Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Systems: A Comprehensive Review of Sources, Distribution, and Ecotoxicological Impacts
Abstract
:1. Introduction
1.1. Polycyclic Aromatic Hydrocarbons
1.2. Freshwater Systems
1.3. Current Research Gaps and Emerging Perspectives in PAH Studies in Freshwater Systems
2. Sources of PAHs in Freshwater Systems
2.1. Rivers
2.2. Streams
2.3. Lakes
2.4. Wetlands
2.5. Groundwater
2.6. Glaciers
3. Distribution of PAHs in Freshwater Systems
3.1. Transport Mechanisms
3.1.1. Physical Transport Mechanisms
3.1.2. Chemical Transport Mechanisms
3.1.3. Biological Transport Mechanisms
3.2. Factors Affecting Distribution of PAHs
3.3. Interactions with Environmental Matrices
4. Ecotoxicological Impacts of PAHs in Freshwater Systems
4.1. Molecular and Cellular Level Effects
4.2. Impacts on Freshwater Biota
4.2.1. Bioaccumulation
4.2.2. Genotoxic, Mutagenic, and Carcinogenic Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAHs | Polycyclic aromatic hydrocarbons |
| LMW-PAHs | Low molecular weight polycyclic aromatic hydrocarbons |
| HMW-PAH | High molecular weight polycyclic aromatic hydrocarbons |
| LRTs | Long-range transport |
| USEPA | United States Environmental Protection Agency |
| WHO | World Health Organization |
| SPM | Suspended particulate |
| OM | Organic matter |
| OC | Organic carbon |
| BaP | Benzo[a]pyrene |
| ROS | Reactive oxygen species |
| OS | Oxidative stress |
| CYP | Cytochrome P450 |
| Log Kow | Octanol-water partitioning |
References
- Okere, U.V. Biodegradation of PAHs in ‘Pristine’ Soils from Different Climatic Regions. J. Bioremed. Biodegrad. 2011, S1, 006. [Google Scholar] [CrossRef]
- Edokpayi, J.; Odiyo, J.; Popoola, O.; Msagati, T. Determination and Distribution of Polycyclic Aromatic Hydrocarbons in Rivers, Sediments and Wastewater Effluents in Vhembe District, South Africa. Int. J. Environ. Res. Public Health 2016, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Boehm, P.D.; Page, D.S.; Brown, J.S.; Neff, J.M.; Burns, W.A. Polycyclic Aromatic Hydrocarbon Levels in Mussels from Prince William Sound, ALASKA, USA, Document the Return to Baseline Conditions. Environ. Toxicol. Chem. 2004, 23, 2916–2929. [Google Scholar] [CrossRef]
- Baali, A.; Yahyaoui, A.; Baali, A.; Yahyaoui, A. Polycyclic Aromatic Hydrocarbons (PAHs) and Their Influence to Some Aquatic Species. In Biochemical Toxicology—Heavy Metals and Nanomaterials; IntechOpen: London, UK, 2019; ISBN 978-1-78984-697-3. [Google Scholar]
- Provencher, J.F.; Thomas, P.J.; Pauli, B.; Braune, B.M.; Franckowiak, R.P.; Gendron, M.; Savard, G.; Sarma, S.N.; Crump, D.; Zahaby, Y.; et al. Polycyclic Aromatic Compounds (PACs) and Trace Elements in Four Marine Bird Species from Northern Canada in a Region of Natural Marine Oil and Gas Seeps. Sci. Total Environ. 2020, 744, 140959. [Google Scholar] [CrossRef]
- Azuma, H.; Toyota, M.; Asakawa, Y.; Kawano, S. Naphthalene—A Constituent of Magnolia Flowers. Phytochemistry 1996, 42, 999–1004. [Google Scholar] [CrossRef]
- Jürgens, A.; Webber, A.C.; Gottsberger, G. Floral Scent Compounds of Amazonian Annonaceae Species Pollinated by Small Beetles and Thrips. Phytochemistry 2000, 55, 551–558. [Google Scholar] [CrossRef]
- Hansen, D.J.; DiToro, D.M.; McGrath, J.A.; Swartz, R.C.; Mount, D.R.; Spehar, R.L.; Burgess, R.M.; Ozretich, R.J.; Bell, H.E.; Reiley, M.C.; et al. Procedures for the Derivation of Equilibrium Partitioning Sediment Benchmarks (ESBs) for the Protection of Benthic Organisms: PAH Mixtures 2003; EPA: Washington, DC, USA, 2003.
- Dhar, K.; Subashchandrabose, S.R.; Venkateswarlu, K.; Krishnan, K.; Megharaj, M. Anaerobic Microbial Degradation of Polycyclic Aromatic Hydrocarbons: A Comprehensive Review. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Ed.; Reviews of Environmental Contamination and Toxicology; Springer International Publishing: Cham, Switzerland, 2020; Volume 251, pp. 25–108. ISBN 978-3-030-27149-7. [Google Scholar]
- Wang, Z.; Yang, C.; Parrott, J.L.; Frank, R.A.; Yang, Z.; Brown, C.E.; Hollebone, B.P.; Landriault, M.; Fieldhouse, B.; Liu, Y.; et al. Forensic Source Differentiation of Petrogenic, Pyrogenic, and Biogenic Hydrocarbons in Canadian Oil Sands Environmental Samples. J. Hazard. Mater. 2014, 271, 166–177. [Google Scholar] [CrossRef]
- Mali, M.; Ragone, R.; Dell’Anna, M.M.; Romanazzi, G.; Damiani, L.; Mastrorilli, P. Improved Identification of Pollution Source Attribution by Using PAH Ratios Combined with Multivariate Statistics. Sci. Rep. 2022, 12, 19298. [Google Scholar] [CrossRef]
- Pang, S.Y.; Suratman, S.; Latif, M.T.; Khan, M.F.; Simoneit, B.R.T.; Mohd Tahir, N. Polycyclic Aromatic Hydrocarbons in Coastal Sediments of Southern Terengganu, South China Sea, Malaysia: Source Assessment Using Diagnostic Ratios and Multivariate Statistic. Environ. Sci. Pollut. Res. 2022, 29, 15849–15862. [Google Scholar] [CrossRef]
- Bralewska, K.; Rakowska, J. Concentrations of Particulate Matter and PM-Bound Polycyclic Aromatic Hydrocarbons Released during Combustion of Various Types of Materials and Possible Toxicological Potential of the Emissions: The Results of Preliminary Studies. Int. J. Environ. Res. Public Health 2020, 17, 3202. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Nohchi, M.; Yang, X.; Sugiyama, T.; Miura, K.; Takami, A.; Sato, K.; Chen, X.; Kato, S.; Kajii, Y.; et al. Degradation of PAHs during Long Range Transport Based on Simultaneous Measurements at Tuoji Island, China, and at Fukue Island and Cape Hedo, Japan. Environ. Pollut. 2020, 260, 113906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, L.; Zhou, Q.; Zhang, X.; Xing, W.; Wei, Y.; Hu, M.; Zhao, L.; Toriba, A.; Hayakawa, K.; et al. Size Distribution of Particulate Polycyclic Aromatic Hydrocarbons in Fresh Combustion Smoke and Ambient Air: A Review. J. Environ. Sci. 2020, 88, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Hoque, R.R.; Balachandran, S.; Medhi, S.; Idris, M.G.; Rahman, M.; Hussain, F.L. Monitoring and Risk Analysis of PAHs in the Environment. In Handbook of Environmental Materials Management; Hussain, C.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 973–1007. ISBN 978-3-319-73645-7. [Google Scholar]
- Bhatti, S.S.; Bhatia, A.; Bhagat, G.; Singh, S.; Dhaliwal, S.S.; Sharma, V.; Verma, V.; Yin, R.; Singh, J. PAHs in Terrestrial Environment and Their Phytoremediation. In Bioremediation for Sustainable Environmental Cleanup; CRC Press: Boca Raton, FL, USA, 2024; ISBN 978-1-003-27794-1. [Google Scholar]
- Ashayeri, N.Y.; Keshavarzi, B.; Moore, F.; Kersten, M.; Yazdi, M.; Lahijanzadeh, A.R. Presence of Polycyclic Aromatic Hydrocarbons in Sediments and Surface Water from Shadegan Wetland—Iran: A Focus on Source Apportionment, Human and Ecological Risk Assessment and Sediment-Water Exchange. Ecotoxicol. Environ. Saf. 2018, 148, 1054–1066. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef]
- Keith, L.H. The Source of U.S. EPA’s Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- World Health Organization. Human Health Effects of Polycyclic Aromatic Hydrocarbons as Ambient Air Pollutants: Report of the Working Group on Polycyclic Aromatic Hydrocarbons of the Joint Task Force on the Health Aspects of Air Pollution; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2021; ISBN 978-92-890-5653-3.
- Canadian Council of Ministers of the Environment. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health: Polycyclic Aromatic Hydrocarbons 2010; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2010.
- Kelly, C.; Santillo, D.; Johnston, P.; Fayad, G.; Baker, K.L.; Law, R.J. Polycyclic Aromatic Hydrocarbons in Oysters from Coastal Waters of the Lebanon 10 Months after the Jiyeh Oil Spill in 2006. Mar. Pollut. Bull. 2008, 56, 1215–1218. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Farrell, C.M.; Feldgarden, M.; Fine, A.M.; Sherry, S.T.; et al. National Center for Biotechnology Information PubChem Dataset 2023. Nucleic Acids Res. 2023, 51, D29–D38. [Google Scholar] [CrossRef]
- Shen, H. Polycyclic Aromatic Hydrocarbons; Springer Theses; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-49678-7. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Polycyclic Aromatic Hydrocarbons; U.S. Department of Health and Human Services: Washington, DC, USA, 1996.
- Mackay, D.; Shiu, W.-Y.; Shiu, W.-Y.; Lee, S.C. Handbook of Physical-Chemical Properties and Environmental Fate for Organic Chemicals, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-15007-4. [Google Scholar]
- Ji, G.; Zou, L.; Guan, W.; Yang, T.; Qiu, H.; Zhu, L. Partition, Transportation and Ecological Risks of Polycyclic Aromatic Hydrocarbons (PAHs) under Heavy Anthropogenic Estuary: A Case Study in the Xiaoqing River Estuary, North China. Reg. Stud. Mar. Sci. 2021, 43, 101664. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, H.; Wang, R.; Tang, Z.; Yang, M. Distribution and Risk Assessment of 80 Polycyclic Aromatic Compounds in Surface Water and Industrial Effluent in the Upper Yangtze River, China. ACS EST Water 2024, 4, 2300–2308. [Google Scholar] [CrossRef]
- Azimi, A.; Riahi Bakhtiari, A.; Tauler, R. Polycyclic Aromatic Hydrocarbon Source Fingerprints in the Environmental Samples of Anzali—South of Caspian Sea. Environ. Sci. Pollut. Res. 2020, 27, 32719–32731. [Google Scholar] [CrossRef] [PubMed]
- Ouro-Sama, K.; Tanouayi, G.; Solitoke, H.D.; Barsan, N.; Mosnegutu, E.; Badassan, T.E.-E.; Agbere, S.; Adje, K.; Nedeff, V.; Gnandi, K. Polycyclic Aromatic Hydrocarbons (PAHs) Contamination in Chrysichthys Nigrodigitatus Lacépède, 1803 from Lake Togo-Lagoon of Aného, Togo: Possible Human Health Risk Suitable to Their Consumption. Int. J. Environ. Res. Public Health 2023, 20, 1666. [Google Scholar] [CrossRef] [PubMed]
- Sahai, H.; García Valverde, M.; Murcia Morales, M.; Hernando, M.D.; Aguilera Del Real, A.M.; Fernández- Alba, A.R. Exploring Sorption of Pesticides and PAHs in Microplastics Derived from Plastic Mulch Films Used in Modern Agriculture. Chemosphere 2023, 333, 138959. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, D.L.; Van Vleet, E.S. Accumulation and Distribution of Petroleum Hydrocarbons Found in Mussels (Mytilus galloprovincialis) in the Canals of Venice, Italy. Mar. Pollut. Bull. 2004, 48, 927–936. [Google Scholar] [CrossRef]
- Ambade, B.; Sethi, S.S. Health Risk Assessment and Characterization of Polycyclic Aromatic Hydrocarbon from the Hydrosphere. J. Hazard. Toxic Radioact. Waste 2021, 25, 05020008. [Google Scholar] [CrossRef]
- Montuori, P.; De Rosa, E.; Di Duca, F.; De Simone, B.; Scippa, S.; Russo, I.; Sarnacchiaro, P.; Triassi, M. Polycyclic Aromatic Hydrocarbons (PAHs) in the Dissolved Phase, Particulate Matter, and Sediment of the Sele River, Southern Italy: A Focus on Distribution, Risk Assessment, and Sources. Toxics 2022, 10, 401. [Google Scholar] [CrossRef]
- Santos, E.; Souza, M.R.R.; Junior, A.R.V.; da Silva Soares, L.; Frena, M.; Alexandre, M.R. Polycyclic Aromatic Hydrocarbons in Suspended Particulate Matter of a Region Influenced by Agricultural Activities in Northeast Brazil. Reg. Stud. Mar. Sci. 2023, 57, 102683. [Google Scholar] [CrossRef]
- Yang, J.; Qadeer, A.; Liu, M.; Zhu, J.-M.; Huang, Y.-P.; Du, W.-N.; Wei, X.-Y. Occurrence, Source, and Partition of PAHs, PCBs, and OCPs in the Multiphase System of an Urban Lake, Shanghai. Appl. Geochem. 2019, 106, 17–25. [Google Scholar] [CrossRef]
- Cathey, A.L.; Watkins, D.J.; Rosario, Z.Y.; Vélez Vega, C.M.; Loch-Caruso, R.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Polycyclic Aromatic Hydrocarbon Exposure Results in Altered CRH, Reproductive, and Thyroid Hormone Concentrations during Human Pregnancy. Sci. Total Environ. 2020, 749, 141581. [Google Scholar] [CrossRef]
- Sun, K.; Song, Y.; He, F.; Jing, M.; Tang, J.; Liu, R. A Review of Human and Animals Exposure to Polycyclic Aromatic Hydrocarbons: Health Risk and Adverse Effects, Photo-Induced Toxicity and Regulating Effect of Microplastics. Sci. Total Environ. 2021, 773, 145403. [Google Scholar] [CrossRef]
- Gupte, A.; Tripathi, A.; Patel, H.; Rudakiya, D.; Gupte, S. Bioremediation of Polycyclic Aromatic Hydrocarbon (PAHs): A Perspective. Open Biotechnol. J. 2016, 10, 363–378. [Google Scholar] [CrossRef]
- Méndez García, M.; García de Llasera, M.P. A Review on the Enzymes and Metabolites Identified by Mass Spectrometry from Bacteria and Microalgae Involved in the Degradation of High Molecular Weight PAHs. Sci. Total Environ. 2021, 797, 149035. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F.; Rocha, B.A.; Souza, M.C.O.; Bocato, M.Z.; Azevedo, L.F.; Adeyemi, J.A.; Santana, A.; Campiglia, A.D. Polycyclic Aromatic Hydrocarbons (PAHs): Updated Aspects of Their Determination, Kinetics in the Human Body, and Toxicity. J. Toxicol. Environ. Health B Crit. Rev. 2023, 26, 28–65. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Klimkowicz-Pawlas, A.; Ukalska-Jaruga, A.; Mierzwa-Hersztek, M.; Gondek, K.; Szara-Bąk, M.; Tarnawski, M.; Spałek, I. Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in the Bottom Sediments of a Dam Reservoir, Their Interaction with Organic Matter and Risk to Benthic Fauna. J. Soils Sediments 2021, 21, 2418–2431. [Google Scholar] [CrossRef]
- Jesus, F.; Pereira, J.L.; Campos, I.; Santos, M.; Ré, A.; Keizer, J.; Nogueira, A.; Gonçalves, F.J.M.; Abrantes, N.; Serpa, D. A Review on Polycyclic Aromatic Hydrocarbons Distribution in Freshwater Ecosystems and Their Toxicity to Benthic Fauna. Sci. Total Environ. 2022, 820, 153282. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N. Forest Fires as Drivers of Contamination of Polycyclic Aromatic Hydrocarbons to the Terrestrial and Aquatic Ecosystems. Curr. Opin. Environ. Sci. Health 2021, 24, 100293. [Google Scholar] [CrossRef]
- Baumard, P.; Budzinski, H.; Michon, Q.; Garrigues, P.; Burgeot, T.; Bellocq, J. Origin and Bioavailability of PAHs in the Mediterranean Sea from Mussel and Sediment Records. Estuar. Coast. Shelf Sci. 1998, 47, 77–90. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Man, Y.B.; Mo, W.Y.; Zhang, F.; Wong, M.H. Health Risk Assessments Based on Polycyclic Aromatic Hydrocarbons in Freshwater Fish Cultured Using Food Waste-Based Diets. Environ. Pollut. 2020, 256, 113380. [Google Scholar] [CrossRef]
- Shiklomanov, I. World Fresh Water Resources. In Water in Crisis: A Guide to the World’s Fresh Water Resources; Gleick, P.H., Ed.; Oxford University Press: New York, NY, USA, 1993; ISBN 0-19-507628-1. [Google Scholar]
- Shiklomanov, I.A. Appraisal and Assessment of World Water Resources. Water Int. 2000, 25, 11–32. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Gleick, P.H. Water Resources. In Encyclopedia of Climate and Weather; Oxford University Press: New York, NY, USA, 1996; pp. 817–823. [Google Scholar]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Irfan, S.; Alatawi, A.M.M. Aquatic Ecosystem and Biodiversity: A Review. Open J. Ecol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ Warning to Humanity on the Freshwater Biodiversity Crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef]
- Meier, S.; Karlsen, Ø.; Goff, J.L.; Sørensen, L.; Sørhus, E.; Pampanin, D.M.; Donald, C.E.; Fjelldal, P.G.; Dunaevskaya, E.; Romano, M.; et al. DNA Damage and Health Effects in Juvenile Haddock (Melanogrammus aeglefinus) Exposed to PAHs Associated with Oil-Polluted Sediment or Produced Water. PLoS ONE 2020, 15, e0240307. [Google Scholar] [CrossRef]
- Yazdani, M. Comparative Toxicity of Selected PAHs in Rainbow Trout Hepatocytes: Genotoxicity, Oxidative Stress and Cytotoxicity. Drug Chem. Toxicol. 2020, 43, 71–78. [Google Scholar] [CrossRef]
- Simão, F.C.P.; Gravato, C.; Machado, A.L.; Soares, A.M.V.M.; Pestana, J.L.T. Toxicity of Different Polycyclic Aromatic Hydrocarbons (PAHs) to the Freshwater Planarian Girardia Tigrina. Environ. Pollut. 2020, 266, 115185. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Zhang, T.; Fang, H.H.-P. Bacteria-Mediated PAH Degradation in Soil and Sediment. Appl. Microbiol. Biotechnol. 2011, 89, 1357–1371. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Shi, K.; Shang, N.; He, Y.; Meng, L.; Huang, T.; Yang, H.; Huang, C. Polycyclic Aromatic Hydrocarbons in Remote Lakes from the Tibetan plateau: Concentrations, Source, Ecological Risk, and Influencing Factors. J. Environ. Manag. 2022, 319, 115689. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Wan Azelee, N.I.; Jeon, B.-H.; et al. Polyaromatic Hydrocarbons (PAHs) in the Water Environment: A Review on Toxicity, Microbial Biodegradation, Systematic Biological Advancements, and Environmental Fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- House, W.A. Sampling Techniques for Organic Substances in Surface Waters. Int. J. Environ. Anal. Chem. 1994, 57, 207–214. [Google Scholar] [CrossRef]
- Manoli, E.; Samara, C. Polycyclic Aromatic Hydrocarbons in Natural Waters: Sources, Occurrence and Analysis. TrAC Trends Anal. Chem. 1999, 18, 417–428. [Google Scholar] [CrossRef]
- Frapiccini, E.; Panfili, M.; Guicciardi, S.; Santojanni, A.; Marini, M.; Truzzi, C.; Annibaldi, A. Effects of Biological Factors and Seasonality on the Level of Polycyclic Aromatic Hydrocarbons in Red Mullet (Mullus barbatus). Environ. Pollut. 2020, 258, 113742. [Google Scholar] [CrossRef] [PubMed]
- Raudonytė-Svirbutavičienė, E.; Jokšas, K.; Stakėnienė, R.; Rybakovas, A.; Nalivaikienė, R.; Višinskienė, G.; Arbačiauskas, K. Pollution Patterns and Their Effects on Biota within Lotic and Lentic Freshwater Ecosystems: How Well Contamination and Response Indicators Correspond? Environ. Pollut. 2023, 335, 122294. [Google Scholar] [CrossRef]
- Men, B.; He, M.; Tan, L.; Lin, C.; Quan, X. Distributions of Polycyclic Aromatic Hydrocarbons in the Daliao River Estuary of Liaodong Bay, Bohai Sea (China). Mar. Pollut. Bull. 2009, 58, 818–826. [Google Scholar] [CrossRef]
- Balmer, J.E.; Hung, H.; Yu, Y.; Letcher, R.J.; Muir, D.C.G. Sources and Environmental Fate of Pyrogenic Polycyclic Aromatic Hydrocarbons (PAHs) in the Arctic. Emerg. Contam. 2019, 5, 128–142. [Google Scholar] [CrossRef]
- Heintzman, L.J.; Anderson, T.A.; Carr, D.L.; McIntyre, N.E. Local and Landscape Influences on PAH Contamination in Urban Stormwater. Landsc. Urban Plan. 2015, 142, 29–37. [Google Scholar] [CrossRef]
- Awonaike, B.; Lei, Y.D.; Parajulee, A.; Mitchell, C.P.J.; Wania, F. Polycyclic Aromatic Hydrocarbons and Quinones in Urban and Rural Stormwater Runoff: Effects of Land Use and Storm Characteristics. ACS EST Water 2021, 1, 1209–1219. [Google Scholar] [CrossRef]
- Gong, X.; Zhao, Z.; Zhang, L.; Yao, S.; Xue, B. North-South Geographic Heterogeneity and Control Strategies for Polycyclic Aromatic Hydrocarbons (PAHs) in Chinese Lake Sediments Illustrated by Forward and Backward Source Apportionments. J. Hazard. Mater. 2022, 431, 128545. [Google Scholar] [CrossRef]
- Rabodonirina, S.; Net, S.; Ouddane, B.; Merhaby, D.; Dumoulin, D.; Popescu, T.; Ravelonandro, P. Distribution of Persistent Organic Pollutants (PAHs, Me-PAHs, PCBs) in Dissolved, Particulate and Sedimentary Phases in Freshwater Systems. Environ. Pollut. 2015, 206, 38–48. [Google Scholar] [CrossRef]
- Salowsky, H.; Schäfer, W.; Schneider, A.-L.; Müller, A.; Dreher, C.; Tiehm, A. Beneficial Effects of Dynamic Groundwater Flow and Redox Conditions on Natural Attenuation of Mono-, Poly-, and NSO-Heterocyclic Hydrocarbons. J. Contam. Hydrol. 2021, 243, 103883. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Gong, X.; Zhang, L.; Jin, M.; Cai, Y.; Wang, X. Riverine Transport and Water-Sediment Exchange of Polycyclic Aromatic Hydrocarbons (PAHs) along the Middle-Lower Yangtze River, China. J. Hazard. Mater. 2021, 403, 123973. [Google Scholar] [CrossRef] [PubMed]
- Semenov, M.Y.; Marinaite, I.I.; Silaev, A.V.; Begunova, L.A. Composition, Concentration and Origin of Polycyclic Aromatic Hydrocarbons in Waters and Bottom Sediments of Lake Baikal and Its Tributaries. Water 2023, 15, 2324. [Google Scholar] [CrossRef]
- Lin, W.; Fan, F.; Xu, G.; Gong, K.; Cheng, X.; Yuan, X.; Zhang, C.; Gao, Y.; Wang, S.; Ng, H.Y.; et al. Microbial Community Assembly Responses to Polycyclic Aromatic Hydrocarbon Contamination across Water and Sediment Habitats in the Pearl River Estuary. J. Hazard. Mater. 2023, 457, 131762. [Google Scholar] [CrossRef]
- Kumar, V.; Kothiyal, N.C.; Saruchi; Vikas, P.; Sharma, R. Sources, Distribution, and Health Effect of Carcinogenic Polycyclic Aromatic Hydrocarbons (PAHs)—Current Knowledge and Future Directions. J. Chin. Adv. Mater. Soc. 2016, 4, 302–321. [Google Scholar] [CrossRef]
- Dat, N.-D.; Chang, M.B. Review on Characteristics of PAHs in Atmosphere, Anthropogenic Sources and Control Technologies. Sci. Total Environ. 2017, 609, 682–693. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive Review of Polycyclic Aromatic Hydrocarbons in Water Sources, Their Effects and Treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbiol. 2020, 11, 562813. [Google Scholar] [CrossRef]
- Ceschin, S.; Bellini, A.; Scalici, M. Aquatic Plants and Ecotoxicological Assessment in Freshwater Ecosystems: A Review. Environ. Sci. Pollut. Res. 2021, 28, 4975–4988. [Google Scholar] [CrossRef]
- Robin, S.L.; Marchand, C. Polycyclic Aromatic Hydrocarbons (PAHs) in Mangrove Ecosystems: A Review. Environ. Pollut. 2022, 311, 119959. [Google Scholar] [CrossRef]
- Behera, B.K.; Das, A.; Sarkar, D.J.; Weerathunge, P.; Parida, P.K.; Das, B.K.; Thavamani, P.; Ramanathan, R.; Bansal, V. Polycyclic Aromatic Hydrocarbons (PAHs) in Inland Aquatic Ecosystems: Perils and Remedies through Biosensors and Bioremediation. Environ. Pollut. 2018, 241, 212–233. [Google Scholar] [CrossRef] [PubMed]
- Ben Othman, H.; Pick, F.R.; Sakka Hlaili, A.; Leboulanger, C. Effects of Polycyclic Aromatic Hydrocarbons on Marine and Freshwater Microalgae—A Review. J. Hazard. Mater. 2023, 441, 129869. [Google Scholar] [CrossRef] [PubMed]
- Famiyeh, L.; Chen, K.; Xu, J.; Sun, Y.; Guo, Q.; Wang, C.; Lv, J.; Tang, Y.-T.; Yu, H.; Snape, C.; et al. A Review on Analysis Methods, Source Identification, and Cancer Risk Evaluation of Atmospheric Polycyclic Aromatic Hydrocarbons. Sci. Total Environ. 2021, 789, 147741. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Lan, J.; Xie, Z.; Pu, J.; Yuan, D.; Yang, H.; Xing, B. Vertical Migration from Surface Soils to Groundwater and Source Appointment of Polycyclic Aromatic Hydrocarbons in Epikarst Spring Systems, Southwest China. Chemosphere 2019, 230, 616–627. [Google Scholar] [CrossRef]
- Birks, S.J.; Cho, S.; Taylor, E.; Yi, Y.; Gibson, J.J. Characterizing the PAHs in Surface Waters and Snow in the Athabasca Region: Implications for Identifying Hydrological Pathways of Atmospheric Deposition. Sci. Total Environ. 2017, 603–604, 570–583. [Google Scholar] [CrossRef]
- Marquès, M.; Mari, M.; Audí-Miró, C.; Sierra, J.; Soler, A.; Nadal, M.; Domingo, J.L. Climate Change Impact on the PAH Photodegradation in Soils: Characterization and Metabolites Identification. Environ. Int. 2016, 89–90, 155–165. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef]
- Akinpelumi, V.K.; Kumi, K.G.; Onyena, A.P.; Sam, K.; Ezejiofor, A.N.; Frazzoli, C.; Ekhator, O.C.; Udom, G.J.; Orisakwe, O.E. A Comparative Study of the Impacts of Polycyclic Aromatic Hydrocarbons in Water and Soils in Nigeria and Ghana: Towards a Framework for Public Health Protection. J. Hazard. Mater. Adv. 2023, 11, 100336. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Ya, M.; Li, Y.; Hong, H. Seasonal Variation and Spatial Transport of Polycyclic Aromatic Hydrocarbons in Water of the Subtropical Jiulong River Watershed and Estuary, Southeast China. Chemosphere 2019, 234, 215–223. [Google Scholar] [CrossRef]
- Schwanen, C.A.; Kronsbein, P.M.; Balik, B.; Schwarzbauer, J. Dynamic Transport and Distribution of Organic Pollutants in Water and Sediments of the Rur River. Water Air Soil. Pollut. 2023, 235, 9. [Google Scholar] [CrossRef]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment. Arch. Environ. Contam. Toxicol. 2019, 76, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, I.N.; Sikoki, F.D.; Semple, K.T. Risk Assessment of PAHs and N-PAH Analogues in Sediment Cores from the Niger Delta. Mar. Pollut. Bull. 2020, 161, 111684. [Google Scholar] [CrossRef] [PubMed]
- Iwegbue, C.M.A.; Irerhievwie, G.O.; Tesi, G.O.; Olisah, C.; Nwajei, G.E.; Martincigh, B.S. Polycyclic Aromatic Hydrocarbons (PAHs) in Surficial Sediments from Selected Rivers in the Western Niger Delta of Nigeria: Spatial Distribution, Sources, and Ecological and Human Health Risks. Mar. Pollut. Bull. 2021, 167, 112351. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.; Villa, S.; Waichman, A.V.; de Souza Nunes, G.S.; de Oliveira, R.; Vighi, M.; Rico, A. Occurrence, Sources, and Ecological Risks of Polycyclic Aromatic Hydrocarbons (PAHs) in the Amazon River. Chemosphere 2023, 336, 139285. [Google Scholar] [CrossRef]
- Umeh, C.T.; Nduka, J.K.; Omokpariola, D.O.; Morah, J.E.; Mmaduakor, E.C.; Okoye, N.H.; Lilian, E.-E.I.; Kalu, I.F. Ecological Pollution and Health Risk Monitoring Assessment of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Surface Water, Southeastern Nigeria. Environ. Anal. Health Toxicol. 2023, 38, e2023007. [Google Scholar] [CrossRef]
- Grmasha, R.A.; Abdulameer, M.H.; Stenger-Kovács, C.; Al-sareji, O.J.; Al-Gazali, Z.; Al-Juboori, R.A.; Meiczinger, M.; Hashim, K.S. Polycyclic Aromatic Hydrocarbons in the Surface Water and Sediment along Euphrates River System: Occurrence, Sources, Ecological and Health Risk Assessment. Mar. Pollut. Bull. 2023, 187, 114568. [Google Scholar] [CrossRef]
- Wakeham, S.G.; Canuel, E.A. Biogenic Polycyclic Aromatic Hydrocarbons in Sediments of the San Joaquin River in California (USA), and Current Paradigms on Their Formation. Environ. Sci. Pollut. Res. Int. 2016, 23, 10426–10442. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Wu, J.; Cui, Z.; Su, P. Sedimentary Polycyclic Aromatic Hydrocarbons (PAHs) along the Mouth Bar of the Yangtze River Estuary: Source, Distribution, and Potential Toxicity. Mar. Pollut. Bull. 2020, 159, 111494. [Google Scholar] [CrossRef]
- Liu, Y.; He, Y.; Han, B.; Liu, H.; Tao, S.; Liu, W. Sewage Discharge and Organic Matter Affect the Partitioning Behaviors of Different Polycyclic Aromatic Hydrocarbons in a River Surface Sediment-Pore Water System. J. Hazard. Mater. 2023, 446, 130757. [Google Scholar] [CrossRef]
- Arowojolu, I.M.; Tongu, S.M.; Itodo, A.U.; Sodre, F.F.; Kyenge, B.A.; Nwankwo, R.C. Investigation of Sources, Ecological and Health Risks of Sedimentary Polycyclic Aromatic Hydrocarbons in River Benue, Nigeria. Environ. Technol. Innov. 2021, 22, 101457. [Google Scholar] [CrossRef]
- Lee, C.-C.; Chen, C.S.; Wang, Z.-X.; Tien, C.-J. Polycyclic Aromatic Hydrocarbons in 30 River Ecosystems, Taiwan: Sources, and Ecological and Human Health Risks. Sci. Total Environ. 2021, 795, 148867. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; He, B.; Wu, X.; Simonich, S.L.M.; Liu, H.; Fu, J.; Chen, A.; Liu, H.; Wang, Q. Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Stream Sediments of Suzhou Industrial Park, an Emerging Eco-Industrial Park in China: Occurrence, Sources and Potential Risk. Ecotoxicol. Environ. Saf. 2021, 214, 112095. [Google Scholar] [CrossRef] [PubMed]
- Van Metre, P.C.; Mahler, B.J.; Qi, S.L.; Gellis, A.C.; Fuller, C.C.; Schmidt, T.S. Sediment Sources and Sealed-Pavement Area Drive Polycyclic Aromatic Hydrocarbon and Metal Occurrence in Urban Streams. Environ. Sci. Technol. 2022, 56, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, N.; Pouch, A.; Matej-Łukowicz, K.; Pazdro, K.; Mohsin, M.; Rezania, S.; Wojciechowska, E. A Multi-Criteria Approach to Investigate Spatial Distribution, Sources, and the Potential Toxicological Effect of Polycyclic Aromatic Hydrocarbons (PAHs) in Sediments of Urban Retention Tanks. Environ. Sci. Pollut. Res. 2023, 30, 27895–27911. [Google Scholar] [CrossRef]
- Carvalho, F.; Pradhan, A.; Abrantes, N.; Campos, I.; Keizer, J.J.; Cássio, F.; Pascoal, C. Wildfire Impacts on Freshwater Detrital Food Webs Depend on Runoff Load, Exposure Time and Burnt Forest Type. Sci. Total Environ. 2019, 692, 691–700. [Google Scholar] [CrossRef]
- Kieta, K.A.; Owens, P.N.; Petticrew, E.L.; French, T.D.; Koiter, A.J.; Rutherford, P.M. Polycyclic Aromatic Hydrocarbons in Terrestrial and Aquatic Environments Following Wildfire: A Review. Environ. Rev. 2023, 31, 141–167. [Google Scholar] [CrossRef]
- Khiari, N.; Charef, A.; Atoui, A.; Azouzi, R.; Khalil, N.; Khadhar, S. Southern Mediterranean Coast Pollution: Long-Term Assessment and Evolution of PAH Pollutants in Monastir Bay (Tunisia). Mar. Pollut. Bull. 2021, 167, 112268. [Google Scholar] [CrossRef]
- dos Santos, P.R.S.; Moreira, L.F.F.; Moraes, E.P.; de Farias, M.F.; Domingos, Y.S. Traffic-Related Polycyclic Aromatic Hydrocarbons (PAHs) Occurrence in a Tropical Environment. Environ. Geochem. Health 2021, 43, 4577–4587. [Google Scholar] [CrossRef]
- Yao, K.; Xie, Z.; Zhi, L.; Wang, Z.; Qu, C. Polycyclic Aromatic Hydrocarbons in the Water Bodies of Dong Lake and Tangxun Lake, China: Spatial Distribution, Potential Sources and Risk Assessment. Water 2023, 15, 2416. [Google Scholar] [CrossRef]
- Keiser, D.A.; Shapiro, J.S. Consequences of the Clean Water Act and the Demand for Water Quality. Q. J. Econ. 2019, 134, 349–396. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, J.; Zhou, N.; Zhang, T.; Zeng, H. Does the “10-Point Water Plan” Reduce the Intensity of Industrial Water Pollution? Quasi-Experimental Evidence from China. J. Environ. Manag. 2021, 295, 113048. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guo, Y.; Wang, Y.; Zeng, W. Spatial and Temporal Evolution of the “Source–Sink” Risk Pattern of NPS Pollution in the Upper Reaches of Erhai Lake Basin under Land Use Changes in 2005–2020. Water Air Soil Pollut. 2022, 233, 202. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, X.; Lu, S.; Zhang, T.; Jin, B.; Wang, Q.; Tang, Z.; Liu, Y.; Guo, X.; Zhou, J.; et al. A Review on Occurrence and Risk of Polycyclic Aromatic Hydrocarbons (PAHs) in Lakes of China. Sci. Total Environ. 2019, 651, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Luo, X.; Cai, H.; Liu, F.; Zhang, T.; Yang, Q. Seasonal Dynamics of Polycyclic Aromatic Hydrocarbons between Water and Sediment in a Tide-Dominated Estuary and Ecological Risks for Estuary Management. Mar. Pollut. Bull. 2021, 162, 111831. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Anthropogenic PAHs in Lake Sediments: A Literature Review (2002–2018). Environ. Sci. Process. Impacts 2018, 20, 1649–1666. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Shi, X.; You, X.; Cao, Z. Polycyclic Aromatic Hydrocarbons (PAHs) in Water from Three Estuaries of China: Distribution, Seasonal Variations and Ecological Risk Assessment. Mar. Pollut. Bull. 2016, 109, 471–479. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Chen, R.-S.; Meng, X.-Z.; Xu, J.; Qadeer, A.; Liu, M. Modeling and Evaluating Spatial Variation of Polycyclic Aromatic Hydrocarbons in Urban Lake Surface Sediments in Shanghai. Environ. Pollut. 2018, 235, 1–10. [Google Scholar] [CrossRef]
- Golobokova, L.; Khodzher, T.; Khuriganova, O.; Marinayte, I.; Onishchuk, N.; Rusanova, P.; Potemkin, V. Variability of Chemical Properties of the Atmospheric Aerosol above Lake Baikal during Large Wildfires in Siberia. Atmosphere 2020, 11, 1230. [Google Scholar] [CrossRef]
- Gorshkov, A.G.; Izosimova, O.N.; Kustova, O.V.; Marinaite, I.I.; Galachyants, Y.P.; Sinyukovich, V.N.; Khodzher, T.V. Wildfires as a Source of PAHs in Surface Waters of Background Areas (Lake Baikal, Russia). Water 2021, 13, 2636. [Google Scholar] [CrossRef]
- Tao, Y.; Xue, B.; Feng, M. Spatial and Historical Occurrence, Sources, and Potential Toxicological Risk of Polycyclic Aromatic Hydrocarbons in Sediments of the Largest Chinese Deep Lake. Arch. Environ. Contam. Toxicol. 2019, 77, 501–513. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, D.; Lei, Y.; Song, J.; Xia, J. Spatiotemporal Distribution of Polycyclic Aromatic Hydrocarbons in Sediments of a Typical River Located in the Loess Plateau, China: Influence of Human Activities and Land-Use Changes. J. Hazard. Mater. 2022, 424, 127744. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Ma, J.; Ren, Y.; Liu, G.-J.; Zhang, Q.; Chen, F. Assessing the Spatial Distribution of Soil PAHs and Their Relationship with Anthropogenic Activities at a National Scale. Int. J. Environ. Res. Public Health 2019, 16, 4928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, J.-Z.; Liang, B.; Zeng, E.Y. Occurrence of Polycyclic Aromatic Hydrocarbons in Surface Sediments of a Highly Urbanized River System with Special Reference to Energy Consumption Patterns. Environ. Pollut. 2011, 159, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, C.; Shen, Z.; Zhang, D.; Crittenden, J.C. Spatial Variation and Sources of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Sediments from the Yangtze Estuary, China. Environ. Sci. Process. Impacts 2015, 17, 1340–1347. [Google Scholar] [CrossRef]
- Grmasha, R.A.; Stenger-Kovács, C.; Bedewy, B.A.H.; Al-sareji, O.J.; Al-Juboori, R.A.; Meiczinger, M.; Hashim, K.S. Ecological and Human Health Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAH) in Tigris River near the Oil Refineries in Iraq. Environ. Res. 2023, 227, 115791. [Google Scholar] [CrossRef]
- Adesina, O.B.; Paul, E.D.; Nuhu, A.A.; Onoyima, C.C.; Okibe, F.G. Spatiotemporal Variation and Health Risk Assessment of Selected Polycyclic Aromatic Hydrocarbons and Pesticides in Ogun River, Lagos, Nigeria. J. Appl. Sci. Environ. Manag. 2024, 28, 1501–1512. [Google Scholar] [CrossRef]
- Qiao, M.; Wang, C.; Huang, S.; Wang, D.; Wang, Z. Composition, Sources, and Potential Toxicological Significance of PAHs in the Surface Sediments of the Meiliang Bay, Taihu Lake, China. Environ. Int. 2006, 32, 28–33. [Google Scholar] [CrossRef]
- Ren, C.; Wu, Y.; Zhang, S.; Wu, L.-L.; Liang, X.-G.; Chen, T.-H.; Zhu, C.-Z.; Sojinu, S.O.; Wang, J.-Z. PAHs in Sediment Cores at Main River Estuaries of Chaohu Lake: Implication for the Change of Local Anthropogenic Activities. Environ. Sci. Pollut. Res. 2015, 22, 1687–1696. [Google Scholar] [CrossRef]
- Garg, J.K. Wetland Assessment, Monitoring and Management in India Using Geospatial Techniques. J. Environ. Manag. 2015, 148, 112–123. [Google Scholar] [CrossRef]
- Oyuela Leguizamo, M.A.; Fernández Gómez, W.D.; Sarmiento, M.C.G. Native Herbaceous Plant Species with Potential Use in Phytoremediation of Heavy Metals, Spotlight on Wetlands—A Review. Chemosphere 2017, 168, 1230–1247. [Google Scholar] [CrossRef]
- Lettoof, D.C.; Bateman, P.W.; Aubret, F.; Gagnon, M.M. The Broad-Scale Analysis of Metals, Trace Elements, Organochlorine Pesticides and Polycyclic Aromatic Hydrocarbons in Wetlands Along an Urban Gradient, and the Use of a High Trophic Snake as a Bioindicator. Arch. Environ. Contam. Toxicol. 2020, 78, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, H.; Tao, Y.; Kou, X.; He, C.; Wang, Z. Community Diversity of Soil Meso-Fauna Indicates the Impacts of Oil Exploitation on Wetlands. Ecol. Indic. 2022, 144, 109451. [Google Scholar] [CrossRef]
- Yancheshmeh, R.A.; Bakhtiari, A.R.; Mortazavi, S.; Savabieasfahani, M. Sediment PAH: Contrasting Levels in the Caspian Sea and Anzali Wetland. Mar. Pollut. Bull. 2014, 84, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Rasta, M.; Sattari, M.; Taleshi, M.S.; Namin, J.I. Identification and Distribution of Microplastics in the Sediments and Surface Waters of Anzali Wetland in the Southwest Caspian Sea, Northern Iran. Mar. Pollut. Bull. 2020, 160, 111541. [Google Scholar] [CrossRef]
- Amini-Birami, F.; Keshavarzi, B.; Esmaeili, H.R.; Moore, F.; Busquets, R.; Saemi-Komsari, M.; Zarei, M.; Zarandian, A. Microplastics in Aquatic Species of Anzali Wetland: An Important Freshwater Biodiversity Hotspot in Iran. Environ. Pollut. 2023, 330, 121762. [Google Scholar] [CrossRef]
- Yang, W.; Cao, Z.; Lang, Y. Pollution Status of Polycyclic Aromatic Hydrocarbons (PAHs) in Northeastern China: A Review and Metanalysis. Environ. Process. 2021, 8, 429–454. [Google Scholar] [CrossRef]
- Shi, C.; Qu, C.; Sun, W.; Zhou, J.; Zhang, J.; Cao, Y.; Zhang, Y.; Guo, J.; Zhang, J.; Qi, S. Multimedia Distribution of Polycyclic Aromatic Hydrocarbons in the Wang Lake Wetland, China. Environ. Pollut. 2022, 306, 119358. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, G.; Ma, X.; Zhou, B.; Yuan, D.; Liu, H.; Wei, Z. Presence of Polycyclic Aromatic Hydrocarbons among Multi-Media in a Typical Constructed Wetland Located in the Coastal Industrial Zone, Tianjin, China: Occurrence Characteristics, Source Apportionment and Model Simulation. Sci. Total Environ. 2021, 800, 149601. [Google Scholar] [CrossRef]
- Cheshmvahm, H.; Keshavarzi, B.; Moore, F.; Zarei, M.; Esmaeili, H.R.; Hooda, P.S. Investigation of the Concentration, Origin and Health Effects of PAHs in the Anzali Wetland: The Most Important Coastal Freshwater Wetland of Iran. Mar. Pollut. Bull. 2023, 193, 115191. [Google Scholar] [CrossRef]
- Rokhbar, M.; Keshavarzi, B.; Moore, F.; Zarei, M.; Hooda, P.S.; Risk, M.J. Occurrence and Source of PAHs in Miankaleh International Wetland in Iran. Chemosphere 2023, 321, 138140. [Google Scholar] [CrossRef]
- Fakhradini, S.S.; Moore, F.; Keshavarzi, B.; Lahijanzadeh, A. Polycyclic Aromatic Hydrocarbons (PAHs) in Water and Sediment of Hoor Al-Azim Wetland, Iran: A Focus on Source Apportionment, Environmental Risk Assessment, and Sediment-Water Partitioning. Environ. Monit. Assess. 2019, 191, 233. [Google Scholar] [CrossRef] [PubMed]
- Bemanikharanagh, A.; Riahi Bakhtiari, A.; Mohammadi, J.; Taghizadeh-Mehrjardi, R. Toxicity and Origins of PAHs in Sediments of Shadegan Wetland, in Khuzestan Province, Iran. J. Maz. Univ. Med. Sci. 2017, 26, 304–317. [Google Scholar]
- Bao, K.; Shen, J.; Zhang, Y.; Wang, J.; Wang, G. A 200-Year Record of Polycyclic Aromatic Hydrocarbons Contamination in an Ombrotrophic Peatland in Great Hinggan Mountain, Northeast China. J. Mt. Sci. 2014, 11, 1085–1096. [Google Scholar] [CrossRef]
- Russkikh, I.V.; Strel’nikova, E.B.; Serebrennikova, O.V.; Voistinova, E.S.; Kharanzhevskaya, Y.A. Identification of Hydrocarbons in the Waters of Raised Bogs in the Southern Taiga of Western Siberia. Geochem. Int. 2020, 58, 447–455. [Google Scholar] [CrossRef]
- Salman, N.A.; Al-Saad, H.T.; Al-Imarah, F.J. The Status of Pollution in the Southern Marshes of Iraq: A Short Review. In Southern Iraq’s Marshes: Their Environment and Conservation; Jawad, L.A., Ed.; Coastal Research Library; Springer International Publishing: Cham, Switzerland, 2021; pp. 505–516. ISBN 978-3-030-66238-7. [Google Scholar]
- International Association of Hydrogeologists Groundwater—More About the Hidden Resource. Available online: https://iah.org/education/general-public/groundwater-hidden-resource (accessed on 16 January 2024).
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Liu, Q. PAHs Behavior in Surface Water and Groundwater of the Yellow River Estuary: Evidence from Isotopes and Hydrochemistry. Chemosphere 2017, 178, 143–153. [Google Scholar] [CrossRef]
- Pan, Z.; Li, B.; Yang, J.; Zhang, D.; Yang, Y.; Zhang, S. Study on the Spatial and Temporal Distribution and Risk Assessment of PAHs between River and Groundwater—Take the Typical Section of Beijing North Canal as an Example. J. Coast. Res. 2020, 115, 361–366. [Google Scholar] [CrossRef]
- Lan, J.; Sun, Y.; Yuan, D. Transport of Polycyclic Aromatic Hydrocarbons in a Highly Vulnerable Karst Underground River System of Southwest China. Environ. Sci. Pollut. Res. 2018, 25, 34519–34530. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Zhu, Y.; Wang, X.; Wang, L.; Xiong, J.; Qian, Z.; Xiong, S.; Zhao, R.; Liu, W.; et al. Distribution, Sources and Transport of Polycyclic Aromatic Hydrocarbons (PAHs) in Karst Spring Systems from Western Hubei, Central China. Chemosphere 2022, 300, 134502. [Google Scholar] [CrossRef]
- Lu, L.; Chen, Y.; Zou, S.; Wang, Z.; Fan, L. The Sources, Diffusion, and Health Risks of Polycyclic Aromatic Hydrocarbons in Water and Sediment of a Typical Underground River in South China. Environ. Earth Sci. 2024, 83, 100. [Google Scholar] [CrossRef]
- Deka, J.; Sarma, K.P.; Gupta, N.; Sahbaz Ahmed, M.; Mazumder, M.A.J.; Hoque, R.R. Polycyclic Aromatic Hydrocarbons in Groundwater of Oil-Rich Regions of Upper Brahmaputra Valley, India: Linkages of Colloidal Transport. Arab. J. Geosci. 2023, 16, 66. [Google Scholar] [CrossRef]
- Mansilha, C.; Melo, A.; Martins, Z.E.; Ferreira, I.M.P.L.V.O.; Pereira, A.M.; Espinha Marques, J. Wildfire Effects on Groundwater Quality from Springs Connected to Small Public Supply Systems in a Peri-Urban Forest Area (Braga Region, NW Portugal). Water 2020, 12, 1146. [Google Scholar] [CrossRef]
- Ilić, P.; Nešković Markić, D.; Stojanović Bjelić, L. Evaluation of Sources and Ecological Risk of PAHs in Different Layers of Soil and Groundwater. Preprints 2020. [Google Scholar] [CrossRef]
- Edet, A.; Nyong, E.; Ukpong, A.; Edet, C. Evaluation and Risk Assessment of Polycyclic Aromatic Hydrocarbons in Groundwater and Soil near a Petroleum Distribution Pipeline Spill Site, Eleme, Nigeria. Sustain. Water Resour. Manag. 2021, 7, 50. [Google Scholar] [CrossRef]
- Qi, X.; Lan, J.; Sun, Y.; Wang, S.; Liu, L.; Wang, J.; Long, Q.; Huang, M.; Yue, K. Linking PAHs Concentration, Risk to PAHs Source Shift in Soil and Water in Epikarst Spring Systems, Southwest China. Ecotoxicol. Environ. Saf. 2023, 264, 115465. [Google Scholar] [CrossRef]
- Montuori, P.; De Rosa, E.; Cerino, P.; Pizzolante, A.; Nicodemo, F.; Gallo, A.; Rofrano, G.; De Vita, S.; Limone, A.; Triassi, M. Estimation of Polycyclic Aromatic Hydrocarbons in Groundwater from Campania Plain: Spatial Distribution, Source Attribution and Health Cancer Risk Evaluation. Toxics 2023, 11, 435. [Google Scholar] [CrossRef]
- Liu, X.; Dong, Z.; Baccolo, G.; Gao, W.; Li, Q.; Wei, T.; Qin, X. Distribution, Composition and Risk Assessment of PAHs and PCBs in Cryospheric Watersheds of the Eastern Tibetan Plateau. Sci. Total Environ. 2023, 890, 164234. [Google Scholar] [CrossRef]
- Pawlak, F.; Koziol, K.; Polkowska, Z. Chemical Hazard in Glacial Melt? The Glacial System as a Secondary Source of POPs (in the Northern Hemisphere). A Systematic Review. Sci. Total Environ. 2021, 778, 145244. [Google Scholar] [CrossRef]
- Marchal, L.; Gateuille, D.; Naffrechoux, E.; Deline, P.; Baudin, F.; Clément, J.-C.; Poulenard, J. Polycyclic Aromatic Hydrocarbon Dynamics in Soils along Proglacial Chronosequences in the Alps. Sci. Total Environ. 2023, 902, 165998. [Google Scholar] [CrossRef]
- Ademollo, N.; Spataro, F.; Rauseo, J.; Pescatore, T.; Fattorini, N.; Valsecchi, S.; Polesello, S.; Patrolecco, L. Occurrence, Distribution and Pollution Pattern of Legacy and Emerging Organic Pollutants in Surface Water of the Kongsfjorden (Svalbard, Norway): Environmental Contamination, Seasonal Trend and Climate Change. Mar. Pollut. Bull. 2021, 163, 111900. [Google Scholar] [CrossRef]
- Li, R.; Gao, H.; Ji, Z.; Jin, S.; Ge, L.; Zong, H.; Jiao, L.; Zhang, Z.; Na, G. Distribution and Sources of Polycyclic Aromatic Hydrocarbons in the Water Column of Kongsfjorden, Arctic. J. Environ. Sci. 2020, 97, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Szopińska, M.; Szumińska, D.; Bialik, R.J.; Dymerski, T.; Rosenberg, E.; Polkowska, Ż. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) and Other Organic Pollutants in Freshwaters on the Western Shore of Admiralty Bay (King George Island, Maritime Antarctica). Environ. Sci. Pollut. Res. 2019, 26, 18143–18161. [Google Scholar] [CrossRef] [PubMed]
- Deelaman, W.; Pongpiachan, S.; Tipmanee, D.; Suttinun, O.; Choochuay, C.; Iadtem, N.; Charoenkalunyuta, T.; Promdee, K. Source Apportionment of Polycyclic Aromatic Hydrocarbons in the Terrestrial Soils of King George Island, Antarctica. J. S. Am. Earth Sci. 2020, 104, 102832. [Google Scholar] [CrossRef]
- Pongpiachan, S.; Hattayanone, M.; Tipmanee, D.; Suttinun, O.; Khumsup, C.; Kittikoon, I.; Hirunyatrakul, P. Chemical Characterization of Polycyclic Aromatic Hydrocarbons (PAHs) in 2013 Rayong Oil Spill-Affected Coastal Areas of Thailand. Environ. Pollut. 2018, 233, 992–1002. [Google Scholar] [CrossRef]
- Colby, G.A. Deposition of Polycyclic Aromatic Hydrocarbons (PAHs) into Northern Ontario Lake Sediments. bioRxiv 2019, 786913. [Google Scholar] [CrossRef]
- Miao, X.; Hao, Y.; Cai, J.; Xie, Y.; Zhang, J. The Distribution, Sources and Health Risk of Polycyclic Aromatic Hydrocarbons (PAHs) in Sediments of Liujiang River Basin: A Field Study in Typical Karstic River. Mar. Pollut. Bull. 2023, 188, 114666. [Google Scholar] [CrossRef]
- Leizou, K.E.; Ashraf, M.A. Distribution, Compositional Pattern and Potential to Human Exposure of PAHs in Water, Amassoma Axis, Nun River, Bayelsa State, Nigeria. Acta Chem. Malays. 2019, 3, 16–20. [Google Scholar] [CrossRef]
- Yuan, Z.; Shi, S.; Wu, X.; Wang, Q.; Wang, S.; Fan, Z. Polycyclic Aromatic Hydrocarbons (PAHs) in Riparian Soils of the Middle Reach of Huaihe River: A Typical Coal Mining Area in China. Soil Sediment Contam. Int. J. 2022, 32, 259–273. [Google Scholar] [CrossRef]
- Nair, M.M.; Sreeraj, M.K.; Rakesh, P.S.; Thomas, J.K.; Kharat, P.Y.; Sukumaran, S. Distribution, Source and Potential Biological Impacts of Polycyclic Aromatic Hydrocarbons in the Core Sediments of a Networked Aquatic System in the Northwest Coast of India—A Special Focus on Thane Creek Flamingo Sanctuary (Ramsar Site). Reg. Stud. Mar. Sci. 2024, 70, 103377. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Corsi, S.R.; Oliver, S.K.; Lenaker, P.L.; Nott, M.A.; Mills, M.A.; Norris, G.A.; Paatero, P. Primary Sources of Polycyclic Aromatic Hydrocarbons to Streambed Sediment in Great Lakes Tributaries Using Multiple Lines of Evidence. Environ. Toxicol. Chem. 2020, 39, 1392–1408. [Google Scholar] [CrossRef]
- Jabali, Y.; Iaaly, A.; Millet, M. Environmental Occurrence, Spatial Distribution, and Source Identification of PAHs in Surface and Groundwater Samples of Abou Ali River-North Lebanon. Environ. Monit. Assess. 2021, 193, 714. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Zheng, B.; Li, X.; Zhao, X.; Dionysiou, D.D.; Liu, Y. Influencing Factors and Health Risk Assessment of Polycyclic Aromatic Hydrocarbons in Groundwater in China. J. Hazard. Mater. 2021, 402, 123419. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, M.A.; Harikumar, P.S. Occurrence, Distribution, and Ecological Risk of Heavy Metals and Persistent Organic Pollutants (OCPs, PCBs, and PAHs) in Surface Sediments of the Ashtamudi Wetland, South-West Coast of India. Reg. Stud. Mar. Sci. 2023, 64, 103044. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Sheng, L.; Liu, X.; Zheng, X. Distribution Characteristics and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Momoge Wetland, China. Int. J. Environ. Res. Public Health 2017, 14, 85. [Google Scholar] [CrossRef]
- Singh, V.; Negi, R.; Jacob, M.; Gayathri, A.; Rokade, A.; Sarma, H.; Kalita, J.; Tasfia, S.T.; Bharti, R.; Wakid, A.; et al. Polycyclic Aromatic Hydrocarbons (PAHs) in Aquatic Ecosystem Exposed to the 2020 Baghjan Oil Spill in Upper Assam, India: Short-Term Toxicity and Ecological Risk Assessment. PLoS ONE 2023, 18, e0293601. [Google Scholar] [CrossRef]
- Nim, N.; Morris, J.; Tekasakul, P.; Dejchanchaiwong, R. Fine and Ultrafine Particle Emission Factors and New Diagnostic Ratios of PAHs for Peat Swamp Forest Fires. Environ. Pollut. 2023, 335, 122237. [Google Scholar] [CrossRef]
- Caumo, S.; Lázaro, W.L.; Sobreira Oliveira, E.; Beringui, K.; Gioda, A.; Massone, C.G.; Carreira, R.; de Freitas, D.S.; Ignacio, A.R.A.; Hacon, S. Human Risk Assessment of Ash Soil after 2020 Wildfires in Pantanal Biome (Brazil). Air Qual Atmos Health 2022, 15, 2239–2254. [Google Scholar] [CrossRef]
- Hites, R.A. Polycyclic Aromatic Hydrocarbons in the Atmosphere near the Great Lakes: Why Do Their Concentrations Vary? Environ. Sci. Technol. 2021, 55, 9444–9449. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Park, M.-K.; Son, J.-M.; Choi, S.-D. Spatial Distribution and Temporal Variation of Polycyclic Aromatic Hydrocarbons in Runoff and Surface Water. Sci. Total Environ. 2021, 793, 148339. [Google Scholar] [CrossRef]
- Wu, Y.; Salamova, A.; Venier, M. Using Diagnostic Ratios to Characterize Sources of Polycyclic Aromatic Hydrocarbons in the Great Lakes Atmosphere. Sci. Total Environ. 2021, 761, 143240. [Google Scholar] [CrossRef]
- Yang, J.; Sun, P.; Zhang, X.; Wei, X.-Y.; Huang, Y.-P.; Du, W.-N.; Qadeer, A.; Liu, M.; Huang, Y. Source Apportionment of PAHs in Roadside Agricultural Soils of a Megacity Using Positive Matrix Factorization Receptor Model and Compound-Specific Carbon Isotope Analysis. J. Hazard. Mater. 2021, 403, 123592. [Google Scholar] [CrossRef] [PubMed]
- Fei, W.; Ying, Z. Pollution Characteristics and Risk Assessment of PAHs in Agricultural Soil from Sewage Irrigation Area of Taiyuan City, Shanxi Province. Ecol. Environ. 2022, 31, 160. [Google Scholar] [CrossRef]
- Meyers, R.A. (Ed.) Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2001; ISBN 978-0-08-091795-5. [Google Scholar]
- Cao, Y.; Lin, C.; Zhang, X.; Liu, X.; He, M.; Ouyang, W. Distribution, Source, and Ecological Risks of Polycyclic Aromatic Hydrocarbons in Lake Qinghai, China. Environ. Pollut. 2020, 266, 115401. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liu, M.; Li, Y.; Zhu, J.; Wei, X.; Yang, J.; Huang, Y.; Zhao, D.; Gao, D.; Qadeer, A. Cross-Interface Transfer of Polycyclic Aromatic Hydrocarbons (PAHs) in a Shallow Urban Lake in Shanghai, China Based on the Fugacity Model. Sci. Total Environ. 2020, 736, 139369. [Google Scholar] [CrossRef]
- Nie, N.; Li, T.; Miao, Y.; Wei, X.; Zhao, D.; Liu, M. Environmental Fate and Health Risks of Polycyclic Aromatic Hydrocarbons in the Yangtze River Delta Urban Agglomeration during the 21st Century. J. Hazard. Mater. 2024, 465, 133407. [Google Scholar] [CrossRef]
- Duttagupta, S.; Mukherjee, A.; Routh, J.; Devi, L.G.; Bhattacharya, A.; Bhattacharya, J. Role of Aquifer Media in Determining the Fate of Polycyclic Aromatic Hydrocarbons in the Natural Water and Sediments along the Lower Ganges River Basin. J. Environ. Sci. Health Part A 2020, 55, 354–373. [Google Scholar] [CrossRef]
- Gregg, T.; Prahl, F.G.; Simoneit, B.R.T. Suspended Particulate Matter Transport of Polycyclic Aromatic Hydrocarbons in the Lower Columbia River and Its Estuary. Limnol. Oceanogr. 2015, 60, 1935–1949. [Google Scholar] [CrossRef]
- Tucca, F.; Luarte, T.; Nimptsch, J.; Woelfl, S.; Pozo, K.; Casas, G.; Dachs, J.; Barra, R.; Chiang, G.; Galbán-Malagón, C. Sources and Diffusive Air–Water Exchange of Polycyclic Aromatic Hydrocarbons in an Oligotrophic North–Patagonian Lake. Sci. Total Environ. 2020, 738, 139838. [Google Scholar] [CrossRef]
- Minick, D.J.; Anderson, K.A. Diffusive Flux of PAHs across Sediment–Water and Water–Air Interfaces at Urban Superfund Sites. Environ. Toxicol. Chem. 2017, 36, 2281–2289. [Google Scholar] [CrossRef]
- Ma, X.; Kong, X.; Xue, B.; Mu, S.; Huang, C.; Huang, T.; Li, S.; Jiang, Q. Sediment Records and Multi-Media Transfer and Fate of Polycyclic Aromatic Hydrocarbons in Dianchi Lake over the Past 100 Years. Ecol. Indic. 2024, 160, 111774. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Z.; Qadeer, A.; Liu, Y.; Qiao, X.; Zheng, B.; Zhao, G.; Zhao, X. The Sediment-Water Diffusion and Risk Assessment of PAHs in Different Types of Drinking Water Sources in the Yangtze River Delta, China. J. Clean. Prod. 2021, 309, 127456. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, C.; Qi, S.; Zhang, Y.; Mao, L.; Liu, J.; Qin, S.; Yang, D. Spatial-Temporal Variations and Transport Process of Polycyclic Aromatic Hydrocarbons in Poyang Lake: Implication for Dry–Wet Cycle Impacts. J. Geochem. Explor. 2021, 226, 106738. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, G.; Wang, R.; Huang, H. Polycyclic Aromatic Hydrocarbons in the Water-SPM-Sediment System from the Middle Reaches of Huai River, China: Distribution, Partitioning, Origin Tracing and Ecological Risk Assessment. Environ. Pollut. 2017, 230, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Athanasiou, D.; Rao, B.; Bejar, M.; Rakowska, M.; Drygiannaki, I.; Chadwick, D.B.; Colvin, M.A.; Hayman, N.T.; Rosen, G.H.; et al. Sediment Recontamination Potential and Biological Impacts of Hydrophobic Organics from Stormwater in a Mixed-Use Watershed. Sci. Total Environ. 2024, 906, 167444. [Google Scholar] [CrossRef]
- Usanase, G.; Azema, N.; Bitouri, Y.E.; Souche, J.-C.; Gonzalez, C. Contribution of Settling Measurements to the Study of Polycyclic Aromatic Hydrocarbons’ (PAHs) Mobilisation during Resuspension of PAHs-Associated Sediment. Environ. Sci. Pollut. Res. 2021, 28, 68349–68363. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, J.; Ma, Q.; Chen, Y.; Ju, H. Polycyclic Aromatic Hydrocarbons (PAHs) in Water and Sediment from a River Basin: Sediment–Water Partitioning, Source Identification and Environmental Health Risk Assessment. Environ. Geochem. Health 2017, 39, 63–74. [Google Scholar] [CrossRef]
- Wan, N.; Gao, J.; Duan, D.; Yang, Y.; Ran, Y. Sedimentation and Resuspension Fluxes of Polycyclic Aromatic Hydrocarbons in Lake and Reservoir, South China 2022. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Hu, T.; Shi, M.; Mao, Y.; Liu, W.; Li, M.; Yu, Y.; Yu, H.; Cheng, C.; Zhang, Z.; Zhang, J.; et al. The Characteristics of Polycyclic Aromatic Hydrocarbons and Heavy Metals in Water and Sediment of Dajiuhu Subalpine Wetland, Shennongjia, Central China, 2018–2020: Insights for Sources, Sediment-Water Exchange, and Ecological Risk. Chemosphere 2022, 309, 136788. [Google Scholar] [CrossRef]
- Mu, G.; Bian, D.; Zou, M.; Wang, X.; Chen, F. Pollution and Risk Assessment of Polycyclic Aromatic Hydrocarbons in Urban Rivers in a Northeastern Chinese City: Implications for Continuous Rainfall Events. Sustainability 2023, 15, 5777. [Google Scholar] [CrossRef]
- Oyo-Ita, I.; Nkom, P.Y.; Ugim, S.U.; Bassey, F.I.; Oyo-Ita, O.E. Seasonal Changes of PAHs in Water and Suspended Particulate Matter from Cross River Estuary, SE Nigeria in Response to Human-Induced Activity and Hydrological Cycle. Polycycl. Aromat. Compd. 2022, 42, 5456–5473. [Google Scholar] [CrossRef]
- Bacosa, H.P.; Steichen, J.; Kamalanathan, M.; Windham, R.; Lubguban, A.; Labonté, J.M.; Kaiser, K.; Hala, D.; Santschi, P.H.; Quigg, A. Polycyclic Aromatic Hydrocarbons (PAHs) and Putative PAH-Degrading Bacteria in Galveston Bay, TX (USA), Following Hurricane Harvey (2017). Environ. Sci. Pollut. Res. 2020, 27, 34987–34999. [Google Scholar] [CrossRef] [PubMed]
- Shang, N.; Wang, C.; Kong, J.; Yu, H.; Li, J.; Hao, W.; Huang, T.; Yang, H.; He, H.; Huang, C. Dissolved Polycyclic Aromatic Hydrocarbons (PAHs-d) in Response to Hydrology Variation and Anthropogenic Activities in the Yangtze River, China. J. Environ. Manag. 2023, 326, 116673. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, X.; Ya, M.; Li, Y.; Liu, Y.; Chen, H. Spatial-Temporal Distribution and Transport Flux of Polycyclic Aromatic Hydrocarbons in a Large Hydropower Reservoir of Southeast China: Implication for Impoundment Impacts. Environ. Pollut. 2020, 257, 113603. [Google Scholar] [CrossRef] [PubMed]
- Skic, K.; Boguta, P.; Klimkowicz-Pawlas, A.; Ukalska-Jaruga, A.; Baran, A. Effect of Sorption Properties on the Content, Ecotoxicity, and Bioaccumulation of Polycyclic Aromatic Hydrocarbons (PAHs) in Bottom Sediments. J. Hazard. Mater. 2023, 442, 130073. [Google Scholar] [CrossRef]
- Soukarieh, B.; Hamieh, M.; Halloum, W.; Budzinski, H.; Jaber, F. The Effect of the Main Physicochemical Properties of Polycyclic Aromatic Hydrocarbons on Their Water/Sediments Distribution. Int. J. Environ. Sci. Technol. 2023, 20, 10261–10270. [Google Scholar] [CrossRef]
- Huang, W.; Peng, P.; Yu, Z.; Fu, J. Effects of Organic Matter Heterogeneity on Sorption and Desorption of Organic Contaminants by Soils and Sediments. Appl. Geochem. 2003, 18, 955–972. [Google Scholar] [CrossRef]
- Sun, Y.; Xie, Z.; Wu, K.; Lan, J.; Li, T.; Yuan, D. Speciation, Distribution and Migration Pathways of Polycyclic Aromatic Hydrocarbons in a Typical Underground River System in Southwest China. J. Hydrol. 2021, 596, 125690. [Google Scholar] [CrossRef]
- Adeola, A.O.; Forbes, P.B.C. Influence of Natural Organic Matter Fractions on PAH Sorption by Stream Sediments and a Synthetic Graphene Wool Adsorbent. Environ. Technol. Innov. 2021, 21, 101202. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Shan, B. Effects of Organic Matter on Polycyclic Aromatic Hydrocarbons in Riverine Sediments Affected by Human Activities. Sci. Total Environ. 2022, 815, 152570. [Google Scholar] [CrossRef]
- Lu, Z.; Tian, W.; Chu, M.; Zhang, S.; Zhao, J.; Liu, B.; Huo, B.; Chen, Z.; Zhang, R. A Novel and Thorough Research into Desorption Behavior of PAHs from Sediments to Seawater: Aging Process, Thermodynamics, Kinetics, Influencing Factors. Chem. Eng. J. 2024, 480, 148322. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Zhu, L. New Insights into Thermal Desorption Remediation of Pyrene-Contaminated Soil Based on an Optimized Numerical Model. J. Hazard. Mater. 2024, 461, 132687. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wu, X.; Song, X.; Shi, C.; Zhang, Z. Sorption and Desorption of Petroleum Hydrocarbons on Biodegradable and Nondegradable Microplastics. Chemosphere 2021, 273, 128553. [Google Scholar] [CrossRef] [PubMed]
- Almouallem, W.; Michel, J.; Dorge, S.; Joyeux, C.; Trouvé, G.; Le Nouen, D. A Comparative Study of the Sorption of O-PAHs and PAHs onto Soils to Understand Their Transport in Soils and Groundwater. J. Environ. Sci. 2023, 124, 61–75. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Qin, N.; He, W.; Xu, F. The Impacts of Algae Biological Pump Effect on the Occurrence, Source Apportionment and Toxicity of SPM-Bound PAHs in Lake Environment. Sci. Total Environ. 2021, 753, 141980. [Google Scholar] [CrossRef]
- Ding, Q.; Gong, X.; Jin, M.; Yao, X.; Zhang, L.; Zhao, Z. The Biological Pump Effects of Phytoplankton on the Occurrence and Benthic Bioaccumulation of Hydrophobic Organic Contaminants (HOCs) in a Hypereutrophic Lake. Ecotoxicol. Environ. Saf. 2021, 213, 112017. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Z.; Wu, H.; Zhang, G.; Zhang, H.; Yang, M. Polycyclic Aromatic Hydrocarbons in Sediments from Typical Algae, Macrophyte Lake Bay and Adjoining River of Taihu Lake, China: Distribution, Sources, and Risk Assessment. Water 2021, 13, 470. [Google Scholar] [CrossRef]
- Ololade, I.A.; Oladoja, N.A.; Ololade, O.O.; Saliu, T.D.; Alabi, A.B.; Obadawo, S.B.; Anifowose, M.M. Bioaccumulation and Toxic Potencies of Polycyclic Aromatic Hydrocarbons in Freshwater Biota from the Ogbese River, Nigeria. Environ. Monit. Assess. 2020, 193, 8. [Google Scholar] [CrossRef]
- Pouch, A.; Zaborska, A.; Dąbrowska, A.M.; Pazdro, K. Bioaccumulation of PCBs, HCB and PAHs in the Summer Plankton from West Spitsbergen Fjords. Mar. Pollut. Bull. 2022, 177, 113488. [Google Scholar] [CrossRef]
- Pastorino, P.; Nocita, A.; Ciccotelli, V.; Zaccaroni, A.; Anselmi, S.; Giugliano, R.; Tomasoni, M.; Silvi, M.; Menconi, V.; Vivaldi, B.; et al. Health Risk Assessment of Potentially Toxic Elements, Persistence of NDL-PCB, PAHs, and Microplastics in the Translocated Edible Freshwater Sinotaia Quadrata (Gasteropoda, Viviparidae): A Case Study from the Arno River Basin (Central Italy). Expo. Health 2021, 13, 583–596. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, B.; Yang, Y.; Yang, S.; Dong, M.; Xu, M. Microbial Carriers Promote and Guide Pyrene Migration in Sediments. J. Hazard. Mater. 2022, 424, 127188. [Google Scholar] [CrossRef]
- Ou, D.; Liu, M.; Xu, S.; Cheng, S.; Hou, L.; Wang, L. Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in overlying waters and surface sediments from the Yangtze estuarine and coastal areas. Huan Jing Ke Xue 2009, 30, 3043–3049. [Google Scholar] [PubMed]
- Hung, H.; Halsall, C.; Ball, H.; Bidleman, T.; Dachs, J.; Silva, A.D.; Hermanson, M.; Kallenborn, R.; Muir, D.; Sühring, R.; et al. Climate Change Influence on the Levels and Trends of Persistent Organic Pollutants (POPs) and Chemicals of Emerging Arctic Concern (CEACs) in the Arctic Physical Environment—A Review. Environ. Sci. Process. Impacts 2022, 24, 1577–1615. [Google Scholar] [CrossRef] [PubMed]
- Na, M.; Zhao, Y.; Rina, S.; Wang, R.; Liu, X.; Tong, Z.; Zhang, J. Residues, Potential Source and Ecological Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Water of the East Liao River, Jilin Province, China. Sci. Total Environ. 2023, 886, 163977. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Xin, M.; Wang, B.; Lin, C. Spatial Distribution and Partition of Polycyclic Aromatic Hydrocarbons (PAHs) in the Water and Sediment of the Southern Bohai Sea: Yellow River and PAH Property Influences. Water Res. 2024, 248, 120873. [Google Scholar] [CrossRef]
- Cai, T.; Ding, Y.; Zhang, Z.; Wang, X.; Wang, T.; Ren, Y.; Dong, Y. Effects of Total Organic Carbon Content and Leaching Water Volume on Migration Behavior of Polycyclic Aromatic Hydrocarbons in Soils by Column Leaching Tests. Environ. Pollut. 2019, 254, 112981. [Google Scholar] [CrossRef]
- Garcia, M.R.; Martins, C.C. A Systematic Evaluation of Polycyclic Aromatic Hydrocarbons in South Atlantic Subtropical Mangrove Wetlands under a Coastal Zone Development Scenario. J. Environ. Manag. 2021, 277, 111421. [Google Scholar] [CrossRef]
- Lin, B.; Qi, F.; An, X.; Zhao, C.; Gao, Y.; Liu, Y.; Zhong, Y.; Qiu, B.; Wang, Z.; Hu, Q.; et al. Review: The Application of Source Analysis Methods in Tracing Urban Non-Point Source Pollution: Categorization, Hotspots, and Future Prospects. Environ. Sci. Pollut. Res. 2024, 31, 23482–23504. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Gong, P. Combined Risk Assessment Method Based on Spatial Interaction: A Case for Polycyclic Aromatic Hydrocarbons and Heavy Metals in Taihu Lake Sediments. J. Clean. Prod. 2021, 328, 129590. [Google Scholar] [CrossRef]
- Tien, C.-J.; Wang, Z.-X.; Chen, C.S. Microplastics in Water, Sediment and Fish from the Fengshan River System: Relationship to Aquatic Factors and Accumulation of Polycyclic Aromatic Hydrocarbons by Fish. Environ. Pollut. 2020, 265, 114962. [Google Scholar] [CrossRef]
- José, S.; Jordao, L. Exploring the Interaction between Microplastics, Polycyclic Aromatic Hydrocarbons and Biofilms in Freshwater. Polycycl. Aromat. Compd. 2022, 42, 2210–2221. [Google Scholar] [CrossRef]
- Ali, M.; Xu, D.; Yang, X.; Hu, J. Microplastics and PAHs Mixed Contamination: An in-Depth Review on the Sources, Co-Occurrence, and Fate in Marine Ecosystems. Water Res. 2024, 257, 121622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Hou, L.; Li, X.; Yin, G.; Sun, P.; Yang, J.; Wei, X.; He, Y.; Zheng, D. Geographical Distribution of Polycyclic Aromatic Hydrocarbons in Estuarine Sediments over China: Human Impacts and Source Apportionment. Sci. Total Environ. 2021, 768, 145279. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, K.; Liu, H.; Yuan, X.; Li, M.; Xiong, B.; Du, R.; Johnson, D.M.; Xi, Y. Distribution, Sources and Risk Assessment of PAHs in Soil from the Water Level Fluctuation Zone of Xiangxi Bay, Three Gorges Reservoir. Environ. Geochem. Health 2022, 44, 2615–2628. [Google Scholar] [CrossRef] [PubMed]
- Łyszczarz, S.; Lasota, J.; Szuszkiewicz, M.M.; Błońska, E. Soil Texture as a Key Driver of Polycyclic Aromatic Hydrocarbons (PAHs) Distribution in Forest Topsoils. Sci. Rep. 2021, 11, 14708. [Google Scholar] [CrossRef]
- Pillay, V.; Moodley, B. Assessment of the Impact of Reforestation on Soil, Riparian Sediment and River Water Quality Based on Polyaromatic Hydrocarbon Pollutants. J. Environ. Manag. 2022, 324, 116331. [Google Scholar] [CrossRef]
- Domínguez, C.; Sarkar, S.K.; Bhattacharya, A.; Chatterjee, M.; Bhattacharya, B.D.; Jover, E.; Albaigés, J.; Bayona, J.M.; Alam, M.A.; Satpathy, K.K. Quantification and Source Identification of Polycyclic Aromatic Hydrocarbons in Core Sediments from Sundarban Mangrove Wetland, India. Arch. Environ. Contam. Toxicol. 2010, 59, 49–61. [Google Scholar] [CrossRef]
- Stephansen, D.A.; Arias, C.A.; Brix, H.; Fejerskov, M.L.; Nielsen, A.H. Relationship between Polycyclic Aromatic Hydrocarbons in Sediments and Invertebrates of Natural and Artificial Stormwater Retention Ponds. Water 2020, 12, 2020. [Google Scholar] [CrossRef]
- Rasheed, R.O. Seasonal Variations of Polycyclic Aromatic Hydrocarbons in the Muscle Tissue of Silurus Triostegus Heckel, 1843 from Derbendikhan Reservoir. Polycycl. Aromat. Compd. 2023, 43, 2144–2151. [Google Scholar] [CrossRef]
- Lécrivain, N.; Duparc, A.; Clément, B.; Naffrechoux, E.; Frossard, V. Tracking Sources and Transfer of Contamination According to Pollutants Variety at the Sediment-Biota Interface Using a Clam as Bioindicator in Peri-Alpine Lakes. Chemosphere 2020, 238, 124569. [Google Scholar] [CrossRef]
- Sheng, Y.; Yan, C.; Nie, M.; Ju, M.; Ding, M.; Huang, X.; Chen, J. The Partitioning Behavior of PAHs between Settled Dust and Its Extracted Water Phase: Coefficients and Effects of the Fluorescent Organic Matter. Ecotoxicol. Environ. Saf. 2021, 223, 112573. [Google Scholar] [CrossRef]
- Li, R.; Hua, P.; Zhang, J.; Krebs, P. Effect of Anthropogenic Activities on the Occurrence of Polycyclic Aromatic Hydrocarbons in Aquatic Suspended Particulate Matter: Evidence from Rhine and Elbe Rivers. Water Res. 2020, 179, 115901. [Google Scholar] [CrossRef] [PubMed]
- Nybom, I.; van Grimbergen, J.; Forsell, M.; Mustajärvi, L.; Martens, J.; Sobek, A. Water Column Organic Carbon Composition as Driver for Water-Sediment Fluxes of Hazardous Pollutants in a Coastal Environment. J. Hazard. Mater. 2024, 465, 133393. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Ren, J.; Li, S.; Rene, E.R.; Zhou, D.; Zhang, P.; Hu, Q.; Ma, W. Prediction of the Impact of Benzo[a]Pyrene on Shallow Groundwater during Natural Infiltration of Reclaimed Water-Receiving Rivers: A Case Study of Liangshui, China. J. Environ. Manag. 2022, 323, 116070. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Lu, L.; Cao, J.; Zhao, L.; Luan, S. Source, Partition and Ecological Risk of Polycyclic Aromatic Hydrocarbons in Karst Underground River Environment, Southern China. Water 2021, 13, 2655. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, T.; Hu, L.; Guo, T.; Guo, Z. Polycyclic Aromatic Hydrocarbons in Sediment–Porewater System from the East China Sea: Occurrence, Partitioning, and Diffusion. Environ. Res. 2022, 209, 112755. [Google Scholar] [CrossRef]
- Hu, S.-Y.; Hsieh, C.-Y.; Dahms, H.-U.; Tseng, Y.-H.; Chen, J.; Wu, M.-C.; Kim, J.-H.; Liu, C.-H. Toxic Effects of Heavy Metals and Organic Polycyclic Aromatic Hydrocarbons in Sediment Porewater on the Amphipod Hyalella Azteca and Zebrafish Brachydanio Rerio Embryos from Different Rivers in Taiwan. Appl. Sci. 2021, 11, 8021. [Google Scholar] [CrossRef]
- MacKay, C.E.; Knock, G.A. Control of Vascular Smooth Muscle Function by Src-Family Kinases and Reactive Oxygen Species in Health and Disease. J. Physiol. 2015, 593, 3815–3828. [Google Scholar] [CrossRef]
- Vondráček, J.; Machala, M. The Role of Metabolism in Toxicity of Polycyclic Aromatic Hydrocarbons and Their Non-Genotoxic Modes of Action. Curr. Drug Metab. 2021, 22, 584–595. [Google Scholar] [CrossRef]
- Cheng, X.-M.; Hu, Y.-Y.; Yang, T.; Wu, N.; Wang, X.-N. Reactive Oxygen Species and Oxidative Stress in Vascular-Related Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7906091. [Google Scholar] [CrossRef]
- Harvey, R.G.; Dai, Q.; Ran, C.; Lim, K.; Blair, I.; Penning, T.M. Syntheses of adducts of active metabolites of carcinogenic polycyclic aromatic hydrocarbons with 2′-deoxyribonucleosides. Polycycl. Aromat. Compd. 2005, 25, 371–391. [Google Scholar] [CrossRef]
- Hrdina, A.I.H.; Kohale, I.N.; Kaushal, S.; Kelly, J.; Selin, N.E.; Engelward, B.P.; Kroll, J.H. The Parallel Transformations of Polycyclic Aromatic Hydrocarbons in the Body and in the Atmosphere. Environ. Health Perspect. 2022, 130, 025004. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.L.; Zubair, M.; John, K.; Poirier, M.C.; Martin, F.L. Carcinogens and DNA Damage. Biochem. Soc. Trans. 2018, 46, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Hochalter, J.B.; Carmella, S.G.; Hecht, S.S. Quantitation of Phenanthrene Dihydrodiols in the Urine of Smokers and Non-Smokers by Gas Chromatography-Negative Ion Chemical Ionization-Tandem Mass Spectrometry. J. Chromatogr. B 2020, 1141, 122023. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Lam, A.K.; Gopalan, V. Diet Derived Polycyclic Aromatic Hydrocarbons and Its Pathogenic Roles in Colorectal Carcinogenesis. Crit. Rev. Oncol./Hematol. 2021, 168, 103522. [Google Scholar] [CrossRef]
- Peng, B.; Dong, Q.; Li, F.; Wang, T.; Qiu, X.; Zhu, T. A Systematic Review of Polycyclic Aromatic Hydrocarbon Derivatives: Occurrences, Levels, Biotransformation, Exposure Biomarkers, and Toxicity. Environ. Sci. Technol. 2023, 57, 15314–15335. [Google Scholar] [CrossRef]
- Penning, T.M.; Burczynski, M.E.; Hung, C.-F.; McCoull, K.D.; Palackal, N.T.; Tsuruda, L.S. Dihydrodiol Dehydrogenases and Polycyclic Aromatic Hydrocarbon Activation: Generation of Reactive and Redox Active o-Quinones. Chem. Res. Toxicol. 1999, 12, 1–18. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Fröhlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol Health Effects from Molecular to Global Scales. Environ. Sci. Technol. 2017, 51, 13545–13567. [Google Scholar] [CrossRef]
- Liu, J.; Hanzhong, J.; Kecheng, Z.; Zhao, S.; Eric, L. Formation of Environmentally Persistent Free Radicals and Reactive Oxygen Species during the Thermal Treatment of Soils Contaminated by Polycyclic Aromatic Hydrocarbons. Environ. Chem. Lett. 2020, 18, 1329–1336. [Google Scholar] [CrossRef]
- Sun, N.; Li, M.; Liu, G.; Jing, M.; He, F.; Cao, Z.; Zong, W.; Tang, J.; Gao, C.; Liu, R. Toxic Mechanism of Pyrene to Catalase and Protective Effects of Vitamin C: Studies at the Molecular and Cell Levels. Int. J. Biol. Macromol. 2021, 171, 225–233. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative Stress and Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Pang, Q.; Huang, C.; Xie, J.; Hu, J.; Wang, L.; Wang, C.; Meng, L.; Fan, R. Environmental Dose of 16 Priority-Controlled PAHs Mixture Induce Damages of Vascular Endothelial Cells Involved in Oxidative Stress and Inflammation. Toxicol. Vitr. 2022, 79, 105296. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Prasad, S.; Kishore, S.; Kumar, A.; Upadhyay, V. A Perspective Review on Impact and Molecular Mechanism of Environmental Carcinogens on Human Health. Biotechnol. Genet. Eng. Rev. 2021, 37, 178–207. [Google Scholar] [CrossRef]
- Sun, Y.; Shi, Z.; Lin, Y.; Zhang, M.; Liu, J.; Zhu, L.; Chen, Q.; Bi, J.; Li, S.; Ni, Z.; et al. Benzo(a)Pyrene Induces MUC5AC Expression through the AhR/Mitochondrial ROS/ERK Pathway in Airway Epithelial Cells. Ecotoxicol. Environ. Saf. 2021, 210, 111857. [Google Scholar] [CrossRef]
- Basu, A.K.; Essigmann, J.M. Establishing Linkages Among DNA Damage, Mutagenesis, and Genetic Diseases. Chem. Res. Toxicol. 2022, 35, 1655–1675. [Google Scholar] [CrossRef]
- Qin, C.; Hu, X.; Yang, B.; Liu, J.; Gao, Y. Amino, Nitro, Chloro, Hydroxyl and Methyl Substitutions May Inhibit the Binding of PAHs with DNA. Environ. Pollut. 2021, 268, 115798. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Dai, Y.; Wu, Y.; Lin, X. Effects of Polycyclic Aromatic Hydrocarbon Structure on PAH Mineralization and Toxicity to Soil Microorganisms after Oxidative Bioremediation by Laccase. Environ. Pollut. 2021, 287, 117581. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, R.; Bhattacharya, P.; Devanesan, S.; AlSalhi, M.S. Concentration, Source Apportionment and Potential Carcinogenic Risks of Polycyclic Aromatic Hydrocarbons (PAHs) in Roadside Soils. Chemosphere 2022, 292, 133413. [Google Scholar] [CrossRef]
- Bukowska, B.; Sicińska, P. Influence of Benzo(a)Pyrene on Different Epigenetic Processes. Int. J. Mol. Sci. 2021, 22, 13453. [Google Scholar] [CrossRef]
- Xie, S.; Zhou, A.; Feng, Y.; Zhang, Y.; Li, J.; Sun, Z.; Fan, L.; Zou, J. Cytochrome P450 1A mRNA in the Gambusia Affinis and Response to Several PAHs. Biochem. Genet. 2020, 58, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Ceccaldi, P.-F.; Carbonnel, M.; Feki, A.; Ayoubi, J.-M. Pollution and Endometriosis: A Deep Dive into the Environmental Impacts on Women’s Health. BJOG Int. J. Obstet. Gynaecol. 2024, 131, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.; Connolly, L.; Igout, A.; Nott, K.; Muller, M.; Scippo, M. In Vitro Profiling of the Potential Endocrine Disrupting Activities Affecting Steroid and Aryl Hydrocarbon Receptors of Compounds and Mixtures Prevalent in Human Drinking Water Resources. Chemosphere 2020, 258, 127332. [Google Scholar] [CrossRef]
- Bozinovic, G.; Shea, D.; Feng, Z.; Hinton, D.; Sit, T.; Oleksiak, M.F. PAH-Pollution Effects on Sensitive and Resistant Embryos: Integrating Structure and Function with Gene Expression. PLoS ONE 2021, 16, e0249432. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Alaekwe, I.O.; Abba, O. Polycyclic Aromatic Hydrocarbons in Water: A Review of the Sources, Properties, Exposure Pathways, Bionetwork and Strategies for Remediation. J. Geosci. Environ. Prot. 2022, 10, 137–144. [Google Scholar] [CrossRef]
- Ramesh, A.; Harris, K.J.; Archibong, A.E. Chapter 38—Reproductive Toxicity of Polycyclic Aromatic Hydrocarbons. In Reproductive and Developmental Toxicology, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 759–778. ISBN 978-0-323-89773-0. [Google Scholar]
- Šimečková, P.; Pěnčíková, K.; Kováč, O.; Slavík, J.; Pařenicová, M.; Vondráček, J.; Machala, M. In Vitro Profiling of Toxic Effects of Environmental Polycyclic Aromatic Hydrocarbons on Nuclear Receptor Signaling, Disruption of Endogenous Metabolism and Induction of Cellular Stress. Sci. Total Environ. 2022, 815, 151967. [Google Scholar] [CrossRef]
- Peng, F.-J.; Palazzi, P.; Viguié, C.; Appenzeller, B.M.R. Measurement of Hair Thyroid and Steroid Hormone Concentrations in the Rat Evidence Endocrine Disrupting Potential of a Low Dose Mixture of Polycyclic Aromatic Hydrocarbons. Environ. Pollut. 2022, 313, 120179. [Google Scholar] [CrossRef]
- Vieira, L.R.; Guilhermino, L. Multiple Stress Effects on Marine Planktonic Organisms: Influence of Temperature on the Toxicity of Polycyclic Aromatic Hydrocarbons to Tetraselmis chuii. J. Sea Res. 2012, 72, 94–98. [Google Scholar] [CrossRef]
- Marlatt, V.L.; Bayen, S.; Castaneda-Cortès, D.; Delbès, G.; Grigorova, P.; Langlois, V.S.; Martyniuk, C.J.; Metcalfe, C.D.; Parent, L.; Rwigemera, A.; et al. Impacts of Endocrine Disrupting Chemicals on Reproduction in Wildlife and Humans. Environ. Res. 2022, 208, 112584. [Google Scholar] [CrossRef]
- Esmaeilbeigi, M.; Kalbassi, M.R.; Seyedi, J.; Tayemeh, M.B.; Moghaddam, J.A. Intra and Extracellular Effects of Benzo [α] Pyrene on Liver, Gill and Blood of Caspian White Fish (Rutilus Frissi Kutum): Cyto-Genotoxicity and Histopathology Approach. Mar. Pollut. Bull. 2021, 163, 111942. [Google Scholar] [CrossRef] [PubMed]
- Black, T.A.; White, M.S.; Blais, J.M.; Hollebone, B.; Orihel, D.M.; Palace, V.P.; Rodriguez-Gil, J.L.; Hanson, M.L. Surface Oil Is the Primary Driver of Macroinvertebrate Impacts Following Spills of Diluted Bitumen in Freshwater. Environ. Pollut. 2021, 290, 117929. [Google Scholar] [CrossRef] [PubMed]
- Indiketi, N.; Lhoste, E.; Grenon, M.C.; Gagnon, M.; Veilleux, É.; Triffault-Bouchet, G.; Couture, P. Toxicity and Risk Management of Oil-Spiked Sediments by Diluted Bitumen for Two Freshwater Benthic Invertebrates. Environ. Pollut. 2023, 327, 121497. [Google Scholar] [CrossRef] [PubMed]
- Canadian Council of Ministers of the Environment. Canadian Sediment Quality Guidelines for the Protection of Aquatic Life—Polycyclic Aromatic Hydrocarbons. Available online: https://ccme.ca/en/res/polycyclic-aromatic-hydrocarbons-pahs-canadian-sediment-quality-guidelines-for-the-protection-of-aquatic-life-en.pdf (accessed on 9 June 2024).
- Asl, A.G.; Nabavi, S.M.B.; Rouzbahani, M.M.; Alipour, S.S.; Monavari, S.M. Persistent Organic Pollutants Influence the Marine Benthic Macroinvertebrate Assemblages in Surface Sediments of Nayband National Park and Bay, Northern Persian Gulf, Iran. Environ. Sci. Pollut. Res. 2023, 30, 30254–30270. [Google Scholar] [CrossRef]
- Yan, Z.; Song, N.; Wang, C.; Jiang, H. Functional Potential and Assembly of Microbes from Sediments in a Lake Bay and Adjoining River Ecosystem for Polycyclic Aromatic Hydrocarbon Biodegradation. Environ. Microbiol. 2021, 23, 628–640. [Google Scholar] [CrossRef]
- Idan, F.S.; Jazza, S.H. Bioaccumulation of Hydrocarbon Compounds in the Muscle of Three Aquatic Birds in Um Alnaaj Marsh, Iraq. Int. J. Aquat. Biol. 2022, 10, 234–241. [Google Scholar]
- Li, H.; Duan, D.; Beckingham, B.; Yang, Y.; Ran, Y.; Grathwohl, P. Impact of Trophic Levels on Partitioning and Bioaccumulation of Polycyclic Aromatic Hydrocarbons in Particulate Organic Matter and Plankton. Mar. Pollut. Bull. 2020, 160, 111527. [Google Scholar] [CrossRef]
- Liu, B.; Gao, L.; Ding, L.; Lv, L.; Yu, Y. Trophodynamics and Bioaccumulation of Polycyclic Aromatic Hydrocarbons (PAHs) in Marine Food Web from Laizhou Bay, China. Mar. Pollut. Bull. 2023, 194, 115307. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Liu, W.; Kong, X.; Xu, F.; Giesy, J.P. Tissue Distribution, Bioaccumulation, and Carcinogenic Risk of Polycyclic Aromatic Hydrocarbons in Aquatic Organisms from Lake Chaohu, China. Sci. Total Environ. 2020, 749, 141577. [Google Scholar] [CrossRef]
- Mai, Y.; Wang, Y.; Geng, T.; Peng, S.; Lai, Z.; Wang, X.; Li, H. A Systematic Toxicologic Study of Polycyclic Aromatic Hydrocarbons on Aquatic Organisms via Food-Web Bioaccumulation. Sci. Total Environ. 2024, 929, 172362. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michałowicz, J. Benzo[a]Pyrene—Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef] [PubMed]
- Bignell, J.P.; Barber, J.; Bateman, K.S.; Etherton, M.; Feist, S.W.; Galloway, T.S.; Katsiadaki, I.; Sebire, M.; Scott, A.P.; Stentiford, G.D.; et al. Insights into the Development of Hepatocellular Fibrillar Inclusions in European Flounder (Platichthys flesus) from UK Estuaries. Chemosphere 2020, 256, 126946. [Google Scholar] [CrossRef]
- Brette, F.; Machado, B.; Cros, C.; Incardona, J.P.; Scholz, N.L.; Block, B.A. Crude Oil Impairs Cardiac Excitation-Contraction Coupling in Fish. Science 2014, 343, 772–776. [Google Scholar] [CrossRef] [PubMed]
- Cherr, G.N.; Fairbairn, E.; Whitehead, A. Impacts of Petroleum-Derived Pollutants on Fish Development. Annu. Rev. Anim. Biosci. 2017, 5, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Uchida, M.; Ishibashi, H.; Hirano, M.; Ichikawa, N.; Arizono, K.; Koyama, J.; Tominaga, N. Potential Mechanisms Underlying Embryonic Developmental Toxicity Caused by Benzo[a]Pyrene in Japanese Medaka (Oryzias latipes). Chemosphere 2020, 242, 125243. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Patnaik, L. Acetylcholinesterase, as a Potential Biomarker of Naphthalene Toxicity in Different Tissues of Freshwater Teleost, Anabas Testudineus. J. Environ. Eng. Landsc. Manag. 2021, 29, 403–409. [Google Scholar] [CrossRef]
- Haverinen, J.; Badr, A.; Korajoki, H.; Hassinen, M.; Vornanen, M. Dual Effect of Polyaromatic Hydrocarbons on Sarco(Endo)Plasmic Reticulum Calcium ATPase (SERCA) Activity of a Teleost Fish (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2024, 276, 109785. [Google Scholar] [CrossRef]
- Bramatti, I.; Matos, B.; Figueiredo, N.; Pousão-Ferreira, P.; Branco, V.; Martins, M. Interaction of Polycyclic Aromatic Hydrocarbon Compounds in Fish Primary Hepatocytes: From Molecular Mechanisms to Genotoxic Effects. Sci. Total Environ. 2023, 855, 158783. [Google Scholar] [CrossRef]
- Khan, S.; Qamar, Z.; Khan, A.; Waqas, M.; Nawab, J.; Khisroon, M.; Khan, A. Genotoxic Effects of Polycyclic Aromatic Hydrocarbons (PAHs) Present in Vehicle-Wash Wastewater on Grass Carp (Ctenopharyngodon idella) and Freshwater Mussels (Anodonta cygnea). Environ. Pollut. 2023, 327, 121513. [Google Scholar] [CrossRef]
- Ho, K.T.; Konovets, I.M.; Terletskaya, A.V.; Milyukin, M.V.; Lyashenko, A.V.; Shitikova, L.I.; Shevchuk, L.I.; Afanasyev, S.A.; Krot, Y.G.; Zorina-Sakharova, K.Y.; et al. Contaminants, Mutagenicity and Toxicity in the Surface Waters of Kyiv, Ukraine. Mar. Pollut. Bull. 2020, 155, 111153. [Google Scholar] [CrossRef]
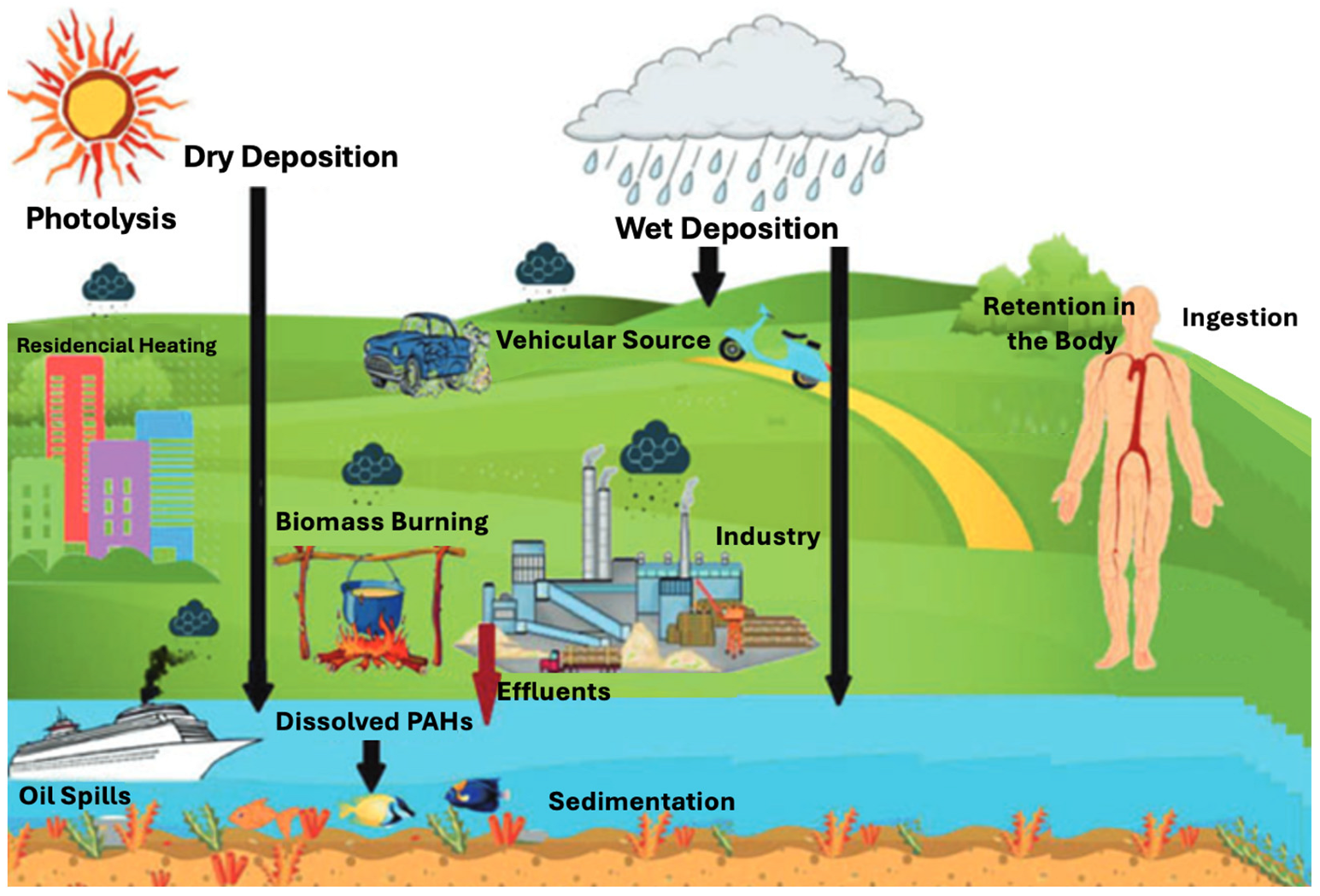
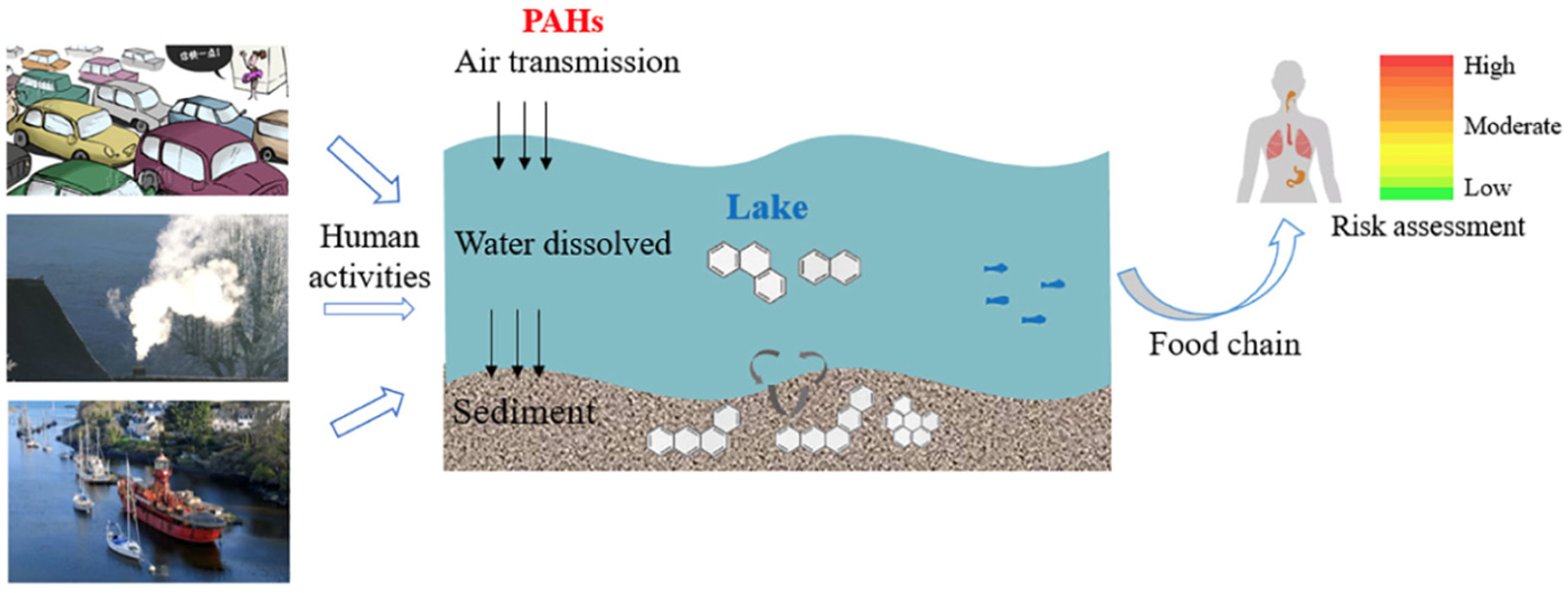

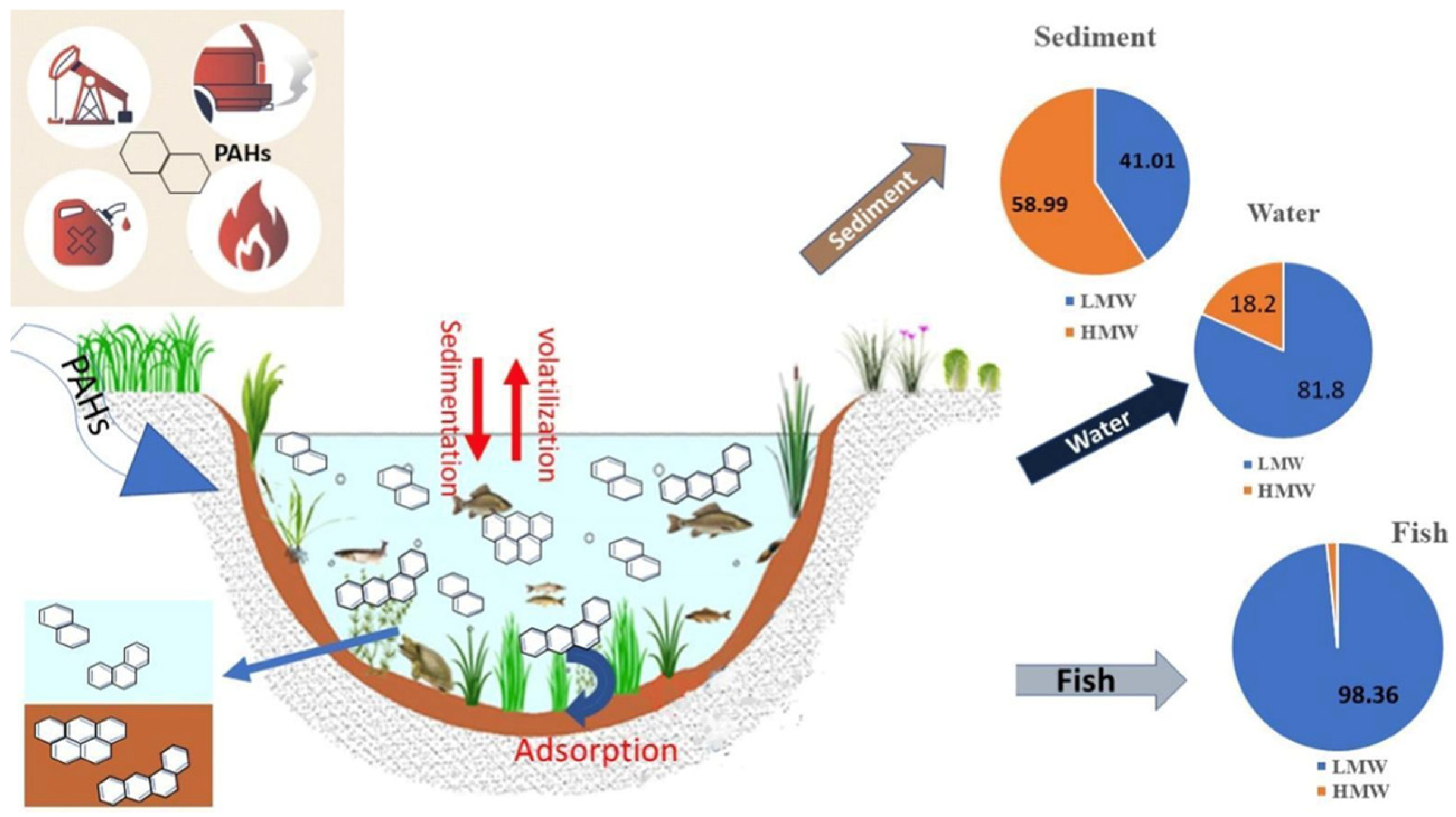
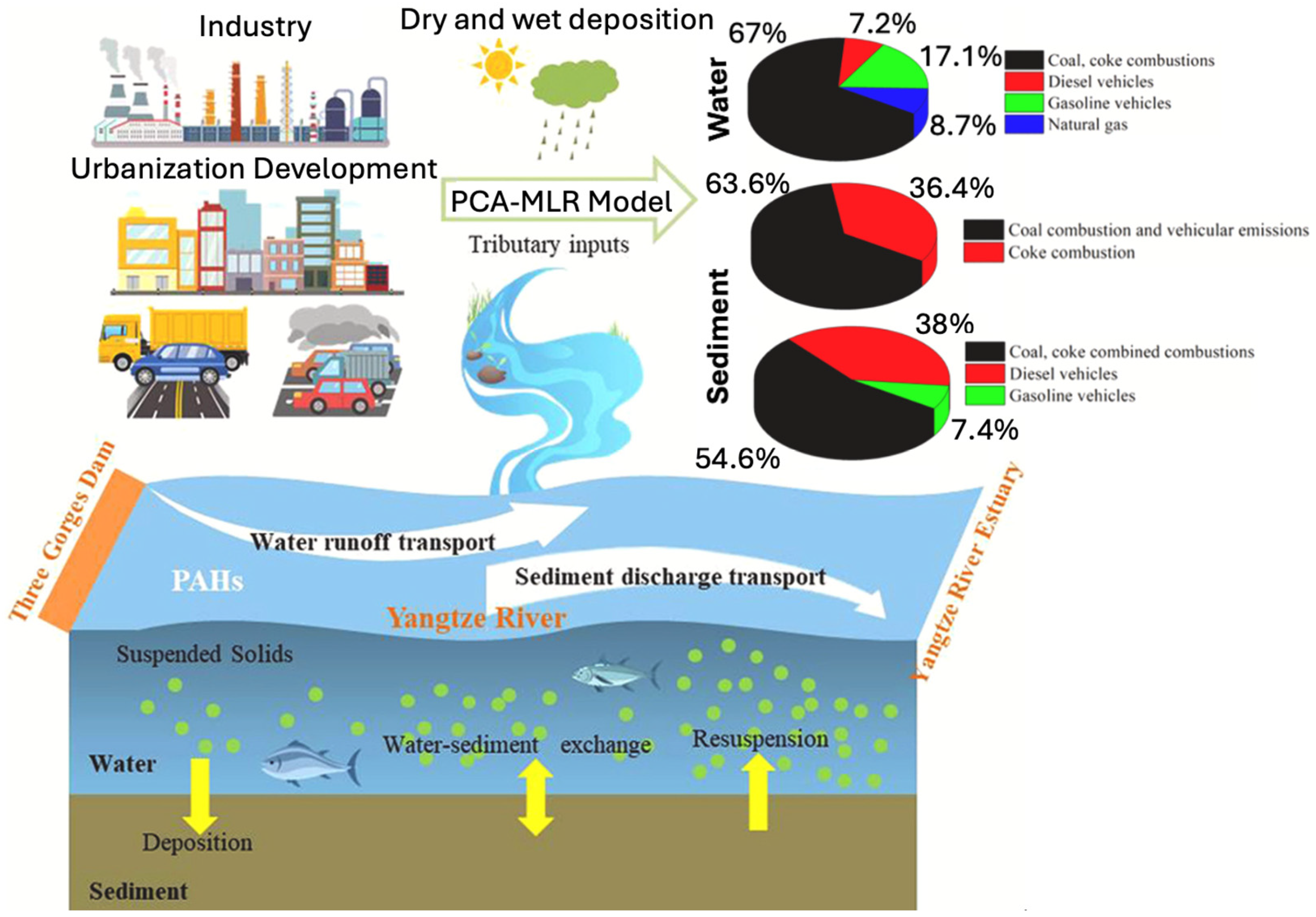
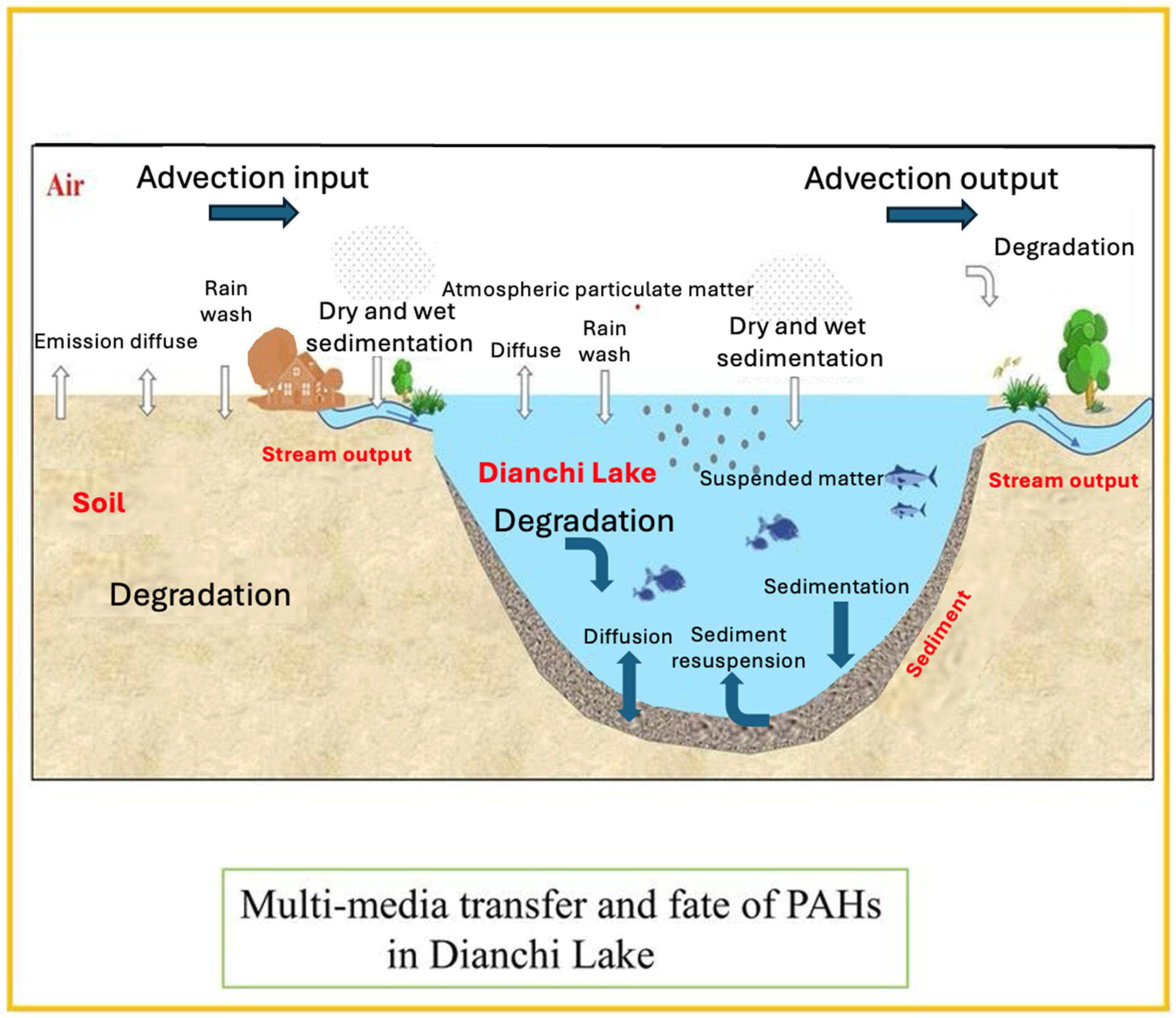
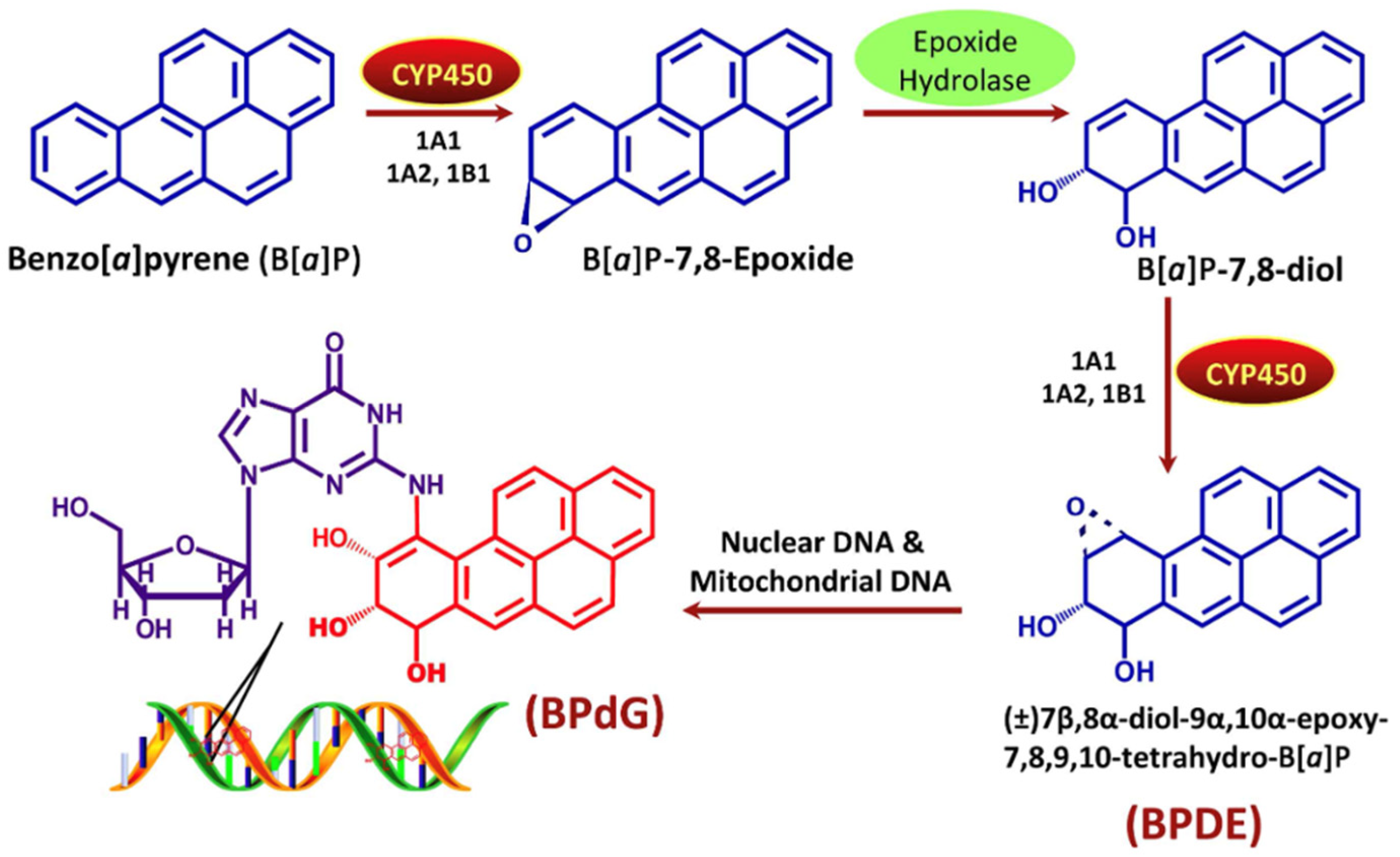
| River System (Region) | Main PAHs Detected | Sources | Ecological Effects | References |
|---|---|---|---|---|
| Pearl River (China) | Fluoranthene, Pyrene, Benzo[a]pyrene | Industrial discharges, urban runoff | Bioaccumulation in fish; risk to benthic fauna | [125] |
| Yangtze River (China) | Phenanthrene, Fluoranthene, Chrysene | Domestic sewage, agricultural runoff | Chronic exposure in mollusks; endocrine disruption | [126] |
| Tigris River (Iraq) | Benzo[a]anthracene, Chrysene | Wastewater discharges, oil refinery pollution | Decreased diversity of benthic invertebrates; moderate ecological risk | [127] |
| Ogun River (Nigeria) | Naphthalene, Acenaphthene | Industrial effluents | Risk to biota | [128] |
| Taihu Lake (China) | Phenanthrene, Pyrene, Benzo[a]anthracene | Urban-industrial interface | Oxidative stress in fish and invertebrates | [129] |
| Chaohu Lake (China) | Fluoranthene, Pyrene, Benzo[a]anthracene | Industrial discharges, urban runoff, atmospheric deposition | Moderate ecological risk; potential impact on aquatic organisms | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berríos-Rolón, P.J.; Cotto, M.C.; Márquez, F. Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Systems: A Comprehensive Review of Sources, Distribution, and Ecotoxicological Impacts. Toxics 2025, 13, 321. https://doi.org/10.3390/toxics13040321
Berríos-Rolón PJ, Cotto MC, Márquez F. Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Systems: A Comprehensive Review of Sources, Distribution, and Ecotoxicological Impacts. Toxics. 2025; 13(4):321. https://doi.org/10.3390/toxics13040321
Chicago/Turabian StyleBerríos-Rolón, Pedro J., María C. Cotto, and Francisco Márquez. 2025. "Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Systems: A Comprehensive Review of Sources, Distribution, and Ecotoxicological Impacts" Toxics 13, no. 4: 321. https://doi.org/10.3390/toxics13040321
APA StyleBerríos-Rolón, P. J., Cotto, M. C., & Márquez, F. (2025). Polycyclic Aromatic Hydrocarbons (PAHs) in Freshwater Systems: A Comprehensive Review of Sources, Distribution, and Ecotoxicological Impacts. Toxics, 13(4), 321. https://doi.org/10.3390/toxics13040321








