4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Cell Viability
2.3. Gene Expression Profiling
2.4. Cell Cycle Analysis
2.5. Annexin-V and Propidium Iodide (PI) Staining
2.6. RT–qPCR
2.7. High-Throughput Screening
2.8. Statistics
3. Results
3.1. Citrinin Decreased the Viability of IPEC-J2 Cells
3.2. Identification and Validation of DEGs
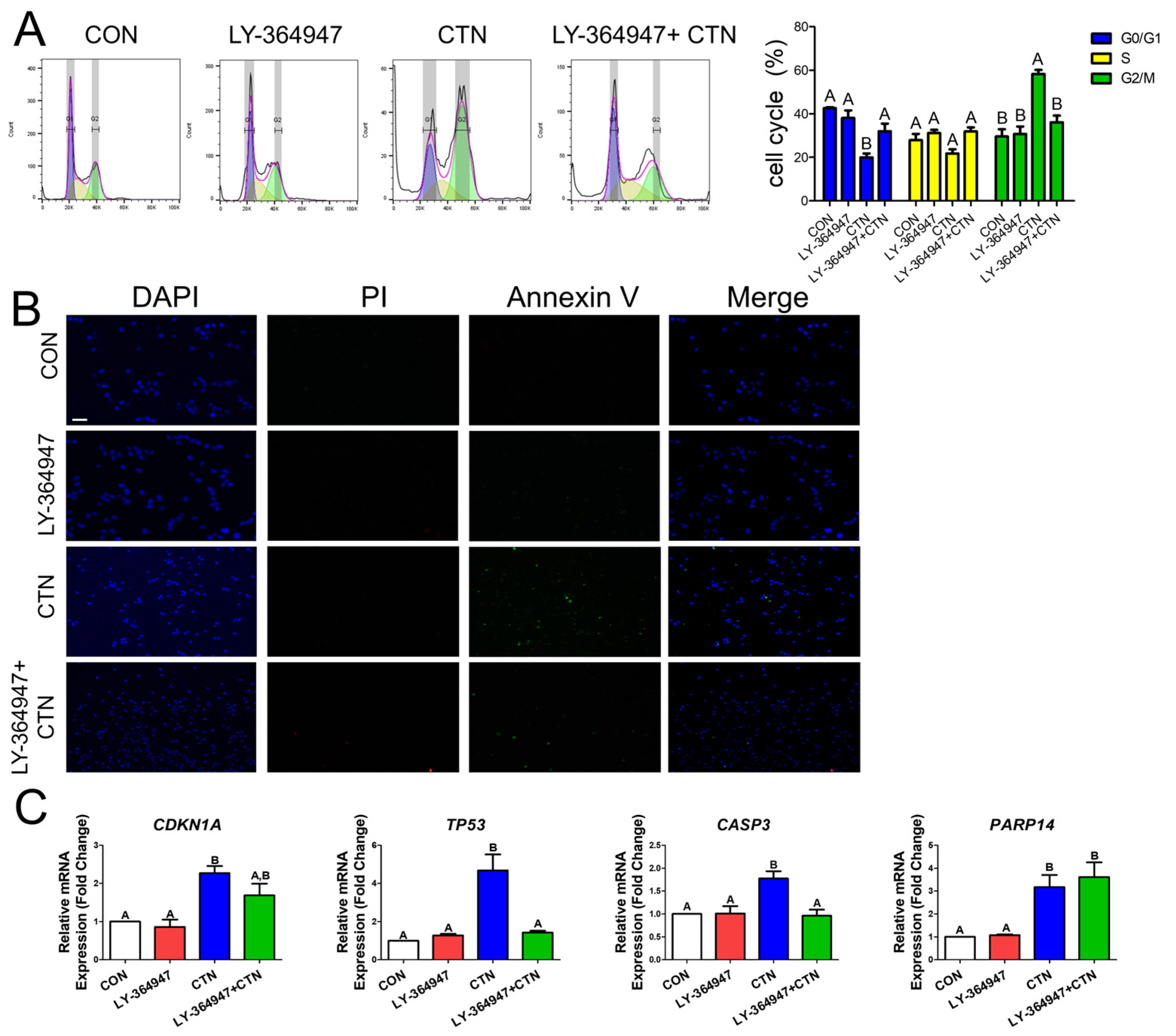
3.3. CTN Induces G2/M Phase Cell Cycle Arrest and Apoptosis in IPEC-J2 Cells
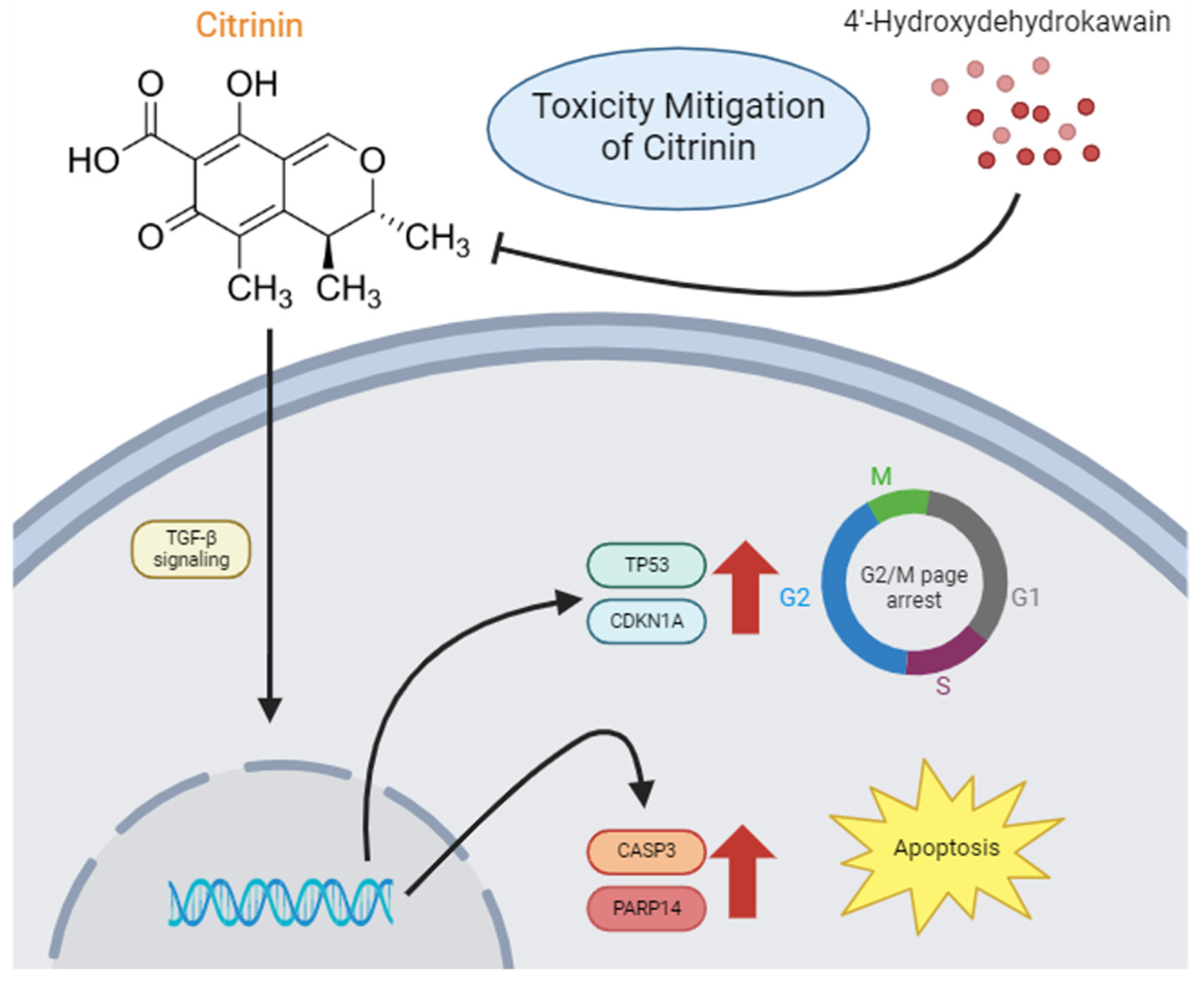
3.4. CTN Induces Apoptosis and G2/M Phase Cell Cycle Arrest Through the TGF-Beta Signaling Pathway
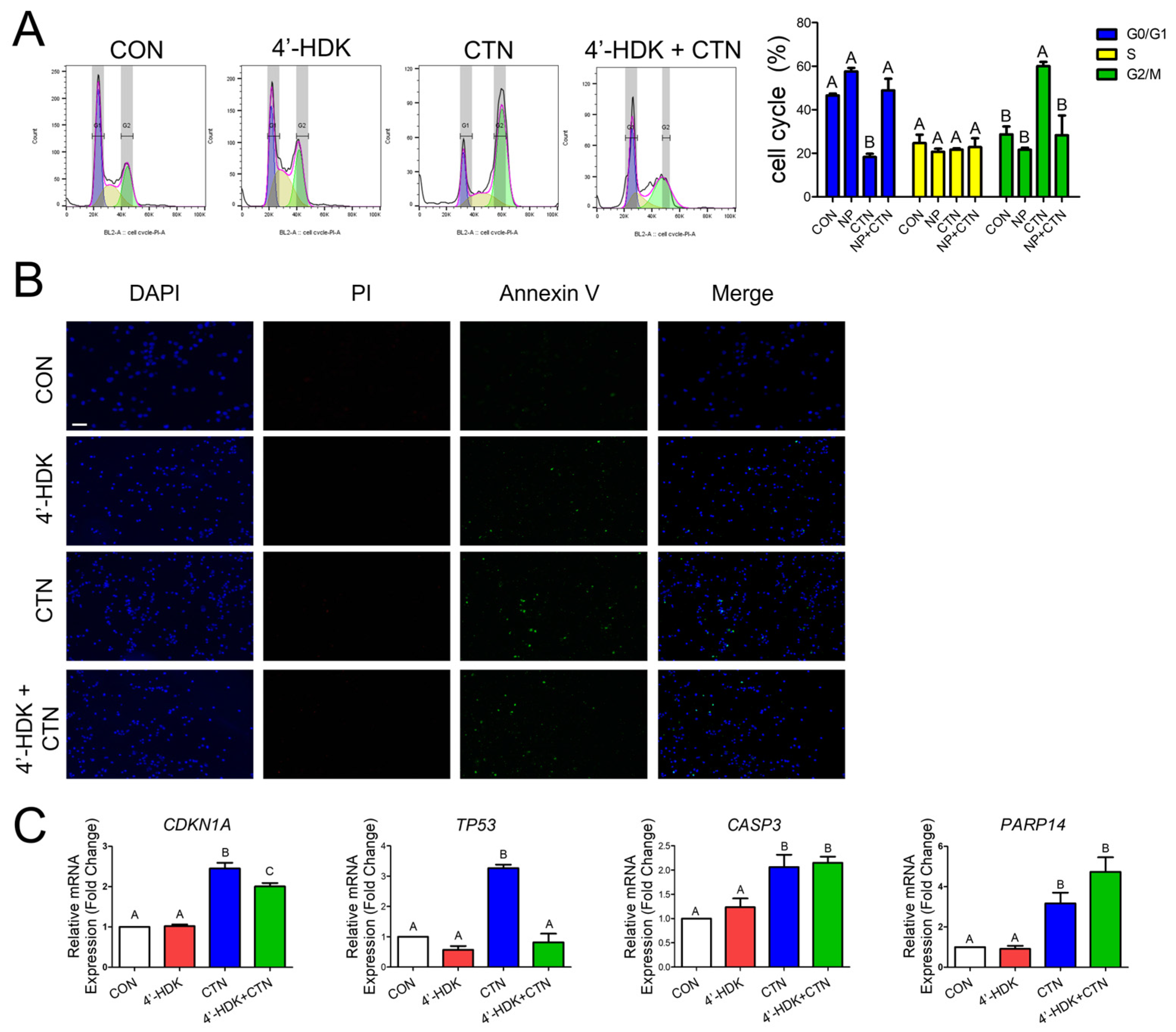
3.5. High-Throughput Screening of NPs to Assess Their Ability to Alleviate Citrinin Toxicity
3.6. 4-HDK Mitigates Citrinin-Induced Toxicity by Modulating Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CTN | Citrinin |
| IPEC-J2 | Intestinal Porcine Epithelial Cells-J2 |
| TGF-β | Transforming Growth Factor-beta |
| 4-HDK | 4′-Hydroxydehydrokawain |
| PI | Propidium iodide |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| DEG | Differentially expressed gene |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FACS | Fluorescence-Activated Cell Sorting |
| NP | Natural product |
References
- Peraica, M.; Radić, B.; Lucić, A.; Pavlović, M. Toxic effects of mycotoxins in humans. Bull. World Health Organ. 1999, 77, 754–766. [Google Scholar] [PubMed]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Chang, C.H.; Yu, F.Y.; Wu, T.S.; Wang, L.T.; Liu, B.H. Mycotoxin citrinin induced cell cycle G2/M arrest and numerical chromosomal aberration associated with disruption of microtubule formation in human cells. Toxicol. Sci. 2011, 119, 84–92. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, C.; Fan, H.; Wang, H.; Wu, Z.; Wu, S.; Bao, W. Analysis of RIOK2 Functions in Mediating the Toxic Effects of Deoxynivalenol in Porcine Intestinal Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 12712. [Google Scholar] [CrossRef] [PubMed]
- Ciacci-Zanella, J.R.; Merrill, A.H., Jr.; Wang, E.; Jones, C. Characterization of cell-cycle arrest by fumonisin B1 in CV-1 cells. Food Chem. Toxicol. 1998, 36, 791–804. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. Int. J. Mol. Sci. 2020, 21, 6517. [Google Scholar] [CrossRef] [PubMed]
- Solcan, C.; Timofte, D.; Floristean, V.C.; Carter, S.D.; Solcan, G. Ultrastructural lesions and immunohistochemical analysis of Bcl-2 protein expression in the kidney of chickens with experimental ochratoxicosis. Acta Vet. Hung. 2013, 61, 344–353. [Google Scholar] [CrossRef]
- Wang, F.; Zuo, Z.; Chen, K.; Gao, C.; Yang, Z.; Zhao, S.; Li, J.; Song, H.; Peng, X.; Fang, J.; et al. Histopathological Injuries, Ultrastructural Changes, and Depressed TLR Expression in the Small Intestine of Broiler Chickens with Aflatoxin B1. Toxins 2018, 10, 131. [Google Scholar] [CrossRef]
- Feng, G.D.; He, J.; Ao, X.; Chen, D.W. Effects of maize naturally contaminated with aflatoxin B1 on growth performance, intestinal morphology, and digestive physiology in ducks. Poult. Sci. 2017, 96, 1948–1955. [Google Scholar] [CrossRef]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Aleo, M.D.; Wyatt, R.D.; Schnellmann, R.G. The role of altered mitochondrial function in citrinin-induced toxicity to rat renal proximal tubule suspensions. Toxicol. Appl. Pharmacol. 1991, 109, 455–463. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; Santos, J.V.O.; de Alencar, M.; Júnior, A.L.G.; Paz, M.; de Brito, M.; JMC, E.S.; et al. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Yu, F.Y.; Wu, T.S.; Li, S.Y.; Su, M.C.; Wang, M.C.; Shih, S.M. Evaluation of genotoxic risk and oxidative DNA damage in mammalian cells exposed to mycotoxins, patulin and citrinin. Toxicol. Appl. Pharmacol. 2003, 191, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Nusrat, A. The Life and Death of Epithelia During Inflammation: Lessons Learned from the Gut. Annu. Rev. Pathol. Mech. Dis. 2012, 7, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, K.; Roth, S.; Nieuwenhuis, E.E.S.; Middendorp, S. Intestinal epithelial cell polarity defects in disease: Lessons from microvillus inclusion disease. Dis. Model. Mech. 2018, 11, dmm031088. [Google Scholar] [CrossRef]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef]
- Okamoto, R.; Watanabe, M. Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J. Gastroenterol. 2004, 39, 1–6. [Google Scholar] [CrossRef]
- Potten, C.S.; Gandara, R.; Mahida, Y.R.; Loeffler, M.; Wright, N.A. The stem cells of small intestinal crypts: Where are they? Cell Prolif. 2009, 42, 731–750. [Google Scholar] [CrossRef]
- Hageman, J.H.; Heinz, M.C.; Kretzschmar, K.; van der Vaart, J.; Clevers, H.; Snippert, H.J.G. Intestinal Regeneration: Regulation by the Microenvironment. Dev. Cell 2020, 54, 435–446. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and deoxynivalenol affect rat intestinal epithelial cells: A concentration related study. PLoS ONE 2012, 7, e52051. [Google Scholar] [CrossRef]
- He, Y.; Yin, X.; Dong, J.; Yang, Q.; Wu, Y.; Gong, Z. Transcriptome Analysis of Caco-2 Cells upon the Exposure of Mycotoxin Deoxynivalenol and Its Acetylated Derivatives. Toxins 2021, 13, 167. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, M.; Peng, X.; Cui, H.; Zhou, Y.; He, M.; Zuo, Z.; Ouyang, P.; Fan, J.; Fang, J. The molecular mechanism of G2M cell cycle arrest induced by AFB1 in the jejunum. Oncotarget 2016, 7, 35592–35606. [Google Scholar] [CrossRef] [PubMed]
- Doug, H.; Chen, S.X.; Xu, H.X.; Kadota, S.; Namba, T. A new antiplatelet diarylheptanoid from Alpinia blepharocalyx. J. Nat. Prod. 1998, 61, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zheng, C. Effects of Alpinetin on Intestinal Barrier Function, Inflammation and Oxidative Stress in Dextran Sulfate Sodium-Induced Ulcerative Colitis Mice. Am. J. Med. Sci. 2018, 355, 377–386. [Google Scholar] [CrossRef]
- Wang, K.; Lv, Q.; Miao, Y.M.; Qiao, S.M.; Dai, Y.; Wei, Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem. Pharmacol. 2018, 155, 494–509. [Google Scholar] [CrossRef]
- Yang, J.; Dai, Y.; Xia, Y.F.; Huang, W.Z.; Wang, Z.T. Alpinia katsumadai Hayata prevents mouse sepsis induced by cecal ligation and puncture through promoting bacterial clearance and downregulating systemic inflammation. Phytother. Res. 2009, 23, 267–273. [Google Scholar] [CrossRef]
- An, W.; Zhang, Y.; Lai, H.; Zhang, Y.; Zhang, H.; Zhao, G.; Liu, M.; Li, Y.; Lin, X.; Cao, S. Alpinia katsumadai Hayata induces growth inhibition and autophagy-related apoptosis by regulating the AMPK and Akt/mTOR/p70S6K signaling pathways in cancer cells. Oncol. Rep. 2022, 48, 142. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, N.; Sharma, B.; Mishra, S.; Arora, S.; Selvakumar, R.; Saurabh, V.; et al. Citrinin Mycotoxin Contamination in Food and Feed: Impact on Agriculture, Human Health, and Detection and Management Strategies. Toxins 2022, 14, 85. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; di Mavungu, J.D.; Huybrechts, B.; Tangni, E.K.; Devreese, M.; Croubels, S.; De Saeger, S. Development and validation of an LC-MS/MS method for the simultaneous determination of citrinin and ochratoxin a in a variety of feed and foodstuffs. J. Chromatogr. A 2018, 1580, 100–109. [Google Scholar] [CrossRef]
- Flajs, D.; Peraica, M. Toxicological properties of citrinin. Arh. Hig. Rada Toksikol. 2009, 60, 457–464. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A. Food Toxicity of Mycotoxin Citrinin and Molecular Mechanisms of Its Potential Toxicity Effects through the Implicated Targets Predicted by Computer-Aided Multidimensional Data Analysis. Life 2023, 13, 880. [Google Scholar] [CrossRef]
- Nakayama, H.; Kitagawa, N.; Otani, T.; Iida, H.; Anan, H.; Inai, T. Ochratoxin A, citrinin and deoxynivalenol decrease claudin-2 expression in mouse rectum CMT93-II cells. Microscopy 2018, 67, 99–111. [Google Scholar] [CrossRef]
- Jiang, W.J.; Liu, W.; Li, Y.H.; Jiang, H.; Xu, Y.N.; Kim, N.H. Citrinin impairs pig oocyte maturation by inducing oxidative stress and apoptosis. Toxicon 2022, 205, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.F.; Wu, T.S.; Huang, Y.T.; Lin, W.J.; Yu, F.Y.; Liu, B.H. Exposure to Mycotoxin Citrinin Promotes Carcinogenic Potential of Human Renal Cells. J. Agric. Food Chem. 2023, 71, 19054–19065. [Google Scholar] [CrossRef]
- Jo, M.K.; Moon, C.M.; Jeon, H.J.; Han, Y.; Lee, E.S.; Kwon, J.H.; Yang, K.M.; Ahn, Y.H.; Kim, S.E.; Jung, S.A.; et al. Effect of aging on the formation and growth of colonic epithelial organoids by changes in cell cycle arrest through TGF-β-Smad3 signaling. Inflamm. Regen. 2023, 43, 35. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W. Recycling the Cell Cycle: Cyclins Revisited. Cell 2004, 116, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chung, D.H.; Kim, Y.B.; Choi, Y.H.; Moon, Y. Ribotoxic mycotoxin deoxynivalenol induces G2/M cell cycle arrest via p21Cip/WAF1 mRNA stabilization in human epithelial cells. Toxicology 2008, 243, 145–154. [Google Scholar] [CrossRef]
- Denicourt, C.; Dowdy, S.F. Cip/Kip proteins: More than just CDKs inhibitors. Genes Dev. 2004, 18, 851–855. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Oyarbide, U.; Shah, A.N.; Amaya-Mejia, W.; Snyderman, M.; Kell, M.J.; Allende, D.S.; Calo, E.; Topczewski, J.; Corey, S.J. Loss of Sbds in zebrafish leads to neutropenia and pancreas and liver atrophy. JCI. Insight 2020, 5, e134309. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; MacFarlane, M.; Cain, K.; Cohen, G.M. Protein complexes activate distinct caspase cascades in death receptor and stress-induced apoptosis. Exp. Cell Res. 2000, 256, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Chen, Q.; Shi, W.; Tang, L.; Fu, Y. PARP14 promotes the proliferation and gemcitabine chemoresistance of pancreatic cancer cells through activation of NF-κB pathway. Mol. Carcinog. 2019, 58, 1291–1302. [Google Scholar] [CrossRef] [PubMed]




| Genes | Description | Accession No. | Sequence (5’-3’) | |
|---|---|---|---|---|
| TGM2 | Transglutaminase 2 | XM_003359989.5 | Forward | CGC CTT CTC TCC GTA TGA CT |
| Reverse | TTT TGT GCT TCT TCC TGT GC | |||
| BEX5 | Brain expressed X-linked 5 | XM_005657871.3 | Forward | TAT CCT CAG CAG GTC CAC GT |
| Reverse | CTT CTT CATVTCC GCA TTT GA | |||
| FOXJ1 | Forkhead box protein J1 | XM_003357959.4 | Forward | CTG TCC TCC CCA GGT CTC TA |
| Reverse | AAA TCT CCT TGC TCC ACC AG | |||
| BCAS1 | Brain-enriched myelin- associated protein 1 | NM_001110175.1 | Forward | GCC CCC GAC AGA GAA TAA TA |
| Reverse | CAC TTG AGC ATC CAA CAT CG | |||
| CASP3 | Caspase3 | NM_214131 | Forward | CTC AGG GAG ACC TTC ACA AC |
| Reverse | GCA CGC AAA TAA AAC TGC TC | |||
| PARP14 | Poly (ADP-ribose) polymerase family member 14 | XM_021070260.1 | Forward | CCA CTC TCT GTG TTC CCG TA |
| Reverse | GGT GAG AGA CAC AAG GGC AT | |||
| CDKN1A | Cyclin-dependent kinase inhibitor 1A | XM_013977858.2 | Forward | GGT TCC CCA GTT CTA CCT CA |
| Reverse | GCG TCT CGC TTC ATC ATT TA | |||
| TP53 | Transformation-related protein 53 | NM_213824.3 | Forward | TGC TGT TTC CGT GTG TTT TT |
| Reverse | ATG GGG AGG GAG GTT ATC A | |||
| GABDH | Glyceraldehyde-3- phosphate dehydrogenase | NM_001206359 | Forward | ACA CCG AGC ATC TCC TGA CT |
| Reverse | GAC GAG GCA GGT CTC CCT AA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, S.J.; Shin, S.; Lee, S.I. 4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics 2025, 13, 315. https://doi.org/10.3390/toxics13040315
Lim SJ, Shin S, Lee SI. 4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics. 2025; 13(4):315. https://doi.org/10.3390/toxics13040315
Chicago/Turabian StyleLim, Seung Joon, Sangsu Shin, and Sang In Lee. 2025. "4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells" Toxics 13, no. 4: 315. https://doi.org/10.3390/toxics13040315
APA StyleLim, S. J., Shin, S., & Lee, S. I. (2025). 4′-Hydroxydehydrokawain Mitigate the Cytotoxicity of Citrinin in Porcine Intestinal Epithelial Cells. Toxics, 13(4), 315. https://doi.org/10.3390/toxics13040315







