Do We Need Titanium Dioxide (TiO2) Nanoparticles in Face Masks?
Abstract
1. Introduction
2. Methodology: Literature Review and Risk Assessment
3. Mask Types, Composition, and Filtration Efficiency
- -
- Cloth masks: Reusable homemade face masks for non-medical use, made of cotton [9]. Sometimes, other fabrics are used, such as silk, flannel, synthetics, and combinations of these [68]. Due to this variation, the performance of cloth masks is very heterogeneous: filtration efficiencies for a single layer of various fabrics ranged from 5 to 80% for particle sizes < 300 nm, and from 5 to 95% for particles > 300 nm [68]. Mechanical filtration can be enhanced by combining multiple layers and using cotton with high weave densities (Table 1) [68]. During the earliest part of the COVID-19 pandemic, shortages of surgical and N95 masks occurred, leading local governments to call on citizens to manufacture cloth masks [9,11,69,70]. Due to the lack of control and standardisation, the safety of these masks raises questions.
- -
- Surgical masks: Disposable, professionally produced face masks consisting of three or four nonwoven layers, mainly intended for medical use by infected patients [71,72]. During the COVID-19 pandemic, surgical masks became widely used by the general public. Both surgical masks and respirators are composed of a variety of thermoplastic materials (e.g., polypropylene, polyurethane, polyacrylonitrile, polystyrene, polycarbonate, polyethylene, and polyester) [47,73]. Three-ply surgical masks consist of a hydrophobic external layer repelling mucosalivary droplets, a filtering middle layer (usually polypropylene), and a skin-friendly inner layer that retains droplets from the user [9,71]. In four-ply masks, an additional filtering layer is added, sometimes with activated carbon [71]. In general, the high-density fibre construct used for the outer and inner layers is produced via melt-spinning, while the filtering middle layer requires finer microfibres produced through melt-blowing [9]. Surgical masks perform better than cloth masks in terms of filtering capacity (Table 1). They are certified according to the American ASTM F2100 and European EN 14 683:2019 standards [11]. European types I and II have a Bacterial Filtration Efficiency (BFE, determined via Staphylococcus aureus aerosol) of >95 and 98%, respectively, while IR and IIR masks are also splash-resistant. Similarly high Viral Filtration Efficiencies (VFE > 98%) were obtained by Whiley et al. [74]. In the latter study, S. aureus (~1 µm) was replaced by bacteriophage MS2 (~27 nm), which is 2–3 times smaller than the SARS-CoV-2 virus [74].
- -
- Respirators. Both reusable and disposable, professionally produced, highly performant protective devices to prevent the inhalation of dust particles, aerosols, and infectious agents. Filtering facepiece (FFP) respirators are intended to protect healthcare workers during contact with patients with airborne diseases, such as COVID-19 or influenza [71,72]. Unlike surgical and cloth masks, respirators are fitted tightly against the face, forcing particles through the filtering material. Filtration is mainly achieved mechanically, due to the polypropylene microfibres, and through electrostatic attraction [71]. In the European Union, three types of disposable Filtering Facepiece respirators exist (FFP1, FFP2, and FFP3), certified under the European Standard EN 149:2001 + A1:2009. They have minimum filtration efficiencies (at 95 L/min air flow) of at least 80%, 94%, and 99%, and a maximum inward leakage of less than 22%, 8%, and 2%, respectively. In the US, the National Institute for Occupational Safety and Health (NIOSH) approves N95 respirators that achieve a minimum of 95% filtration efficiency at approximately 300 nm NaCl aerosol size, certified under the NIOSH 42 CFR 84 standard [75,76]. Chinese KN95 respirators also match similar criteria under the GB2626 standard, filtering at least 95% of particles around 300 nm. Hence, N95, K95, and FPP2 respirators are very similar [11,71]. Despite some product-specific exceptions [77], measurements confirm the very high filtration requirements, often performing > 99% for particles > 300 nm (Table 1). Zhou et al. [78] demonstrated a > 99.7% efficiency of a new N95 mask for the exclusion of the influenza A virus, rhinovirus 14, and S. aureus. While the filtration requirements of FFP2, N95, and K95 respirators are fixed, their structure and composition may vary by brand [11,70,78].
| Mask Type | Study | Particle Size (nm) | Filtration Efficiency (%) | Remarks |
|---|---|---|---|---|
| Cloth masks | Rengasamy et al. [87] | 20–1000, median 75 ± 20 | 10–26 | Polydisperse NaCl aerosol. Face velocity: 5.5 cm/s. |
| Konda et al. [68] | <300 | 9 ± 13 | 1 layer quilter’s cotton (80 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |
| 38 ± 11 | 2 layers quilter’s cotton (80 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |||
| 79 ± 23 | 1 layer cotton (600 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |||
| 82 ± 19 | 2 layers cotton (600 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |||
| >300 | 14 ± 1 | 1 layer quilter’s cotton (80 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | ||
| 49 ± 3 | 2 layers quilter’s cotton (80 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |||
| 98.4 ± 0.2 | 1 layer cotton (600 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |||
| 99.5 ± 0.1 | 2 layers cotton (600 TPI). Polydisperse NaCl aerosol, 1.2 CFM. | |||
| Sankhyan et al. [86] | 300 | 16–23 | Ammonium sulphate aerosol. NIOSH N95 filtration efficiency procedure. | |
| Surgical masks | Konda et al. [68] | <300 | 76 ± 22 | No gap. Polydisperse NaCl aerosol, 1.2 CFM. |
| 50 ± 7 | With gap. Polydisperse NaCl aerosol, 1.2 CFM | |||
| >300 | 99.6 ± 0.1 | No gap. Polydisperse NaCl aerosol, 1.2 CFM. | ||
| 44 ± 3 | With gap. Polydisperse NaCl aerosol, 1.2 CFM | |||
| Sankhyan et al. [86] | 300 | 42–88 | Ammonium sulphate aerosol. NIOSH N95 filtration efficiency procedure. | |
| Whiley et al. [74] | Average: 2600 | 98.5, 99.5 | Average VFE (2.6 µm) calculated with larger aerosols excluded. Adapted ASTM F201-14 method with MS2 bacteriophage. | |
| Average: 6000 | 99.6, 99.9 | Average VFE (6.0 µm). Adapted ASTM F201-14 method with MS2 bacteriophage. | ||
| Respirators (N95, K95) | Rengasamy et al. [87] | 20–1000, median: 75 ± 20 | 99.88 | Polydisperse NaCl aerosol. Face velocity: 5.5 cm/s. |
| 20–1000, median: 75 ± 20 | >95 | Polydisperse NaCl aerosol. Face velocity: 16.5 cm/s. | ||
| Konda et al. [68] | <300 | 85 ± 15 | No gap. Polydisperse NaCl aerosol, 1.2 CFM. | |
| >300 | 99.9 ± 0.1 | No gap. Polydisperse NaCl aerosol, 1.2 CFM. | ||
| Sankhyan et al. [86] | 300 | 83–99 | Ammonium sulphate aerosol. NIOSH N95 filtration efficiency procedure. | |
| Whiley et al. [74] | Average: 2600 | 99.3 | Average VFE (2.6 µm) calculated with larger aerosols excluded. Adapted ASTM F201-14 method with MS2 bacteriophage. | |
| Average: 6000 | 99.9 | Average VFE (6.0 µm). Adapted ASTM F201-14 method with MS2 bacteriophage. |
4. Metal (Nano)Particles in Face Masks: Application and Antimicrobial Properties
4.1. Silver
4.2. Copper
4.3. Zinc
4.4. Titanium Dioxide
5. Metal (Nano)Particles in Face Masks: Legal Status in the European Union
6. Hazard Identification and Characterisation of TiO2 Nanoparticles
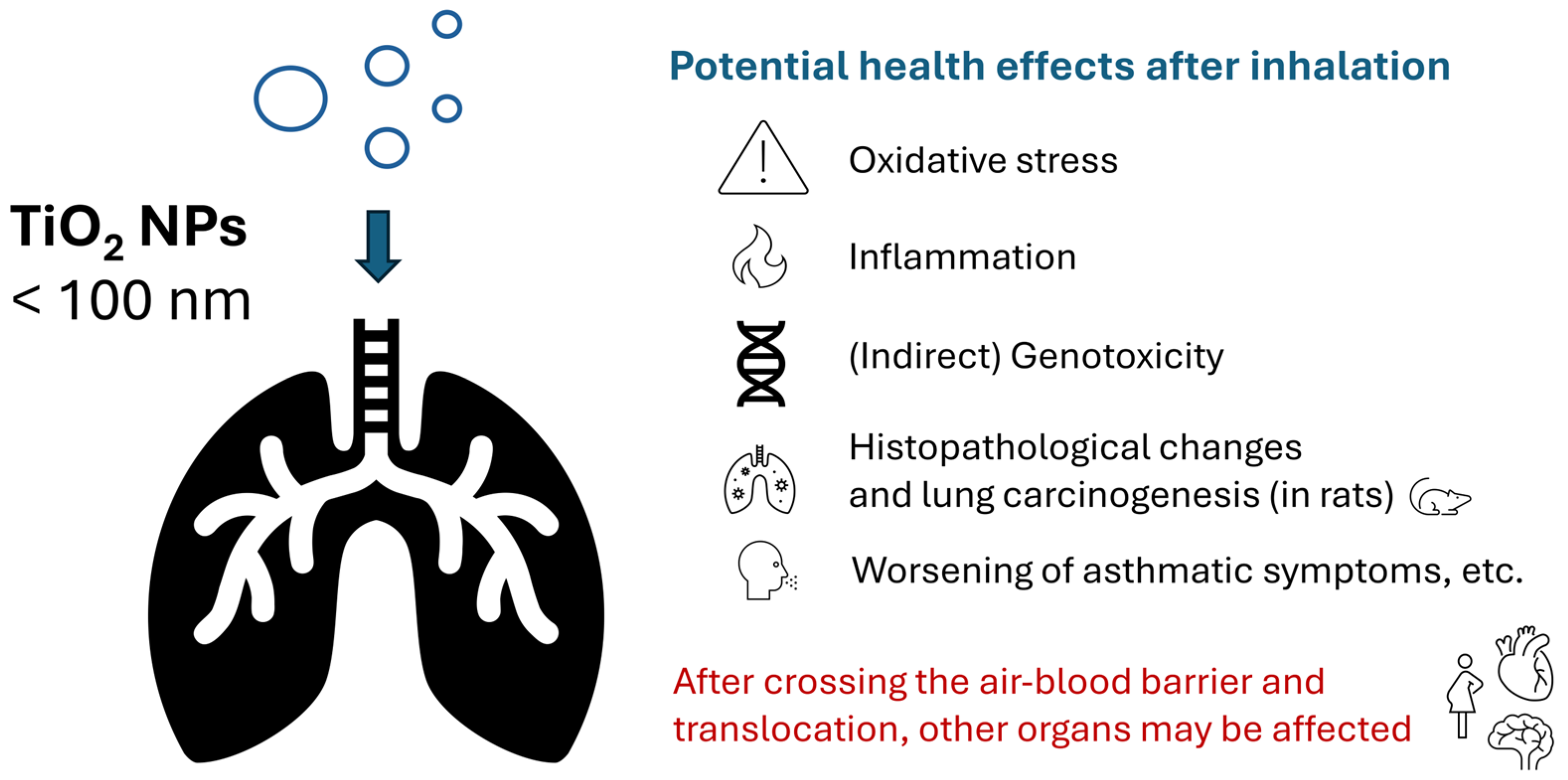
6.1. Oxidative Stress
6.2. Genotoxicity
6.3. Respiratory Toxicity (Non-Carcinogenic)
6.4. Lung Carcinogenesis
6.5. Other Health Effects
6.6. Health-Based Inhalation Exposure Limits for TiO2 NPs
7. Exposure Assessment: How Many TiO2 (Nano)Particles Are Released?
8. Risk Characterisation of Different Exposure Scenarios
9. Discussion
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEL | Acceptable exposure limit |
| AELmask | Acceptable exposure limit for one face mask |
| AF | Assessment factor |
| ANSES | Agence nationale de sécurité sanitaire, de l’ alimentation, de l’ environnement et du travail |
| AOP | Adverse outcome pathway |
| aOR | Adjusted odds ratio |
| ASTM | American Society for Testing and Materials |
| ATP | Adenosine triphosphate |
| BFE | Bacterial filtration efficiency |
| bw | Body weight |
| CB | Conduction band |
| CFM | Cubic feet per minute |
| CI | Confidence Interval |
| COVID-19 | Coronavirus disease 2019 |
| CRA | Cumulative risk assessment |
| DNA | Deoxyribonucleic acid |
| EC50 | Half maximal effective concentration |
| ECHA | European Chemicals Agency |
| EFSA | European Food Safety Authority |
| Eg | Energy band gap |
| EN | Europäische Norm |
| ENRHES | Engineered Nanoparticles: Review of Health and Environmental Safety |
| RNA | Ribonucleic acid |
| EPA | Environmental Protection Agency |
| FFP | Filtering facepiece |
| HEC | Human equivalent concentration |
| IARC | International Agency for Research on Cancer |
| INEL | Indicative no-effect level |
| MP | Microparticle (fine particle > 100 nm) |
| NIOSH | National Institute for Occupational Safety and Health |
| NOAEC | No Observed Adverse Effect Concentration |
| NP | Nanoparticle (ultrafine particle, <100 nm) |
| NRCWE | National Research Centre for the Working Environment |
| OEL | Occupational Exposure Limit |
| OPE | Organophosphorus ester |
| OPFR | Organophosphate flame retardant |
| OSHA | Occupational Safety and Health Administration |
| PAH | Polycyclic aromatic hydrocarbon |
| PEL | Permissible exposure limit |
| PFAS | Per- and polyfluoroalkyl substances |
| POD | Point of departure |
| PPE | Personal protective equipment |
| PSLT | Poorly soluble low toxicity |
| QSAR | Quantitative structure-activity relationship |
| RAC | Committee for risk assessment |
| ROS | Reactive oxygen species |
| RR | Relative risk |
| SHC | Superior Health Council of Belgium |
| SMR | Standardised mortality ratio |
| STEL | Short-term exposure limit |
| TiO2 | Titanium dioxide |
| TWA | Time-weighted average |
| TPI | Threads per inch |
| TRV | Toxicity reference value |
| UF | Uncertainty factor |
References
- Msemburi, W.; Karlinsky, A.; Knutson, V.; Aleshin-Guendel, S.; Chatterji, S.; Wakefield, J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature 2023, 613, 130–137. [Google Scholar] [CrossRef]
- Jurcevic, J.; Ekelson, R.; Nganda, S.; Bustos Sierra, N.; Vernemmen, C. Epidemiology of COVID-19 Mortality in Belgium, from Wave 1 to Wave 7 (March 2020–11 September 2022); Sciensano: Brussels, Belgium, 2023. [Google Scholar]
- Ward, I.L.; Bermingham, C.; Ayoubkhani, D.; Gethings, O.J.; Pouwels, K.B.; Yates, T.; Khunti, K.; Hippisley-Cox, J.; Banerjee, A.; Walker, A.S.; et al. Risk of COVID-19 related deaths for SARS-CoV-2 Omicron (B.1.1.529) compared with Delta (B.1.617.2). BMJ 2022, 378, e070695. [Google Scholar] [CrossRef] [PubMed]
- Adjei, S.; Hong, K.; Molinari, N.M.; Bull-Otterson, L.; Ajani, U.A.; Gundlapalli, A.V.; Harris, A.M.; Hsu, J.; Kadri, S.S.; Starnes, J.; et al. Mortality Risk Among Patients Hospitalized Primarily for COVID-19 During the Omicron and Delta Variant Pandemic Periods—United States, April 2020–June 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1182–1189. [Google Scholar] [CrossRef]
- Du, J.; Lang, H.-M.; Ma, Y.; Chen, A.-W.; Qin, Y.-Y.; Zhang, X.-P.; Huang, C.-Q. Global trends in COVID-19 incidence and case fatality rates (2019–2023): A retrospective analysis. Front. Public Health 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Pullangott, G.; Kannan, U.; Kiran, D.V.; Maliyekkal, S.M. A comprehensive review on antimicrobial face masks: An emerging weapon in fighting pandemics. RSC Adv. 2021, 11, 6544–6576. [Google Scholar] [CrossRef]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Chua, M.H.; Cheng, W.; Goh, S.S.; Kong, J.; Li, B.; Lim, J.Y.C.; Mao, L.; Wang, S.; Xue, K.; Yang, L.; et al. Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [Google Scholar] [CrossRef]
- Wang, A.B.; Zhang, X.; Gao, L.J.; Zhang, T.; Xu, H.J.; Bi, Y.J. A Review of Filtration Performance of Protective Masks. Int. J. Environ. Res. Public Health 2023, 20, 2346. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Hoque, M.E.; Alam, M.R.; Rouf, M.A.; Khan, S.I.; Xu, H.; Ramakrishna, S. Face Masks to Combat Coronavirus (COVID-19)—Processing, Roles, Requirements, Efficacy, Risk and Sustainability. Polymers 2022, 14, 1296. [Google Scholar] [CrossRef]
- Kim, M.S.; Seong, D.; Li, H.; Chung, S.K.; Park, Y.; Lee, M.; Lee, S.W.; Yon, D.K.; Kim, J.H.; Lee, K.H.; et al. Comparative effectiveness of N95, surgical or medical, and non-medical facemasks in protection against respiratory virus infection: A systematic review and network meta-analysis. Rev. Med. Virol. 2022, 32, e2336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, M.; Gao, L.; Ayaz Ahmed, M.; Uy, J.P.; Cheng, C.; Zhou, Q.; Sun, C. Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis. Am. J. Infect. Control 2021, 49, 900–906. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.-A. Airborne transmission of COVID-19 and the role of face mask to prevent it: A systematic review and meta-analysis. Eur. J. Med. Res. 2021, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, G.; Thiede, B.; Hejazi, B.; Schlenczek, O.; Bodenschatz, E. An upper bound on one-to-one exposure to infectious human respiratory particles. Proc. Natl. Acad. Sci. USA 2021, 118, e2110117118. [Google Scholar] [CrossRef]
- Hansen, N.H.; Mano, R.C. Mask mandates save lives. J. Health Econ. 2023, 88, 102721. [Google Scholar] [CrossRef] [PubMed]
- ANSES. Avis Complété de l’Anses Relatif à L’évaluation des Risques Sanitaires liés à la Présence de Substances Chimiques dans des Masques Chirurgicaux mis à la Disposition du Grand Public; ANSES: Maisons-Alfort, France, 2021; 73p. [Google Scholar]
- Wijnhoven, S.; Brand, W.; Hendriks, H.; Huiberts, E.; van Kesteren, P.; Visser, M. Chemische Veiligheid Mondkapjes. Voortgangsrapportage. Chemical Safety of Face Masks. Progress Report; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- SHC. Gezondheidsrisico’s van Stoffen Mondmaskers Behandeld met Biocide op Basis van Zilver ter Bescherming Tegen COVID-19 Infectie; SHC 9654; Superior Health Council of Belgium: Brussels, Belgium, 2021; p. 26. [Google Scholar]
- Vandenbroucke, F. Naar een Strikte Regelgeving Voor Álle Mondmaskers op de Belgische Markt. Available online: https://web.archive.org/web/20240703183954/https://vandenbroucke.belgium.be/nl/naar-een-strikte-regelgeving-voor-%C3%A1lle-mondmaskers-op-de-belgische-markt (accessed on 3 December 2024).
- SHC. The Potential Impact of Face Masks on Belgian Public Health and the Environment: Evaluation and Policy Recommendations; SHC 9765; Superior Health Council of Belgium: Brussels, Belgium, 2024. [Google Scholar]
- Testaankoop. Onderzoek naar Schadelijke Stoffen in Mondmaskers niet Helemaal Geruststellend; Testaankoop: Sint-Gillis, Belgium, 2021. [Google Scholar]
- Bhangare, R.C.; Tiwari, M.; Ajmal, P.Y.; Rathod, T.D.; Sahu, S.K. Exudation of microplastics from commonly used face masks in COVID-19 pandemic. Environ. Sci. Pollut. Res. 2023, 30, 35258–35268. [Google Scholar] [CrossRef]
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Dobaradaran, S.; Spitz, J.; Nabipour, I.; Keshtkar, M.; Akhbarizadeh, R.; Tangestani, M.; Abedi, D.; Javanfekr, F. Release of phthalate esters (PAEs) and microplastics (MPs) from face masks and gloves during the COVID-19 pandemic. Environ. Res. 2022, 215, 114337. [Google Scholar] [CrossRef]
- De-la-Torre, G.E.; Pizarro-Ortega, C.I.; Dioses-Salinas, D.C.; Ammendolia, J.; Okoffo, E.D. Investigating the current status of COVID-19 related plastics and their potential impact on human health. Curr. Opin. Toxicol. 2021, 27, 47–53. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Li, Z.; Song, K. COVID-19: Performance study of microplastic inhalation risk posed by wearing masks. J. Hazard. Mater. 2021, 411, 124955. [Google Scholar] [CrossRef]
- Jiang, H.; Luo, D.; Wang, L.; Zhang, Y.; Wang, H.; Wang, C. A review of disposable facemasks during the COVID-19 pandemic: A focus on microplastics release. Chemosphere 2023, 312, 137178. [Google Scholar] [CrossRef]
- Li, M.; Hou, Z.; Meng, R.; Hao, S.; Wang, B. Unraveling the potential human health risks from used disposable face mask-derived micro/nanoplastics during the COVID-19 pandemic scenario: A critical review. Environ. Int. 2022, 170, 107644. [Google Scholar] [CrossRef] [PubMed]
- Cabrejos-Cardeña, U.; De-la-Torre, G.E.; Dobaradaran, S.; Rangabhashiyam, S. An ecotoxicological perspective of microplastics released by face masks. J. Hazard. Mater. 2023, 443, 130273. [Google Scholar] [CrossRef]
- Aerts, O.; Dendooven, E.; Foubert, K.; Stappers, S.; Ulicki, M.; Lambert, J. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermat. 2020, 83, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Clawson, R.C.; Pariser, R. Formaldehyde-Induced Contact Dermatitis from an N95 Respirator Mask. Cutis 2021, 108, E11–E14. [Google Scholar] [CrossRef]
- Kawakami, T.; Obama, T.; Sakai, S.; Takagi, M.; Takahashi, N.; Oshima, N.; Tahara, M.; Ikarashi, Y. Free formaldehyde in non-medical face masks purchased from the Japanese market since the COVID-19 outbreak. J. Environ. Sci. Health Part A 2022, 57, 193–197. [Google Scholar] [CrossRef]
- Wang, X.; Okoffo, E.D.; Banks, A.P.W.; Li, Y.; Thomas, K.V.; Rauert, C.; Aylward, L.L.; Mueller, J.F. Phthalate esters in face masks and associated inhalation exposure risk. J. Hazard. Mater. 2022, 423, 127001. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Han, W.; Xie, Q.; Xu, T.; Zhu, M.; Chen, J. Face mask—A potential source of phthalate exposure for human. J. Hazard. Mater. 2022, 422, 126848. [Google Scholar] [CrossRef]
- Leoni, C.; Majorani, C.; Cresti, R.; Marcello, I.; Berardi, E.; Fava, L.; Attias, L.; D’Ilio, S. Determination and risk assessment of phthalates in face masks. An Italian study. J. Hazard. Mater. 2023, 443, 130176. [Google Scholar] [CrossRef]
- Kisielinski, K.; Hockertz, S.; Hirsch, O.; Korupp, S.; Klosterhalfen, B.; Schnepf, A.; Dyker, G. Wearing face masks as a potential source for inhalation and oral uptake of inanimate toxins—A scoping review. Ecotoxicol. Environ. Saf. 2024, 275, 115858. [Google Scholar] [CrossRef]
- Jin, L.; Griffith, S.M.; Sun, Z.; Yu, J.Z.; Chan, W. On the Flip Side of Mask Wearing: Increased Exposure to Volatile Organic Compounds and a Risk-Reducing Solution. Environ. Sci. Technol. 2021, 55, 14095–14104. [Google Scholar] [CrossRef]
- Fernández-Arribas, J.; Moreno, T.; Bartrolí, R.; Eljarrat, E. COVID-19 face masks: A new source of human and environmental exposure to organophosphate esters. Environ. Int. 2021, 154, 106654. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Du, J.; Han, W.; Tang, J.; Li, X.; Chen, J. Occurrence and health risks of semi-volatile organic compounds in face masks. Sci. Bull. 2021, 66, 1601–1603. [Google Scholar] [CrossRef]
- Muensterman, D.J.; Cahuas, L.; Titaley, I.A.; Schmokel, C.; De la Cruz, F.B.; Barlaz, M.A.; Carignan, C.C.; Peaslee, G.F.; Field, J.A. Per- and Polyfluoroalkyl Substances (PFAS) in Facemasks: Potential Source of Human Exposure to PFAS with Implications for Disposal to Landfills. Environ. Sci. Technol. Lett. 2022, 9, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Huang, R.-J.; Cheng, K.; Lin, C.; Ling, Q.; Haque, M.M.; Ovadnevaite, J.; O’Dowd, C. Highly Time-Resolved and Nontargeted Characterization of Volatile Organic Compound Emissions from Face Masks. Environ. Sci. Technol. Lett. 2022, 9, 1007–1013. [Google Scholar] [CrossRef]
- Huang, Q.; Pan, L.; Luo, G.; Jiang, R.; Ouyang, G.; Ye, Y.; Cai, J.a.; Guo, P. Exploring the release of hazardous volatile organic compounds from face masks and their potential health risk. Environ. Pollut. 2023, 333, 122042. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.V.; Martins, A.O.; Martins, S.D.S.; Mata, T.M. Low-VOC Emission Label Proposal for Facemask Safety Based on Respiratory and Skin Health Criteria. Environments 2023, 10, 10. [Google Scholar] [CrossRef]
- Mast, J.; Van Miert, E.; Siciliani, L.; Cheyns, K.; Blaude, M.-N.; Wouters, C.; Waegeneers, N.; Bernsen, R.; Vleminckx, C.; Van Loco, J.; et al. Application of silver-based biocides in face masks intended for general use requires regulatory control. Sci. Total Environ. 2023, 870, 161889. [Google Scholar] [CrossRef]
- Estevan, C.; Vilanova, E.; Sogorb, M.A. Case study: Risk associated to wearing silver or graphene nanoparticle-coated facemasks for protection against COVID-19. Arch. Toxicol. 2022, 96, 105–119. [Google Scholar] [CrossRef]
- Verleysen, E.; Ledecq, M.; Siciliani, L.; Cheyns, K.; Vleminckx, C.; Blaude, M.-N.; De Vos, S.; Brassinne, F.; Van Steen, F.; Nkenda, R.; et al. Titanium dioxide particles frequently present in face masks intended for general use require regulatory control. Sci. Rep. 2022, 12, 2529. [Google Scholar] [CrossRef]
- Bussan, D.D.; Snaychuk, L.; Bartzas, G.; Douvris, C. Quantification of trace elements in surgical and KN95 face masks widely used during the SARS-COVID-19 pandemic. Sci. Total Environ. 2022, 814, 151924. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. The latest strategies in the fight against the COVID-19 pandemic: The role of metal and metal oxide nanoparticles. New J. Chem. 2021, 45, 6167–6179. [Google Scholar] [CrossRef]
- The Danish Environmental Protection Agency. Survey and Risk Assessment of Chemicals in Textile Face Masks; The Danish Environmental Protection Agency: Odense, Denmark, 2021; p. 123. [Google Scholar]
- Palmieri, V.; De Maio, F.; De Spirito, M.; Papi, M. Face masks and nanotechnology: Keep the blue side up. Nano Today 2021, 37, 101077. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Laffon, B. The impact of nanotechnology in the current universal COVID-19 crisis. Let’s not forget nanosafety! Nanotoxicology 2020, 14, 1013–1016. [Google Scholar] [CrossRef]
- Pollard, Z.A.; Karod, M.; Goldfarb, J.L. Metal leaching from antimicrobial cloth face masks intended to slow the spread of COVID-19. Sci. Rep. 2021, 11, 19216. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Honarvar, N.; Seiffert, S.B.; Oesch, B.; Oesch, F. Genotoxicity testing of nanomaterials. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1833. [Google Scholar] [CrossRef]
- Liu, L.; Kong, L. Research progress on the carcinogenicity of metal nanomaterials. J. Appl. Toxicol. 2021, 41, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health—Where are we? Part. Fibre Toxicol. 2019, 16, 19. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Laird Forrest, M.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Bergamaschi, A. Toxicological Effects of Titanium Dioxide Nanoparticles: A Review of In Vivo Studies. J. Nanomater. 2012, 2012, 964381. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and environmental impacts of nanoparticles: A scoping review of the current literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef]
- Shabbir, S.; Kulyar, M.F.-e.-A.; Bhutta, Z.A.; Boruah, P.; Asif, M. Toxicological Consequences of Titanium Dioxide Nanoparticles (TiO2NPs) and Their Jeopardy to Human Population. BioNanoScience 2021, 11, 621–632. [Google Scholar] [CrossRef]
- Rashid, M.M.; Forte Tavčer, P.; Tomšič, B. Influence of Titanium Dioxide Nanoparticles on Human Health and the Environment. Nanomaterials 2021, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef]
- IARC. Carbon Black, Titanium Dioxide, and Talc. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; Volume 93, p. 452. [Google Scholar]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Cogliano, V. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 2006, 7, 295–296. [Google Scholar] [CrossRef] [PubMed]
- RAC. Opinion Proposing Harmonised Classification and Labelling at EU Level of Titanium Dioxide EC Number 236-675-5; CAS Number: 13463-67-7; European Chemicals Agency: Helsinki, Finland, 2017; p. 50. [Google Scholar]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef]
- VRTNWS. We Moeten “Nadenken” om Zélf Mondmaskers te Maken, Maar Hoe Doet u Dat? 2020. Available online: https://www.vrt.be/vrtnws/nl/2020/03/18/zelf-mondmaskers-maken/ (accessed on 3 March 2025).
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight Against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, S.; Das, A.; Das, S.; Chakraborty, P.; Sarkar, J. A comprehensive review of various categories of face masks resistant to COVID-19. Clin. Epidemiol. Glob. Health 2021, 12, 100835. [Google Scholar] [CrossRef]
- Bałazy, A.; Toivola, M.; Adhikari, A.; Sivasubramani, S.K.; Reponen, T.; Grinshpun, S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am. J. Infect. Control 2006, 34, 51–57. [Google Scholar] [CrossRef]
- Armentano, I.; Barbanera, M.; Carota, E.; Crognale, S.; Marconi, M.; Rossi, S.; Rubino, G.; Scungio, M.; Taborri, J.; Calabrò, G. Polymer Materials for Respiratory Protection: Processing, End Use, and Testing Methods. ACS Appl. Polym. Mater. 2021, 3, 531–548. [Google Scholar] [CrossRef]
- Whiley, H.; Keerthirathne, T.P.; Nisar, M.A.; White, M.A.F.; Ross, K.E. Viral Filtration Efficiency of Fabric Masks Compared with Surgical and N95 Masks. Pathogens 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. Community Respirators and Masks. Available online: https://www.cdc.gov/niosh/topics/publicppe/community-ppe.html (accessed on 2 December 2024).
- NIOSH. NIOSH Guide to the Selection and Use of Particulate Respirators. Available online: https://www.cdc.gov/niosh/docs/96-101/default.html (accessed on 3 December 2024).
- Lee, S.-A.; Hwang, D.-C.; Li, H.-Y.; Tsai, C.-F.; Chen, C.-W.; Chen, J.-K. Particle Size-Selective Assessment of Protection of European Standard FFP Respirators and Surgical Masks against Particles-Tested with Human Subjects. J. Healthc. Eng. 2016, 2016, 8572493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Lukula, S.; Chiossone, C.; Nims, R.W.; Suchmann, D.B.; Ijaz, M.K. Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac. Dis. 2018, 10, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef]
- Tellier, R.; Li, Y.; Cowling, B.J.; Tang, J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect. Dis. 2019, 19, 101. [Google Scholar] [CrossRef]
- Tcharkhtchi, A.; Abbasnezhad, N.; Zarbini Seydani, M.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- Lee, B.U. Minimum Sizes of Respiratory Particles Carrying SARS-CoV-2 and the Possibility of Aerosol Generation. Int. J. Environ. Res. Public Health 2020, 17, 6960. [Google Scholar] [CrossRef]
- Alsved, M.; Nygren, D.; Thuresson, S.; Fraenkel, C.-J.; Medstrand, P.; Löndahl, J. Size distribution of exhaled aerosol particles containing SARS-CoV-2 RNA. Infect. Dis. 2023, 55, 158–163. [Google Scholar] [CrossRef]
- Jefferson, T.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; van Driel, M.L.; Bawazeer, G.A.; Jones, M.A.; Hoffmann, T.C.; Clark, J.; Beller, E.M.; et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2023. [Google Scholar] [CrossRef]
- Lu, Y.; Okpani, A.I.; McLeod, C.B.; Grant, J.M.; Yassi, A. Masking strategy to protect healthcare workers from COVID-19: An umbrella meta-analysis. Infect. Dis. Health 2023, 28, 226–238. [Google Scholar] [CrossRef]
- Sankhyan, S.; Heinselman, K.N.; Ciesielski, P.N.; Barnes, T.; Himmel, M.E.; Teed, H.; Patel, S.; Vance, M.E. Filtration Performance of Layering Masks and Face Coverings and the Reusability of Cotton Masks after Repeated Washing and Drying. Aerosol Air Qual. Res. 2021, 21, 210117. [Google Scholar] [CrossRef]
- Rengasamy, S.; Eimer, B.; Shaffer, R.E. Simple Respiratory Protection—Evaluation of the Filtration Performance of Cloth Masks and Common Fabric Materials Against 20–1000 nm Size Particles. Ann. Occup. Hyg. 2010, 54, 789–798. [Google Scholar] [CrossRef]
- Montalvo, D.; Mercier, G.M.; Mast, J.; Cheyns, K. Release of silver and titanium from face masks traded for the general population. Sci. Total Environ. 2023, 901, 165616. [Google Scholar] [CrossRef]
- Montalvo, D.; Wouters, C.; Siciliani, L.; Vleminckx, C.; Van Miert, E.; Waegeneers, N.; Van Loco, J.; Verleysen, E.; Cheyns, K.; Mast, J. Silver-Based Biocides and Titanium Dioxide Particles from Face Masks for General Use. Final Report of the TiO2Mask and AgMask COVID-19 Projects; Sciensano: Brussels, Belgium, 2023; p. 48. [Google Scholar]
- Delgado-Gallardo, J.; Sullivan, G.L.; Tokaryk, M.; Russell, J.E.; Davies, G.R.; Johns, K.V.; Hunter, A.P.; Watson, T.M.; Sarp, S. Disposable FFP2 and Type IIR Medical-Grade Face Masks: An Exhaustive Analysis into the Leaching of Micro- and Nanoparticles and Chemical Pollutants Linked to the COVID-19 Pandemic. ACS EST Water 2022, 2, 527–538. [Google Scholar] [CrossRef]
- Sullivan, G.L.; Delgado-Gallardo, J.; Watson, T.M.; Sarp, S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks—Linked to the COVID-19 pandemic. Water Res. 2021, 196, 117033. [Google Scholar] [CrossRef] [PubMed]
- Toledo, G.G.; Toledo, V.H.; Lanfredi, A.J.C.; Escote, M.; Champi, A.; Silva, M.; Nantes-Cardoso, I.L. Promising Nanostructured Materials against Enveloped Virus. An. Acad. Bras. Cienc. 2020, 92, e20200718. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, V.; Jose, S.; Badanayak, P.; Sankaran, A.; Anandan, V. Antimicrobial Finishing of Metals, Metal Oxides, and Metal Composites on Textiles: A Systematic Review. Ind. Eng. Chem. Res. 2022, 61, 86–101. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, F.; Zhao, Q.; Ji, R.; Qie, Y.; Zhou, Y.; Hu, Y.; Pang, C.; Hristozov, D.; Giesy, J.P.; et al. Predicting toxic potencies of metal oxide nanoparticles by means of nano-QSARs. Nanotoxicology 2016, 10, 1207–1214. [Google Scholar] [CrossRef]
- Hussain, F.S.; Abro, N.Q.; Ahmed, N.; Memon, S.Q.; Memon, N. Nano-antivirals: A comprehensive review. Front. Nanotechnol. 2022, 4, 1064615. [Google Scholar] [CrossRef]
- Alayande, A.B.; Kang, Y.; Jang, J.; Jee, H.; Lee, Y.-G.; Kim, I.S.; Yang, E. Antiviral Nanomaterials for Designing Mixed Matrix Membranes. Membranes 2021, 11, 458. [Google Scholar] [CrossRef]
- Ahmed, T.; Ogulata, R.T.; Sezgin Bozok, S. Silver nanoparticles against SARS-CoV-2 and its potential application in medical protective clothing—A review. J. Text. Inst. 2022, 113, 2825–2838. [Google Scholar] [CrossRef]
- Canalli Bortolassi, A.C.; Guerra, V.G.; Aguiar, M.L.; Soussan, L.; Cornu, D.; Miele, P.; Bechelany, M. Composites Based on Nanoparticle and Pan Electrospun Nanofiber Membranes for Air Filtration and Bacterial Removal. Nanomaterials 2019, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Botelho, C.M.; Fernandes, M.M.; Souza, J.M.; Dias, N.; Sousa, A.M.; Teixeira, J.A.; Fangueiro, R.; Zille, A. New Textile for Personal Protective Equipment—Plasma Chitosan/Silver Nanoparticles Nylon Fabric. Fibers 2021, 9, 3. [Google Scholar] [CrossRef]
- López-Martín, R.; Rodrigo, I.; Ballesta, C.; Arias, A.; Mas, A.; Santos Burgos, B.; Normile, P.S.; De Toro, J.A.; Binns, C. Effectiveness of Silver Nanoparticles Deposited in Facemask Material for Neutralising Viruses. Nanomaterials 2022, 12, 2662. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Cortes, A. The role of additive manufacturing and antimicrobial polymers in the COVID-19 pandemic. Expert Rev. Med. Devices 2020, 17, 477–481. [Google Scholar] [CrossRef]
- Borkow, G.; Zhou, S.S.; Page, T.; Gabbay, J. A Novel Anti-Influenza Copper Oxide Containing Respiratory Face Mask. PLoS ONE 2010, 5, e11295. [Google Scholar] [CrossRef]
- Giedraitienė, A.; Ruzauskas, M.; Šiugždinienė, R.; Tučkutė, S.; Milcius, D. Antimicrobial Properties of CuO Particles Deposited on a Medical Mask. Materials 2022, 15, 7896. [Google Scholar] [CrossRef]
- Kalpana, V.N.; Devi Rajeswari, V. A Review on Green Synthesis, Biomedical Applications, and Toxicity Studies of ZnO NPs. Bioinorg. Chem. Appl. 2018, 2018, 3569758. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Katuwal, S.; Tettey, F.; Gupta, A.; Bhattarai, S.; Jaisi, S.; Bhandari, D.P.; Shah, A.K.; Bhattarai, N.; Parajuli, N. Current Research on Zinc Oxide Nanoparticles: Synthesis, Characterization, and Biomedical Applications. Nanomaterials 2022, 12, 3066. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Roy, J.; Nayak, P.S.; Mallick, B.; Jha, S. Zinc oxide nanoparticle energy band gap reduction triggers the oxidative stress resulting into autophagy-mediated apoptotic cell death. Free Radic. Biol. Med. 2017, 110, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Athinarayanan, J.; Periyasamy, V.S.; Alshuniaber, M.A.; Alshammari, G.; Hakeem, M.J.; Ahmed, M.A.; Alshatwi, A.A. Antibacterial Mechanisms of Zinc Oxide Nanoparticle against Bacterial Food Pathogens Resistant to Beta-Lactam Antibiotics. Molecules 2022, 27, 2489. [Google Scholar] [CrossRef]
- O’Neill, S.; Robertson, J.M.C.; Héquet, V.; Chazarenc, F.; Pang, X.; Ralphs, K.; Skillen, N.; Robertson, P.K.J. Comparison of Titanium Dioxide and Zinc Oxide Photocatalysts for the Inactivation of Escherichia coli in Water Using Slurry and Rotating-Disk Photocatalytic Reactors. Ind. Eng. Chem. Res. 2023, 62, 18952–18959. [Google Scholar] [CrossRef]
- Abou Zeid, S.; Perez, A.; Bastide, S.; Le Pivert, M.; Rossano, S.; Remita, H.; Hautière, N.; Leprince-Wang, Y. Antibacterial and Photocatalytic Properties of ZnO Nanostructure Decorated Coatings. Coatings 2024, 14, 41. [Google Scholar] [CrossRef]
- McQuerry, M.; Dodson, A. An antimicrobial zinc ion fiber for COVID-19 prevention in nonwoven face coverings for healthcare settings. J. Occup. Environ. Hyg. 2024, 21, 239–246. [Google Scholar] [CrossRef]
- d’Alessandro, N.; Coccia, F.; Vitali, L.A.; Rastelli, G.; Cinosi, A.; Mascitti, A.; Tonucci, L. Cu-ZnO Embedded in a Polydopamine Shell for the Generation of Antibacterial Surgical Face Masks. Molecules 2024, 29, 4512. [Google Scholar] [CrossRef]
- Megha, T.; Jenny, M. Titanium Dioxide in Sunscreen. In Application of Titanium Dioxide; Magdalena, J., Ed.; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Windler, L.; Lorenz, C.; von Goetz, N.; Hungerbühler, K.; Amberg, M.; Heuberger, M.; Nowack, B. Release of Titanium Dioxide from Textiles during Washing. Environ. Sci. Technol. 2012, 46, 8181–8188. [Google Scholar] [CrossRef]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Kisch, H. Semiconductor Photocatalysis—Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef]
- Charbonnier, P.; Jouneau, P.-H.; Deniaud, A. The endocrine disruptor effect of metal nanoparticles mainly depends on their capacity to release metal ions. Environ. Sci. Nano 2024, 11, 3192–3201. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Yetisen, A.K.; Qu, H.; Manbachi, A.; Butt, H.; Dokmeci, M.R.; Hinestroza, J.P.; Skorobogatiy, M.; Khademhosseini, A.; Yun, S.H. Nanotechnology in Textiles. ACS Nano 2016, 10, 3042–3068. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Margarucci, L.M.; Gianfranceschi, G.; Romano Spica, V.; D’Ermo, G.; Refi, C.; Podico, M.; Vitali, M.; Romano, F.; Valeriani, F. Photocatalytic Treatments for Personal Protective Equipment: Experimental Microbiological Investigations and Perspectives for the Enhancement of Antimicrobial Activity by Micrometric TiO2. Int. J. Environ. Res. Public Health 2021, 18, 8662. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Yang, Z. Toxicology of nanosized titanium dioxide: An update. Arch. Toxicol. 2015, 89, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future approaches. Comptes Rendus Chimie 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Verbruggen, S.W.; Keulemans, M.; Filippousi, M.; Flahaut, D.; Van Tendeloo, G.; Lacombe, S.; Martens, J.A.; Lenaerts, S. Plasmonic gold–silver alloy on TiO2 photocatalysts with tunable visible light activity. Appl. Catal. B Environ. 2014, 156–157, 116–121. [Google Scholar] [CrossRef]
- Han, C.; Lalley, J.; Namboodiri, D.; Cromer, K.; Nadagouda, M.N. Titanium dioxide-based antibacterial surfaces for water treatment. Curr. Opin. Chem. Eng. 2016, 11, 46–51. [Google Scholar] [CrossRef]
- Zahid, M.; Papadopoulou, E.L.; Suarato, G.; Binas, V.D.; Kiriakidis, G.; Gounaki, I.; Moira, O.; Venieri, D.; Bayer, I.S.; Athanassiou, A. Fabrication of Visible Light-Induced Antibacterial and Self-Cleaning Cotton Fabrics Using Manganese Doped TiO2 Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1154–1164. [Google Scholar] [CrossRef]
- Ahmed, O.B.; Alamro, T. Evaluation of the antibacterial activities of face masks coated with titanium dioxide nanoparticles. Sci. Rep. 2022, 12, 18739. [Google Scholar] [CrossRef]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.W.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef]
- Blevens, M.S.; Pastrana, H.F.; Mazzotta, H.C.; Tsai, C.S.-J. Cloth Face Masks Containing Silver: Evaluating the Status. ACS Chem. Health Saf. 2021, 28, 171–182. [Google Scholar] [CrossRef]
- EPA. Inert Reassessment for Titanium Dioxide—CAS No. 13463-67-7; U.S. Environmental Protection Agency: Washington, DC, USA, 2005. [Google Scholar]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Luo, Z.; Li, Z.; Xie, Z.; Sokolova, I.M.; Song, L.; Peijnenburg, W.J.G.M.; Hu, M.; Wang, Y. Rethinking Nano-TiO2 Safety: Overview of Toxic Effects in Humans and Aquatic Animals. Small 2020, 16, 2002019. [Google Scholar] [CrossRef]
- Iavicoli, I.; Leso, V.; Fontana, L.; Bergamaschi, A. Toxicological effects of titanium dioxide nanoparticles: A review of in vitro mammalian studies. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 481–508. [Google Scholar]
- Ayorinde, T.; Sayes, C.M. An updated review of industrially relevant titanium dioxide and its environmental health effects. J. Hazard. Mater. Lett. 2023, 4, 100085. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Ling, C.; An, H.; Li, L.; Wang, J.; Lu, T.; Wang, H.; Hu, Y.; Song, G.; Liu, S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles In Vitro: A Systematic Review of the Literature and Meta-analysis. Biol. Trace Elem. Res. 2021, 199, 2057–2076. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide; NIOSH: Cincinnati, OH, USA, 2011. [Google Scholar]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ali, M. What function of nanoparticles is the primary factor for their hyper-toxicity? Adv. Colloid Interface Sci. 2023, 314, 102881. [Google Scholar] [CrossRef]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Hankin, S.M.; Peters, S.A.; Tran, C.L.; Christensen, F.M. Review of carbon nanotubes toxicity and exposure—Appraisal of human health risk assessment based on open literature. Crit. Rev. Toxicol. 2010, 40, 759–790. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Gurr, J.R.; Wang, A.S.; Chen, C.H.; Jan, K.Y. Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology 2005, 213, 66–73. [Google Scholar] [CrossRef]
- Luijten, M.; van Benthem, J.; Morita, T.; Corvi, R.; Escobar, P.A.; Fujita, Y.; Hemmerich, J.; Honarvar, N.; Kirkland, D.; Koyama, N.; et al. Evaluation of the standard battery of in vitro genotoxicity tests to predict in vivo genotoxicity through mathematical modeling: A report from the 8th International Workshop on Genotoxicity Testing. Environ. Mol. Mutagen. 2024. [Google Scholar] [CrossRef]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
- EFSA; Younes, M.; Aquilina, G.; Castle, L.; Engel, K.-H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- Charles, S.; Jomini, S.; Fessard, V.; Bigorgne-Vizade, E.; Rousselle, C.; Michel, C. Assessment of the in vitro genotoxicity of TiO2 nanoparticles in a regulatory context. Nanotoxicology 2018, 12, 357–374. [Google Scholar] [CrossRef]
- Thoustrup Saber, A.; Søs Poulsen, S.; Hadrup, N.; Sørig Hougaard, K.; Jacobsen Raun, N.; Vogel, U. Titanium Dioxide Nanomaterials: Scientific Basis for Setting a Health-Based Occupational Exposure Limit; The National Research Centre for the Working Environment: Copenhagen, Denmark, 2018. [Google Scholar]
- Li, N.; Ma, L.; Wang, J.; Zheng, L.; Liu, J.; Duan, Y.; Liu, H.; Zhao, X.; Wang, S.; Wang, H.; et al. Interaction Between Nano-Anatase TiO2 and Liver DNA from Mice In Vivo. Nanoscale Res. Lett. 2009, 5, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Simkó, M.; Mattsson, M.-O. Risks from accidental exposures to engineered nanoparticles and neurological health effects: A critical review. Part. Fibre Toxicol. 2010, 7, 42. [Google Scholar] [CrossRef]
- Kim, C.S.; Jaques, P.A. Respiratory dose of inhaled ultrafine particles in healthy adults. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2000, 358, 2693–2705. [Google Scholar] [CrossRef]
- Ananda Rao, A.; Johncy, S. Tennis Courts in the Human Body: A Review of the Misleading Metaphor in Medical Literature. Cureus 2022, 14, e21474. [Google Scholar] [CrossRef]
- Oberdörster, G. Lung Dosimetry: Pulmonary Clearance of Inhaled Particles. Aerosol Sci. Technol. 1993, 18, 279–289. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Gosens, I.; Heringa, M.B.; Oomen, A.G.; Vandebriel, R.J.; Groenewold, M.; Cassee, F.R. Mechanism of Action of TiO2: Recommendations to Reduce Uncertainties Related to Carcinogenic Potential. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 203–223. [Google Scholar] [CrossRef]
- Chang, X.; Fu, Y.; Zhang, Y.; Tang, M.; Wang, B. Effects of Th1 and Th2 cells balance in pulmonary injury induced by nano titanium dioxide. Environ. Toxicol. Pharmacol. 2014, 37, 275–283. [Google Scholar] [CrossRef]
- Chen, H.W.; Su, S.F.; Chien, C.T.; Lin, W.H.; Yu, S.L.; Chou, C.C.; Chen, J.J.; Yang, P.C. Titanium dioxide nanoparticles induce emphysema-like lung injury in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2006, 20, 2393–2395. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.; Yu, C.P. Two-Phase Pulmonary Clearance of Insoluble Particles in Mammalian Species. Inhal. Toxicol. 1998, 10, 121–130. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Reed, K.L.; Frerichs, S.; Sayes, C.M. Pulmonary toxicity study in rats with three forms of ultrafine-TiO2 particles: Differential responses related to surface properties. Toxicology 2007, 230, 90–104. [Google Scholar] [CrossRef]
- Hsiao, I.L.; Huang, Y.-J. Effects of various physicochemical characteristics on the toxicities of ZnO and TiO2 nanoparticles toward human lung epithelial cells. Sci. Total Environ. 2011, 409, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Wahi, R.; Kurian, P.A.; Liu, Y.; West, J.L.; Ausman, K.D.; Warheit, D.B.; Colvin, V.L. Correlating Nanoscale Titania Structure with Toxicity: A Cytotoxicity and Inflammatory Response Study with Human Dermal Fibroblasts and Human Lung Epithelial Cells. Toxicol. Sci. 2006, 92, 174–185. [Google Scholar] [CrossRef]
- Lee, K.P.; Trochimowicz, H.J.; Reinhardt, C.F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol. Appl. Pharmacol. 1985, 79, 179–192. [Google Scholar] [CrossRef]
- Lee, K.P.; Henry, N.W.; Trochimowicz, H.J.; Reinhardt, C.F. Pulmonary response to impaired lung clearance in rats following excessive TiO2 dust deposition. Environ. Res. 1986, 41, 144–167. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Fuhst, R.; Rittinghausen, S.; Creutzenberg, O.; Bellmann, B.; Koch, W.; Levsen, K. Chronic Inhalation Exposure of Wistar Rats and two Different Strains of Mice to Diesel Engine Exhaust, Carbon Black, and Titanium Dioxide. Inhal. Toxicol. 1995, 7, 533–556. [Google Scholar] [CrossRef]
- Warheit, D.B.; Frame, S.R. Characterization and Reclassification of Titanium Dioxide-Related Pulmonary Lesions. J. Occup. Environ. Med. 2006, 48, 1308–1313. [Google Scholar] [CrossRef]
- Pott, F.; Roller, M. Carcinogenicity study with nineteen granular dusts in rats. Eur. J. Oncol. 2005, 10, 249–281. [Google Scholar]
- Thompson, C.M.; Suh, M.; Mittal, L.; Wikoff, D.S.; Welsh, B.; Proctor, D.M. Development of linear and threshold no significant risk levels for inhalation exposure to titanium dioxide using systematic review and mode of action considerations. Regul. Toxicol. Pharmacol. 2016, 80, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Soutar, A.; Cherrie, J.W.; Granath, F.; Andersen, A.; Anttila, A.; Blettner, M.; Gaborieau, V.; Klug, S.J.; Langard, S.; et al. Mortality Among Workers Employed in the Titanium Dioxide Production Industry in Europe. Cancer Causes Control 2004, 15, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Fryzek, J.P.; Chadda, B.; Marano, D.; White, K.; Schweitzer, S.; McLaughlin, J.K.; Blot, W.J. A cohort mortality study among titanium dioxide manufacturing workers in the United States. J. Occup. Environ. Med. 2003, 45, 400–409. [Google Scholar] [CrossRef]
- Chen, J.L.; Fayerweather, W.E. Epidemiologic Study of Workers Exposed to Titanium Dioxide. J. Occup. Environ. Med. 1988, 30, 937–942. [Google Scholar] [CrossRef]
- Siemiatycki, J. Risk Factors for Cancer in the Workplace; CRC Press: Boca Rato, FL, USA, 1991. [Google Scholar]
- Boffetta, P.; Gaborieau, V.; Nadon, L.; Parent, M.-E.; Weiderpass, E.; Siemiatycki, J. Exposure to titanium dioxide and risk of lung cancer in a population-based study from Montreal. Scand. J. Work Environ. Health 2001, 27, 227–232. [Google Scholar] [CrossRef]
- Ramanakumar, A.V.; Parent, M.-É.; Latreille, B.; Siemiatycki, J. Risk of lung cancer following exposure to carbon black, titanium dioxide and talc: Results from two case–control studies in Montreal. Int. J. Cancer 2008, 122, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.D.; Watkins, J.; Tankersley, W.; Phillips, J.; Girardi, D. Mortality among titanium dioxide workers at three DuPont plants. J. Occup. Environ. Med. 2010, 52, 303–309. [Google Scholar] [CrossRef]
- Ellis, E.D.; Watkins, J.P.; Tankersley, W.G.; Phillips, J.A.; Girardi, D.J. Occupational exposure and mortality among workers at three titanium dioxide plants. Am. J. Ind. Med. 2013, 56, 282–291. [Google Scholar] [CrossRef]
- Le, H.Q.; Tomenson, J.A.; Warheit, D.B.; Fryzek, J.P.; Golden, A.P.; Ellis, E.D. A Review and Meta-Analysis of Occupational Titanium Dioxide Exposure and Lung Cancer Mortality. J. Occup. Environ. Med. 2018, 60, e356–e367. [Google Scholar] [CrossRef]
- ANSES. Valeurs Toxicologiques de Référence. Le Dioxyde de Titane sous Forme Nanoparticulaire; ANSES: Maisons-Alfort, France, 2019; p. 94. [Google Scholar]
- ANSES. Valeurs Limites D’exposition en Milieu Professionnel. Le Dioxyde de Titane sous Forme Nanométrique (TiO2-NP, P25); ANSES: Maisons-Alfort, France, 2020; p. 115. [Google Scholar]
- Kirkland, D.; Aardema, M.J.; Battersby, R.V.; Beevers, C.; Burnett, K.; Burzlaff, A.; Czich, A.; Donner, E.M.; Fowler, P.; Johnston, H.J.; et al. A weight of evidence review of the genotoxicity of titanium dioxide (TiO2). Regul. Toxicol. Pharmacol. 2022, 136, 105263. [Google Scholar] [CrossRef]
- Wolf, S.; Sriram, K.; Camassa, L.M.A.; Pathak, D.; Bing, H.L.; Mohr, B.; Zienolddiny-Narui, S.; Samulin Erdem, J. Systematic review of mechanistic evidence for TiO2 nanoparticle-induced lung carcinogenicity. Nanotoxicology 2024, 18, 437–463. [Google Scholar] [CrossRef]
- Nymark, P.; Karlsson, H.L.; Halappanavar, S.; Vogel, U. Adverse Outcome Pathway Development for Assessment of Lung Carcinogenicity by Nanoparticles. Front. Toxicol. 2021, 3, 653386. [Google Scholar] [CrossRef]
- Bos, P.M.J.; Gosens, I.; Geraets, L.; Delmaar, C.; Cassee, F.R. Pulmonary toxicity in rats following inhalation exposure to poorly soluble particles: The issue of impaired clearance and the relevance for human health hazard and risk assessment. Regul. Toxicol. Pharmacol. 2019, 109, 104498. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Leso, V.; Bergamaschi, A. The Effects of Nanomaterials as Endocrine Disruptors. Int. J. Mol. Sci. 2013, 14, 16732–16801. [Google Scholar] [CrossRef]
- Minghui, F.; Ran, S.; Yuxue, J.; Minjia, S. Toxic effects of titanium dioxide nanoparticles on reproduction in mammals. Front. Bioeng. Biotechnol. 2023, 11, 1183592. [Google Scholar] [CrossRef]
- OSHA. Permissible Exposure Limits—Annotated Tables. OSHA Annotated Table Z-1. Available online: https://www.osha.gov/annotated-pels/table-z-1 (accessed on 23 January 2024).
- Bermudez, E.; Mangum, J.B.; Wong, B.A.; Asgharian, B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. Off. J. Soc. Toxicol. 2004, 77, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Christensen, F.M.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Hankin, S.; Peters, S.; Aschberger, K. Nano-TiO2—Feasibility and challenges for human health risk assessment based on open literature. Nanotoxicology 2011, 5, 110–124. [Google Scholar] [CrossRef]
- Stockmann-Juvala, H.; Taxell, P.; Santonen, T. Formulating Occupational Exposure Limits Values (OELs) (Inhalation & Dermal); Scaffold SPD7; Finnish Institute of Occupational Health: Helsinki, Finland, 2014. [Google Scholar]
- Hougaard, K.S.; Jackson, P.; Jensen, K.A.; Sloth, J.J.; Löschner, K.; Larsen, E.H.; Birkedal, R.K.; Vibenholt, A.; Boisen, A.M.; Wallin, H.; et al. Effects of prenatal exposure to surface-coated nanosized titanium dioxide (UV-Titan). A study in mice. Part. Fibre Toxicol. 2010, 7, 16. [Google Scholar] [CrossRef]
- von Goetz, N.; Lorenz, C.; Windler, L.; Nowack, B.; Heuberger, M.; Hungerbühler, K. Migration of Ag- and TiO2-(Nano)particles from Textiles into Artificial Sweat under Physical Stress: Experiments and Exposure Modeling. Environ. Sci. Technol. 2013, 47, 9979–9987. [Google Scholar] [CrossRef]
- Rovira, J.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Trace elements in skin-contact clothes and migration to artificial sweat: Risk assessment of human dermal exposure. Text. Res. J. 2017, 87, 726–738. [Google Scholar] [CrossRef]
- Franz, R.; Bott, J.; Störmer, A. Considerations for and Guidance to Testing and Evaluating Migration/Release of Nanoparticles from Polymer Based Nanocomposites. Nanomaterials 2020, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- NIOSH. Current Intelligence Bulletin 70: Health Effects of Occupational Exposure to Silver Nanomaterials; NIOSH: Cincinnnati, OH, USA, 2021. [Google Scholar]
- Hadrup, N.; Sharma, A.K.; Loeschner, K.; Jacobsen, N.R. Pulmonary toxicity of silver vapours, nanoparticles and fine dusts: A review. Regul. Toxicol. Pharmacol. 2020, 115, 104690. [Google Scholar] [CrossRef]
- Hadrup, N.; Sahlgren, N.; Jacobsen, N.R.; Saber, A.T.; Hougaard, K.S.; Vogel, U.; Jensen, K.A. Toxicity dose descriptors from animal inhalation studies of 13 nanomaterials and their bulk and ionic counterparts and variation with primary particle characteristics. Nanotoxicology 2023, 17, 338–371. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)—Critical Review: State of the Art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Tulinska, J.; Mikusova, M.L.; Liskova, A.; Busova, M.; Masanova, V.; Uhnakova, I.; Rollerova, E.; Alacova, R.; Krivosikova, Z.; Wsolova, L.; et al. Copper Oxide Nanoparticles Stimulate the Immune Response and Decrease Antioxidant Defense in Mice After Six-Week Inhalation. Front. Immunol. 2022, 13, 874253. [Google Scholar] [CrossRef]


| Properties | Silver | Copper | Zinc | TiO2 | Remarks |
|---|---|---|---|---|---|
| Release of ions | Yes | Yes | Yes | No | Zn2+, Ag+, Cu2+ are released. These ions contribute to cellular disruption, and oxidative stress. |
| Light-dependent | No | No | Partial | Yes | TiO2 requires UV light for ROS generation through photocatalysis, while ZnO also acts by releasing ions. |
| ROS generation and oxidative stress | Yes | Yes | Yes | Yes | ROS generation and increased oxidative stress are key mechanisms for cytotoxicity and antimicrobial activity. |
| Disruption of cell membrane/viral envelope | Yes | Yes | Yes | Yes | Direct interaction leads to structural damage, increased permeability, and cellular leakage. |
| DNA damage | Yes | Yes | Yes | Yes | Primarily indirect genotoxicity by ROS. Conclusions regarding their direct genotoxicity require further study for each NP type. |
| Protein damage | Yes | Yes | Yes | Yes | Protein damage occurs via ROS or direct interactions with released metal ions (e.g., Ag+ binds thiol groups). |
| E. coli cytotoxicity: Predicted log(1/EC50) | 4.07 | 3.35 | 3.39 | 1.95 | Values derived using the nano-QSAR model of Mu et al. [94] for nano- Ag2O, CuO, ZnO, and TiO2. Higher values indicate higher cytotoxicity and vice versa. Hence, the cytotoxicity of TiO2 NPs is relatively weak. |
| Institute or Project | Limit | Value | Exposure Details | Remarks | Source |
|---|---|---|---|---|---|
| NIOSH | REL | 300 µg/m3 | Chronic. 10 h/day TWA, 40 h work week. | Reduces the excess human lung cancer risk to below 1:1000. Benchmark dose approach with model averaging, based on chronic rat inhalation studies (e.g., [166]). | [141] |
| ENRHES EU project | INEL | 17 µg/m3 | Chronic. 8 h/day. | Prevents pulmonary inflammation. Threshold-based. Derived with AFtotal = 15 from corrected NOAEC (0.25 mg/m3) in a sub-chronic rat inhalation study [189]. | [190] |
| Scaffold EU project | OEL | 100 µg/m3 | Chronic. 8 h/day. | Prevents pulmonary inflammation. Threshold-based. Derived with AFtotal = 2.5 from corrected NOAEC (0.25 mg/m3) in a sub-chronic rat inhalation study [189]. | [191] |
| NRCWE | OEL | 10 µg/m3 | Chronic. 8 h/day. | Prevents pulmonary inflammation. Threshold-based. Derived with AFtotal = 25 from corrected NOAEC (0.25 mg/m3) in a sub-chronic rat inhalation study [189]. | [151] |
| OEL 1:100,000 | 0.04 µg/m3 | Chronic. 8 h/day, 40 h work week, 45 years. | Reduces the excess human lung cancer risk to 1:100,000. Non-threshold based, assuming linear-dose–response. Based on estimated human lung burden, derived from chronic rat inhalation study [166] and pulmonary deposition fraction in mice [192]. | [151] | |
| ANSES | TRV | 0.12 µg/m3 | Chronic (general population) | Prevents pulmonary inflammation. Threshold-based. Derived with AFtotal = 225 from corrected NOAECHEC (0.028 mg/m3) in a sub-chronic rat inhalation study [189]. Applicable to Aeroxide TiO2 P25 (80% anatase/20% rutile; 21 nm). | [179] |
| OEL | 0.80 µg/m3 | Chronic. 8 h/day TWA, 240 days/year, life-long. | Prevents pulmonary inflammation. Threshold-based. Derived with AFtotal = 81 from corrected NOAECHEC (0.065 mg/m3) in a sub-chronic rat inhalation study [189]. Applicable to Aeroxide TiO2 P25 (80% anatase/20% rutile; 21 nm). | [180] | |
| STEL | 4 µg/m3 | 15 min TWA. | Threshold-based. Maximum 5× 8 h OEL. | [180] | |
| Sciensano | AEL | 0.72 µg/m3 | Subchronic. 8 h/day. | Prevents pulmonary inflammation. Threshold-based. Derived with AFtotal = 90 from corrected NOAECHEC (0.065 mg/m3) in a sub-chronic rat inhalation study [189]. Applicable to Aeroxide TiO2 P25 (80% anatase/20% rutile; 21 nm). | [46] |
| Theoretical Exposure Scenario | Ti (µg) Leached from Mask | Converted to TiO2 (µg) | Simulated TWA TiO2 Inhalation (µg/m3) | Exposure Limit | RCR |
|---|---|---|---|---|---|
| Scenario 1: Adult wearing 1 × face mask 2 of Sullivan et al. [91] for 8 h/day; air inhalation rate 1.25 m3/h. Assumption: inhaled TiO2 during 8 h equals the measured amount of TiO2 in water leachate (=0.64 µg Ti/L × 0.25 L/mask × 1.668) after a contact time of 24 h. | 0.16 | 0.27 | 0.027 | NIOSH—REL (300 µg/m3) | 8.9 × 10−5 |
| NRCWE—OEL 1:100,000 (0.04 µg/m3) | 0.67 | ||||
| ANSES—OEL (0.8 µg/m3) | 0.03 | ||||
| Sciensano—AEL (0.72 µg/m3) | 0.04 | ||||
| ANSES—TRV (0.12 µg/m3) | 0.22 | ||||
| Scenario 2: Adult wearing 2 × face mask 2 of Sullivan et al. [91]; each mask worn for 4 h/day; air inhalation rate 1.25 m3/h. Assumption: inhaled TiO2 during 8 h equals 2 × the measured amount of TiO2 in water leachate after a contact time of 24 h. | 0.32 | 0.53 | 0.053 | NIOSH—REL (300 µg/m3) | 1.8 × 10−4 |
| NRCWE—OEL 1:100,000 (0.04 µg/m3) | 1.33 | ||||
| ANSES—OEL (0.8 µg/m3) | 0.07 | ||||
| Sciensano—AEL (0.72 µg/m3) | 0.07 | ||||
| ANSES—TRV (0.12 µg/m3) | 0.44 | ||||
| Scenario 3: Adult wearing 1 × AgMask18 of Montalvo et al. [88] for 8 h/day; inhalation rate 1.25 m3/h. Assumption: inhaled TiO2 during 8 h equals the measured amount of TiO2 in artificial sweat leachate after a contact time of 8 h (=47 µg Ti/mask × 1.668). | 47 | 78.40 | 7.840 | NIOSH—REL (300 µg/m3) | 0.03 |
| NRCWE—OEL 1:100,000 (0.04 µg/m3) | 195.99 | ||||
| ANSES—OEL (0.8 µg/m3) | 9.80 | ||||
| Sciensano—AEL (0.72 µg/m3) | 10.89 | ||||
| ANSES—TRV (0.12 µg/m3) | 65.33 | ||||
| Scenario 4: Adult wearing 2 × AgMask18 of Montalvo et al. [88]; each mask worn for 4 h/day; air inhalation rate 1.25 m3/h. Assumption: inhaled TiO2 during 8 h equals 2 × the measured amount of TiO2 in artificial sweat leachate after a contact time of 1 h (=2 masks × 34 µg Ti/mask × 1.668). | 68 | 113.42 | 11.342 | NIOSH—REL (300 µg/m3) | 0.04 |
| NRCWE—OEL 1:100,000 (0.04 µg/m3) | 283.56 | ||||
| ANSES—OEL (0.8 µg/m3) | 14.18 | ||||
| Sciensano—AEL (0.72 µg/m3) | 15.75 | ||||
| ANSES—TRV (0.12 µg/m3) | 94.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Everaert, S.; Godderis, L.; Raquez, J.-M.; Schoeters, G.; Spanoghe, P.; Moens, J.; Hens, L.; Michel, O.; Adang, D.; Fraeyman, N. Do We Need Titanium Dioxide (TiO2) Nanoparticles in Face Masks? Toxics 2025, 13, 244. https://doi.org/10.3390/toxics13040244
Everaert S, Godderis L, Raquez J-M, Schoeters G, Spanoghe P, Moens J, Hens L, Michel O, Adang D, Fraeyman N. Do We Need Titanium Dioxide (TiO2) Nanoparticles in Face Masks? Toxics. 2025; 13(4):244. https://doi.org/10.3390/toxics13040244
Chicago/Turabian StyleEveraert, Stijn, Lode Godderis, Jean-Marie Raquez, Greet Schoeters, Pieter Spanoghe, Jonas Moens, Luc Hens, Olivier Michel, Dirk Adang, and Norbert Fraeyman. 2025. "Do We Need Titanium Dioxide (TiO2) Nanoparticles in Face Masks?" Toxics 13, no. 4: 244. https://doi.org/10.3390/toxics13040244
APA StyleEveraert, S., Godderis, L., Raquez, J.-M., Schoeters, G., Spanoghe, P., Moens, J., Hens, L., Michel, O., Adang, D., & Fraeyman, N. (2025). Do We Need Titanium Dioxide (TiO2) Nanoparticles in Face Masks? Toxics, 13(4), 244. https://doi.org/10.3390/toxics13040244











