Benchmark Dose of Urinary Cadmium for Assessing Renal Tubular and Glomerular Function in a Cadmium-Polluted Area of Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Measurement
2.2. Statistical Analysis
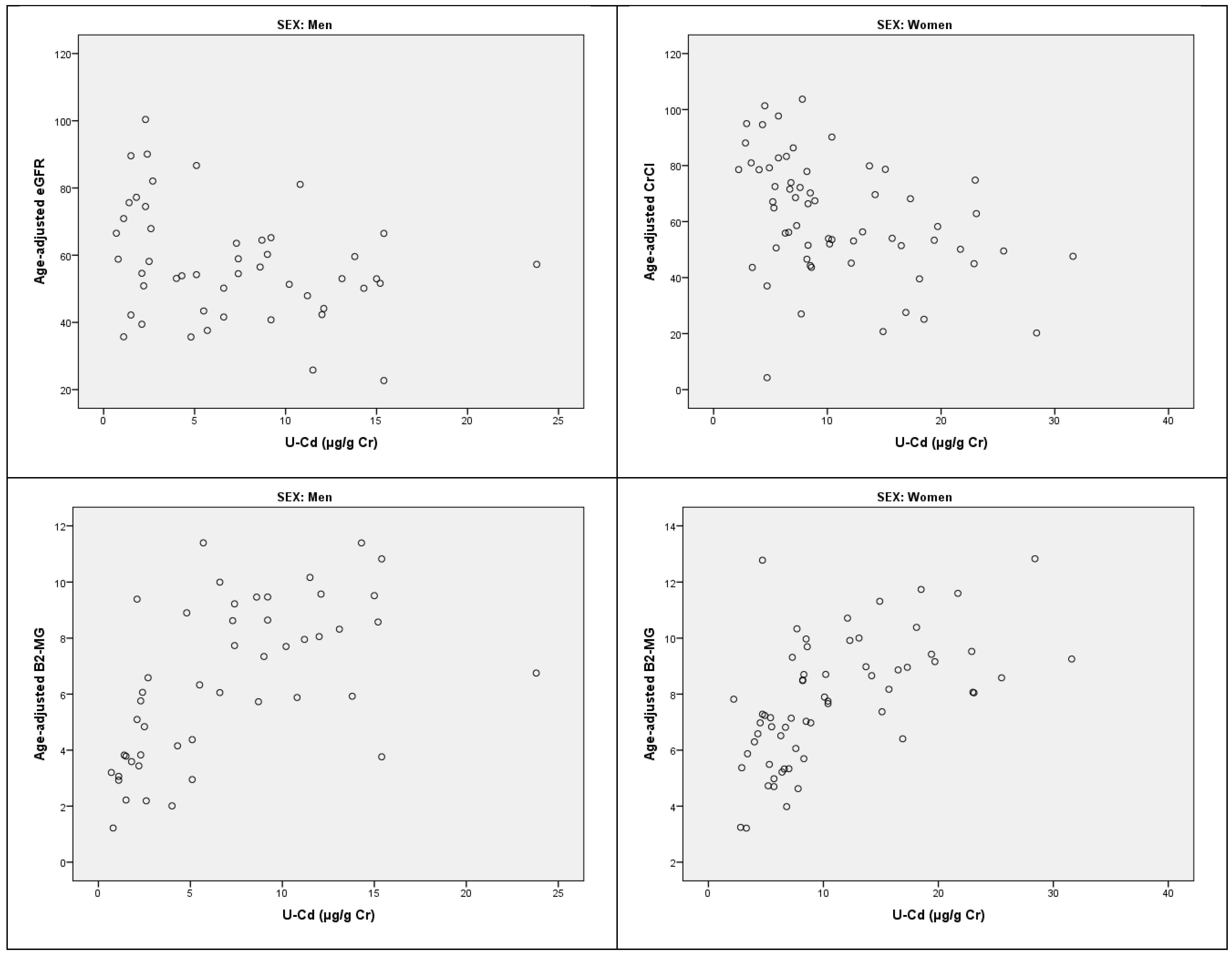
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | Benchmark dose |
| BMDL | Benchmark dose low |
| BMR | Benchmark response |
| Cd | Cadmium |
| CKD | Chronic kidney disease |
| Cr | Creatinine |
| CrCl | Creatinine clearance |
| eGFR | Estimated glomerular filtration rate |
| EFSA | European Food Safety Authority |
| %TRP | Fraction of tubular reabsorption of filtered phosphorus |
| %TRβ2-MG | Fraction of tubular reabsorption of filtered β2-MG |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| PTWI | Provisional tolerable weekly intake |
| CONTAM | Scientific Panel on Contaminants in the Food Chain |
| U-Cd | Urinary cadmium |
| β2-MG | Β2-microglobulin |
References
- Nordberg Gunnar, F.; Bernard, A.; Diamond Gary, L.; Duffus John, H.; Illing, P.; Nordberg, M.; Bergdahl Ingvar, A.; Jin, T.; Skerfving, S. Risk assessment of effects of cadmium on human health (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 755–808. [Google Scholar] [CrossRef]
- Suwazono, Y.; Watanabe, Y.; Nogawa, K.; Nogawa, K. Itai-Itai Disease. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 712–719. [Google Scholar]
- Imura, J.; Tsuneyama, K.; Ueda, Y. Novel Pathological Study of Cadmium Nephropathy of Itai-itai Disease. In Cadmium Toxicity: New Aspects in Human Disease, Rice Contamination, and Cytotoxicity; Himeno, S., Aoshima, K., Eds.; Springer Singapore: Singapore, 2019; pp. 39–50. [Google Scholar]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L. Health hazards in the manufacture of alkaline accumulators with special reference to chronic cadmium poisoning; a clinical and experimental study. Acta Med. Scand. Suppl. 1950, 240, 1–124. [Google Scholar] [PubMed]
- Nordberg, G.F.; Nogawa, K.; Nordberg, M. Chapter 32—Cadmium. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 667–716. [Google Scholar]
- Järup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Åkesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and Glomerular Kidney Effects in Swedish Women with Low Environmental Cadmium Exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef]
- Crump, K.S. A new method for determining allowable daily intakes. Fundam. Appl. Toxicol. 1984, 4, 854–871. [Google Scholar] [CrossRef]
- U.S. EPA. The Use of the Benchmark Dose (BMD) Approach in Health Risk Assessment, Final Report; EPA/630/R-94/007; Risk Assessment Forum, U.S. Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- Suwazono, Y.; Uetani, M.; Akesson, A.; Vahter, M. Recent applications of benchmark dose method for estimation of reference cadmium exposure for renal effects in man. Toxicol. Lett. 2010, 198, 40–43. [Google Scholar] [CrossRef]
- Suwazono, Y.; Sand, S.; Vahter, M.; Filipsson, A.F.; Skerfving, S.; Lidfeldt, J.; Akesson, A. Benchmark dose for cadmium-induced renal effects in humans. Environ. Health Perspect. 2006, 114, 1072–1076. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Đorđević, A.B. The Validity of Benchmark Dose Limit Analysis for Estimating Permissible Accumulation of Cadmium. Int. J. Environ. Res. Public. Health 2022, 19, 15697. [Google Scholar] [CrossRef]
- Crump, K. Calculation of benchmark doses from continuous data. Risk Anal. 1995, 15, 79–89. [Google Scholar] [CrossRef]
- Sand, S.; Victorin, K.; Filipsson, A.F. The current state of knowledge on the use of the benchmark dose concept in risk assessment. J. Appl. Toxicol. 2008, 28, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, D.W.; Slikker, W.T., Jr. Risk assessment for neurotoxic effects. Neurotoxicology 1990, 11, 211–218. [Google Scholar] [PubMed]
- Kodell, R.L.; West, R.W. Upper confidence limits on excess risk for quantitative responses. Risk Anal. 1993, 13, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Sand, S.; von Rosen, D.; Victorin, K.; Filipsson, A.F. Identification of a critical dose level for risk assessment: Developments in benchmark dose analysis of continuous endpoints. Toxicol. Sci. 2006, 90, 241–251. [Google Scholar] [CrossRef]
- Ishikawa Prefectural Health and Welfare Department. Report on the Health Survey of the Inhabitants of the Kakehashi River Basin; Ishikawa Prefectural Health and Welfare Department: Ishikawa, Japan, 1984; pp. 1–56. (In Japanese) [Google Scholar]
- Kido, T.; Nogawa, K.; Ohmichi, M.; Honda, R.; Tsuritani, I.; Ishizaki, M.; Yamada, Y. The renal handling of sodium and potassium in environmental cadmium-exposed subjects with renal dysfunction. Toxicol. Lett. 1992, 61, 205–212. [Google Scholar] [CrossRef]
- Bonness, R.W.; Taussky, M.H. On the calorimetric determination of creatinine by the Jaffe reaction. J. Biol. Chem. 1945, 158, 581–591. [Google Scholar] [CrossRef]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989, 5, 303–311, discussion 312–303. [Google Scholar]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Japanese Society of Nephrology. Essential points from evidence-based clinical practice guideline for chronic kidney disease 2023. Clin. Exp. Nephrol. 2024, 28, 473–495. [Google Scholar] [CrossRef]
- Imai, E.; Horio, M.; Iseki, K.; Yamagata, K.; Watanabe, T.; Hara, S.; Ura, N.; Kiyohara, Y.; Hirakata, H.; Moriyama, T.; et al. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clin. Exp. Nephrol. 2007, 11, 156–163. [Google Scholar] [CrossRef]
- Karlsson, F.A.; Wibell, L.; Evrin, P.E. beta 2-Microglobulin in clinical medicine. Scand. J. Clin. Lab. Invest. Suppl. 1980, 154, 27–37. [Google Scholar]
- EFSA. Scientific Opinion of the Panel on Contaminants in the Food Chain on a request from the European Commission on cadmium in food. EFSA J. 2009, 7, 980. [Google Scholar]
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants (Thirty-Third Report of the Joint FAO/WHO Expert Committee on Food Additives); WHO Technical Report Series, No. 776; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants. (Fifty-Fifth Report of the Joint FAO/WHO Expert Committee on Food Additives); WHO Technical Report Series, No. 901; World Health Organization (WHO): Geneva, Switzerland, 2001. [Google Scholar]
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants: Seventy-Third [73rd] Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series, No. 960; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
| Cd-Polluted Area | Non-Polluted Area | All | ||||
|---|---|---|---|---|---|---|
| Men (N = 30) | Women (N = 44) | Men (N = 18) | Women (N = 18) | Men (N = 48) | Women (N = 62) | |
| Variable | Mean (SD 1) | Mean (SD 1) | Mean (SD 1) | Mean (SD 1) | Mean (SD 1) | Mean (SD 1) |
| Age, y | 74.1 (7.8) | 73.2 (7.2) | 62.9 (9.9) | 63.9 (9.0) | 69.9 (10.1) | 70.5 (8.8) |
| CrCl, mL/min | 52.8 (23.0) | 48.6 (17.8) | 96.7 (30.3) | 90.9 (18.4) | 69.3 (33.5) | 60.9 (26.3) |
| eGFR, mL/min | 46.9 (12.4) | 46.7 (17.7) | 75.5 (18.4) | 83.7 (15.8) | 58.1 (20.6) | 58.1 (24.5) |
| GM 2 (GSD 3) | GM 2 (GSD 3) | GM 2 (GSD 3) | GM 2 (GSD 3) | GM 2 (GSD 3) | GM 2 (GSD 3) | |
| U-Cd, μg/g Cr | 9.0 (1.6) | 11.6 (1.7) | 1.9 (1.7) | 4.8 (1.5) | 5.0 (2.5) | 9.0 (1.9) |
| U-β2-MG, μg/g Cr | 5537.9 (7.2) | 10,029.4 (5.9) | 14.8 (6.5) | 95.6 (7.1) | 600.3 (32.2) | 2597.5 (16.4) |
| %TRβ2-MG 4, % | 91.0 (1.1) | 82.7 (1.4) | 100.0 (1.0) | 99.8 (1.0) | 94.3 (1.1) | 87.4 (1.4) |
| Prevalence of Decreased %TRβ2-MG 4 | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| %TRβ2-MG 4 < 95% | 16 (53.3%) | 23 (52.3%) | 0 (0.0%) | 0 (0.0%) | 16 (33.3) | 23 (37.1) |
| %TRβ2-MG 4 < 90% | 9 (30.0%) | 16 (36.4%) | 0 (0.0%) | 0 (0.0%) | 9 (18.8) | 16 (25.8) |
| Renal Effect Marker | Men | Women | |||
|---|---|---|---|---|---|
| Multiple Regression Analysis | Explanatory Variable | B 1 (95% CI 2) | p | B 1 (95% CI 2) | p |
| CrCl (mL/min) | U-Cd, μg/g Cr | −1.37 (−3.11, 0.36) | 0.118 | −1.24 (−1.99, −0.48) | 0.002 |
| Age, y | −1.32 (−2.23, −0.41) | 0.005 | −1.55 (−2.14, −0.96) | <0.001 | |
| eGFR (mL/min) | U-Cd, μg/g Cr | −1.02 (−2.01, −0.04) | 0.041 | −0.65 (−1.39, 0.09) | 0.082 |
| Age, y | −0.97 (−1.49, −0.46) | <0.001 | −1.56 (−2.14, −0.98) | <0.001 | |
| β2-MG (μg/g cr) 3 | U-Cd, μg/g Cr | 0.30 (0.16, 0.44) | <0.001 | 0.18 (0.11, 0.25) | <0.001 |
| Age, y | 0.15 (0.08, 0.23) | <0.001 | 0.16 (0.11, 0.22) | <0.001 | |
| Logistic Regression Analysis | Explanatory Variable | OR 4 (95% CI 2) | p | OR 4 (95% CI 2) | p |
| %TRβ2-MG 5 (<95%) | U-Cd, μg/g Cr | 1.17 (1.02, 1.35) | 0.022 | 1.17 (1.05, 1.29) | 0.003 |
| Age, y | 1.04 (0.97, 1.13) | 0.280 | 1.08 (1.00, 1.17) | 0.061 | |
| %TRβ2-MG 5 (<90%) | U-Cd, μg/g Cr | 1.21 (1.03, 1.42) | 0.021 | 1.12 (1.02, 1.23) | 0.017 |
| Age, y | 1.07 (0.96, 1.18) | 0.213 | 1.09 (1.00, 1.20) | 0.057 |
| BMR = 5% | BMR = 10% | |||
|---|---|---|---|---|
| Hybrid Approach | P (0) 1 | Cut-Off Value 2 | BMDL (BMD) | BMDL (BMD) |
| CrCl (women) | 5.0% | 43.3 mL/min | 3.5 (5.7) μg/g Cr | 5.9 (9.6) μg/g Cr |
| eGFR (men) | 5.0% | 38.8 mL/min | 2.9 (5.7) μg/g Cr | 4.9 (9.6) μg/g Cr |
| U-β2-MG (men) | 5.0% | 3094 μg/g Cr | 1.8 (2.8) μg/g Cr | 3.1 (4.6) μg/g Cr |
| U-β2-MG (women) | 5.0% | 6194 μg/g Cr | 2.5 (3.6) μg/g Cr | 4.2 (6.0) μg/g Cr |
| Logistic Regression Analysis | Cut-Off Value | P (0) 3 | BMDL (BMD) | BMDL (BMD) |
| %TRβ2-MG (men) | <95% | 11.8% | 1.8 (2.6) μg/g Cr | 3.1 (4.6) μg/g Cr |
| %TRβ2-MG (women) | <95% | 8.1% | 2.6 (3.5) μg/g Cr | 4.5 (6.0) μg/g Cr |
| %TRβ2-MG (men) | <90% | 3.4% | 3.6 (5.0) μg/g Cr | 5.6 (7.7) μg/g Cr |
| %TRβ2-MG (women) | <90% | 6.6% | 3.9 (5.4) μg/g Cr | 6.6 (9.1) μg/g Cr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, T.; Nogawa, K.; Watanabe, Y.; Kido, T.; Sakurai, M.; Nakagawa, H.; Suwazono, Y. Benchmark Dose of Urinary Cadmium for Assessing Renal Tubular and Glomerular Function in a Cadmium-Polluted Area of Japan. Toxics 2024, 12, 836. https://doi.org/10.3390/toxics12120836
Hayashi T, Nogawa K, Watanabe Y, Kido T, Sakurai M, Nakagawa H, Suwazono Y. Benchmark Dose of Urinary Cadmium for Assessing Renal Tubular and Glomerular Function in a Cadmium-Polluted Area of Japan. Toxics. 2024; 12(12):836. https://doi.org/10.3390/toxics12120836
Chicago/Turabian StyleHayashi, Takuya, Kazuhiro Nogawa, Yuuka Watanabe, Teruhiko Kido, Masaru Sakurai, Hideaki Nakagawa, and Yasushi Suwazono. 2024. "Benchmark Dose of Urinary Cadmium for Assessing Renal Tubular and Glomerular Function in a Cadmium-Polluted Area of Japan" Toxics 12, no. 12: 836. https://doi.org/10.3390/toxics12120836
APA StyleHayashi, T., Nogawa, K., Watanabe, Y., Kido, T., Sakurai, M., Nakagawa, H., & Suwazono, Y. (2024). Benchmark Dose of Urinary Cadmium for Assessing Renal Tubular and Glomerular Function in a Cadmium-Polluted Area of Japan. Toxics, 12(12), 836. https://doi.org/10.3390/toxics12120836







