Toxicological Effects of Combined Exposure of Cadmium and Enrofloxacin on Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Experimental Process
2.3. Analytical Method
2.3.1. Joint Toxicity Assessment Method
2.3.2. Determination of Cd in Tissues (ng/L) and Organs of Zebrafish
2.3.3. Determination of Antioxidant Stress Index
2.3.4. Determination of 16S rRNA Gene of Intestinal Flora of Zebrafish
2.3.5. Data Analysis
3. Results
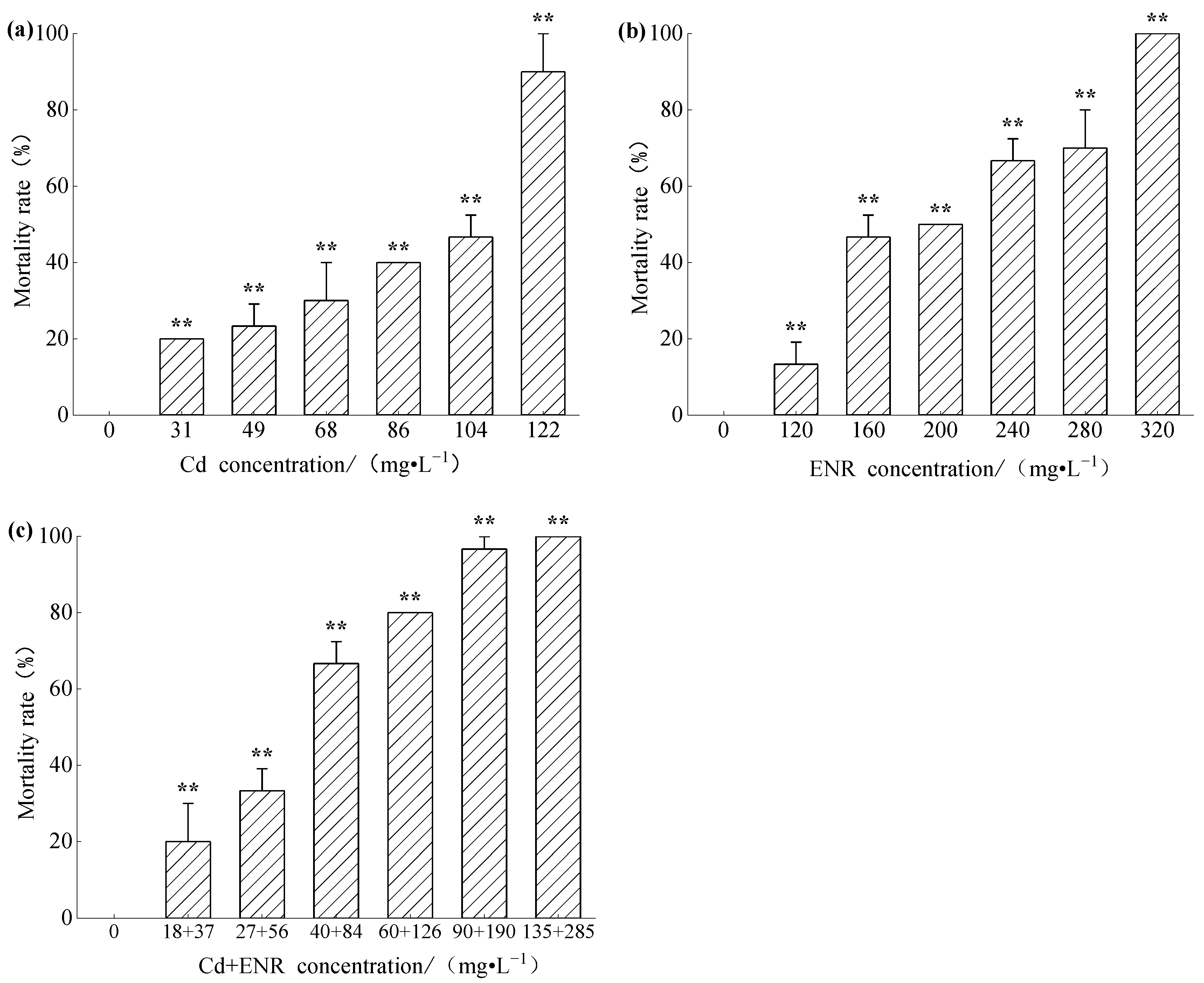
3.1. Acute Toxicity of Cd and ENR to Zebrafish
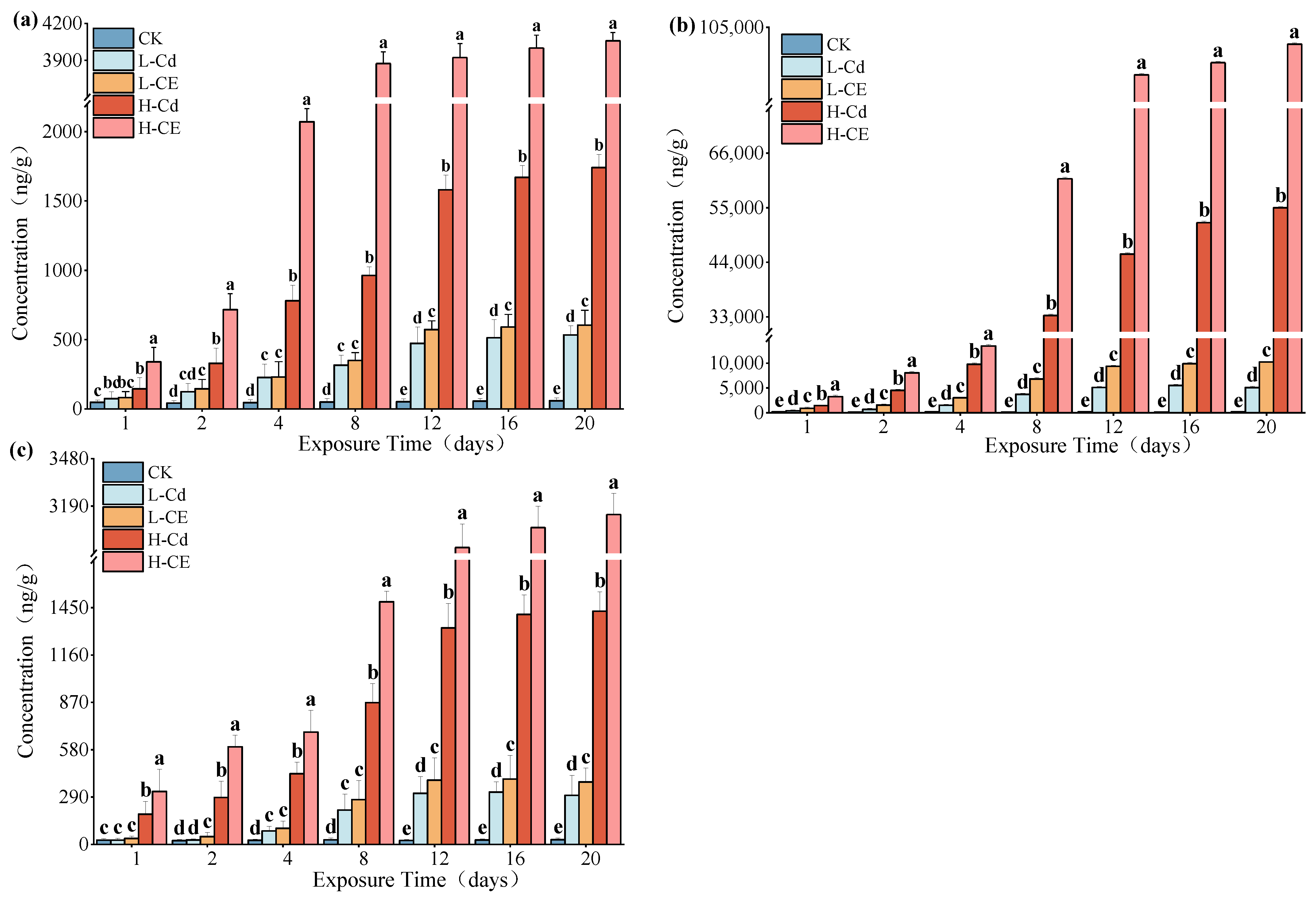
3.2. Cd Accumulation in Zebrafish Tissues Under ENR Conditions
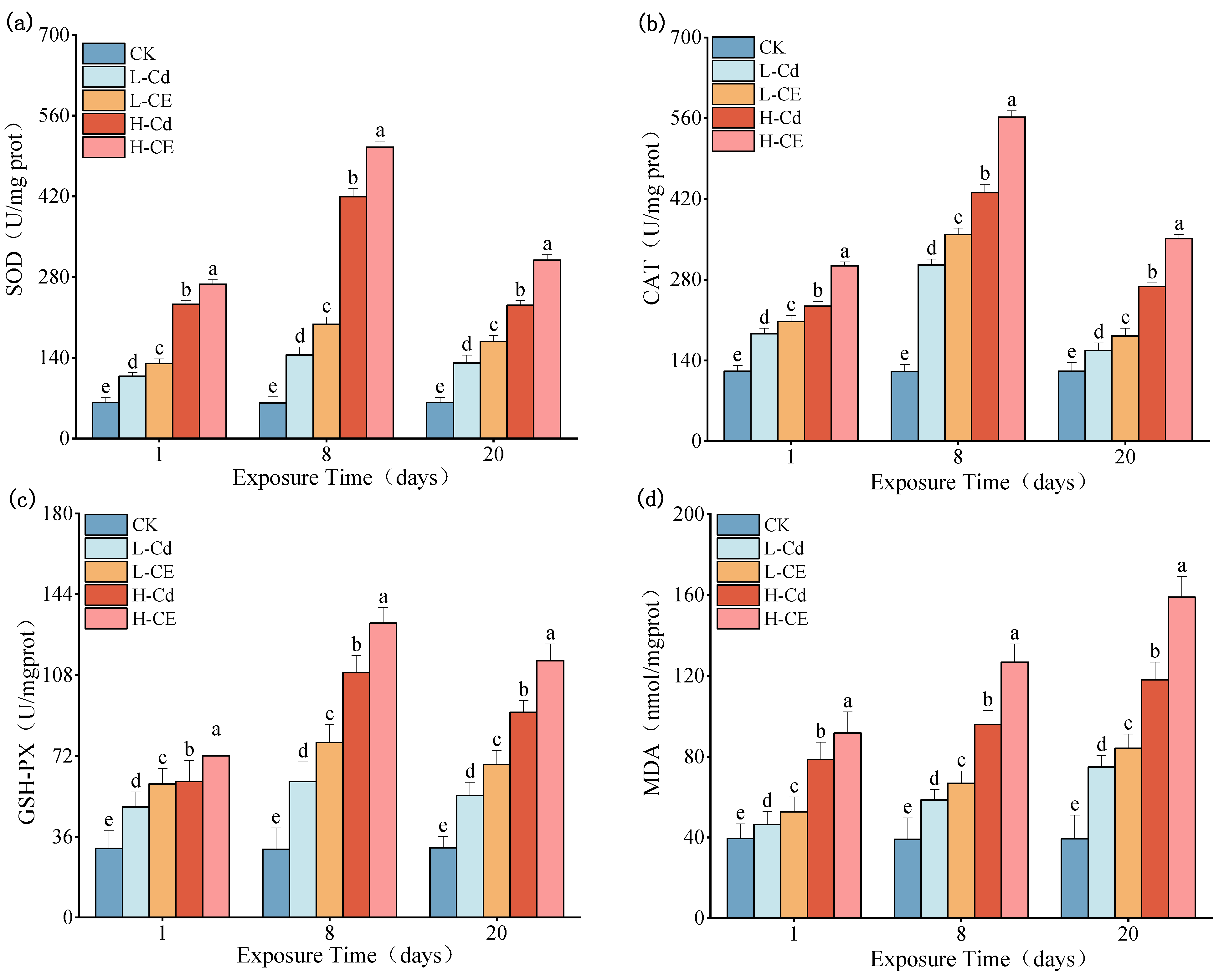
3.3. Effect of Cd on Oxidative Stress Indexes in Liver Under ENR
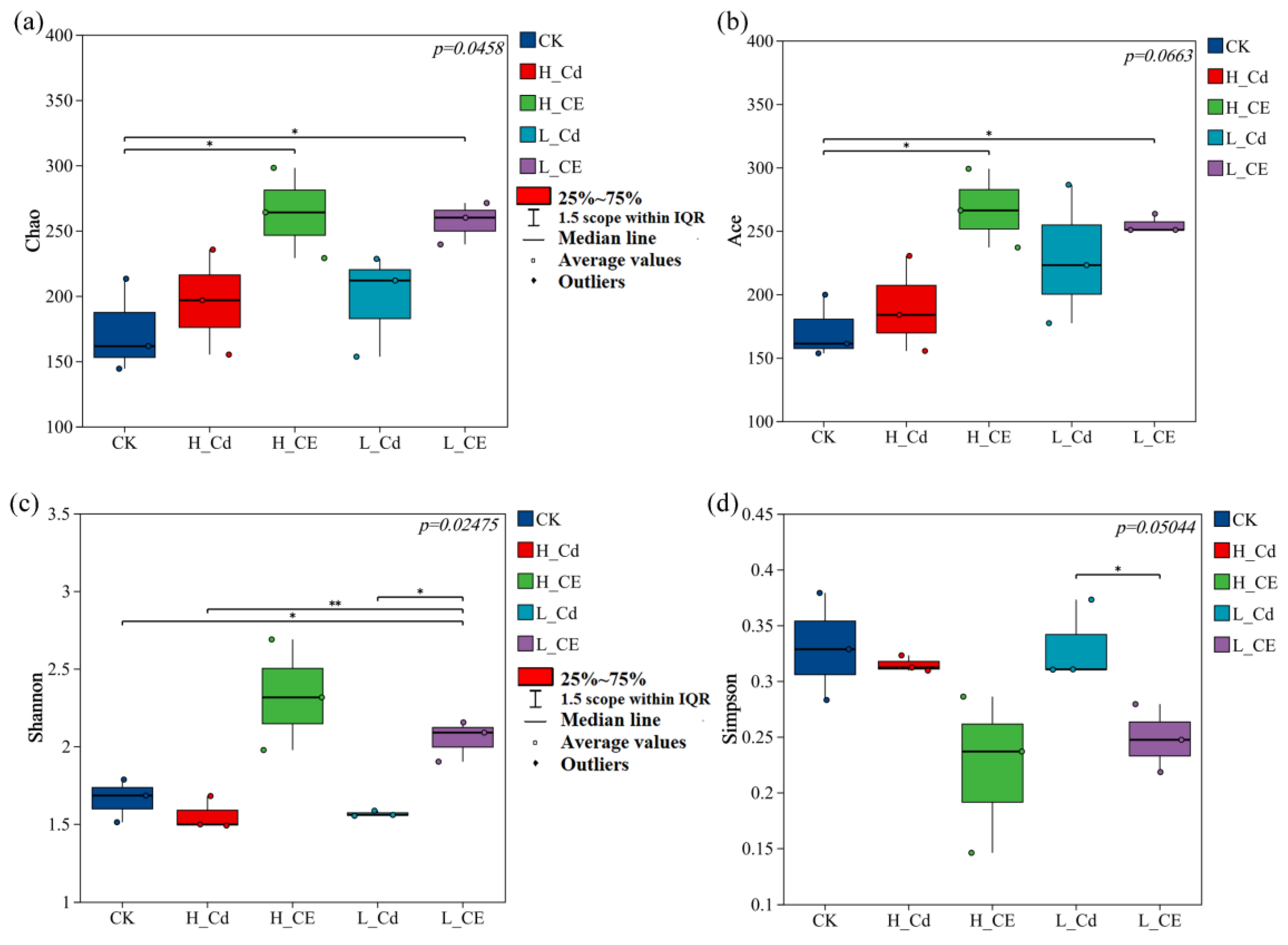
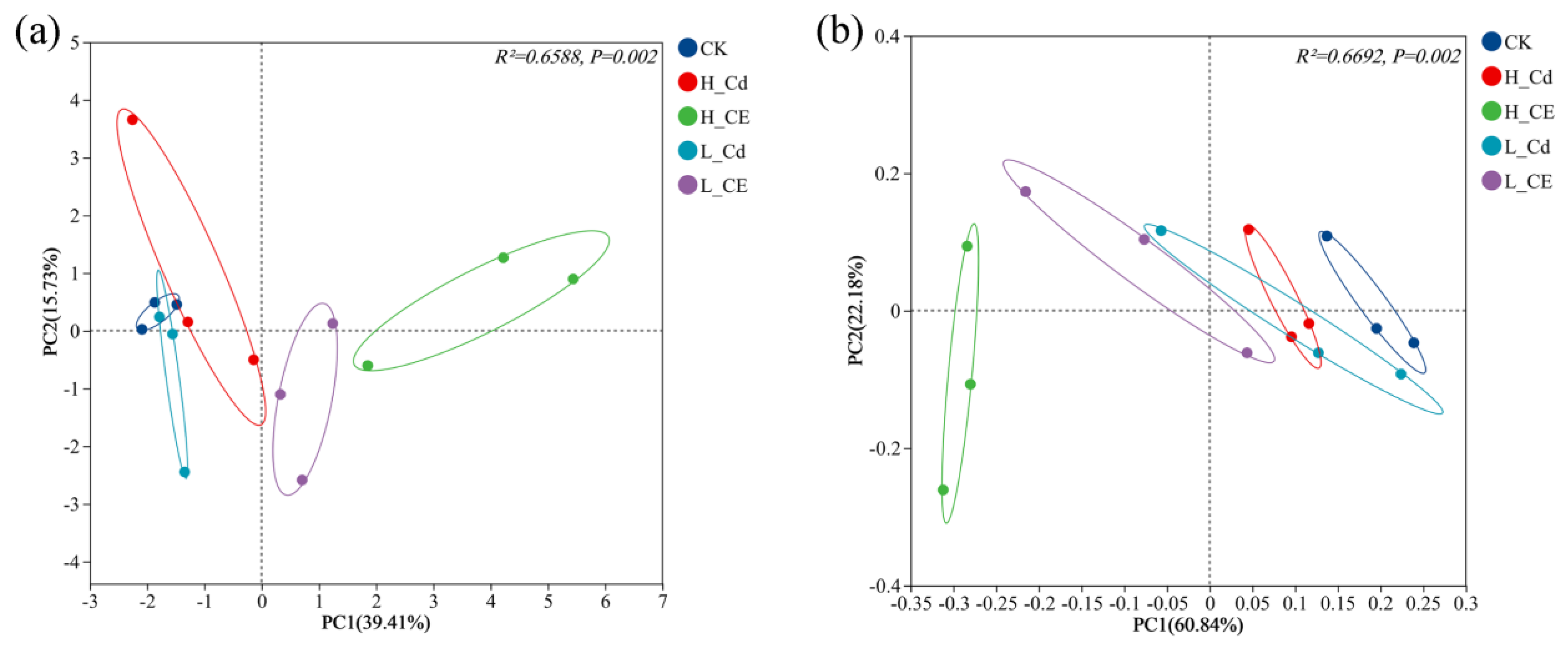
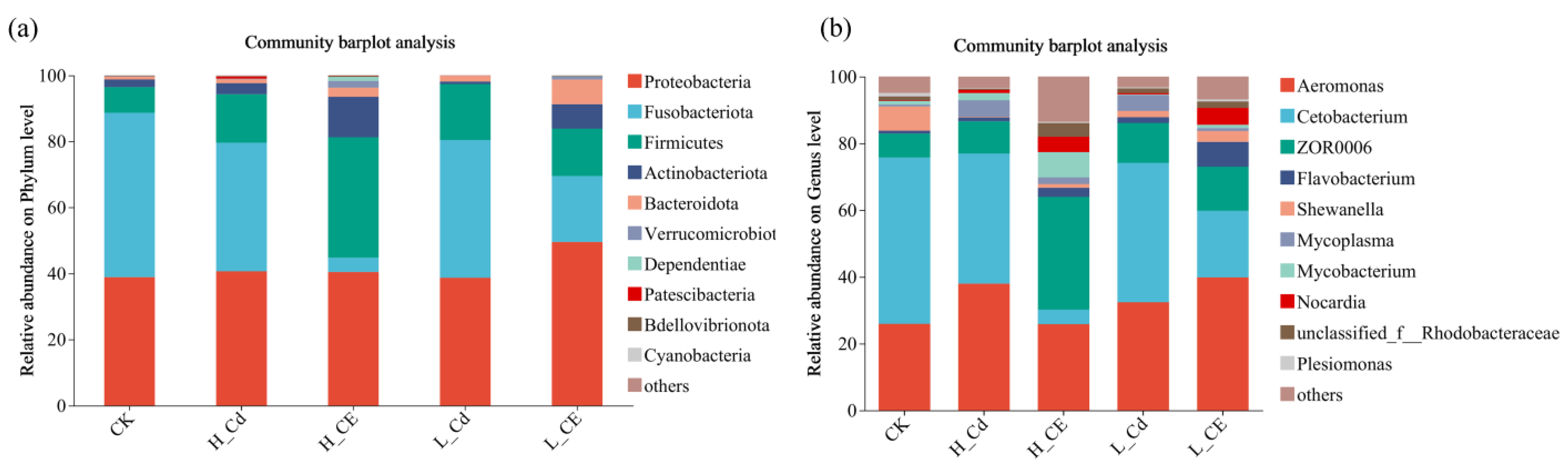
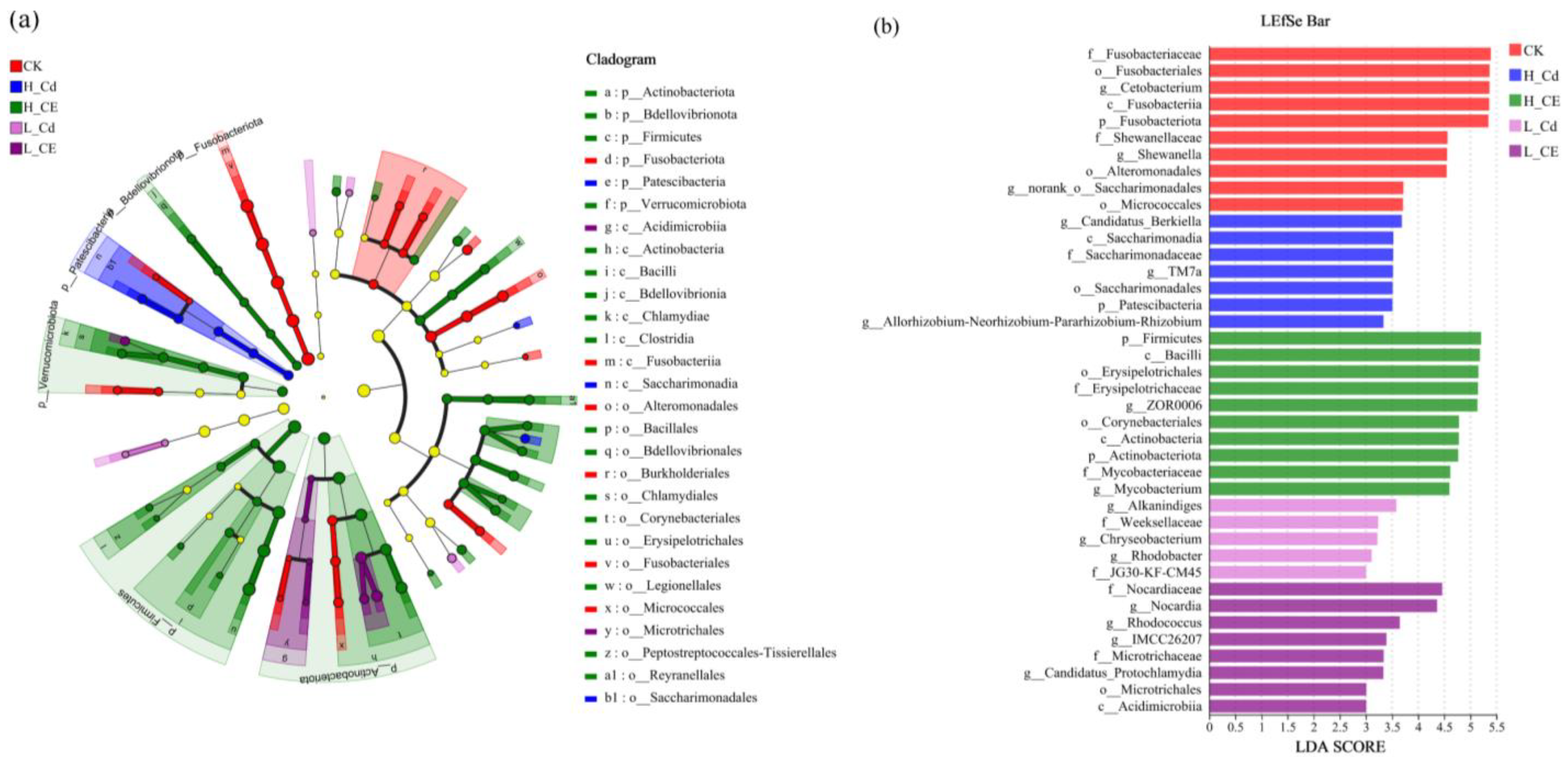
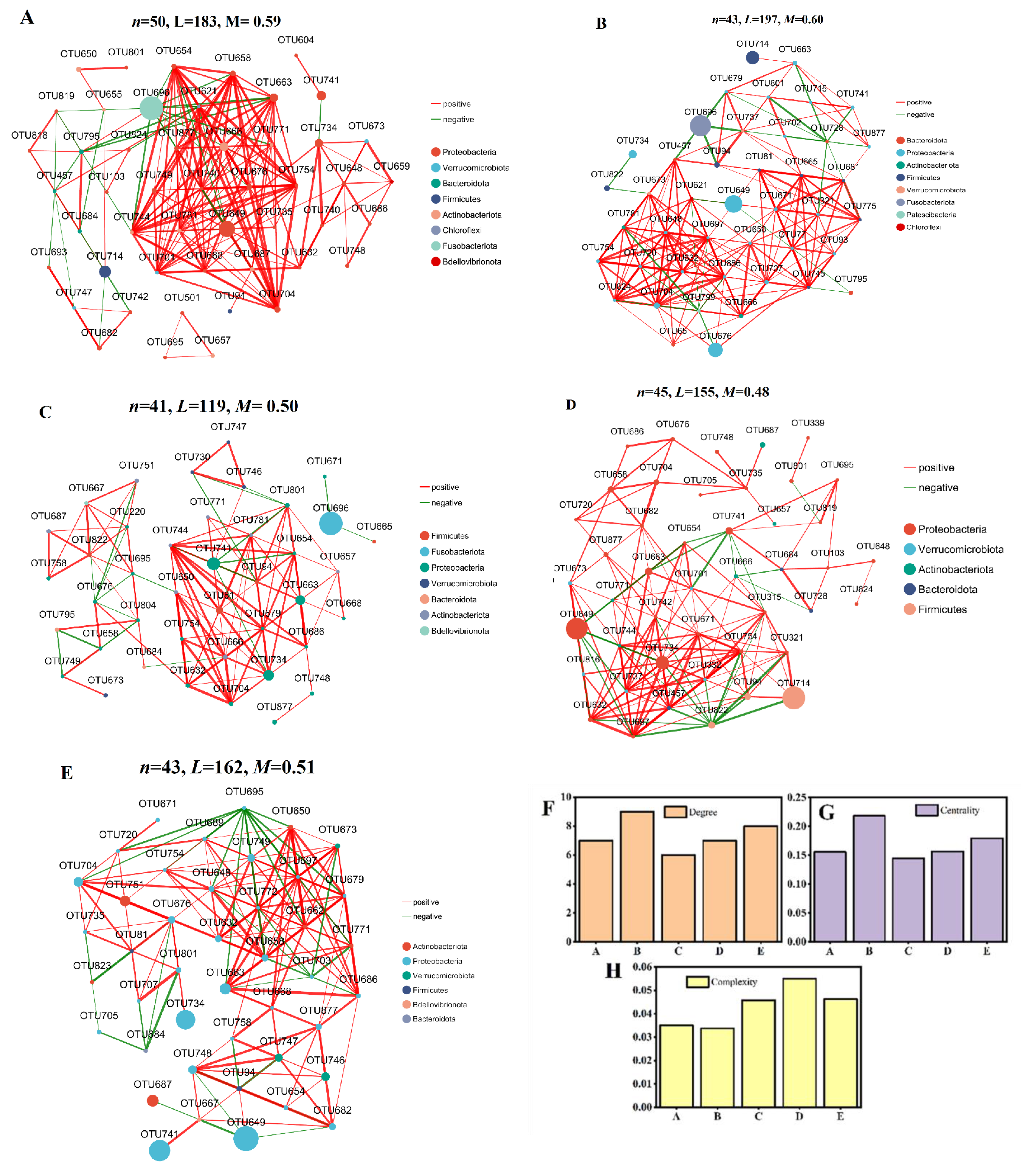
3.4. Effect of Cd and ENR on Intestinal Flora Diversity
4. Discussion
4.1. Acute Toxicity of Cd and ENR to Zebrafish and Accumulation of Cd in Zebrafish Tissues Under ENR Stress
4.2. Effects of Cd on Hepatic Antioxidant Enzymes and Oxidative Damage Markers Under ENR Exposure
4.3. Effect of Cd on Intestinal Flora Under ENR
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuccato, E.; Castiglioni, S.; Bagnati, R.; Melis, M.; Fanelli, R. Source, occurrence and fate of antibiotics in the Italian aquatic environment. J. Hazard. Mater. 2010, 179, 1042–1048. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Thunders, M.; Matthew, C.; Wang, X.; Ai, X.; Zhou, X.; Qiu, J. Effect of enrofloxacin on the proteome of earthworms. Sci. Total. Environ. 2018, 616, 531–542. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Wu, X.; Zou, H.; Zhu, R.; Wang, J. Analysis of Antibiotic Pollution Characteristics and Ecological Risk Assessment in the Waters of Gonghu Bay, Taihu Lake. Environ. Sci. 2016, 37, 4596–4604. [Google Scholar]
- Wang, Q.J.; Mo, C.H.; Li, Y.W.; Gao, P.; Tai, Y.P.; Zhang, Y.; Ruan, Z.L.; Xu, J.W. Determination of four fluoroquinolone antibiotics in tap water in Guangzhou and Macao. Environ. Pollut. 2010, 158, 2350–2358. [Google Scholar]
- Teglia, C.M.; Perez, F.A.; Michlig, N.; Repetti, M.R.; Goicoechea, H.C.; Culzoni, M.J. Occurrence, Distribution, and Ecological Risk of Fluoroquinolones in Rivers and Wastewaters. Environ. Toxicol. Chem. 2019, 38, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Ohore, O.E.; Qin, Z.; Sanganyado, E.; Wang, Y.; Jiao, X.; Liu, W.; Wang, Z. Ecological impact of antibiotics on bioremediation performance of constructed wetlands: Microbial and plant dynamics, and potential antibiotic resistance genes hotspots. J. Hazard. Mater. 2022, 424, 127495. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.; Sun, Y.; Chen, B. Contribution of enrofloxacin and Cu2+ to the antibiotic resistance of bacterial community in a river biofilm. Environ. Pollut. 2021, 291, 118156. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, T.; Liu, X.; Chen, H.; Luo, S.; Chen, Q.; Magnuson, J.T.; Zheng, C.; Xu, E.G.; Schlenk, D. Enrofloxacin Induces Intestinal Microbiota-Mediated Immunosuppression in Zebrafish. Environ. Sci. Technol. 2022, 56, 8428–8437. [Google Scholar] [CrossRef]
- Bona, M.D.; Lizzi, F.; Borgato, A.; De Liguoro, M. Increasing toxicity of enrofloxacin over four generations of Daphnia magna. Ecotoxicol. Environ. Saf. 2016, 132, 397–402. [Google Scholar] [CrossRef]
- Jin, X.; Liu, S.; Zhang, Z.; Liu, T.; Li, N.; Liang, Y.; Zheng, J.; Peng, N. Enrofloxacin-induced transfer of multiple-antibiotic resistance genes and emergence of novel resistant bacteria in red swamp crayfish guts and pond sediments. J. Hazard. Mater. 2023, 443, 130261. [Google Scholar] [CrossRef] [PubMed]
- Curpan, A.; Impellitteri, F.; Plavan, G.; Ciobica, A.; Faggio, C. Review: Mytilus galloprovincialis: An essential, low-cost model organism for the impact of xenobiotics on oxidative stress and public health. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 256, 109302. [Google Scholar] [CrossRef]
- Jyoti, D.; Sinha, R.; Faggio, C. Advances in biological methods for the sequestration of heavy metals from water bodies: A review. Environ. Toxicol. Pharmacol. 2022, 94, 103927. [Google Scholar] [CrossRef]
- Tóth, T.; Zsiros, O.; Kis, M.; Garab, G.; Kovács, L. Cadmium exerts its toxic effects on photosynthesis via a cascade mechanism in the cyanobacterium, Synechocystis PCC 6803. Plant Cell Environ. 2012, 35, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tong, Q.; He, C.; Fan, R. Investigation and Research on Soil Cadmium Pollution. Sichuan Environ. 2004, 8–10. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, G.; Luo, Z.; Sun, X.; Xu, J. Spatial distribution, source identification, and risk assessment of heavy metals in seawater and sediments from Meishan Bay, Zhejiang coast, China. Mar. Pollut. Bull. 2020, 156, 111217. [Google Scholar] [CrossRef]
- El-Sorogy, A.; Al-Kahtany, K.; Youssef, M.; Al-Kahtany, F.; Al-Malky, M. Distribution and metal contamination in the coastal sediments of Dammam Al-Jubail area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2018, 128, 8–16. [Google Scholar] [CrossRef] [PubMed]
- GB 3838-2002; Environmental Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- Zhu, B.; Shi, Z.; Wang, X.; Xu, W.; Yang, W.; Liao, C. Spatial Distribution Characteristics and Source Identification of Heavy Metals in the Water Body of Anning River. Sichuan Metall. 2018, 40, 24–31. [Google Scholar]
- Lee, S.; Kim, C.; Liu, X.; Lee, S.; Kho, Y.; Kim, W.; Kim, P.; Choi, K. Ecological Risk Assessment of Amoxicillin, Enrofloxacin, and Neomycin: Are Their Current Levels in the Freshwater Environment Safe? Toxics 2021, 9, 196. [Google Scholar] [CrossRef]
- Mo, L.; Yang, Y.; Zhao, D.; Qin, L.; Yuan, B.; Liang, N. Time-Dependent Toxicity and Health Effects Mechanism of Cadmium to Three Green Algae. Int. J. Environ. Res. Public Health 2022, 19, 10974. [Google Scholar] [CrossRef]
- Ros, M.T.L.; Al-Enezi, E.; Cesarini, E.; Canonico, B.; Bucci, C.; Martins, M.V.A.; Papa, S.; Frontalini, F. Assessing the Cadmium Effects on the Benthic Foraminifer Ammonia cf. parkinsoniana: An Acute Toxicity Test. Water 2020, 12, 1018. [Google Scholar] [CrossRef]
- Gao, D.; Xu, Z.; Zhang, X.; Zhu, C.; Wang, Y.; Min, W. Cadmium triggers kidney cell apoptosis of purse red common carp (Cyprinus carpio) without caspase-8 activation. Dev. Comp. Immunol. 2013, 41, 728–737. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Raghib, F.; Dar, M.I.; Khan, F.A.; Hessini, K.; Ahmad, P. Uptake, accumulation and elimination of cadmium in a soil-Faba bean (Vicia faba)-Aphid (Aphis fabae)-Ladybird (Coccinella transversalis) food chain. Chemosphere 2021, 279, 130522. [Google Scholar] [CrossRef] [PubMed]
- Sehonova, P.; Tokanova, N.; Hodkovicova, N.; Kocour Kroupova, H.; Tumova, J.; Blahova, J.; Marsalek, P.; Plhalova, L.; Doubkova, V.; Dobsikova, R.; et al. Oxidative stress induced by fluoroquinolone enrofloxacin in zebrafish (Danio rerio) can be ameliorated after a prolonged exposure. Environ. Toxicol. Pharmacol. 2019, 67, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Khoei, A.J.; Parhizkar, S.; Rad, F.T.; Salimi, B. Assessment of Some Heavy Metals and Their Relationship with Oxidative Stress and Immunological Parameters in Aquatic Animal Species. Biol. Trace Elem. Res. 2023, 201, 4547–4557. [Google Scholar] [CrossRef]
- Wang, N.; Guo, Z.; Zhang, Y.; Zhang, P.; Liu, J.; Cheng, Y.; Zhang, L.; Li, Y. Effect on intestinal microbiota, bioaccumulation, and oxidative stress of Carassius auratus gibelio under waterborne cadmium exposure. Fish. Physiol. Biochem. 2020, 46, 2299–2309. [Google Scholar] [CrossRef]
- Yeşilbudak, B.; Erdem, C. Cadmium Accumulation in Gill, Liver, Kidney and Muscle Tissues of Common Carp, Cyprinus carpio, and Nile Tilapia, Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2014, 92, 546–550. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Xu, X.; Diao, Z.; Sun, K.; Hao, Q.; Liu, S.; Ying, G. Tissue distribution, bioaccumulation characteristics and health risk of antibiotics in cultured fish from a typical aquaculture area. J. Hazard. Mater. 2018, 343, 140–148. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Mukherjee, S.; Mandal, S.M. Fluoroquinolone antibiotics show genotoxic effect through DNA-binding and oxidative damage. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117634. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Jin, L.; Huang, J.; Pu, D.; Wang, D.; Zhang, Y. Effects of subchronic exposure to waterborne cadmium on H-P-I axis hormones and related genes in rare minnows (Gobiocypris rarus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Alvarado, C.; Ramirez, J.M.; Herrera-Lopez, E.J.; Cortez-Valladolid, D.; Ramirez, G. Bioaccumulation of Metals in Cultured Carp (Cyprinus carpio) from Lake Chapala, Mexico. Biol. Trace Elem. Res. 2020, 195, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H.; Wang, Y.; Bekele, T.G.; Liu, W.; Chen, J. Uptake and depuration of eight fluoroquinolones (FQs) in common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2019, 180, 202–207. [Google Scholar] [CrossRef]
- Chen, X.; Guo, J.; Huang, Y.; Li, Z.; Yuan, W.; Zeng, S.; Zhu, H.; Zhong, Y.; Lin, W.; Lu, H.; et al. Toxicity of o-phenylphenol on craniofacial cartilage development through ROS-induced oxidative stress in zebrafish embryos. Sci. Total Environ. 2023, 892, 164396. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Wang, J.; Zhu, L.; Wang, J.; Yan, S.; Ahmad, Z. Toxicity of enrofloxacin and cadmium alone and in combination to enzymatic activities and microbial community structure in soil. Environ. Geochem. Health 2019, 41, 2593–2606. [Google Scholar] [CrossRef]
- Gao, M.; Lv, M.; Han, M.; Song, W.; Wang, D. Avoidance behavior of Eisenia fetida in oxytetracycline- and heavy metal-contaminated soils. Environ. Toxicol. Pharmacol. 2016, 47, 119–123. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Y.; Luo, J.; Yan, S.; Wang, T.; Liu, L.; Zhang, Z. Interactive Effects of Tetracyclines and Copper on Plant Growth and Nutrient Uptake by Eichhornia crassipes. Clean-Soil Air Water 2016, 44, 96–104. [Google Scholar] [CrossRef]
- Li, Y.; Tang, H.; Hu, Y.; Wang, X.; Ai, X.; Tang, L.; Matthew, C.; Cavanagh, J.; Qiu, J. Enrofloxacin at environmentally relevant concentrations enhances uptake and toxicity of cadmium in the earthworm Eisenia fetida in farm soils. J. Hazard. Mater. 2016, 308, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Pan, B.; Gao, G.; Liu, Y.; Liu, S.; Liang, N.; Zhou, D.; Vijver, M.G.; Peijnenburg, W.J.G.M. Tannic acid promotes ion release of copper oxide nanoparticles: Impacts from solution pH change and complexation reactions. Water Res. 2017, 127, 59–67. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Wang, Q.; Lv, M.; Zhao, X.; Ji, Y.; Han, X.; Wang, X.; Chen, L. The combined toxic effects of polyvinyl chloride microplastics and di(2-ethylhexyl) phthalate on the juvenile zebrafish (Danio rerio). J. Hazard. Mater. 2022, 440, 129711. [Google Scholar] [CrossRef]
- GB/T 27861-2011; Chemicals-Fish Acute Toxicity Test. Standards Press of China: Beijing, China, 2011.
- Belden, J.B.; Gilliom, R.J.; Lydy, M.J. How Well Can We Predict the Toxicity of Pesticide Mixtures to Aquatic Life. Integr. Environ. Assess. Manag. 2007, 3, 364–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Song, X.; Ge, H. Prediction for the mixture toxicity of six organophosphorus pesticides to the luminescent bacterium Q67. Ecotoxicol. Environ. Saf. 2008, 71, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Liang, L.; Qin, L.; Qin, M.; Gao, H. Qualitative and Quantitative Assessment of the Toxicity Interactions of Mixtures of Four Heavy Metals and Two Pesticides on Vibrio fischeri. Asian J. Ecotoxicol. 2018, 13, 251–260. [Google Scholar]
- Vijver, M.G.; Peijnenburg, W.J.G.M.; De Snoo, G.R. Toxicological Mixture Models are Based on Inadequate Assumptions. Environ. Sci. Technol. 2010, 44, 4841–4842. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lei, L.; Liu, M.; Song, Y.; Lu, S.; Li, D.; Shi, H.; Raley-Susman, K.M.; He, D. Single and mixture toxicity of strobilurin and SDHI fungicides to Xenopus tropicalis embryos. Ecotoxicol. Environ. Saf. 2018, 153, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Li, J.; Wang, M.; Zhang, X.; Mi, Y.; Xiang, J.; Gong, S.; Zhou, Y.; Ma, T. Chronic toxicity effects of sediment-associated polystyrene nanoplastics alone and in combination with cadmium on a keystone benthic species Bellamya aeruginosa. J. Hazard. Mater. 2022, 433, 128800. [Google Scholar] [CrossRef]
- Bautista, C.J.; Arango, N.; Plata, C.; Mitre-Aguilar, I.B.; Trujillo, J.; Ramírez, V. Mechanism of cadmium-induced nephrotoxicity. Toxicology 2024, 502, 153726. [Google Scholar] [CrossRef]
- Branca, J.J.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-Induced Oxidative Stress: Focus on the Central Nervous System. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Du, J.; Liu, Q. Enrofloxacin induces intestinal disorders of metabolome and microbiome in American shad (Alosa sapidissima). Aquac. Res. 2021, 52, 6571–6580. [Google Scholar] [CrossRef]
- Wei, X.; Xu, Y.; Tan, X.; Lv, W.; Zhang, D.; He, Y.; Luo, Z. Enrofloxacin (ENR) exposure induces lipotoxicity by promoting mitochondrial fragmentation via dephosphorylation of DRP1 at S627 site. Chemosphere 2023, 340, 139892. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef]
- Bo, H.; Feng, J.; Zhang, X.; Wang, W.; Li, Q.; Liu, L.; He, C.; Tian, L. Research on Enrichment—Excretion Laws and Regulation of Strontium Ions in Zebrafish. Radiat. Prot. 2024, 44, 46–52. [Google Scholar]
- Isani, G.; Andreani, G.; Cocchioni, F.; Fedeli, D.; Carpene, E.; Falcioni, G. Cadmium accumulation and biochemical responses in Sparus aurata following sub-lethal Cd exposure. Ecotoxicol. Environ. Saf. 2009, 72, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Luo, K.; Ji, K.; Wang, L.; Chen, W. ABC transporter slr0982 affects response of Synechocystis sp. PCC 6803 to oxidative stress caused by methyl viologen. Res. Microbiol. 2022, 173, 103888. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, D.; Yi, X.; Su, J.; Duan, G.; Tang, X.; Zhu, Y. Combined pollution of arsenic and Polymyxin B enhanced arsenic toxicity and enriched ARG abundance in soil and earthworm gut microbiotas. J. Environ. Sci. 2021, 109, 171–180. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Hajam, M.E.; Plavan, G.; Kandri, N.I.; Dumitru, G.; Nicoara, M.N.; Zerouale, A.; Faggio, C. Evaluation of softwood and hardwood sawmill wastes impact on the common carp “Cyprinus carpio” and its aquatic environment: An oxidative stress study. Environ. Toxicol. Pharmacol. 2020, 75, 103327. [Google Scholar] [CrossRef]
- Mukherjee, D.; Saha, S.; Chukwuka, A.; Ghosh, B.; Dhara, K.; Saha, N.C.; Pal, P.; Faggio, C. Antioxidant enzyme activity and pathophysiological responses in the freshwater walking catfish, Clarias batrachus Linn under sub-chronic and chronic exposures to the neonicotinoid, Thiamethoxam®. Sci. Total Environ. 2022, 836, 155716. [Google Scholar] [CrossRef] [PubMed]
- Muthulakshmi, S.; Maharajan, K.; Habibi, H.R.; Kadirvelu, K.; Venkataramana, M. Zearalenone induced. embryo and neurotoxicity in zebrafish model (Danio rerio): Role of oxidative stress revealed by a multi biomarker study. Chemosphere 2018, 198, 111–121. [Google Scholar] [CrossRef]
- Plhalova, L.; Sehonova, P.; Blahova, J.; Doubkova, V.; Tichy, F.; Faggio, C.; Berankova, P.; Svobodova, Z. Evaluation of Tramadol Hydrochloride Toxicity to Juvenile Zebrafish-Morphological, Antioxidant and Histological Responses. Appl. Sci. 2020, 10, 2349. [Google Scholar] [CrossRef]
- Sula, E.; Aliko, V.; Barcelo, D.; Faggio, C. Combined effects of moderate hypoxia, pesticides and PCBs upon crucian carp fish, Carassius carassius, from a freshwater lake—in situ ecophysiological approach. Aquat. Toxicol. 2020, 228, 105644. [Google Scholar] [CrossRef]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological Effects of Pesticides on Hematological Parameters and Oxidative Enzymes in Freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Freitas, R.; Silvestro, S.; Coppola, F.; Meucci, V.; Battaglia, F.; Intorre, L.; Soares, A.M.V.M.; Pretti, C.; Faggio, C. Biochemical and physiological responses induced in Mytilus galloprovincialis after a chronic exposure to salicylic acid. Aquat. Toxicol. 2019, 214, 105258. [Google Scholar] [CrossRef]
- Pagano, M.; Savoca, S.; Impellitteri, F.; Albano, M.; Capillo, G.; Faggio, C. Toxicological Evaluation of Acetylsalicylic Acid in Non-Target Organisms: Chronic Exposure on Mytilus galloprovincialis (Lamarck, 1819). Front. Physiol. 2022, 13, 920952. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Capillo, G.; Fabrello, J.; Sandova, M.; Albano, M.; Zuskova, E.; Velisek, J.; Matozzo, V.; Faggio, C. Acute effects of neonicotinoid insecticides on Mytilus galloprovincialis: A case study with the active compound thiacloprid and the commercial formulation calypso 480 SC. Ecotoxicol. Environ. Saf. 2020, 203, 110980. [Google Scholar] [CrossRef]
- Yan, Z.; Li, S.; Xing, Q.; Nan, N.; Qin, G.; Sang, N. Research on the Differences in the Effects of PM2.5 from Different Cities on Liver Fibrosis in Mice. China Environ. Sci. 2022, 42, 2878–2885. [Google Scholar]
- Chen, S.; Zhou, Q.; Gao, S.; Liu, X.; Huang, D.; Yang, H. Effects of Heavy Metal Ions of Zinc, Chromium and Mercury on Antioxidant Enzyme Activities in Zebrafish. Guangzhou Chem. Ind. 2019, 47, 117–118. [Google Scholar]
- Chen, L.; Zhang, G.; Chen, J.; Ren, Z. Effects of Mercury and Selenium Exposure on the Antioxidant Enzyme System of Mytilus edulis. Asian J. Ecotoxicol. 2011, 6, 383–388. [Google Scholar]
- Nirwane, A.; Sridhar, V.; Majumdar, A. Neurobehavioural Changes and Brain Oxidative Stress Induced by Acute Exposure to GSM900 Mobile Phone Radiations in Zebrafish (Danio rerio). Toxicol. Res. 2016, 32, 123–132. [Google Scholar] [CrossRef]
- Chen, M.; Huang, C.; Pu, D.; Zheng, Z.; Yuan, K.; Jin, X.; Zhang, Y.; Jin, L. Toxic Effects of CdSe/ZnS Quantum Dots on the Embryonic Development of Zebrafish. Environ. Sci. 2015, 36, 719–726. [Google Scholar]
- Wang, X.; Bian, L.; Hu, Q.; Qin, B.; Chang, Q.; Ying, N.; Wu, Y.; Yang, L.; Chen, S. Effects of Cadmium on Acute Toxicity, Hepatic Antioxidant Capacity and Tissue Structure of Juvenile Thamnaconus modestus. Prog. Fish. Sci. 2023, 44, 74–84. [Google Scholar]
- Souid, G.; Souayed, N.; Yaktiti, F.; Maaroufi, K. Effect of acute cadmium exposure on metal accumulation and oxidative stress biomarkers of Sparus aurata. Ecotoxicol. Environ. Saf. 2013, 89, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bao, X.; Yue, Y.; Yang, K.; Liu, H.; Yang, Y.; Yu, H.; Yu, Y.; Duan, N. Combined effects of cadmium and nanoplastics on oxidative stress, histopatholog, and intestinal microbiota in largemouth bass (Micropterus salmoides). Aquaculture 2023, 569, 739363. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Y.; Yang, M.; Sun, S. Research on the Ecotoxicological Effects of the Combined Exposure of Cadmium and Prometryn on Earthworms. J. Southwest For. Univ. (Nat. Sci.) 2025, 45, 122–131. [Google Scholar]
- Backhed, F.; Manchester, J.K.; Semenkovich, C.F.; Gordon, J.I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 2007, 104, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Stephens, W.Z.; Stagaman, K.; Wong, S.; Rawls, J.F.; Guillemin, K.; Bohannan, B.J.M. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016, 10, 655–664. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; O’Sullivan, O.; Lucey, A.J.; Humphreys, M.; Hogan, A.; Hayes, P.; O’Reilly, M.; Jeffery, I.B.; Wood-Martin, R.; et al. Exercise and associated dietary extremes impact on gut microbial diversity. Gut 2014, 63, 1913–1920. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, A.; Wei, T.; Li, S.; Yang, B.; Xu, G.; Zou, J. Nanoplastics Induce More Serious Microbiota Dysbiosis and Inflammation in the Gut of Adult Zebrafish than Microplastics. Bull. Environ. Contam. Toxicol. 2021, 107, 640–650. [Google Scholar] [CrossRef]
- Liao, H.; Liu, S.; Junaid, M.; Gao, D.; Ai, W.; Chen, G.; Wang, J. Di-(2-ethylhexyl) phthalate exacerbated the toxicity of polystyrene nanoplastics through histological damage and intestinal microbiota dysbiosis in freshwater Micropterus salmoides. Water Res. 2022, 219, 118608. [Google Scholar] [CrossRef]
- Shin, N.; Whon, T.W.; Bae, J. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Li, Z.; Yan, L.; Junaid, M.; Chen, X.; Liao, H.; Gao, D.; Wang, Q.; Zhang, Y.; Wang, J. Impacts of polystyrene nanoplastics at the environmentally relevant and sub-lethal concentrations on the oxidative stress, immune responses, and gut microbiota to grass carp (Ctenopharyngodon idella). J. Hazard. Mater. 2023, 441, 129995. [Google Scholar] [CrossRef]
- Teame, T.; Wu, X.; Hao, Q.; Ding, Q.; Liu, H.; Ran, C.; Yang, Y.; Xia, L.; Wei, S.; Zhou, Z.; et al. Dietary SWF® enhanced growth performance and disease resistance in hybrid sturgeon (Acipenser baerii x Acipenser schrenckii) mediated by the gut microbiota. Aquac. Rep. 2020, 17, 100346. [Google Scholar] [CrossRef]
- Zheng, Q.; Cui, L.; Liao, H.; Junaid, M.; Li, Z.; Liu, S.; Gao, D.; Zheng, Y.; Lu, S.; Qiu, J.; et al. Combined exposure to polystyrene nanoplastics and bisphenol A induces hepato- and intestinal-toxicity and disturbs gut microbiota in channel catfish (Ictalurus punctatus). Sci. Total Environ. 2023, 891, 164319. [Google Scholar] [CrossRef]
- Liu, P.; Wan, Y.; Zhang, Z.; Ji, Q.; Lian, J.; Yang, C.; Wang, X.; Qin, B.; Zhu, L.; Yu, J. Toxic effects of combined exposure to cadmium and nitrate on intestinal morphology, immune response, and microbiota in juvenile Japanese flounder (Paralichthys olivaceus). Aquat. Toxicol. 2023, 264, 106704. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, F.; Derome, N. Vertically and horizontally transmitted microbial symbionts shape the gut microbiota ontogenesis of a skin-mucus feeding discus fish progeny. Sci. Rep. 2017, 7, 5263. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, X.; Qiu, Q.; Chen, J.; Xiong, J. Identifying Potential Polymicrobial Pathogens: Moving Beyond Differential Abundance to Driver Taxa. Microb. Ecol. 2020, 80, 447–458. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Wei, W.; Yang, Q.; Xiang, D.; Chen, X.; Wen, Z.; Wang, X.; Xu, X.; Peng, C.; Yang, L.; Luo, M.; et al. Combined impacts of microplastics and cadmium on the liver function, immune response, and intestinal microbiota of crucian carp (Carassius carassius). Ecotoxicol. Environ. Saf. 2023, 261, 115104. [Google Scholar] [CrossRef]
- Zhang, F.; Li, D.; Yang, Y.; Zhang, H.; Zhu, J.; Liu, J.; Bu, X.; Li, E.; Qin, J.; Yu, N.; et al. Combined effects of polystyrene microplastics and copper on antioxidant capacity, immune response and intestinal microbiota of Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2022, 808, 152099. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; He, Y.; Hou, C.; Liao, C.; Chen, M. Toxicological Effects of Combined Exposure of Cadmium and Enrofloxacin on Zebrafish. Toxics 2025, 13, 378. https://doi.org/10.3390/toxics13050378
Ren L, He Y, Hou C, Liao C, Chen M. Toxicological Effects of Combined Exposure of Cadmium and Enrofloxacin on Zebrafish. Toxics. 2025; 13(5):378. https://doi.org/10.3390/toxics13050378
Chicago/Turabian StyleRen, Lingfei, Yu He, Chao Hou, Chaoxuan Liao, and Miao Chen. 2025. "Toxicological Effects of Combined Exposure of Cadmium and Enrofloxacin on Zebrafish" Toxics 13, no. 5: 378. https://doi.org/10.3390/toxics13050378
APA StyleRen, L., He, Y., Hou, C., Liao, C., & Chen, M. (2025). Toxicological Effects of Combined Exposure of Cadmium and Enrofloxacin on Zebrafish. Toxics, 13(5), 378. https://doi.org/10.3390/toxics13050378





