Effects of Benzo[k]fluoranthene at Two Temperatures on Viability, Structure, and Detoxification-Related Genes in Rainbow Trout RTL-W1 Cell Spheroids
Abstract
1. Introduction
2. Materials and Methods
2.1. RTL-W1 Cell Culture and Spheroid Formation
2.2. Exposure Assays
2.3. Viability Assays
2.3.1. alamarBlue
2.3.2. Lactate Dehydrogenase (LDH) Assay
2.4. Biometric Parameters of Spheroids
2.5. Optical Microscopy
2.6. Processing for Transmission Electron Microscopy [3]
2.7. Semi-Quantitative Ultrastructure Analysis
2.8. Immunocytochemistry (ICC)
2.9. RNA Extraction
2.10. cDNA Synthesis and RT-qPCR
2.11. Statistical Analysis
3. Results
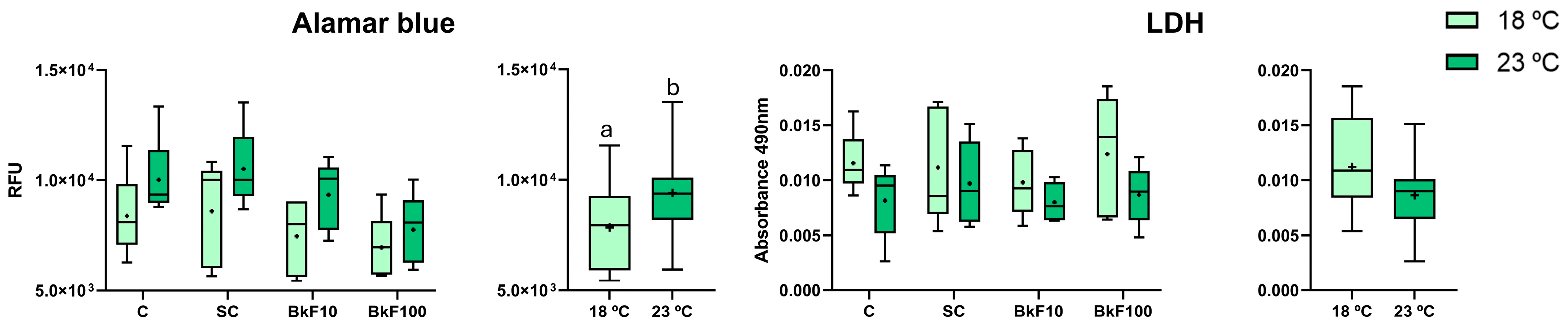
3.1. Viability Assays
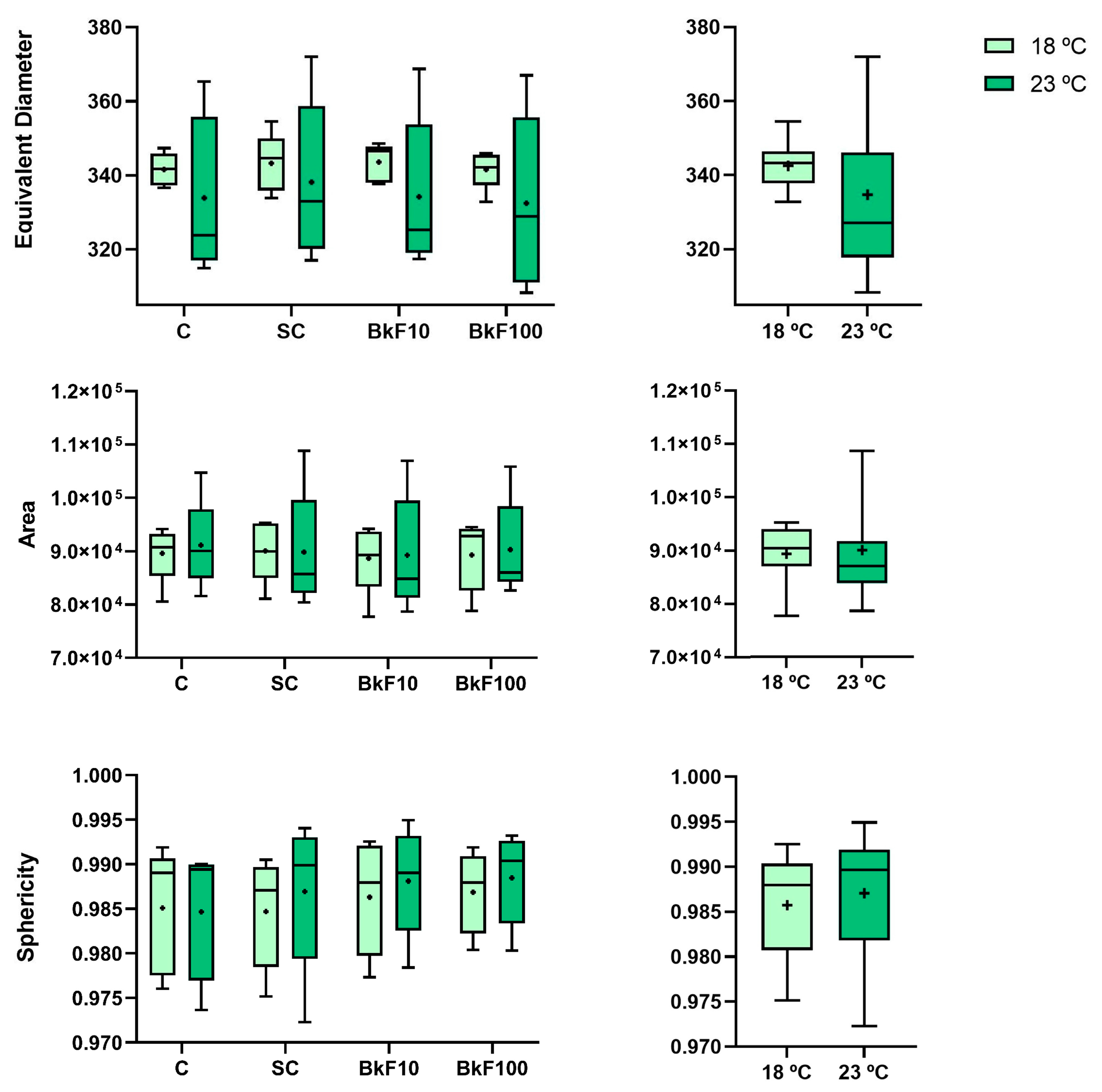
3.2. Biometric Parameters
3.3. Optical Microscopy of Paraffin Sections
3.4. Optical Microscopy of Semithin Sections and Transmission Electron Microscopy
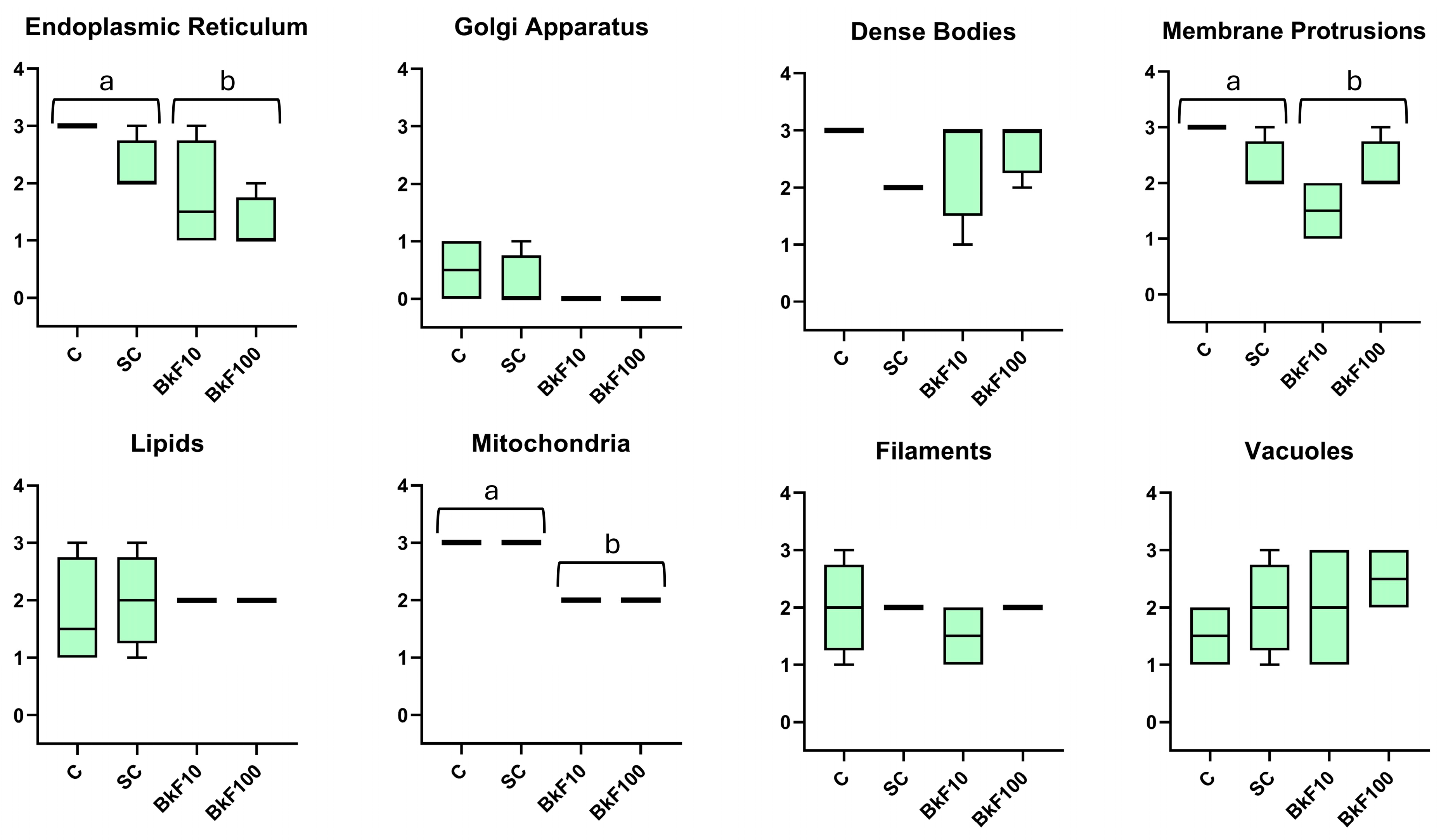
3.5. Semi-Quantitative Ultrastructural Analysis
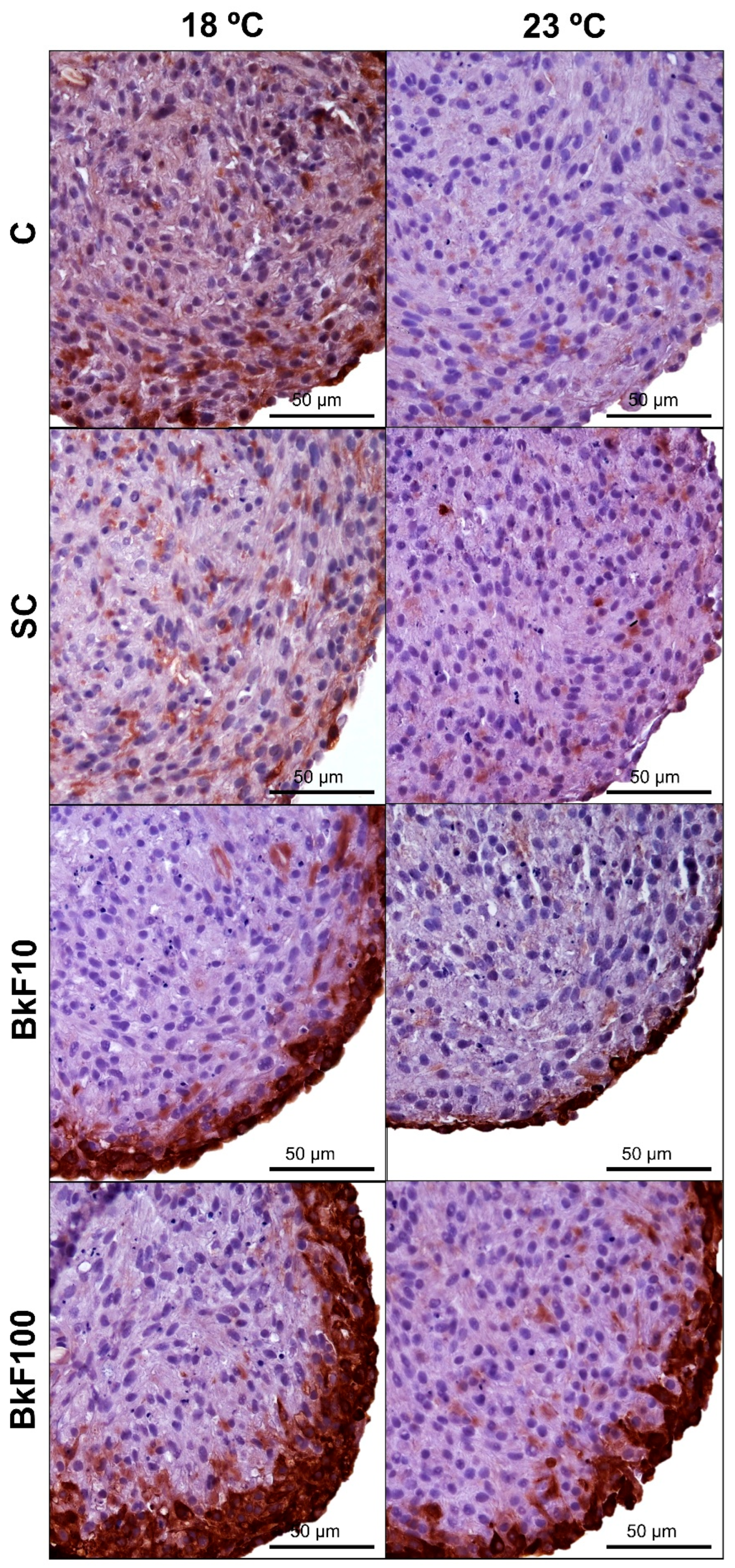
3.6. CYP1A Immunocytochemistry
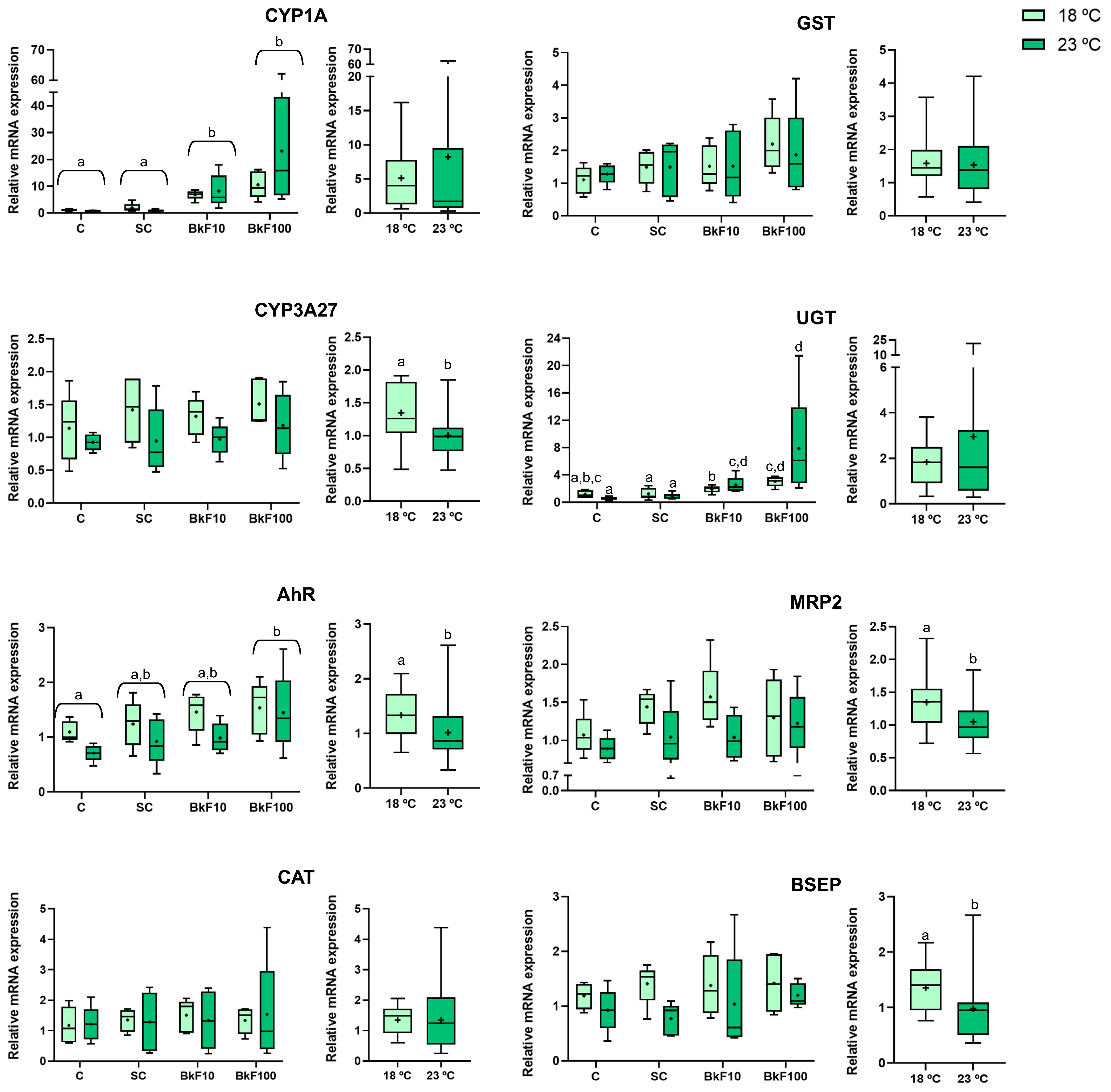
3.7. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Möller, T.; Högner, A.E.; Schleussner, C.-F.; Bien, S.; Kitzmann, N.H.; Lamboll, R.D.; Rogelj, J.; Donges, J.F.; Rockström, J.; Wunderling, N. Achieving net zero greenhouse gas emissions critical to limit climate tipping risks. Nat. Commun. 2024, 15, 6192. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.; Simpson, N.; Johnson, F.; Birt, A. Climate Change 2023: Synthesis Report (Full Volume) Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Forster, P.M.; Smith, C.; Walsh, T.; Lamb, W.F.; Lamboll, R.; Hall, B.; Hauser, M.; Ribes, A.; Rosen, D.; Gillett, N.P.; et al. Indicators of Global Climate Change 2023: Annual update of key indicators of the state of the climate system and human influence. Earth Syst. Sci. Data 2024, 16, 2625–2658. [Google Scholar] [CrossRef]
- WMO. WMO Confirms 2024 as Warmest Year on Record at About 1.55 °C Above Pre-Industrial Level. 2024. Available online: https://wmo.int/news/media-centre/wmo-confirms-2024-warmest-year-record-about-155degc-above-pre-industrial-level (accessed on 10 March 2025).
- Dupuy, J.-L.; Fargeon, H.; Martin-StPaul, N.; Pimont, F.; Ruffault, J.; Guijarro, M.; Hernando, C.; Madrigal, J.; Fernandes, P. Climate change impact on future wildfire danger and activity in southern Europe: A review. Ann. For. Sci. 2020, 77, 35. [Google Scholar] [CrossRef]
- van Hamel, A.; Brunner, M.I. Trends and Drivers of Water Temperature Extremes in Mountain Rivers. Water Resour. Res. 2024, 60, e2024WR037518. [Google Scholar] [CrossRef]
- Kazmi, S.S.U.H.; Wang, Y.Y.L.; Cai, Y.-E.; Wang, Z. Temperature effects in single or combined with chemicals to the aquatic organisms: An overview of thermo-chemical stress. Ecol. Indic. 2022, 143, 109354. [Google Scholar] [CrossRef]
- Ohlberger, J.; Buhle, E.R.; Buehrens, T.W.; Kendall, N.W.; Harbison, T.; Claiborne, A.M.; Losee, J.P.; Whitney, J.; Scheuerell, M.D. Declining Marine Survival of Steelhead Trout Linked to Climate and Ecosystem Change. Fish Fish. 2025, 26, 331–345. [Google Scholar] [CrossRef]
- Roychowdhury, P.; Aftabuddin, M.; Pati, M.K. Thermal stress–induced oxidative damages in the liver and associated death in fish, Labeo rohita. Fish Physiol. Biochem. 2020, 47, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-H.; Guo, Z.-X.; Luo, S.-W.; Wang, A.-L. Effects of high temperature on biochemical parameters, oxidative stress, DNA damage and apoptosis of pufferfish (Takifugu obscurus). Ecotoxicol. Environ. Saf. 2018, 150, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Campos, I.; Abrantes, N. Forest fires as drivers of contamination of polycyclic aromatic hydrocarbons to the terrestrial and aquatic ecosystems. Curr. Opin. Environ. Sci. Heal. 2021, 24, 100293. [Google Scholar] [CrossRef]
- Gomez Isaza, D.F.; Cramp, R.L.; Franklin, C.E. Fire and rain: A systematic review of the impacts of wildfire and associated runoff on aquatic fauna. Glob. Chang. Biol. 2022, 28, 2578–2595. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Heal. 2020, 17, 1363. [Google Scholar] [CrossRef] [PubMed]
- Farwell, A.; Nero, V.; Croft, M.; Bal, P.; Dixon, D.G. Modified Japanese Medaka Embryo-Larval Bioassay for Rapid Determination of Developmental Abnormalities. Arch. Environ. Contam. Toxicol. 2006, 51, 600–607. [Google Scholar] [CrossRef]
- Chen, K.; Tsutsumi, Y.; Yoshitake, S.; Qiu, X.; Xu, H.; Hashiguchi, Y.; Honda, M.; Tashiro, K.; Nakayama, K.; Hano, T.; et al. Alteration of development and gene expression induced by in ovo -nanoinjection of 3-hydroxybenzo[ c ]phenanthrene into Japanese medaka (Oryzias latipes) embryos. Aquat. Toxicol. 2017, 182, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, L.; Seiwert, B.; Huppertsberg, S.; Knepper, T.P.; Reemtsma, T.; Braunbeck, T. Biomarker responses in zebrafish (Danio rerio) following long-term exposure to microplastic-associated chlorpyrifos and benzo(k)fluoranthene. Aquat. Toxicol. 2022, 245, 106120. [Google Scholar] [CrossRef]
- Valdehita, A.; Fernández-Cruz, M.L.; Navas, J.M. The Potentiating Effect of Graphene Oxide on the Arylhydrocarbon Receptor (AhR)–Cytochrome P4501A (Cyp1A) System Activated by Benzo(k)fluoranthene (BkF) in Rainbow Trout Cell Line. Nanomaterials 2023, 13, 2501. [Google Scholar] [CrossRef] [PubMed]
- Derakhshesh, N.; Salamat, N.; Movahedinia, A.; Hashemitabar, M.; Bayati, V. Exposure of liver cell culture from the orange-spotted grouper, Epinephelus coioides, to benzo[a]pyrene and light results in oxidative damage as measured by antioxidant enzymes. Chemosphere 2019, 226, 534–544. [Google Scholar] [CrossRef]
- IARC. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr. Identif. Carcinog. Hazards Hum. 2010, 92, 853. [Google Scholar]
- Rocha, M.J.; Dores-Sousa, J.L.; Cruzeiro, C.; Rocha, E. PAHs in water and surface sediments from Douro River estuary and Porto Atlantic coast (Portugal)—Impacts on human health. Environ. Monit. Assess. 2017, 189, 425. [Google Scholar] [CrossRef]
- Grmasha, R.A.; Stenger-Kovács, C.; Al-Sareji, O.J.; Al-Juboori, R.A.; Meiczinger, M.; Andredaki, M.; Idowu, I.A.; Majdi, H.S.; Hashim, K.; Al-Ansari, N. Temporal and spatial distribution of polycyclic aromatic hydrocarbons (PAHs) in the Danube River in Hungary. Sci. Rep. 2024, 14, 8318. [Google Scholar] [CrossRef]
- Montuori, P.; De Rosa, E.; Cerino, P.; Pizzolante, A.; Nicodemo, F.; Gallo, A.; Rofrano, G.; De Vita, S.; Limone, A.; Triassi, M. Estimation of Polycyclic Aromatic Hydrocarbons in Groundwater from Campania Plain: Spatial Distribution, Source Attribution and Health Cancer Risk Evaluation. Toxics 2023, 11, 435. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Brinkmann, M.; Hudjetz, S.; Kammann, U.; Hennig, M.; Kuckelkorn, J.; Chinoraks, M.; Cofalla, C.; Wiseman, S.; Giesy, J.P.; Schäffer, A.; et al. How flood events affect rainbow trout: Evidence of a biomarker cascade in rainbow trout after exposure to PAH contaminated sediment suspensions. Aquat. Toxicol. 2013, 128–129, 13–24. [Google Scholar] [CrossRef]
- Lyons, M.C.; Wong, D.K.H.; Mulder, I.; Lee, K.; Burridge, L.E. The influence of water temperature on induced liver EROD activity in Atlantic cod (Gadus morhua) exposed to crude oil and oil dispersants. Ecotoxicol. Environ. Saf. 2011, 74, 904–910. [Google Scholar] [CrossRef]
- Andersen, Ø.; Frantzen, M.; Rosland, M.; Timmerhaus, G.; Skugor, A.; Krasnov, A. Effects of crude oil exposure and elevated temperature on the liver transcriptome of polar cod (Boreogadus saida). Aquat. Toxicol. 2015, 165, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.M. Detoxification pathways in the liver. J. Inherit. Metab. Dis. 1991, 14, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Sevior, D.K.; Pelkonen, O.; Ahokas, J.T. Hepatocytes: The powerhouse of biotransformation. Int. J. Biochem. Cell Biol. 2012, 44, 257–261. [Google Scholar] [CrossRef]
- Santana, M.S.; Sandrini-Neto, L.; Filipak Neto, F.; Oliveira Ribeiro, C.A.; Di Domenico, M.; Prodocimo, M.M. Biomarker responses in fish exposed to polycyclic aromatic hydrocarbons (PAHs): Systematic review and meta-analysis. Environ. Pollut. 2018, 242, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Kyffin, J.A.; Sharma, P.; Leedale, J.; Colley, H.E.; Murdoch, C.; Mistry, P.; Webb, S.D. Impact of cell types and culture methods on the functionality of in vitro liver systems–A review of cell systems for hepatotoxicity assessment. Toxicol. Vitr. 2018, 48, 262–275. [Google Scholar] [CrossRef]
- Yang, S.; Ooka, M.; Margolis, R.J.; Xia, M. Liver three-dimensional cellular models for high-throughput chemical testing. Cell Reports Methods 2023, 3. [Google Scholar] [CrossRef]
- Hultman, M.T.; Løken, K.B.; Grung, M.; Reid, M.J.; Lillicrap, A. Performance of three-dimensional rainbow trout (Oncorhynchus mykiss) hepatocyte spheroids for evaluating biotransformation of pyrene. Environ. Toxicol. Chem. 2019, 38, 1738–1747. [Google Scholar] [CrossRef]
- Alves, R.F.; Lopes, C.; Rocha, E.; Madureira, T.V. A step forward in the characterization of primary brown trout hepatocytic spheroids as experimental models. Animals 2023, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Uchea, C.; Owen, S.F.; Chipman, J.K. Functional xenobiotic metabolism and efflux transporters in trout hepatocyte spheroid cultures. Toxicol. Res. 2015, 4, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Holgersson, V.; Joyce, S.; Brookman-Amissah, M.; Lammel, T. Comparative analysis of 3D and 2D in vitro models of the permanent fish liver cell line RTL-W1: Metabolic capabilities and responses to xenobiotics. Ecotoxicol. Environ. Saf. 2024, 288, 117327. [Google Scholar] [CrossRef]
- Lammel, T.; Tsoukatou, G.; Jellinek, J.; Sturve, J. Development of three-dimensional (3D) spheroid cultures of the continuous rainbow trout liver cell line RTL-W1. Ecotoxicol. Environ. Saf. 2019, 167, 250–258. [Google Scholar] [CrossRef] [PubMed]
- de Souza, I.R.; Canavez, A.; Schuck, D.C.; Gagosian, V.S.C.; de Souza, I.R.; Vicari, T.; da Silva Trindade, E.; Cestari, M.M.; Lorencini, M.; Leme, D.M. Development of 3D cultures of zebrafish liver and embryo cell lines: A comparison of different spheroid formation methods. Ecotoxicology 2021, 30, 1893–1909. [Google Scholar] [CrossRef]
- Park, C.G.; Ryu, C.S.; Sung, B.; Manz, A.; Kong, H.; Kim, Y.J. Transcriptomic and physiological analysis of endocrine disrupting chemicals Impacts on 3D Zebrafish liver cell culture system. Aquat. Toxicol. 2022, 245, 106105. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Z.; Quan, J.; Li, L.; Zhao, G.; Lu, J. Protective effects of different concentrations of selenium nanoparticles on rainbow trout (Oncorhynchus mykiss) primary hepatocytes under heat stress. Ecotoxicol. Environ. Saf. 2022, 230, 113121. [Google Scholar] [CrossRef]
- Vilaça, M.; Lopes, C.; Seabra, R.; Rocha, E. 17α-Ethynylestradiol and levonorgestrel exposure of rainbow trout RTL-W1 cells at 18 °C and 21 °C mainly reveals thermal tolerance, absence of estrogenic effects, and progestin-induced upregulation of detoxification genes. Genes 2024, 15, 1189. [Google Scholar] [CrossRef]
- Soto, B. Climate-induced changes in river water temperature in North Iberian Peninsula. Theor. Appl. Climatol. 2018, 133, 101–112. [Google Scholar] [CrossRef]
- Estrela-Segrelles, C.; Gómez-Martínez, G.; Pérez-Martín, M.Á. Climate change risks on mediterranean river ecosystems and adaptation measures (Spain). Water Resour. Manag. 2023, 37, 2757–2770. [Google Scholar] [CrossRef]
- Molés, G.; Valdehita, A.; Connolly, M.; Navas, J.M. Involvement of ahr-dependent Cyp1a detoxification activity, oxidative stress and inflammatory regulation in response to graphene oxide exposure in rainbow trout (Oncorhynchus mykiss). Chemosphere 2024, 364, 143005. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F. AnaSP: A software suite for automatic image analysis of multicellular spheroids. Comput. Methods Programs Biomed. 2015, 119, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Seabra, R.; Malhão, F.; Correia, A.; Costa, C.; Kijjoa, A.; Rocha, E. Effects and mechanisms of action of preussin, a marine fungal metabolite, against the triple-negative breast cancer cell line, MDA-MB-231, in 2D and 3D cultures. Marine Drugs 2023, 21, 166. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- De Anna, J.S.; Darraz, L.A.; Painefilú, J.C.; Cárcamo, J.G.; Moura-Alves, P.; Venturino, A.; Luquet, C.M. The insecticide chlorpyrifos modifies the expression of genes involved in the PXR and AhR pathways in the rainbow trout, Oncorhynchus mykiss. Pestic. Biochem. Physiol. 2021, 178, 104920. [Google Scholar] [CrossRef]
- Batista-Pinto, C. Peroxisomes in Brown trout (Salmo trutta f. fario): Regulation by Estrogens; Institute of Biomedical Sciences Abel Salazar: Porto, Portugal, 2007; pp. 121–135. [Google Scholar]
- Madureira, T.V.; Pinheiro, I.; Malhão, F.; Lopes, C.; Urbatzka, R.; Castro, L.F.C.; Rocha, E. Cross-interference of two model peroxisome proliferators in peroxisomal and estrogenic pathways in brown trout hepatocytes. Aquat. Toxicol. 2017, 187, 153–162. [Google Scholar] [CrossRef]
- Madureira, T.V.; Pinheiro, I.; de Paula Freire, R.; Rocha, E.; Castro, L.F.; Urbatzka, R. Genome specific PPARαB duplicates in salmonids and insights into estrogenic regulation in brown trout. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 208–209, 94–101. [Google Scholar] [CrossRef]
- Jimenez, B.D.; Cirmo, C.P.; McCarthy, J.F. Effects of feeding and temperature on uptake, elimination and metabolism of benzo(a)pyrene in the bluegill sunfish (Lepomis macrochirus). Aquat. Toxicol. 1987, 10, 41–57. [Google Scholar] [CrossRef]
- Malhão, F.; Urbatzka, R.; Navas, J.M.; Cruzeiro, C.; Monteiro, R.A.F.; Rocha, E. Cytological, immunocytochemical, ultrastructural and growth characterization of the rainbow trout liver cell line RTL-W1. Tissue Cell 2013, 45, 159–174. [Google Scholar] [CrossRef]
- Hampton, J.A.; McCuskey, P.A.; McCuskey, R.S.; Hinton, D.E. Functional units in rainbow trout (Salmo gairdneri) liver: I. Arrangement and histochemical properties of hepatocytes. Anat. Rec. 1985, 213, 166–175. [Google Scholar] [CrossRef]
- Rocha, E.; Monteiro, R.A.; Pereira, C.A. The liver of the brown trout, Salmo trutta fario: A light and electron microscope study. J. Anat. 1994, 185, 241–249. [Google Scholar] [PubMed]
- Thyberg, J.; Moskalewski, S. Disorganization of the Golgi complex and the cytoplasmic microtubule system in CHO cells exposed to okadaic acid. J. Cell Sci. 1992, 103, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.S.; Malhotra, V. The curious status of the Golgi apparatus. Cell 1998, 95, 883–889. [Google Scholar] [CrossRef]
- Machamer, C.E. Golgi disassembly in apoptosis: Cause or effect? Trends Cell Biol. 2003, 13, 279–281. [Google Scholar] [CrossRef]
- Machamer, C.E. The Golgi complex in stress and death. Front. Neurosci. 2015, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Cai, F.; Li, Y.; Wang, Y.; Liu, C.; Wang, R.; Gao, P. Biomarker responses and histological damage in the gill, liver, and gonad of Cyprinus carpio with benzo(a)pyrene exposure. Environ. Sci. Pollut. Res. 2021, 28, 61290–61301. [Google Scholar] [CrossRef]
- Mahmoud, A.B.; Al-Salahy, M.B. Physiological and histological studies on the effect of lupine seeds on the fish, Clarias lazera treated with glucose and alloxan. Fish Physiol. Biochem. 2004, 30, 213–229. [Google Scholar] [CrossRef]
- Laguens, R.P.; Candela, S.; Hernández, R.E.; Gagliardino, J.J. Streptozotocin-induced liver damage in mice. Horm. Metab. Res. 1980, 12, 197–201. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Lu, J.; Zhao, G.; Sun, J. Dietary nanoselenium supplementation for heat-stressed rainbow trout: Effects on organizational structure, lipid changes, and biochemical parameters as well as heat-shock-protein- and selenoprotein-related gene expression. Fish Physiol. Biochem. 2022, 48, 707–722. [Google Scholar] [CrossRef]
- Bols, N.C.; Schirmer, K.; Joyce, E.M.; Dixon, D.G.; Greenberg, B.M.; Whyte, J.J. Ability of polycyclic aromatic hydrocarbons to induce 7-Ethoxyresorufin-o-deethylase activity in a trout liver cell line. Ecotoxicol. Environ. Saf. 1999, 44, 118–128. [Google Scholar] [CrossRef]
- Bramatti, I.; Matos, B.; Figueiredo, N.; Pousão-Ferreira, P.; Branco, V.; Martins, M. Interaction of polycyclic aromatic hydrocarbon compounds in fish primary hepatocytes: From molecular mechanisms to genotoxic effects. Sci. Total Environ. 2023, 855, 158783. [Google Scholar] [CrossRef] [PubMed]
- Rodd, A.L.; Messier, N.J.; Vaslet, C.A.; Kane, A.B. A 3D fish liver model for aquatic toxicology: Morphological changes and Cyp1a induction in PLHC-1 microtissues after repeated benzo(a)pyrene exposures. Aquat. Toxicol. 2017, 186, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, P.G.; Resende-de-Oliveira, R.; Rocha, E. Combined effects of increased temperature and levonorgestrel exposure on zebrafish female liver, using stereology and immunohistochemistry against catalase, CYP1A, HSP90 and vitellogenin. Environ. Pollut. 2019, 252, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Pan, L.; Wang, L. Metabolic enzyme activities, metabolism-related genes expression and bioaccumulation in juvenile white shrimp Litopenaeus vannamei exposed to benzo[a]pyrene. Ecotoxicol. Environ. Saf. 2014, 104, 79–86. [Google Scholar] [CrossRef]
- Allmon, E.; Serafin, J.; Chen, S.; Rodgers, M.L.; Griffitt, R.; Bosker, T.; de Guise, S.; Sepúlveda, M.S. Effects of polycyclic aromatic hydrocarbons and abiotic stressors on Fundulus grandis cardiac transcriptomics. Sci. Total Environ. 2021, 752, 142156. [Google Scholar] [CrossRef]
- Bagnyukova, T.V.; Lushchak, O.V.; Storey, K.B.; Lushchak, V.I. Oxidative stress and antioxidant defense responses by goldfish tissues to acute change of temperature from 3 to 23°C. J. Therm. Biol. 2007, 32, 227–234. [Google Scholar] [CrossRef]
- Nahrgang, J.; Camus, L.; Gonzalez, P.; Goksøyr, A.; Christiansen, J.S.; Hop, H. PAH biomarker responses in polar cod (Boreogadus saida) exposed to benzo(a)pyrene. Aquat. Toxicol. 2009, 94, 309–319. [Google Scholar] [CrossRef]



| Gene | Sequence | Protocol | Efficiency | References |
|---|---|---|---|---|
| CYP1A | F: GATGTCAGTGGCAGCTTTGA R: TCCTGGTCATCATGGCTGTA | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 97.7 | [34] |
| CYP3A27 | F: GACGGTGGAGATCAACG R: GAGGATCTCGACCATGG | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 99.0 | [34] |
| GST | F: AGCTGCTCCCAGCTGATCC R:CAAACCACGGCCACATCATGTAATC | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 93.9 | [47] |
| CAT | F: CACTGATGAGGGCAACTGGG R: CTTGAAGTGGAACTTGCAG | 95 °C—3 min (95 °C—10 s; 58.0 °C—30 s; 72 °C—30 s) 40× 95 °C—30 s | 104.5 | [48] |
| UGT | F: ATAAGGACCGTCCCATCGAG R: ATCCAGTTGAGGTCGTGAGC | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 94.9 | [34] |
| MRP2 | F: CCATTCTGTTCGCTGTCTCA R: CTCGTAGCAGGGTCTGGAAG | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 100.2 | [34] |
| BSEP | F: CCGACCAGGGCAAAGTGATT R: CAGAATGGGCTCCTGGGATAC | 94 °C—3 min (94 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 95.3 | [34] |
| AhR | F: GGATGCCACTGAGTTCCAAACCAA R: AATGCCTGGTCTATGGGTAGCTGA | 95 °C—3 min (95 °C—20 s; 60.0 °C—20 s; 72 °C—20 s) 40× 95 °C—1 min | 101.6 | [47] |
| Ef1α | F: TGCCACACTGCTCACATC R: TCTCCAGACTTCAGGAACTTG | 94 °C—3 min (94 °C—20 s; 55.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 97.4 | [49] |
| β-actin | F: TCTGGCATCACACCTTCTAC R: TTCTCCCTGTTGGCTTTGG | 94 °C—3 min (94 °C—20 s; 55.0 °C—20 s; 72 °C—20 s) 40× 94 °C—1 min | 99.7 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves, T.; Malhão, F.; Rocha, E.; Lopes, C. Effects of Benzo[k]fluoranthene at Two Temperatures on Viability, Structure, and Detoxification-Related Genes in Rainbow Trout RTL-W1 Cell Spheroids. Toxics 2025, 13, 302. https://doi.org/10.3390/toxics13040302
Esteves T, Malhão F, Rocha E, Lopes C. Effects of Benzo[k]fluoranthene at Two Temperatures on Viability, Structure, and Detoxification-Related Genes in Rainbow Trout RTL-W1 Cell Spheroids. Toxics. 2025; 13(4):302. https://doi.org/10.3390/toxics13040302
Chicago/Turabian StyleEsteves, Telma, Fernanda Malhão, Eduardo Rocha, and Célia Lopes. 2025. "Effects of Benzo[k]fluoranthene at Two Temperatures on Viability, Structure, and Detoxification-Related Genes in Rainbow Trout RTL-W1 Cell Spheroids" Toxics 13, no. 4: 302. https://doi.org/10.3390/toxics13040302
APA StyleEsteves, T., Malhão, F., Rocha, E., & Lopes, C. (2025). Effects of Benzo[k]fluoranthene at Two Temperatures on Viability, Structure, and Detoxification-Related Genes in Rainbow Trout RTL-W1 Cell Spheroids. Toxics, 13(4), 302. https://doi.org/10.3390/toxics13040302








