Mechanistic Insights into 3-Isopropylphenol-Induced Neurotoxicity in Zebrafish: A Network Toxicology and Molecular Docking Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Breed and Rearing of Zebrafish
2.3. Construction of Targets for 3-Isopropylphenol and Nerve Injury
2.4. Construction of the PPI Network
2.5. GO and KEGG Pathway Analysis
2.6. Molecular Docking
2.7. 3-Isopropylphenol Exposure and General Developmental Toxicity Testing
2.8. Behavioral Testing of Zebrafish Larvae
2.9. Neurodevelopmental Toxicity Studies in Zebrafish Larvae
2.10. Real-Time Fluorescence Quantitative PCR
2.11. Statistical Analysis
3. Results
3.1. Prediction of Neurotoxicity Induced by 3-Isopropylphenol
3.2. The Main Interaction Network and Core Genes of Potential Targets
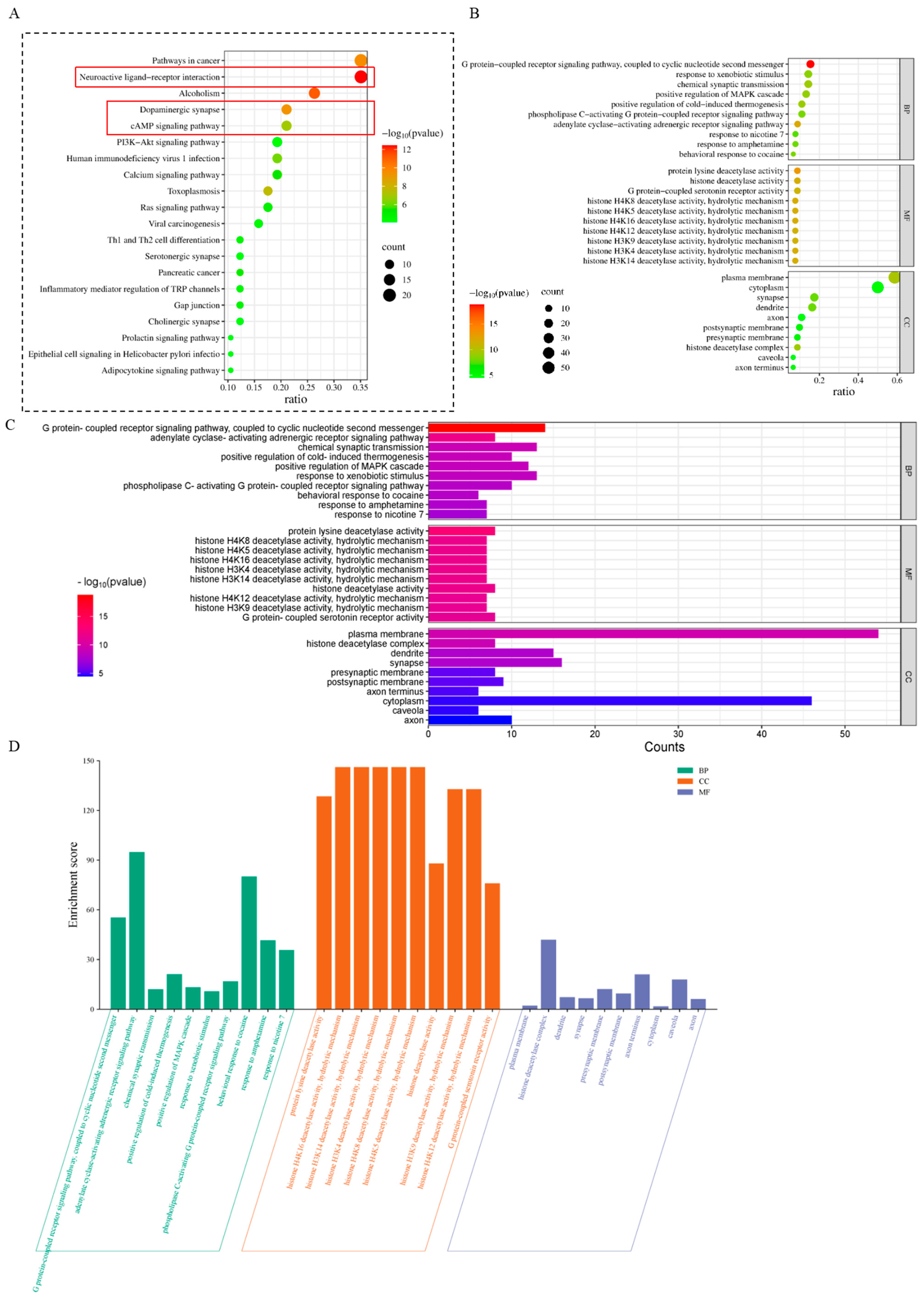
3.3. Target Functional Analysis and Pathway Enrichment Analysis
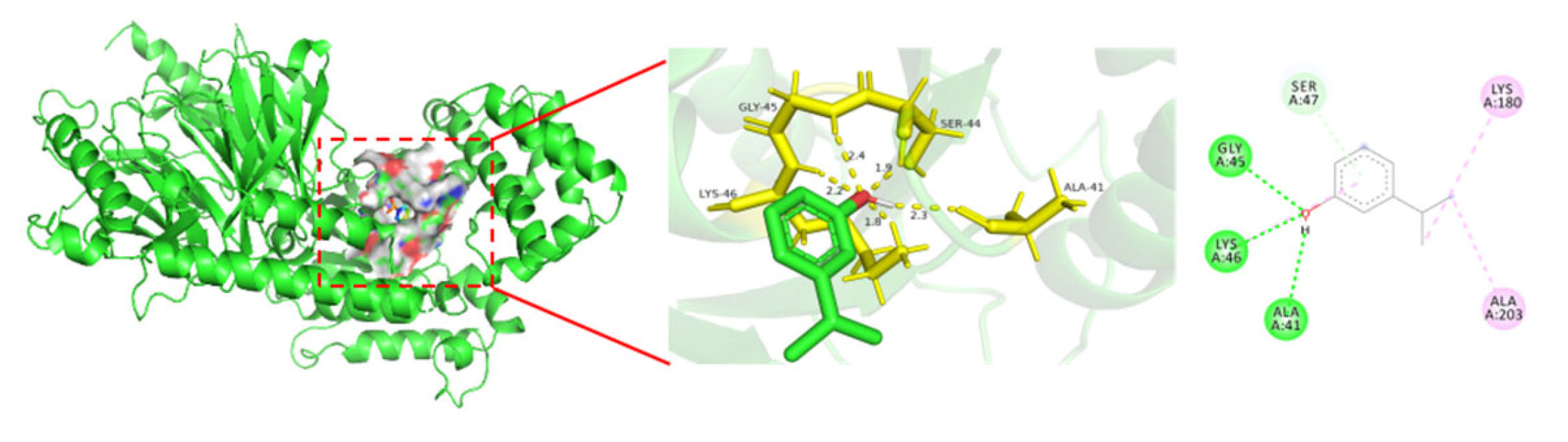
3.4. Molecular Docking of 3-Isopropylphenol and the Core Target Proteins Related to Nerve Injury
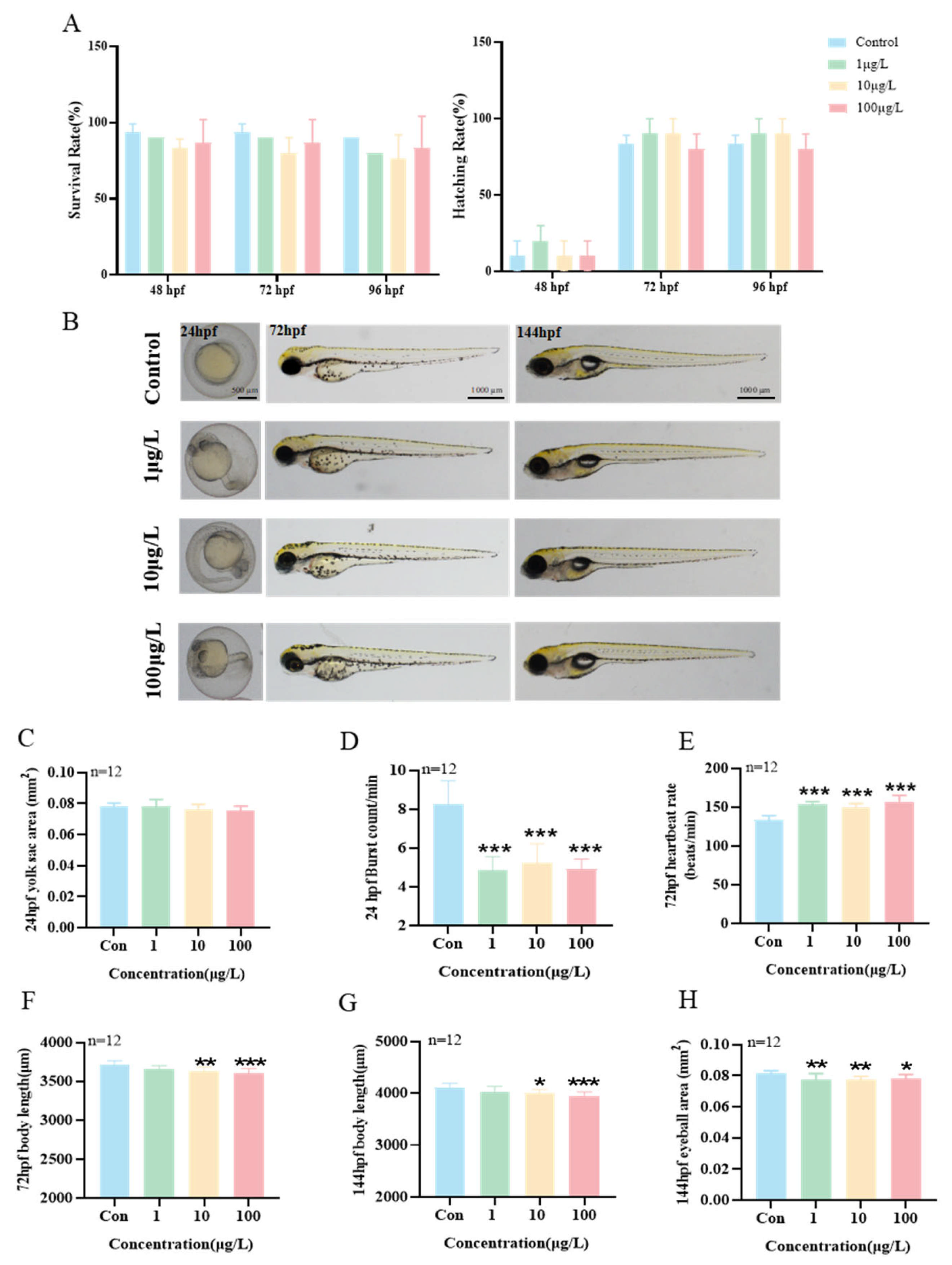
3.5. Effects of Exposure to 3-Isopropylphenol on the Early Development of Zebrafish Larvae
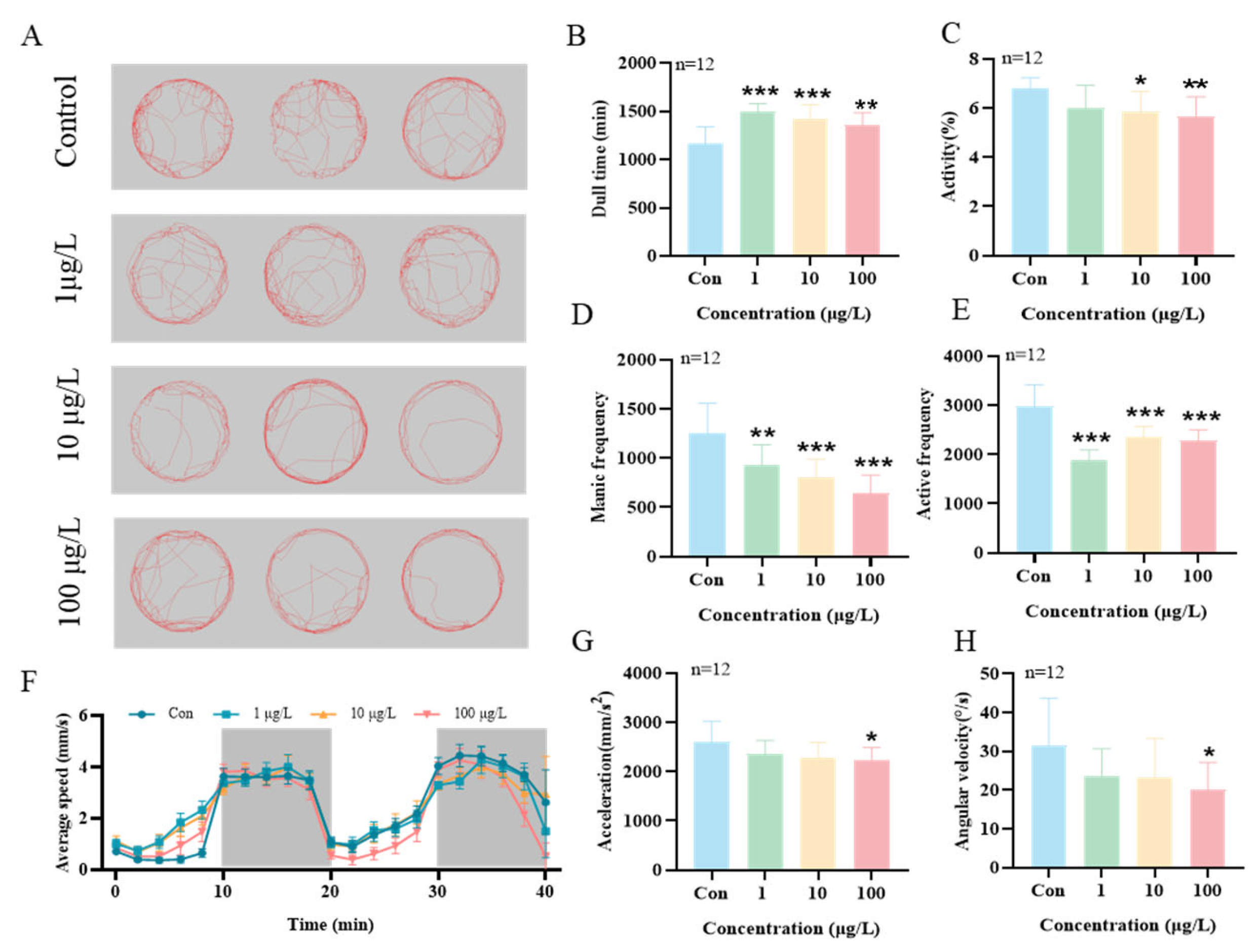
3.6. The Effects of 3-Isopropylphenol on the Motor Behavior of Zebrafish Larvae
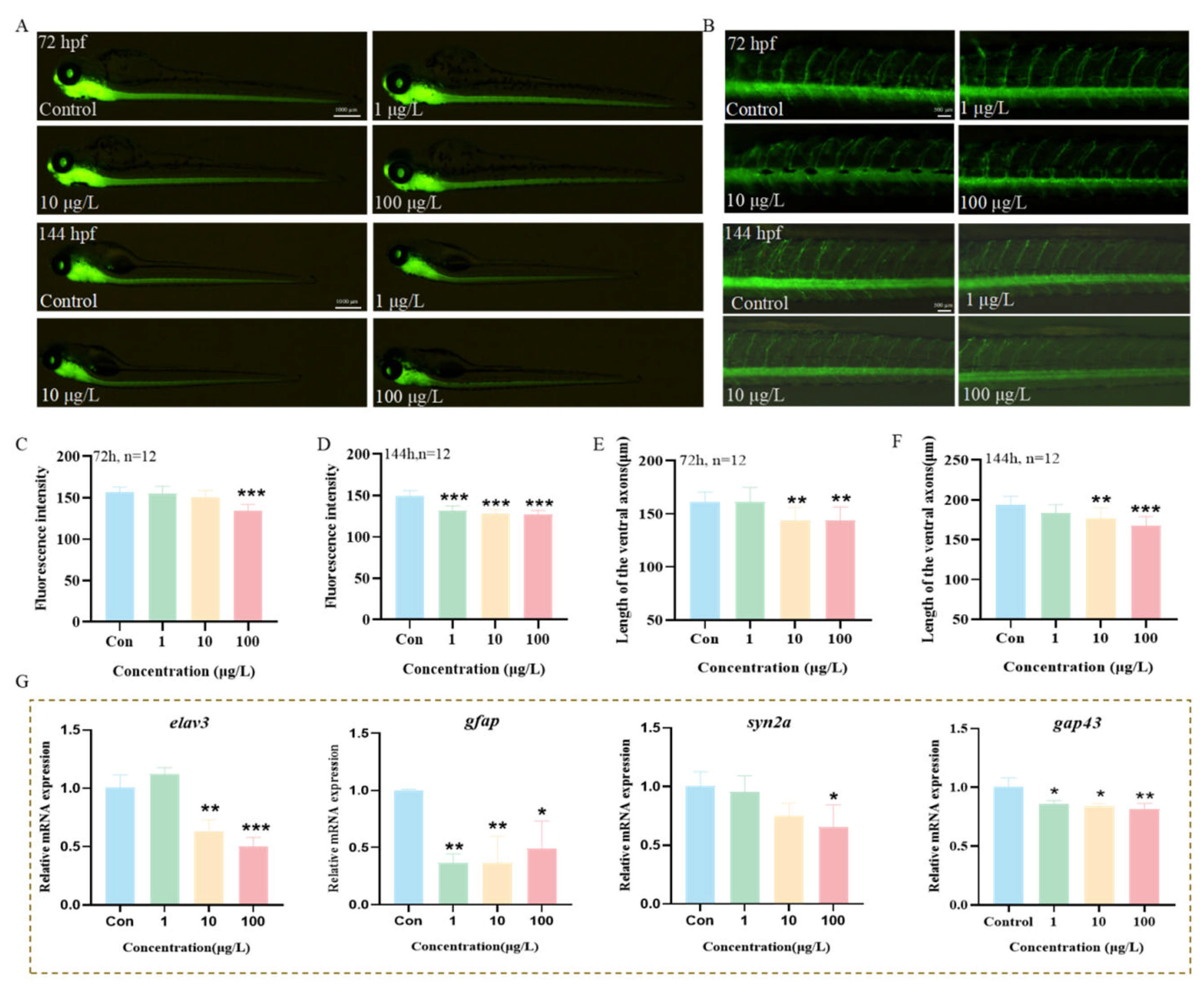
3.7. The Effects of 3-Isopropylphenol on the Nervous System of Zebrafish Larvae
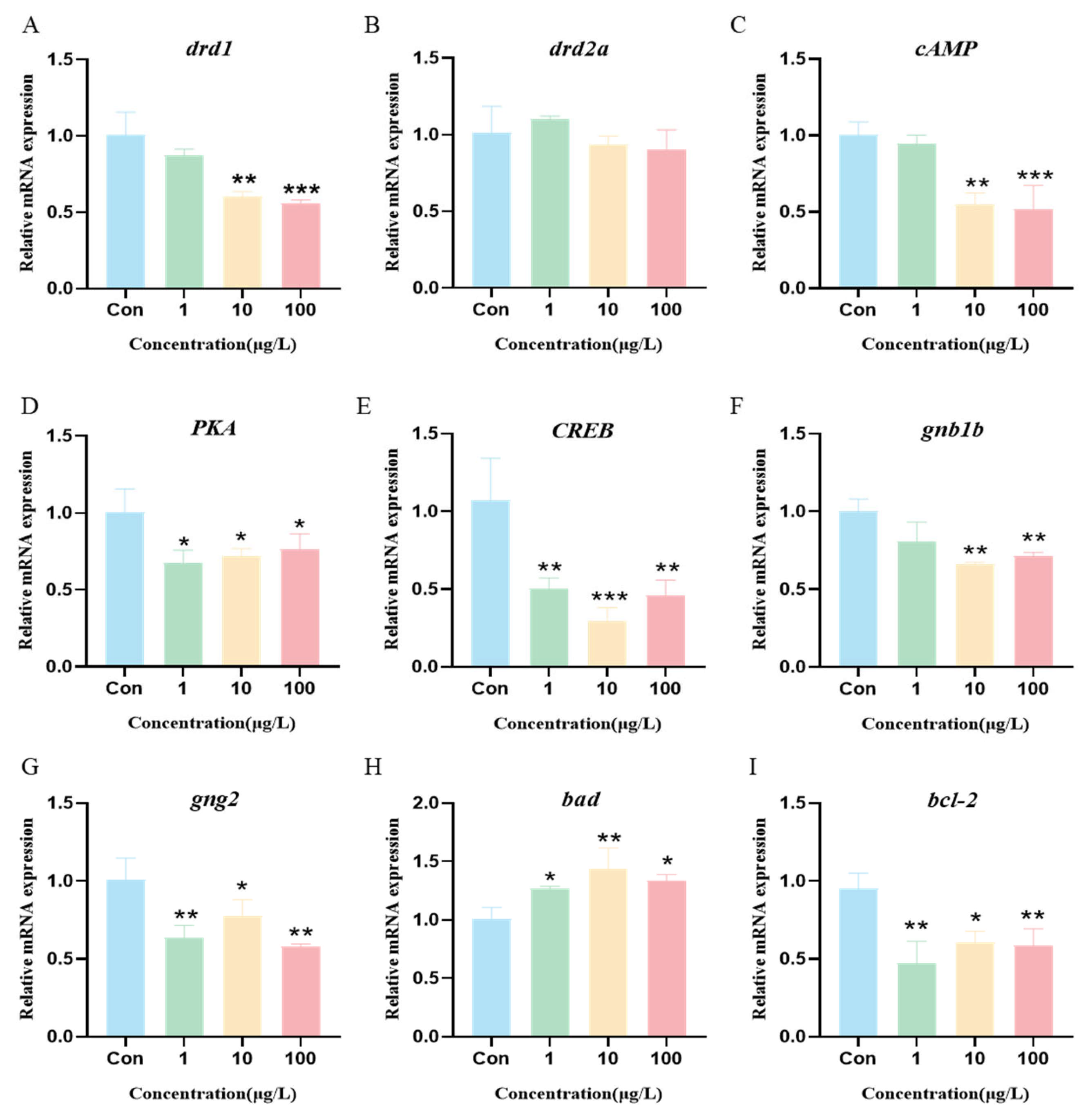
3.8. 3-Isopropylphenol Affects the cAMP/PKA Signaling Pathway in the Body of Zebrafish Larvae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Han, D.; Currell, M.; Song, X.; Zhang, Y. Review of Endocrine Disrupting Compounds (EDCs) in China’s water environments: Implications for environmental fate, transport and health risks. Water Res. 2023, 245, 120645. [Google Scholar] [CrossRef] [PubMed]
- Priac, A.; Morin-Crini, N.; Druart, C.; Gavoille, S.; Bradu, C.; Lagarrigue, C.; Torri, G.; Winterton, P.; Crini, G. Alkylphenol and alkylphenol polyethoxylates in water and wastewater: A review of options for their elimination. Arab. J. Chem. 2017, 10, S3749–S3773. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, J.; Blahova, J.; Divisova, L.; Svobodova, Z. Alkylphenol ethoxylates and alkylphenols--update information on occurrence, fate and toxicity in aquatic environment. Pol. J. Vet. Sci. 2013, 16, 763–772. [Google Scholar] [CrossRef]
- Choi, K.; Sweet, L.I.; Meier, P.G.; Kim, P.G. Aquatic toxicity of four alkylphenols (3-tert-butylphenol, 2-isopropylphenol, 3-isopropylphenol, and 4-isopropylphenol) and their binary mixtures to microbes, invertebrates, and fish. Environ. Toxicol. 2004, 19, 45–50. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Xu, K.; Li, Y.; Zhou, J. Network toxicological and molecular docking to investigate the mechanisms of toxicity of agricultural chemical Thiabendazole. Chemosphere 2024, 363, 142711. [Google Scholar] [CrossRef]
- Huang, S. Efficient analysis of toxicity and mechanisms of environmental pollutants with network toxicology and molecular docking strategy: Acetyl tributyl citrate as an example. Sci. Total Environ. 2023, 905, 167904. [Google Scholar] [CrossRef]
- Tao, W.; Xu, X.; Wang, X.; Li, B.; Wang, Y.; Li, Y.; Yang, L. Network pharmacology-based prediction of the active ingredients and potential targets of Chinese herbal Radix Curcumae formula for application to cardiovascular disease. J. Ethnopharmacol. 2013, 145, 1–10. [Google Scholar] [CrossRef]
- Li, S. Exploring traditional chinese medicine by a novel therapeutic concept of network target. Chin. J. Integr. Med. 2016, 22, 647–652. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- d’Amora, M.; Giordani, S. The Utility of Zebrafish as a Model for Screening Developmental Neurotoxicity. Front. Neurosci. 2018, 12, 976. [Google Scholar] [CrossRef] [PubMed]
- Charron, F.; Stein, E.; Jeong, J.; McMahon, A.P.; Tessier-Lavigne, M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 2003, 113, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gibert, Y.; Trengove, M.C.; Ward, A.C. Zebrafish as a genetic model in pre-clinical drug testing and screening. Curr. Med. Chem. 2013, 20, 2458–2466. [Google Scholar] [CrossRef]
- Planchart, A.; Mattingly, C.J.; Allen, D.; Ceger, P.; Casey, W.; Hinton, D.; Kanungo, J.; Kullman, S.W.; Tal, T.; Bondesson, M.; et al. Advancing toxicology research using in vivo high throughput toxicology with small fish models. ALTEX 2016, 33, 435–452. [Google Scholar] [CrossRef]
- Higashijima, S. Transgenic zebrafish expressing fluorescent proteins in central nervous system neurons. Dev. Growth Differ. 2008, 50, 407–413. [Google Scholar] [CrossRef]
- Bernardos, R.L.; Raymond, P.A. GFAP transgenic zebrafish. Gene Expr. Patterns 2006, 6, 1007–1013. [Google Scholar] [CrossRef]
- Gu, J.; Guo, M.; Huang, C.; Wang, X.; Zhu, Y.; Wang, L.; Wang, Z.; Zhou, L.; Fan, D.; Shi, L.; et al. Titanium dioxide nanoparticle affects motor behavior, neurodevelopment and axonal growth in zebrafish (Danio rerio) larvae. Sci. Total Environ. 2021, 754, 142315. [Google Scholar] [CrossRef]
- Lai, K.P.; Gong, Z.; Tse, W.K.F. Zebrafish as the toxicant screening model: Transgenic and omics approaches. Aquat. Toxicol. 2021, 234, 105813. [Google Scholar] [CrossRef]
- Wallwork, S.B.; Bellan, V.; Catley, M.J.; Moseley, G.L. Neural representations and the cortical body matrix: Implications for sports medicine and future directions. Br. J. Sports Med. 2016, 50, 990–996. [Google Scholar] [CrossRef]
- Li, M.; Pang, X.; Liu, G.; Chen, D.; Meng, L.; Pang, H.; Pang, B.; Guo, F.; Xu, Z. The influences of sedimentary environments on organic matter enrichment in fine-grained rocks of the Paleogene Shahejie formation in Nanpu Sag, Huanghua Depression, Bohai Bay Basin. Energy Explor. Exploit. 2021, 40, 359–380. [Google Scholar] [CrossRef]
- Petrovski, S.; Kury, S.; Myers, C.T.; Anyane-Yeboa, K.; Cogne, B.; Bialer, M.; Xia, F.; Hemati, P.; Riviello, J.; Mehaffey, M.; et al. Germline De Novo Mutations in GNB1 Cause Severe Neurodevelopmental Disability, Hypotonia, and Seizures. Am. J. Hum. Genet. 2016, 98, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Reddy, H.P.; Petri, S.; Williams, D.J.; Shalomov, B.; Dhindsa, R.S.; Gelfman, S.; Krizay, D.; Bera, A.K.; Yang, M.; et al. Epilepsy in a mouse model of GNB1 encephalopathy arises from altered potassium (GIRK) channel signaling and is alleviated by a GIRK inhibitor. Front. Cell. Neurosci. 2023, 17, 1175895. [Google Scholar] [CrossRef]
- Wagnon, J.L. Neurons Are GIRKed in GNB1 Encephalopathy: Unraveling Pathogenic Mechanisms in a Complex Neurodevelopmental Disorder. Epilepsy Curr. 2023, 23, 381–382. [Google Scholar] [CrossRef]
- Reddy, H.P.; Yakubovich, D.; Keren-Raifman, T.; Tabak, G.; Tsemakhovich, V.A.; Pedersen, M.H.; Shalomov, B.; Colombo, S.; Goldstein, D.B.; Javitch, J.A.; et al. Encephalopathy-causing mutations in Gbeta(1) (GNB1) alter regulation of neuronal GIRK channels. iScience 2021, 24, 103018. [Google Scholar] [CrossRef]
- Clapham, D.E.; Neer, E.J. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 167–203. [Google Scholar] [CrossRef]
- Smrcka, A.V. G protein betagamma subunits: Central mediators of G protein-coupled receptor signaling. Cell Mol. Life Sci. 2008, 65, 2191–2214. [Google Scholar] [CrossRef]
- Oldham, W.M.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef]
- Mohammad Nezhady, M.A.; Modaresinejad, M.; Zia, A.; Chemtob, S. Versatile lactate signaling via HCAR1: A multifaceted GPCR involved in many biological processes. Am. J. Physiol.-Cell Physiol. 2023, 325, C1502–C1515. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Liu, Y.; Kou, G.; Chen, Y.; Wu, X.; Lv, Y.; Cai, J.; Chen, R.; Luo, J.; et al. Silver Nanoparticles Cause Neural and Vascular Disruption by Affecting Key Neuroactive Ligand-Receptor Interaction and VEGF Signaling Pathways. Int. J. Nanomed. 2023, 18, 2693–2706. [Google Scholar] [CrossRef]
- Uchigashima, M.; Ohtsuka, T.; Kobayashi, K.; Watanabe, M. Dopamine synapse is a neuroligin-2-mediated contact between dopaminergic presynaptic and GABAergic postsynaptic structures. Proc. Natl. Acad. Sci. USA 2016, 113, 4206–4211. [Google Scholar] [CrossRef]
- Li, X., Sr.; Liu, W.; Jiang, G.; Lian, J.; Zhong, Y.; Zhou, J.; Li, H.; Xu, X.; Liu, Y.; Cao, C.; et al. Celastrol Ameliorates Neuronal Mitochondrial Dysfunction Induced by Intracerebral Hemorrhage via Targeting cAMP-Activated Exchange Protein-1. Adv. Sci. 2024, 11, e2307556. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yue, Y.X.; Shi, S.J.; Xue, J.Z. Investigation on toxicity mechanism of halogenated aromatic disinfection by-products to zebrafish based on molecular docking and QSAR model. Chemosphere 2023, 341, 139916. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Peng, J.; Wu, M.; Zhan, X.; Wang, D.; Zhu, G.; Wang, W.; An, N.; Pan, X. Analyzing the potential targets and mechanisms of chronic kidney disease induced by common synthetic Endocrine Disrupting Compounds (EDCs) in Chinese surface water environment using network toxicology and molecular docking techniques. Sci. Total Environ. 2025, 958, 177980. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, Y.; Wang, D.; Yuan, Q.; Yang, Z.; Deng, C. Assessing male reproductive toxicity of environmental pollutant di-ethylhexyl phthalate with network toxicology and molecular docking strategy. Reprod. Toxicol. 2024, 130, 108749. [Google Scholar] [CrossRef]
- Wang, R.; Lin, Y.; Sun, Y.; Zhao, B.; Chen, L. Insight into the molecular recognition of human and polar bear pregnane X receptor by three organic pollutants using molecular docking and molecular dynamics simulations. Environ. Int. 2024, 190, 108926. [Google Scholar] [CrossRef]
- Bambino, K.; Chu, J. Zebrafish in Toxicology and Environmental Health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.S.; Kho, Y.; Ji, K. Effects of methylisothiazolinone and octylisothiazolinone on development and thyroid endocrine system in zebrafish larvae. J. Hazard. Mater. 2022, 425, 127994. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, S.; Shi, X.; Luo, C.; Huang, W.; Lin, H.; Peng, J.; Tan, W.; Wu, K. Neurodevelopmental toxicity of organophosphate flame retardant triphenyl phosphate (TPhP) on zebrafish (Danio rerio) at different life stages. Environ. Int. 2023, 172, 107745. [Google Scholar] [CrossRef]
- Liang, X.; Souders, C.L., 2nd; Zhang, J.; Martyniuk, C.J. Tributyltin induces premature hatching and reduces locomotor activity in zebrafish (Danio rerio) embryos/larvae at environmentally relevant levels. Chemosphere 2017, 189, 498–506. [Google Scholar] [CrossRef]
- Bailey, J.; Oliveri, A.; Levin, E.D. Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 14–23. [Google Scholar] [CrossRef]
- Goldman, D.; Hankin, M.; Li, Z.; Dai, X.; Ding, J. Transgenic zebrafish for studying nervous system development and regeneration. Transgenic Res. 2001, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Millet, V.; Marder, M.; Pasquini, L.A. Adult CNP::EGFP transgenic mouse shows pronounced hypomyelination and an increased vulnerability to cuprizone-induced demyelination. Exp. Neurol. 2012, 233, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, C.; Wang, J.; Zou, L.; Yang, F.; Chi, X.; Zhu, J. Nano-TiO2 aggravates bioaccumulation and developmental neurotoxicity of difenoconazole in zebrafish larvae via oxidative stress and apoptosis: Protective role of vitamin C. Ecotoxicol. Environ. Saf. 2023, 251, 114554. [Google Scholar] [CrossRef]
- Gu, J.; Guo, L.; Zhu, Y.; Qian, L.; Shi, L.; Zhang, H.; Ji, G. Neurodevelopmental Toxicity of Emamectin Benzoate to the Early Life Stage of Zebrafish Larvae (Danio rerio). Int. J. Mol. Sci. 2023, 24, 3757. [Google Scholar] [CrossRef]
- Tran, C.M.; Do, T.N.; Kim, K.T. Comparative Analysis of Neurotoxicity of Six Phthalates in Zebrafish Embryos. Toxics 2021, 9, 5. [Google Scholar] [CrossRef]
- Pullaguri, N.; Grover, P.; Abhishek, S.; Rajakumara, E.; Bhargava, Y.; Bhargava, A. Triclosan affects motor function in zebrafish larva by inhibiting ache and syn2a genes. Chemosphere 2021, 266, 128930. [Google Scholar] [CrossRef]
- Benowitz, L.I.; Routtenberg, A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997, 20, 84–91. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, J.; Hou, Y.; Shi, X.; Liu, K. Prediction of clinical progression in nervous system diseases: Plasma glial fibrillary acidic protein (GFAP). Eur. J. Med. Res. 2024, 29, 51. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, C.; Li, Q.; Yan, X.; Yang, L.; Li, M.; Cao, X.; Xie, X.; Chen, D.; Rao, C.; et al. Dopamine Homeostasis Imbalance and Dopamine Receptors-Mediated AC/cAMP/PKA Pathway Activation are Involved in Aconitine-Induced Neurological Impairment in Zebrafish and SH-SY5Y Cells. Front. Pharmacol. 2022, 13, 837810. [Google Scholar] [CrossRef]
- Floresco, S.B.; Tse, M.T. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J. Neurosci. 2007, 27, 2045–2057. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Z.; Jiang, Z.; Pu, W.; Chen, X.; Wang, S.; Liu, A.; Zhang, H.; Xu, Z. Long Mu Qing Xin mixture improves behavioral performance in spontaneously hypertensive rats (SHR/NCrl) by upregulating catecholamine neurotransmitters in prefrontal cortex and striatum via DRD1/cAMP/PKA-CREB signaling pathway. Front. Pharmacol. 2024, 15, 1387359. [Google Scholar] [CrossRef] [PubMed]
- Aarnisalo, P.; Palvimo, J.J.; Janne, O.A. CREB-binding protein in androgen receptor-mediated signaling. Proc. Natl. Acad. Sci. USA 1998, 95, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Liu, Y.; Ding, Y.; Mao, G.; Zhao, T.; Chen, K.; Qiu, X.; Xu, T.; Zhao, X.; Wu, X.; et al. Typical neurobehavioral methods and transcriptome analysis reveal the neurotoxicity and mechanisms of di(2-ethylhexyl) phthalate on pubertal male ICR mice with type 2 diabetes mellitus. Arch. Toxicol. 2020, 94, 1279–1302. [Google Scholar] [CrossRef] [PubMed]
- Belgacem, Y.H.; Borodinsky, L.N. CREB at the Crossroads of Activity-Dependent Regulation of Nervous System Development and Function. Adv. Exp. Med. Biol. 2017, 1015, 19–39. [Google Scholar] [CrossRef]
- Liu, X.; Fan, L.; Li, J.; Bai, Z.; Wang, Y.; Liu, Y.; Jiang, H.; Tao, A.; Li, X.; Zhang, H.; et al. Mailuoning oral liquid attenuates convalescent cerebral ischemia by inhibiting AMPK/mTOR-associated apoptosis and promoting CREB/BDNF-mediated neuroprotection. J. Ethnopharmacol. 2023, 317, 116731. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Jin, H.; Hu, J.; Wang, J.; Yin, D. Mechanistic Insights into 3-Isopropylphenol-Induced Neurotoxicity in Zebrafish: A Network Toxicology and Molecular Docking Approach. Toxics 2025, 13, 274. https://doi.org/10.3390/toxics13040274
Gu J, Jin H, Hu J, Wang J, Yin D. Mechanistic Insights into 3-Isopropylphenol-Induced Neurotoxicity in Zebrafish: A Network Toxicology and Molecular Docking Approach. Toxics. 2025; 13(4):274. https://doi.org/10.3390/toxics13040274
Chicago/Turabian StyleGu, Jie, Huilin Jin, Jun Hu, Jian Wang, and Daqiang Yin. 2025. "Mechanistic Insights into 3-Isopropylphenol-Induced Neurotoxicity in Zebrafish: A Network Toxicology and Molecular Docking Approach" Toxics 13, no. 4: 274. https://doi.org/10.3390/toxics13040274
APA StyleGu, J., Jin, H., Hu, J., Wang, J., & Yin, D. (2025). Mechanistic Insights into 3-Isopropylphenol-Induced Neurotoxicity in Zebrafish: A Network Toxicology and Molecular Docking Approach. Toxics, 13(4), 274. https://doi.org/10.3390/toxics13040274





