kobayashi, N.; Kurahara, S.; Huang, H.Y.; Yuan, H.R.; He, Z.H. Removal of sulfur dioxide from diesel exhaust gases by using dry desulfurization MnO2 filter. Sep. Purif. Technol. 2015, 150, 80–85. [Google Scholar] [CrossRef]
- Du, C.C.; Yi, H.H.; Tang, X.L.; Zhao, S.Z.; Gao, F.Y.; Yu, Q.J.; Yang, Z.Y.; Yang, K.; Xie, X.Z.; Ma, Y.Q. Desulfurization and denitrification experiments in SDA system: A new high efficient semi-dry process by NaClO2. Separation and Purification Technology 2020, 230, 115873–115881. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Zhang, M.C.; Wang, D.F.; Wang, L. Study on a novel semidry flue gas desulfurization with multi fluid alkaline spray generator. Ind. Eng. Chem. Res. 2005, 44, 8830–8836. [Google Scholar] [CrossRef]
- Wang, W.Z. Study on Gas-Liquid Mass Transfer and Reaction Characteristic of Absorption Process in Sodium Alkali FGD; Tianjin University: Tianjin, China, 2007. [Google Scholar]
- Song, H.; Li, X.F.; Jin, H.; Liang, X.Q.; Kang, R.L. Research on the technology of flue gas desulfurization by spray dryer absorber (SDA). China Environ. Prot. Ind. 2021, 4, 50–53. [Google Scholar]
- Peng, G.Y. Discussion on energy saving technology and methods of surrounding structure wall of high-rise building. Shanxi Archit. 2018, 44, 195–197. [Google Scholar]
- Xiang, Y.; Lv, W.H.; Yang, G.H.; Lv, G.; Gou, Y.B. Design and analysis of marine exhaust gas treatment system based on wet desulfurization process. Sichuan Environ. 2020, 39, 97–103. [Google Scholar]
- Zhao, Y.; Ma, S.; Wang, X.; Zhang, Q. Experimental and mechanism studies on seawater flue gas desulfurization. J. Environ. Sci. 2003, 15, 123–128. [Google Scholar]
- Shi, N.; Zhang, X.; Lei, L. Sulfite oxidation in seawater flue gas desulfurization by a pulsed corona discharge process. Sep. Purif. Technol. 2009, 70, 212–218. [Google Scholar] [CrossRef]
- Zhao, J.X.; Yang, Y.; Liu, H.R.; Xu, S.C.; Wei, Q.F.; Ren, X.L. The application of two-phase composite absorbent systems consisting of BAD and seawater resources in the wet treatment of ship exhaust gas. iScience 2023, 26, 106472–106490. [Google Scholar] [CrossRef]
- Bian, J.J.; Zhang, S.; Zhang, J.W.; Min, X.; Li, C.H. Supported manganese dioxide catalyst for seawater flue gas desulfurization application. Chem. Eng. J. 2012, 189–190, 57–61. [Google Scholar] [CrossRef]
- Flagiello, D.; Natale, F.D.; Erto, A.; Lancia, A. Wet oxidation scrubbing (WOS) for flue-gas desulphurization using sodium chlorite seawater solutions. Fuel 2020, 277, 118055. [Google Scholar] [CrossRef]
- Jiang, Q.; He, Y.M.; Wu, Y.L.; Jin, Y.J.; Dian, B.; Li, T.G.; Guo, J.F.; Jiang, M. Research progress of seawater flue gas desulfurization technology. J. Salt Sci. Chem. Ind. 2022, 51, 9–12. [Google Scholar]
- Qi, C.B. Comparison between magnesium oxide and limestone-gypsum wet flue gas desulfurization technology. Silicon Val. 2010, 9, 056–057. [Google Scholar]
- Luo, Z.; Peng, J.J.; Wang, D.H.; Yang, J. Recovery of phosphate from piggery biogas slurry by ultrasonication, aeration and addition of MgO desulfurization waste residue. J. Clean. Prod. 2019, 211, 865–873. [Google Scholar] [CrossRef]
- Tian, H.J.; Wan, D.H.; Che, Y.X.; Chang, J.; Zhao, J.; Hu, X.D.; Guo, Q.J.; Wang, Z.W.; Wang, L.Y. Simultaneous magnesia regeneration and sulfur dioxide generation in magnesium-based flue gas desulfurization process. J. Clean. Prod. 2020, 284, 124720–124734. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.T.; Osaka, Y.; He, Z.H.; Deng, L.S.; Huang, H.Y. Preparation and desulfurization performance of various MnOx materials for ship exhaust emissions control. Sep. Purif. Technol. 2020, 253, 117182–117189. [Google Scholar] [CrossRef]
- Fang, D.A.; Zhang, X.F.; Dong, M.G.; Xue, X.X. A novel method to remove chromium, vanadium and ammonium from vanadium industrial wastewater using a byproduct of magnesium based wet flue gas desulfurization. J. Hazard. Mater. 2017, 336, 8–20. [Google Scholar] [CrossRef]
- Cho, K.J.; Keener, T.C.; Khang, S.-J. A study on the conversion of trona to sodium bicarbonate. Powder Technol. 2008, 184, 58–63. [Google Scholar] [CrossRef]
- Ning, H.Y.; Tang, R.J.; Li, C.M.; Gu, X.L.; Gong, Z.J.; Zhu, C.Q.; Li, J.L.; Wang, K.J.; Yu, J. Recent advances in process and materials for dry desulfurization of industrial flue gas: An overview. Sep. Purif. Technol. 2025, 353, 128425–128443. [Google Scholar] [CrossRef]
- Shu, S.; Huang, Y.; Zou, L.; Zhang, X.; Li, J. Mechanism of synergistic removal of NO and SO2 by sodium bicarbonate. RSC Adv. 2023, 13, 32589–32595. [Google Scholar] [CrossRef]
- Kong, C.J. Research on the Characteristics of Alkaline Combined Desulfurization and Decarbonization for Ocean-Going Ships; Chongqing Jiao Tong University: Chongqing, China, 2024. [Google Scholar]
- Liang, E.S. The role of sodium-alkali waste gas washing and desulfurization technology in the exhaust gas treatment of marine diesel engines. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 032036–032044. [Google Scholar] [CrossRef]
- Kumar, L.; Dhakad, V.K.; Jana, S.K. Recycling of marble waste for desulfurization of flue gas accompanied by synthesis of gypsum and PoP. Chem. Eng. Res. Des. 2022, 184, 577–591. [Google Scholar] [CrossRef]
- Pan, D.P.; Zhang, D.P.; Zhang, W.D. Investigation on removal characteristics of SO3 acid mist during limestone-gypsum desulfurization Process. Energy Fuels 2018, 32, 12949–12954. [Google Scholar] [CrossRef]
- Yang, D. Experimental Study on Absorber Resistance Characteristic of WFGD. China Environ. Prot. Ind. 2018, 31–36. [Google Scholar]
- Ye, C.W.; Lv, L.; Liu, B.S. Experimental study on the simultaneous desulfurization and denitrification by using urea treatment. J. Wuhan Univ. Technol. (Transp. Sci. Eng.) 2018, 42, 478–482. [Google Scholar]
- Zhang, H.; Zhang, T. Measures and applications on cost decreasing and benefit increasing of ammonia desulfurization process. Yunnan Metall. 2023, 52, 156–160. [Google Scholar]
- Kim, D.; Lee, C. SCR performance evaluations in relation to experimental parameters in a marine generator engine. J. Mar. Sci. Eng. 2019, 7, 67. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Liu, Y.; Dou, Z.; Zhang, T. Summary of research progress on industrial flue gas desulfurization technology. Sep. Purif. Technol. 2022, 281, 119849–119867. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.M.; Meng, B.B.; Zhang, H.; Zhang, B.; Zou, L.Y. Improvement and application of ammonia flue-gas desulfurization evaporating crystallization system. Yunnan Metall. 2024, 53, 155–160. [Google Scholar]
- Register, L. Understanding Exhaust Gas Treatment Systems–Guidance for Ship Owners and Operators; Lloyd’s Register: London, UK, 2012. [Google Scholar]
- Lin, R.; Peng, J.J.; He, T.Z.; Wang, S.X.; Tong, Y.Z.; Cheng, Y.; Dai, L.B. State-of-the-art and development of marine SCR technology. Ship Boat 2023, 34, 69–80. [Google Scholar]
- Chiker, F.; Nogier, J.P.; Bonardet, J.L. Sub-monolayer V2O5-anatase TiO2 and Eurocat catalysts: IR, Raman and XPS characterisation of VOx dispersion. Catal. Today 2003, 78, 139–147. [Google Scholar] [CrossRef]
- Mladenović, M.; Paprika, M.; Marinković, A. Denitrification techniques for biomass combustion. Renew. Sustain. Energy Rev. 2018, 82, 3350–3364. [Google Scholar] [CrossRef]
- Lehtoranta, K.; Vesala, H.; Koponen, P.; Korhonen, S. Selective Catalytic Reduction Operation with Heavy Fuel Oil: nOx, NH3, and Particle Emissions. Environ. Sci. Technol. 2015, 49, 4735–4741. [Google Scholar] [CrossRef]
- Magnusson, M.; Fridell, E.; Ingelsten, H.H. The influence of sulfur dioxide and water on the performance of a marine SCR catalyst. Appl. Catal. B Environ. Energy 2012, 111–112, 20–26. [Google Scholar] [CrossRef]
- Zheng, G.Y.; Kotrba, A.; Golin, M.; Gardner, T.; Wang, A. Overview of large diesel engine aftertreatment system development. SAE Tech. Paper. 2012, 8, 1960–1972. [Google Scholar]
- Zhang, Y.Q.; Ding, L.X.; Li, B.Y. Analysis and application of SCR technology for exhaust gas aftertreatment of marine diesel engines. Nav. Archit. Ocean. Eng. 2015, 31, 36–40. [Google Scholar]
- Winnes, H.; Fridell, E. Emissions of NOX and particles from maneuvering ships. Transp. Res. Part D Transp. Environ. 2010, 15, 204–211. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhao, Z.H.; Tan, D.L.; Zhang, B.; Yin, Z.B.; Cui, S.W.; Li, J.M. Multi-objective optimization of chemical reaction characteristics of selective catalytic reduction in denitrification of diesel engine using ELM-MOPSO methodology. Energy 2024, 311, 133386. [Google Scholar] [CrossRef]
- Nuszkowski, J.; Clark, N.N.; Spencer, T.K.; Carder, D.K.; Gautam, M.; Balon, T.H.; Moynihan, P.J. Atmospheric emissions from a passenger ferry with selective catalytic reduction. J. Air Waste Manag. Assoc. 2009, 59, 18–30. [Google Scholar] [CrossRef][Green Version]
- Normann, F.; Andersson, K.; Leckner, B.O.; Johnsson, F. Emission control of nitrogen oxides in the oxy-fuel process. Prog. Energy Combust. Science. 2009, 35, 385–397. [Google Scholar] [CrossRef]
- Caneghem, J.V.; Greef, J.D.; Block, C.; Vandecasteele, C. NOx reduction in waste incinerators by selective catalytic reduction (SCR) instead of selective non catalytic reduction (SNCR) compared from a life cycle perspective: A case study. J. Clean. Prod. 2016, 112, 4452–4460. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Air Pollution Control Technology Fact Sheet. 2003. Available online: https://www3.epa.gov/ttncatc1/dir1/fsncr.pdf (accessed on 1 May 2025).
- Onda, K.; Kasuga, Y.; Kato, K.; Fujiwara, M.; Tanimoto, M. Electric discharge removal of SO2 and NOX from combustion flue gas by pulsed corona discharge. Energy Convers. Manag. 1997, 38, 1377–1387. [Google Scholar] [CrossRef]
- Obradović, B.M.; Sretenović, G.B.; Kuraica, M.M. A dual-use of DBD plasma for simultaneous NOX and SO2 removal from coal-combustion flue gas. J. Hazard. Mater. 2010, 185, 1280–1286. [Google Scholar] [CrossRef]
- Yoon, H.J.; Park, H.W.; Park, D.W. Simultaneous oxidation and absorption of NOX and SO2 in an integrated O3 oxidation/wet atomizing system. Energy Fuels 2016, 30, 3289–3297. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Zhu, Y.; Wen, Z.; Liu, J.; Cen, K. Simultaneous removal of NOX, SO2 and Hg in nitrogen flow in a narrow reactor by ozone injection: Experimental results. Fuel Process. Technol. 2007, 88, 817–823. [Google Scholar] [CrossRef]
- Wen, Z.; Wang, Z.; Xu, J.; Zhou, J.; Cen, K. Quantum chemistry study on the mechanism of the reaction between ozone and 2,3,7,8-TCDD. Int. J. Quantum Chem. 2011, 111, 1081–1091. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, Z.; Zhou, Z.; Chen, W.; Zhou, J.; Cen, K. Effect of additive agents on the simultaneous absorption of NO2 and SO2 in the calcium sulfite slurry. Energy Fuels 2012, 26, 5583–5589. [Google Scholar] [CrossRef]
- Xia, P.F. Removal Experimental on NO from marine exhaust gas through in-Site combined Oxidation by dual oxidants of O3 and NaClO. Ship Eng. 2022, 44, 84–88+102. [Google Scholar]
- Li, Y.; Che, D.F.; Yang, C.L.; Yao, M.Y.; Zhao, T.W.; Fu, K.L.; Zhao, H.C. Engineering practice and economic analysis of ozone oxidation wet denitrification technology. Chin. J. Chem. Eng. 2020, 29, 401–408. [Google Scholar] [CrossRef]
- Sławomir, J.; Marcel, Z. Energy efficiency of an ozone generation process in oxygen. Analysis of a pulsed DBD system. Vacuum 2018, 155, 29–37. [Google Scholar]
- Sung, T.; Teii, S.; Liu, C.; Hsiao, R.; Chen, P.; Wu, Y.; Yang, C.; Teii, K.; Ono, S.; Ebihara, K. Effect of pulse power characteristics and gas flow rate on ozone production in a cylindrical dielectric barrier discharge ozonizer. Vacuum 2013, 90, 65–69. [Google Scholar] [CrossRef]
- Xu, G.Q. Integrated treatment device for desulfurization and denitrification of ship exhaust gas based on electrostatic spray technology. Equip. Manuf. Technol. 2023, 2, 137–140. [Google Scholar]
- Cui, S.; Hao, R.; Fu, D. An integrated system of dielectric barrier discharge combined with wet electrostatic precipitator for simultaneous removal of NO and SO2: Key factors assessments, products analysis and mechanism. Fuel 2018, 221, 12–20. [Google Scholar] [CrossRef]
- Oh, Y.-H.; Jo, S.-H.; Son, J.; Jeong, H.; Seo, S.; Kim, T.-H.; Son, Y.-S. Innovative electrostatic spray technology for control of particulate matter emitted from electron beam flue gas treatment process. Process Saf. Environ. Prot. 2025, 196, 106932–106942. [Google Scholar] [CrossRef]
- Li, K.X. Integrated technology of pulsed corona plasma flue gas desulphurisation, denitrification and dust removal and its power supply. Telecom World 2017, 189–190. [Google Scholar]
- Kim, H.H. Nonthermal plasma processing for air-pollution control: A historical review, current issues, and future prospects. Plasma Process. Polym. 2004, 1, 91–110. [Google Scholar] [CrossRef]
- Balachandran, W.; Manivannan, N.; Beleca, R.; Abbod, M.F.; Brennen, D.; Alozie, N.S.; Ganippa, L.C. Nonthermal plasma system for marine diesel engine emission control. IEEE Trans. Ind. Appl. 2016, 52, 2496–2505. [Google Scholar] [CrossRef]
- Park, H.W.; Choi, S.; Park, D.W. Simultaneous treatment of NO and SO2 with aqueous NaClO2 solution in a wet scrubber combined with a plasma electrostatic precipitator. J. Hazard. Mater. 2015, 285, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wu, H.; Liu, X.L.; Zhu, T.Y.; Liu, F.G.; Zhao, X.T. Simultaneous removal of SO2 and NO using a novel method with red mud as absorbent combined with O3 oxidation. J. Hazard. Mater. 2020, 392, 122270–122279. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H. Nitric oxide (NO) separation from flue gas by chemical modified mesoporous silica. Sep. Purif. Technol. 2019, 229, 115833–115840. [Google Scholar] [CrossRef]
- Guo, L.; Han, C.; Zhang, S.; Zhong, Q.; Ding, J.; Zhang, B.; Zeng, Y.Q. Enhancement effects of O2−and OH radicals on NOx removal in the presence of SO2 by using an O3/H2O2 AOP system with inadequate O3 (O3/NO molar ratio = 0.5). Fuel 2018, 233, 769–777. [Google Scholar] [CrossRef]
- Chen, L.; Lin, K.F.; Yang, C.L. Pilot study of absorption of NO2 with Na2S aqueous solutions. Environ. Prog. Sustain. Energy 2011, 30, 632–639. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Chen, X.; Tong, M.; Kang, W.; Guo, S.P.; Zhou, Y.B.; Lu, J. Simultaneous removal of NO and SO2 from flue gas by ozone oxidation and NaOH absorption. Ind. Eng. Chem. Res. 2014, 53, 6450–6456. [Google Scholar] [CrossRef]
- Shi, F.; Li, K.; Ying, D.; Jia, J.; Yan, N.; Zhang, X.J. An improved Wellman-lord process for simultaneously recovering SO2 and removing NOx from non-ferrous metal smelting flue gas. Chem. Eng. J. 2020, 399, 125658–125668. [Google Scholar] [CrossRef]
- Guo, Q.; He, Y.; Sun, T.; Wang, Y.; Jia, J. Simultaneous removal of NOx and SO2 from flue gas using combined Na2SO3 assisted electrochemical reduction and direct electrochemical reduction. J. Hazard. Mater. 2014, 276, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.C.; Dong, K.; Zhao, W.; Wang, J.W.; Chu, G.W.; Zhang, L.L.; Zou, H.K.; Chen, J.F. Simultaneous absorption of NOx and SO2 into Na2SO3 solution in rotating packed bed with preoxidation by ozone. Ind. Eng. Chem. Res. 2019, 58, 8332–8341. [Google Scholar] [CrossRef]
- Chin, T.; Tam, I.; Yin, C.Y. Wet scrubbing process with oxidation and reduction in series for removal of SO2 and NO from marine diesel engine exhaust. Chem. Eng. J. 2023, 464, 142299–142324. [Google Scholar] [CrossRef]
- Hong, L.; Chen, D.Z.; Yin, L.J.; Chen, H.; Wang, D.; Hu, Y.Y. Hydrazine-enhanced NO conversion in a pulsed corona discharge plasma (PCDP) reactor: Behaviors and mechanism. AIP Adv. 2016, 6, 095108–095119. [Google Scholar] [CrossRef]
- Yang, S.L.; Pan, X.X.; Han, Z.T.; Zhao, D.S.; Liu, B.J.; Zheng, D.K.; Yan, Z.J. Kinetics of nitric oxide absorption from simulated flue gas by a wet UV/Chlorine advanced oxidation process. Energy Fuels 2017, 31–37, 7263–7271. [Google Scholar] [CrossRef]
- Li, S.C.; Huang, W.J.; Xu, H.M.; Liu, K.; Wang, J.N.; Sun, Y.N.; Qu, Z.; Yan, N.Q. Enhanced simultaneous absorption of NOx and SO2 in oxidation-reduction-absorption process with a compounded system based on Na2SO3. J. Environ. Sci. 2022, 111, 1–10. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, T.S.; Guo, Y.Q.; Wang, J.W.; Wei, J.X.; Yu, Q.J. Recent advances in simultaneous removal of SO2 and NOX from exhaust gases: Removal process, mechanism and kinetics. Chem. Eng. J. 2021, 420, 127588–127631. [Google Scholar] [CrossRef]
- Li, C.J.; Zhao, R.; Peng, M.P.; Hao, L.; Yu, G.; Xia, D.S. Study on desulfurization and denitrification by modified activated carbon fibers with visible-light photocatalysis. J. Fuel Chem. Technol. 2015, 43, 1516–1522. [Google Scholar] [CrossRef]
- Yao, L.Y.; Zhang, L.; Dong, L.H. Investigation on technology of desulfurization performance from ship emissions. Sci. Technol. Inf. 2012, 11, 196–197+192. [Google Scholar]
- Baboukai, Z.K.; Chermahini, A.N.; Farrokhpour, H. Photocatalytic oxidative desulfurization of a model fuel using S-doped TiO2/BiVO4 composites: A combination of experimental and theoretical study. J. Alloys Compd. 2024, 1002, 175478–175484. [Google Scholar] [CrossRef]
- Xia, D.H.; Hu, L.L.; He, C.; Pan, W.Q.; Yang, T.S.; Yang, Y.C.; Shu, D. Simultaneous photocatalytic elimination of gaseous NO and SO2 in a BiOI/Al2O3-padded trickling scrubber under visible light. Chem. Eng. J. 2015, 279, 929–938. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, W. Experiment and mechanism analysis of desulfurization with a MgO-based desulfurizer fixed bed method. ACS Omega 2020, 5, 30740–30745. [Google Scholar] [CrossRef]
- Jamil, R.; Ming, L.; Jamil, I.; Jamil, R. Application and development trend of flue gas desulfurization FGD process: A Review. Int. J. Innov. Appl. Stud. 2013, 4, 286–297. [Google Scholar]
- Bian, J.J.; Zhang, Q.; Min, X.; Zhang, S.; Feng, L.J.; Li, C.H. Modified clinoptilolite catalysts for seawater flue gas desulfurization application: Preparation, characterization and kinetic evaluation. Process Saf. Environ. Prot. 2016, 101, 117–123. [Google Scholar] [CrossRef]
- Tong, Y. Application status and development prospect of seawater flue gas desulphurisation process in China. Constr. Des. Eng. 2004, 8, 6–8. [Google Scholar]
- Liu, J.; Lu, S.; Wang, L.D.; Qi, T.Y.; Qi, D.; Xing, X.; Zhang, Y.Y.; Xiao, H.N.; Zhang, S.H. Co-site substitution by Mn supported on biomass-derived active carbon for enhancing magnesia desulfurization. J. Hazard. Mater. 2019, 365, 531–537. [Google Scholar] [CrossRef]
- Sun, Y.N.; Xie, J.K.; Huang, W.J.; Li, G.; Li, S.C.; Cui, P.; Xu, H.M.; Qu, Z.; Yan, N.Q. Novel product-adjustable technology using wellman-lord method coupled with sodium-alkali for SO2 removal and regeneration from smelting gas. Fuel 2021, 288, 119714–119720. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Luo, L.Y.; Yuan, Z.L.; Yang, L.J.; Wu, H. Improving the removal of fine particles by chemical agglomeration during the limestone-gypsum wet flue gas desulfurization process. J. Environ. Sci. 2019, 80, 35–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Y.J.; Wang, C.B.; Guo, Y.S.; Liu, X.; Dai, H.; Bai, T. Removal of gas-phase arsenic and selenium in flue gas by a new combined spray-and-scattered-bubble technology based on ammonia desulphurization. Sci. Total Environ. 2021, 772, 145622–145631. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Li, Z.; Hu, X.Y.; Wang, Z.J.; Yang, H.Y.; Elsharkawy, E.R.; El-bahy, S.M.; Wu, M.M.; Hu, M.M.; Guo, Z.H. Simulation and experiment on improving NOX conversion efficiency of ship selective catalytic reduction system. Alex. Eng. J. 2024, 103, 237–250. [Google Scholar] [CrossRef]
- Wang, H.X. Design and application of SCR flue gas denitration system in thermal power plant under background of energy conservation and emission reduction. Energy Energy Conserv. 2024, 56–59. [Google Scholar] [CrossRef]
- Zhao, M.J.; Xue, P.; Liu, J.J.; Liao, J.H.; Guo, J.M. A review of removing SO2 and NOX by wet scrubbing. Sustain. Energy Technol. Assess. 2021, 47, 101451–101463. [Google Scholar] [CrossRef]
- Cui, S.P.; Hao, R.L.; Fu, D. Integrated method of non-thermal plasma combined with catalytical oxidation for simultaneous removal of SO2 and NO. Fuel 2019, 246, 365–374. [Google Scholar] [CrossRef]
- Adewuyi, Y.G.; Khan, M.A. Modeling the synchronous absorption and oxidation of NO and SO2 by activated peroxydisulfate in a lab-scale bubble reactor. Sep. Purif. Technol. 2022, 300, 12841–12854. [Google Scholar] [CrossRef]
- Hao, R.L.; Luo, Y.C.; Qian, Z.; Ma, Z.; Ding, Y.Q.; Gong, Y.P.; Wang, Z.; Zhao, Y. Simultaneous removal of SO2, NO and Hg0 using an enhanced gas phase UV-AOP method. Sci. Total Environ. 2020, 734, 139266–139276. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.Y.; Li, H.L.; Li, Y.; Zhao, Y.C.; Zheng, C.G. Simultaneous removal of SO2, NO and mercury using TiO2-aluminum silicate fiber by photocatalysis. Chem. Eng. J. 2012, 192, 21–28. [Google Scholar] [CrossRef]

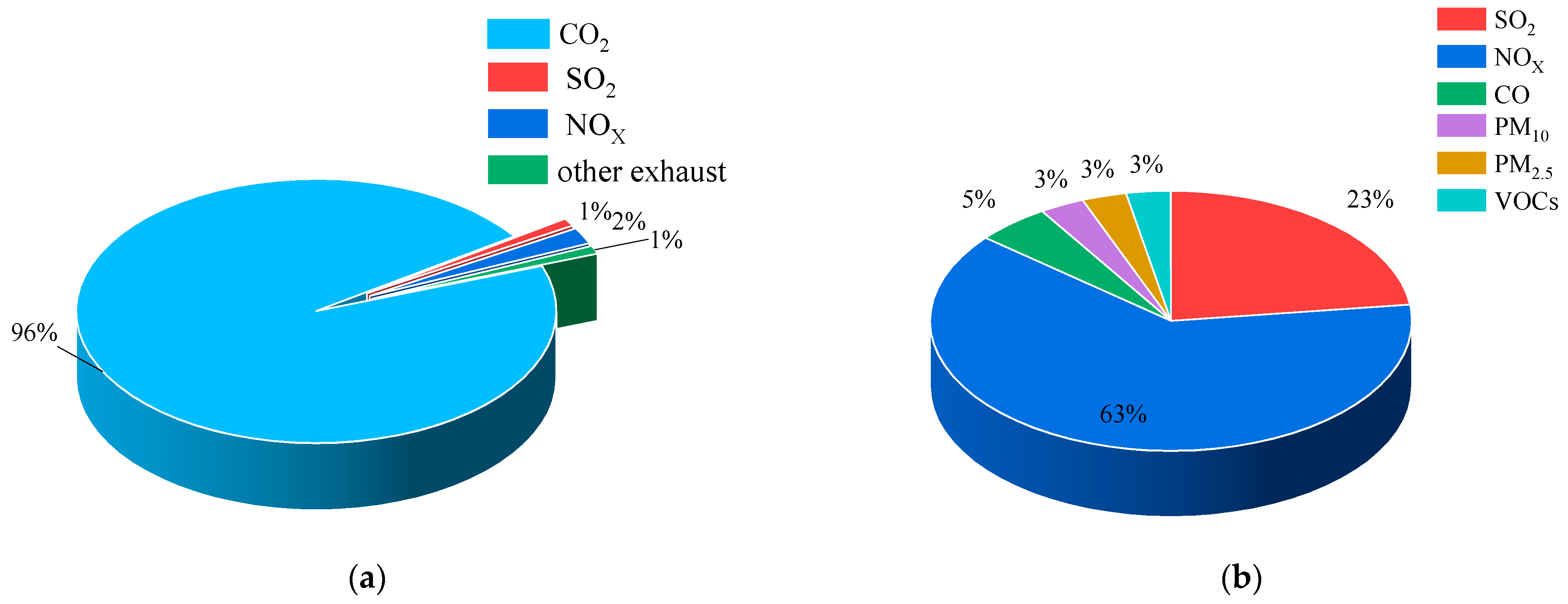






| Vintages | Districts | NOX | SO2 | PM | VOCS | CO | SO2 | PM10 | PM2.5 |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | Jiangsu | 18% | 2.1% | 4% | 3.5% | ||||
| 2018 | Dalian | 2.4% | 7.5% | 7.1% | |||||
| 2018 | Shenzhen | 63% | 23% | 3% | 5% | 3% | 3% | ||
| 2015 | Zhuhai | 20% | 17% | 3% | 10% | ||||
| 2012 | Hong Kong | 32% | 11% | 17% | 50% | 37% | 43% | ||
| 2007 | Hong Kong | 17% | 11% | 16% | |||||
| 2010 | Shanghai | 11.6% | 12.4% | 5.6% |
| Processing | Advantages | Disadvantages | Removal Efficiency | Ref. |
|---|---|---|---|---|
| Dry desulphurization | No water consumption, environmentally friendly | Takes up a lot of space and by-products are difficult to handle | SO2: 85% | [144] |
| Semi-dry desulphurization | Low water consumption, environmentally friendly, simple process, small footprint | Lower desulfurization efficiency, higher calcium-to-sulfur ratio, higher raw material costs | SO2: 85% | [145] |
| Open type Seawater method | Uses seawater, no chemicals added | For low-sulfur fuels only Emissions fail to meet new standards | SO2: 95% | [146] |
| Magnesium method | Low up-front investment, simple process, easy maintenance, no scaling of products [147] | The use of alkaline chemicals increases costs. | SO2: 95–98% | [148] |
| Closed type Sodium alkali method | Low fouling, high SO2 absorption rate, avoid blockage of absorption tower | Not suitable for large amounts of use, large investment, large area, high operating cost | SO2: 99% | [149] |
| limestone-gypsum method | Mature technology, not easy to clog | The device covers a large area, the equipment is easy to wear and block | SO2: >90% | [150] |
| Ammonia method | It has a certain economic value and does not emit carbon dioxide or cause secondary pollution | High cost, takes up a lot of space, ammonia is a hazardous material and easy to corrode pipelines. | SO2: 99% | [151] |
| SCR denitrification technology | Mature technology, very high denitrification efficiency, easy maintenance, no secondary pollution, simple equipment design | Highly affected by SO2, only suitable for low-sulfur fuels | NOX: 96.1% | [152] |
| SNCR denitrification technology | Easy to install, simplicity, the catalyst-free system, wide range of applications, lower cost | High emission temperatures, low reductant utilization, and high window temperatures | NOX: 88.2% | [153] |
| Ozone oxidation denitrification | Occupying less space, easy to obtain the raw materials for the reaction, no fouling of the reaction products, strong selectivity, and fast oxidation speed | High energy consumption, high consumption of O2, and high cost | NOX: 90.3% | [117] |
| Electrostatic spray technology | Strong ability to remove particles, strong ability to remove sulfur and denitrification, occupies little space, environmental protection, the raw materials are inexhaustible, saves costs | High energy consumption, requires electrolysis of seawater. | NOX: 52% SO2: 43% | [154] |
| Plasma oxidation process | High desulphurization and denitrification rates, produce economic products | The equipment is expensive and energy-consuming, radioactive, and the product takes up a lot of space, O2, CO2, and H2O have a negative impact on the effectiveness | NOX: 86.9% SO2: 100% | [155] |
| Oxidative absorption | Strong oxidation capacity, economic and environmental protection, simple process flow, high removal efficiency, and easy modification of wet flue gas desulfurization equipment | Chemically unstable and difficult to handle with high-temperature gases, competitive absorption between NOX and SO2 with oxidants, over oxidation | NOX: 85.0% SO2: 97.6% | [156] |
| Integrated UV-enhanced active chlorine process | Desulphurization rate of 100%, low system complexity, high degree of automation, wide range of raw material sources, no need to store, save ship space, safety. The strong and non-selective oxidation characteristics | The denitrification rate is 50–61 percent, requiring secondary denitrification. large energy consumption, competition for pollutant removal | NOX: 89% SO2: 99% | [157] |
| Photocatalysis | No need to add additional chemicals, selective specificity for more precise and efficient treatment, a compact structure, with no secondary pollution, and a high removal efficiency. | Influenced by the light factor, the absence of light is the need for additional energy, high requirements for catalyst stability, and high reaction conditions. | NOX: 98.9% SO2: 87.1% | [122,158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Xian, B.; Wang, M.; Xiao, Y.; Zhou, X.; Xu, D.; Zhang, Y.; Liu, H.; Bai, S. Progress of Ship Exhaust Emissions in China’s Lijiang River: Current Status and Aftertreatment Technologies. Toxics 2025, 13, 396. https://doi.org/10.3390/toxics13050396
Liu P, Xian B, Wang M, Xiao Y, Zhou X, Xu D, Zhang Y, Liu H, Bai S. Progress of Ship Exhaust Emissions in China’s Lijiang River: Current Status and Aftertreatment Technologies. Toxics. 2025; 13(5):396. https://doi.org/10.3390/toxics13050396
Chicago/Turabian StyleLiu, Pengyu, Bensen Xian, Mei Wang, Yong Xiao, Xiaobin Zhou, Dandan Xu, Yanan Zhang, Huili Liu, and Shaoyuan Bai. 2025. "Progress of Ship Exhaust Emissions in China’s Lijiang River: Current Status and Aftertreatment Technologies" Toxics 13, no. 5: 396. https://doi.org/10.3390/toxics13050396
APA StyleLiu, P., Xian, B., Wang, M., Xiao, Y., Zhou, X., Xu, D., Zhang, Y., Liu, H., & Bai, S. (2025). Progress of Ship Exhaust Emissions in China’s Lijiang River: Current Status and Aftertreatment Technologies. Toxics, 13(5), 396. https://doi.org/10.3390/toxics13050396






