Associations of Environmental Pollutant Mixtures and Red Blood Cell Folate Concentrations: A Mixture Analysis of the U.S. Adult Population Based on NHANES Data, 2007–2016
Abstract
1. Introduction
2. Methods
2.1. Population and Data Collection
2.2. RBC Folate Measurement
2.3. Assessment of Environmental Exposure Biomarkers
2.4. Phthalates (ng/mL)
2.5. Heavy Metals
2.6. PFAS (ng/mL)
2.7. Phenols and Parabens (ng/mL)
2.8. PAH (ng/L)
2.9. Cotinine (ng/mL)
3. Covariates
4. Statistical Methods
5. Results
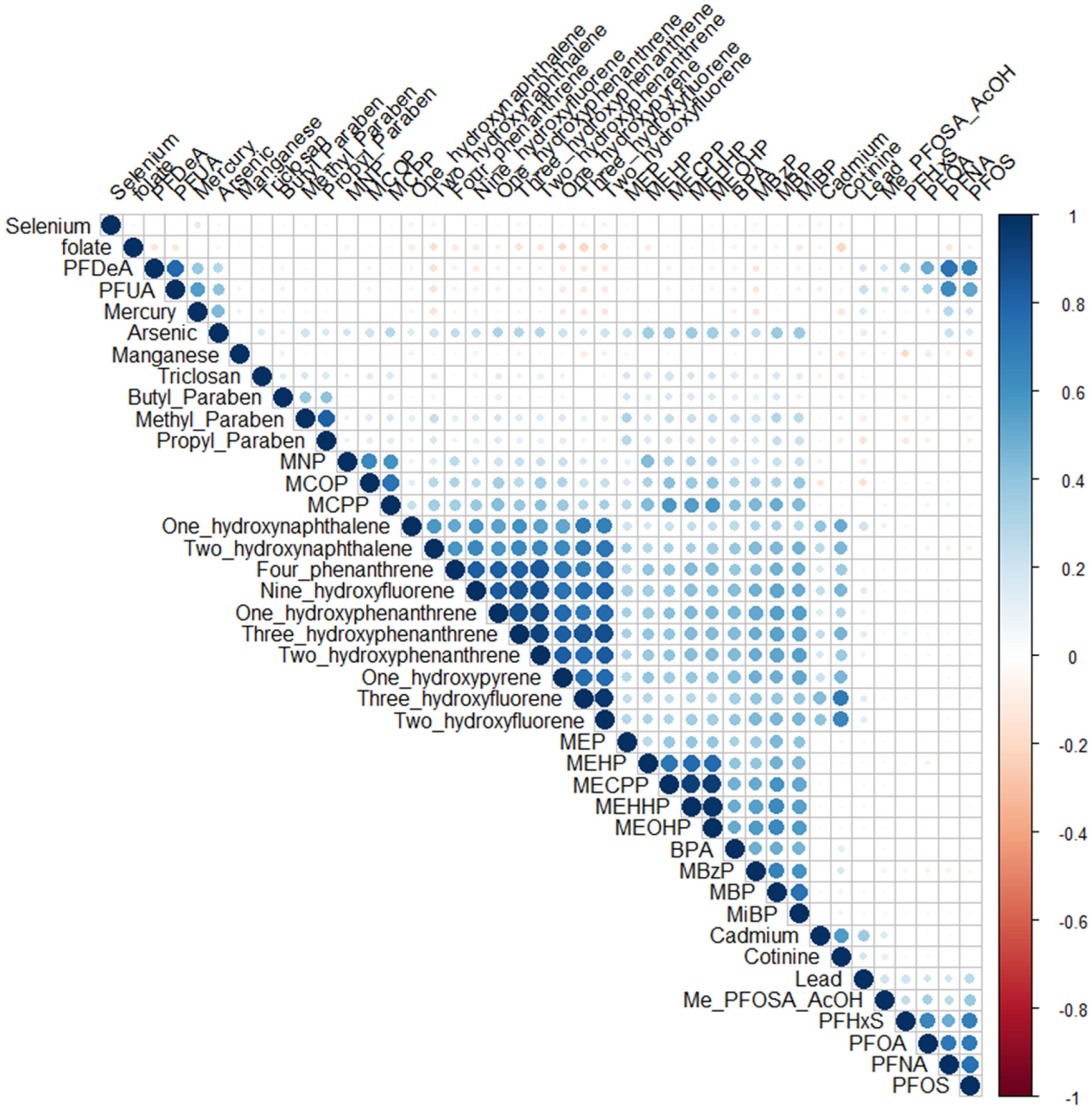
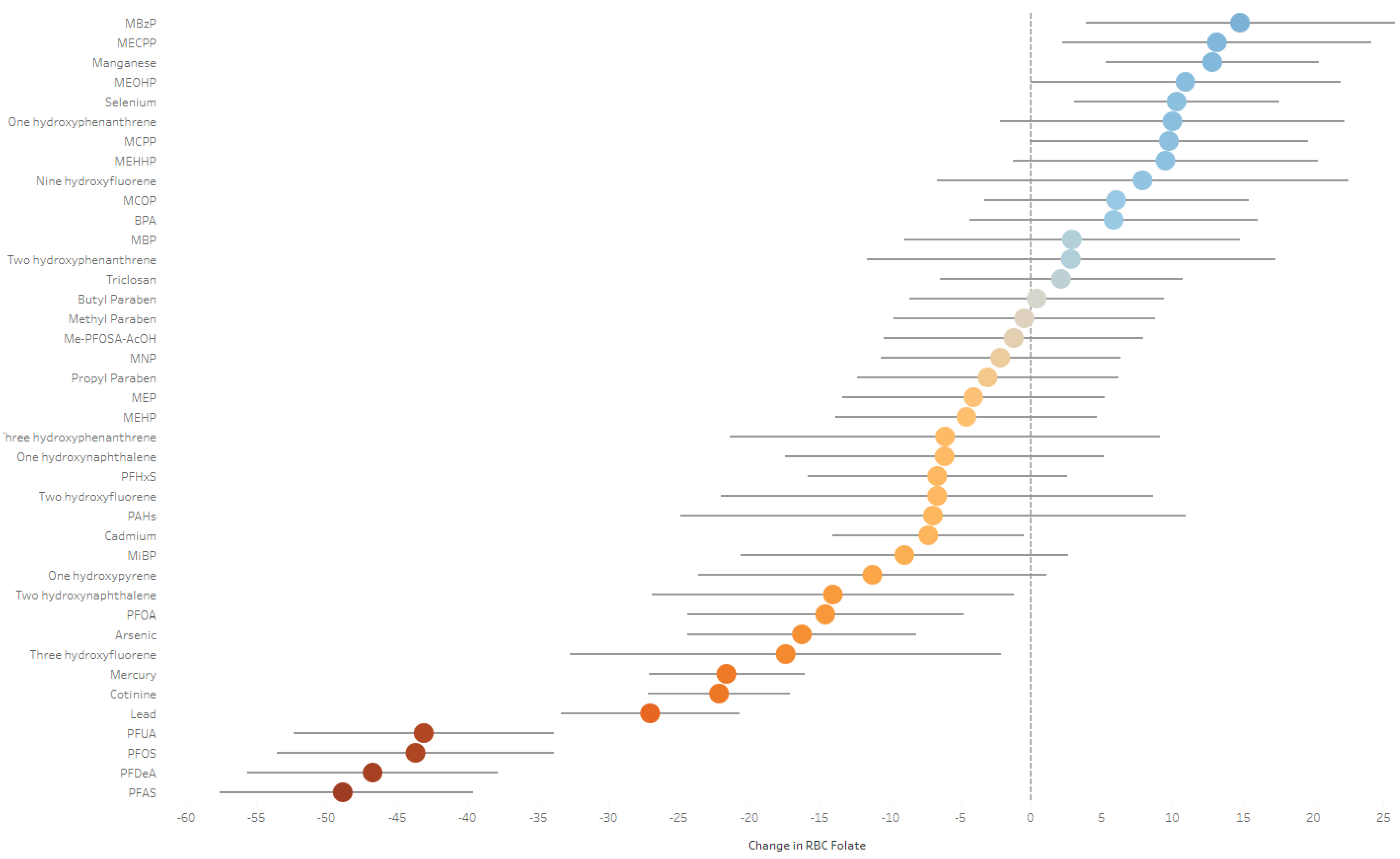
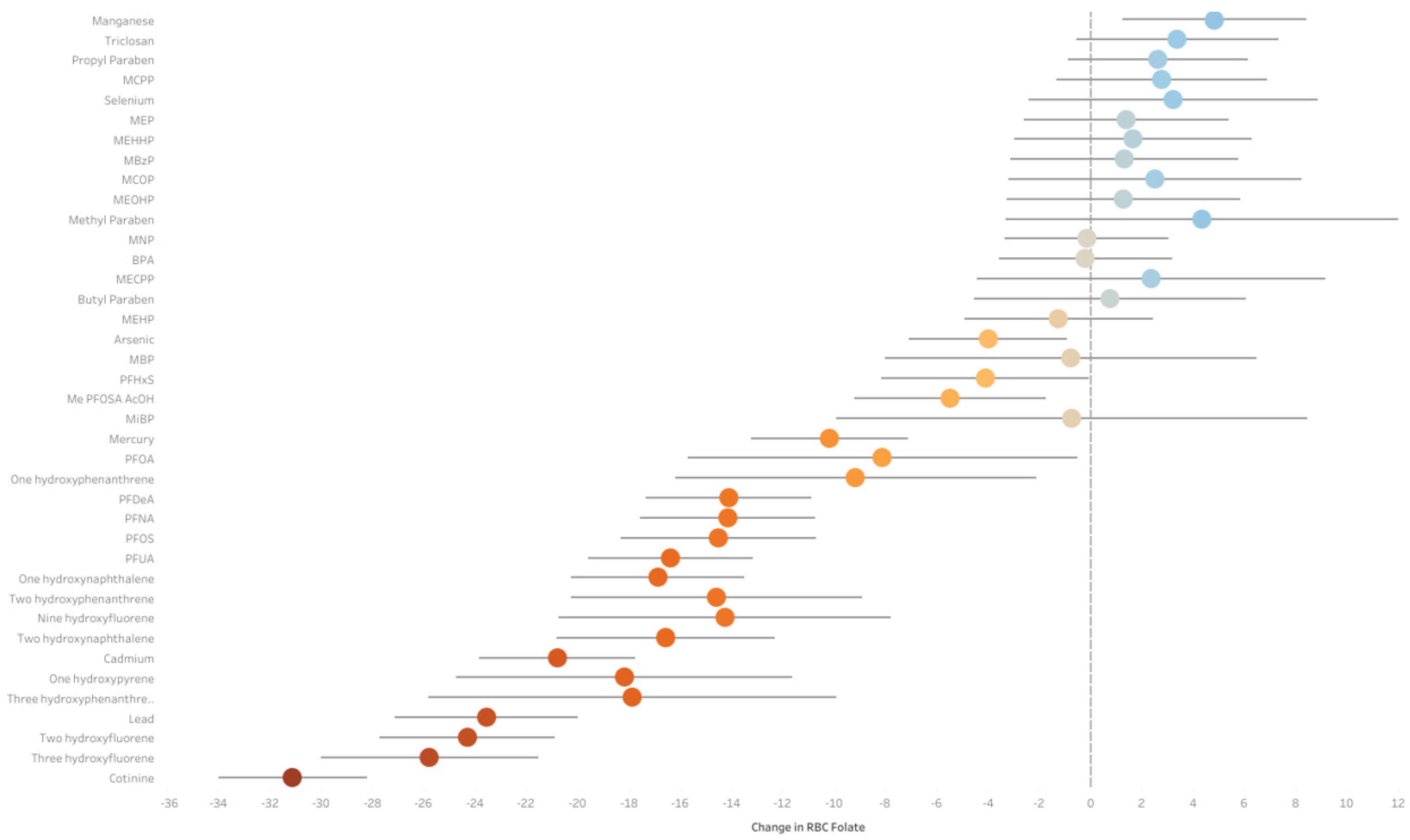
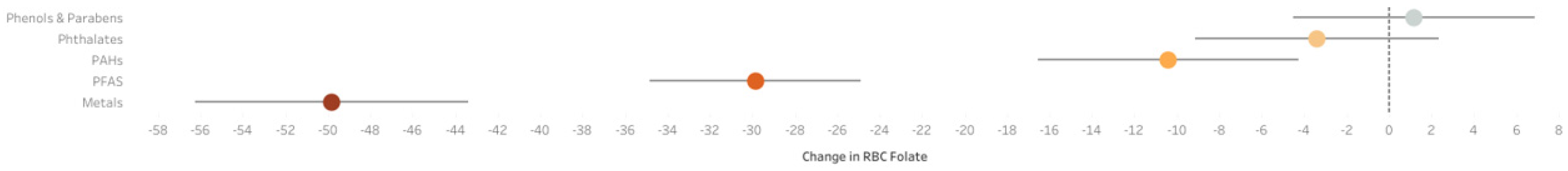
5.1. ExWAS Analysis
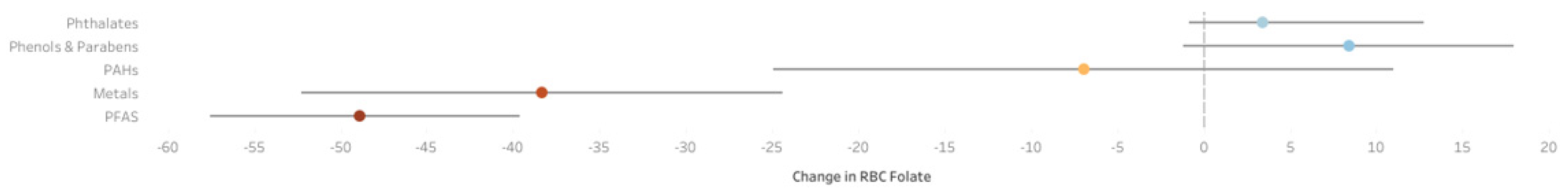
5.2. Q-Gcomp Analysis
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B. The folate cycle and disease in humans. Kidney Int. 2001, 59, 221–229. [Google Scholar] [CrossRef]
- Son, P.; Lewis, L. Hyperhomocysteinemia. [Updated 2020 May 21]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554408/ (accessed on 24 November 2024).
- Keil, K.P.; Lein, P.J. DNA methylation: A mechanism linking environmental chemical exposures to risk of autism spectrum disorders? Environ. Epigenetics 2016, 2, dvv012. [Google Scholar] [CrossRef]
- Kim, Y. Folate and carcinogenesis: Evidence, mechanisms, and implications. J. Nutr. Biochem. 1999, 10, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Nutritional Epigenetics: Impact of Folate Deficiency on DNA Methylation and Colon Cancer Susceptibility. J. Nutr. 2005, 135, 2703–2709. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Zhang, M.; Fazili, Z.; Storandt, R.J.; Crider, K.S.; Lavoie, D.J. Folate status in the US population 20 y after the introduction of folic acid fortification. Am. J. Clin. Nutr. 2019, 110, 1088–1097. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.-H. Folic Acid Supplementation and Pregnancy: More Than Just Neural Tube Defect Prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar] [PubMed] [PubMed Central]
- Botto, L.D.; Moore, C.A.; Khoury, M.J.; Erickson, J.D. Neural-Tube Defects. N. Engl. J. Med. 1999, 341, 1509–1519. [Google Scholar] [CrossRef]
- Czeizel, A.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate Deficiency and Folic Acid Supplementation: The Prevention of Neural-Tube Defects and Congenital Heart Defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef]
- George, L.; Mills, J.L.; Johannson, A.L.V.; Nordmark, A.; Olander, B.; Granath, F.; Cnattingius, S. Plasma Folate Levels and Risk of Spontaneous Abortion. JAMA 2002, 288, 1867. [Google Scholar] [CrossRef]
- Liu, X.; Lv, L.; Zhang, H.; Zhao, N.; Qiu, J.; He, X.; Zhang, Y. Folic acid supplementation, dietary folate intake and risk of preterm birth in China. Eur. J. Nutr. 2015, 55, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Naninck EF, G.; Stijger, P.C.; Brouwer-Brolsma, E.M. The Importance of Maternal Folate Status for Brain Development and Function of Offspring. Adv. Nutr. 2019, 10, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Fukuoka, H.; Sμgiyama, T.; Nagai, Y.; Ogasawara, K.; Yoshiike, N. Dietary folate intake during pregnancy and birth weight in Japan. Eur. J. Nutr. 2008, 47, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wei, L.; Cao, D.; Liu, C.; Tian, J.; Long, Y.; Mo, Z. Low serum folate status in the second trimester increase the risk of low birthweight in Chinese women. J. Obstet. Gynaecol. Res. 2018, 44, 2037–2044. [Google Scholar] [CrossRef]
- Oulhote, Y.; Lanphear, B.; Braun, J.M.; Webster, G.M.; Arbuckle, T.E.; Etzel, T.; Forget-Dubois, N.; Seguin, J.R.; Bouchard, M.F.; MacFarlane, A.; et al. Gestational exposures to phthalates and folic acid, and autistic traits in Canadian children. Environ. Health Perspect. 2020, 128, 027004. [Google Scholar] [CrossRef]
- Weggemans, R.M.; Groot, L.C.D.; Haller, J. Factors Related to Plasma Folate and Vitamin B12. The Seneca Study. Int. J. Food Sci. Nutr. 1997, 48, 141–150. [Google Scholar] [CrossRef]
- Sanvisens, A.; Zuluaga, P.; Pineda, M.; Fuster, D.; Bolao, F.; Juncà, J.; Mμga, R. Folate deficiency in patients seeking treatment of alcohol use disorder. Drug Alcohol Depend. 2017, 180, 417–422. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Yu, Y.; Li, Y.; Zhang, Y.; Liu, L.; Huo, Y. Effect of Smoking and Folate Levels on the Efficacy of Folic Acid Therapy in Prevention of Stroke in Hypertensive Men. Stroke 2018, 49, 114–120. [Google Scholar] [CrossRef]
- Stamm, R.; Hoμghton, L. Nutrient Intake Values for Folate during Pregnancy and Lactation Vary Widely around the World. Nutrients 2013, 5, 3920–3947. [Google Scholar] [CrossRef]
- Zhao, R.; Aluri, S.; Goldman, I.D. The proton-coupled folate transporter (PCFT-SLC46A1) and the syndrome of systemic and cerebral folate deficiency of infancy: Hereditary folate malabsorption. Mol. Asp. Med. 2017, 53, 57–72. [Google Scholar] [CrossRef]
- Kishi, R.; Grandjean, P. Health Impacts of Developmental Exposure to Environmental Chemicals. In Health Impacts of Developmental Exposure to Environmental Chemicals; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data [2007–2016]; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA. Available online: https://www.cdc.gov/nchs/nhanes/index.html (accessed on 24 November 2024).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Protocol 4000.04 [2015–2016]; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Hyattsville, MD, USA. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/FOLATE-I-MET-508.pdf (accessed on 24 November 2024).
- Li, M.X.; Yeung, J.M.; Cherny, S.S.; Sham, P.C. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum. Genet. 2012, 131, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Warembourg, C.; Maitre, L.; Tamayo-Uria, I.; Fossati, S.; Roumeliotaki, T.; Aasvang, G.M.; Basagaña, X. Early-Life Environmental Exposures and Blood Pressure in Children. J. Am. Coll. Cardiol. 2019, 74, 1317–1328. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 047004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X. Comparison of four methods for handing missing data in longitudinal data analysis throμgh a simulation study. Open J. Stat. 2014, 4, 933–944. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley and Son: New York, NY, USA, 2004. [Google Scholar]
- Duncan, K.; Erickson, A.C.; Egeland, G.M.; Weiler, H.; Arbour, L.T. Red blood cell folate levels in Canadian Inuit women of childbearing years: Influence of food security, body mass index, smoking, education, and vitamin use. Can. J. Public Health 2018, 109, 684–691. [Google Scholar] [CrossRef]
- Bal, C.; Hocaoglu, A.; Büyükşekerci, M.; Alagüney, M.E.; Yılmaz, O.H.; Tutkun, E. Evaluation of folate and vitamin B12 status in lead exposed workers. Toxicol. Lett. 2015, 42, 294–298. [Google Scholar] [CrossRef]
- Tian, Y.; Luan, M.; Zhang, J.; Yang, H.; Wang, Y.; Chen, H. Associations of single and multiple perfluoroalkyl substances exposure with folate among adolescents in Nhanes 2007–2010. Chemosphere 2022, 307, 135995. [Google Scholar] [CrossRef]
- Lee, J.E.; Choi, K. Perfluoroalkyl substances exposure and thyroid hormones in humans: Epidemiological observations and implications. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 6–14. [Google Scholar] [CrossRef]
- Catargi, B.; Parrot-Roulaud, F.; Cochet, C.; Ducassou, D.; Roger, P.; Tabarin, A. Homocysteine, hypothyroidism, and effect of thyroid hormone replacement. Thyroid. Off. J. Am. Thyroid. Assoc. 1999, 9, 1163–1166. [Google Scholar] [CrossRef]
- Barjaktarovic, M.; Steegers, E.A.P.; Jaddoe, V.W.V.; de Rijke, Y.B.; Visser, T.J.; Korevaar, T.I.M.; Peeters, R.P. The Association of Thyroid Function with Maternal and Neonatal Homocysteine Concentrations. J. Clin. Endocrinol. Metab. 2017, 102, 4548–4556. [Google Scholar] [CrossRef]
- Tandon, S.K.; Flora, S.J.; Behari, J.R.; Ashquin, M. Vitamin B complex in treatment of cadmium intoxication. Ann. Clin. Lab. Sci. 1984, 14, 487–492. [Google Scholar] [PubMed]
- Sobrino-Plata, J.; Meyssen, D.; Cuypers, A.; Escobar, C.; Hernández, L. Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 2014, 377, 369–381. Available online: https://doi-org.silk.library.umass.edu/10.1007/s11104-013-2006-4 (accessed on 24 November 2024). [CrossRef]
- Branco, V.; Matos, B.; Mourato, C.; Diniz, M.; Carvalho, C.; Martins, M. Synthesis of glutathione as a central aspect of PAH toxicity in liver cells: A comparison between phenanthrene, benzo[b]fluoranthene and their mixtures. Ecotoxicol. Environ. Saf. 2021, 208, 111637. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.J.P.; Das, A.; Calcagnotto, A.M.; Aliaga, C.A.; El-Bayoumy, K. Age related changes in selenium and glutathione levels in different lobes of the rat prostate. Exp. Gerontol. 2012, 47, 223–228. Available online: https://doi-org.silk.library.umass.edu/10.1016/j.exger.2011.11.015 (accessed on 24 November 2024). [CrossRef]
- Mallah, M.A.; Mallah, M.A.; Liu, Y.; Xi, H.; Wang, W.; Feng, F.; Zhang, Q. Relationship Between Polycyclic Aromatic Hydrocarbons and Cardiovascular Diseases: A Systematic Review. Front. Public Health 2021, 9, 763706. [Google Scholar] [CrossRef]
- Min, J.-Y.; Lee, K.-J.; Park, J.-B.; Min, K.-B. Perfluorooctanoic acid exposure is associated with elevated homocysteine and hypertension in US adults. Occup. Environ. Med. 2012, 69, 658–662. Available online: http://www.jstor.org/stable/23567816 (accessed on 24 November 2024). [CrossRef]
- Ledda, C.; Cannizzaro, E.; Lovreglio, P.; Vitale, E.; Stufano, A.; Montana, A.; Li Volti, G.; Rapisarda, V. Exposure to Toxic Heavy Metals Can Influence Homocysteine Metabolism? Antioxidants 2019, 9, 30. [Google Scholar] [CrossRef]
- Blom, H.J.; Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011, 34, 75–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Mustieles, V.; Wang, Y.-X.; Sun, Y.; Agudelo, J.; Bibi, Z.; Torres, N.; Oulhote, Y.; Slitt, A.; Messerlian, C. Folate concentrations and serum perfluoroalkyl and polyfluoroalkyl substance concentrations in adolescents and adults in the USA (National Health and Nutrition Examination Study 2003–2016): An observational study. Lancet Planet. Health 2023, 7, e449–e458. [Google Scholar] [CrossRef]
| N | Mean | SD | p-Value | |
|---|---|---|---|---|
| Overall RBC Folate | 27,938 | 515.7 | 243.7 | N/A |
| Sex | <0.001 | |||
| Male | 13,599 | 500.1 | 232.5 | - |
| Female | 14,339 | 530.6 | 252.9 | <0.001 |
| Age | <0.001 | |||
| 18 to 34 | 8022 | 439.1 | 172.1 | - |
| 35 to 64 | 13,650 | 506.3 | 220.4 | <0.001 |
| 65 and Older | 6266 | 634.4 | 315.0 | <0.001 |
| Race | <0.001 | |||
| Mexican American | 4441 | 478.4 | 199.2 | - |
| Other Hispanic | 3012 | 483.1 | 200.7 | 0.918 |
| Non-Hispanic White | 11,605 | 584.4 | 273.4 | <0.001 |
| Non-Hispanic Black | 5768 | 440.6 | 210.3 | <0.001 |
| Other | 3112 | 483.9 | 211.4 | 0.861 |
| Education | <0.001 | |||
| Less than 9th Grade | 3107 | 489.8 | 236.0 | - |
| 9th to 11th Grade | 4094 | 495.5 | 242.6 | 0.921 |
| High School Graduate/GED | 6309 | 514.4 | 255.4 | <0.001 |
| Some College or AA | 8074 | 519.6 | 246.0 | <0.001 |
| College Graduate or Above | 6254 | 539.0 | 229.1 | <0.001 |
| Missing | 100 | 473.0 | 301.1 | 0.984 |
| Smoking Status | <0.001 | |||
| Has not Smoked in Last 30 Days | 22,200 | 533.6 | 250.6 | - |
| Smoked in Last 30 Days | 5729 | 446.7 | 200.6 | 0.540 |
| Missing | 9 | 448.5 | 134.0 | 0.999 |
| FPR | <0.001 | |||
| Lowest Quartile | 6335 | 475.5 | 223.7 | - |
| 2nd Quartile | 6349 | 516.3 | 251.2 | <0.001 |
| 3rd Quartile | 6365 | 528.9 | 242.9 | <0.001 |
| Highest Quartile | 6401 | 547.5 | 244.1 | <0.001 |
| Missing | 2488 | 501.3 | 258.4 | <0.001 |
| Urinary Creatinine | <0.001 | |||
| Lowest Quartile | 6376 | 548.9 | 258.1 | - |
| 2nd Quartile | 6372 | 531.8 | 253.3 | <0.001 |
| 3rd Quartile | 6473 | 500.0 | 225.0 | <0.001 |
| Highest Quartile | 6469 | 471.4 | 210.1 | <0.001 |
| Missing | 2248 | 549.4 | 288.3 | 0.999 |
| Cycle | 0.251 | |||
| 2007–2008 | 5600 | 538.0 | 265.0 | - |
| 2009–2010 | 6019 | 496.2 | 232.8 | <0.001 |
| 2011–2012 | 5269 | 486.0 | 224.5 | <0.001 |
| 2013–2014 | 5636 | 533.7 | 247.4 | 0.88 |
| 2015–2016 | 5414 | 524.7 | 242.1 | 0.03 |
| HEI | <0.001 | |||
| Lowest Quartile | 6534 | 474.7 | 229.2 | - |
| 2nd Quartile | 6533 | 498.7 | 237.6 | <0.001 |
| 3rd Quartile | 6534 | 528.8 | 251.1 | <0.001 |
| Highest Quartile | 6534 | 565.4 | 249.5 | <0.001 |
| Missing | 1803 | 499.0 | 232.5 | <0.001 |
| Exposure | N | % < LOD | GM | GSD | IQR |
|---|---|---|---|---|---|
| Phthalates (ng/mL) | |||||
| Mono(carboxyisoctyl) phthalate (MCOP) | 9012 | 1.22% | 11.34 | 4.17 | 23.98 |
| Mono-2-ethyl-5-carboxypentyl phthalate (MECPP) | 9012 | 0.23% | 15.19 | 3.25 | 22.90 |
| Mono-n-butyl phthalate (MBP) | 9012 | 2.24% | 11.83 | 3.44 | 19.94 |
| Mono-(3-carboxypropyl) phthalate (MCPP) | 9012 | 9.55% | 2.10 | 3.67 | 3.70 |
| Mono-ethyl phthalate (MEP) (ng/mL) | 9012 | 0.13% | 61.78 | 4.94 | 153.48 |
| Mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | 9012 | 0.67% | 9.68 | 3.51 | 15.90 |
| Mono-(2-ethyl)-hexyl phthalate (MEHP) | 9012 | 33.60% | 1.61 | 3.10 | 2.63 |
| Mono-isobutyl phthalate (MiBP) | 9012 | 2.00% | 7.49 | 3.12 | 12.00 |
| Mono-isononyl phthalate (MNP) | 9012 | 67.63% | 1.05 | 2.74 | 0.61 |
| Mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) | 9012 | 0.95% | 5.96 | 3.40 | 9.45 |
| Mono-benzyl phthalate (MBzP) | 9012 | 2.66% | 4.88 | 3.72 | 9.80 |
| Metals (µg/L) | |||||
| Cadmium (µg/L) | 22,401 | 15.71% | 0.36 | 2.24 | 0.38 |
| Lead | 22,401 | 0.29% | 12.0 | 20.1 | 11.4 |
| Manganese | 10,787 | 0.00% | 9.38 | 1.43 | 4.31 |
| Mercury | 22,401 | 9.85% | 0.90 | 2.69 | 1.22 |
| Selenium | 10,787 | 0.00% | 192.83 | 1.14 | 29.97 |
| Total arsenic | 9170 | 0.87% | 8.35 | 3.17 | 12.40 |
| PFAS (ng/mL) | |||||
| Perfluorodecanoic acid (PFDeA) | 8963 | 16.82% | 0.23 | 2.25 | 0.26 |
| Perfluorohexane sulfonic acid (PFHxS) | 8963 | 1.91% | 1.39 | 2.61 | 1.79 |
| 2-(N-methylperfluoroctanesulfonamido)acetic acid (Me-PFOSA-AcOH) | 8963 | 53.33% | 0.16 | 2.52 | 0.23 |
| Perfluorononanoic acid (PFNA) | 8963 | 1.53% | 0.91 | 2.11 | 0.88 |
| Perfluoroundecanoic acid (PFUA) | 8963 | 59.88% | 0.15 | 2.27 | 0.13 |
| Perfluorooctanoic acid (PFOA) | 8961 | 0.22% | 2.34 | 2.14 | 2.40 |
| Perfluorooctane sulfonic acid (PFOS) | 8961 | 0.39% | 7.49 | 2.61 | 9.59 |
| Phenols and parabens (ng/mL) | |||||
| Bisphenol A (BPA) | 9013 | 0.65% | 1.53 | 3.03 | 2.40 |
| Triclosan | 9013 | 7.51% | 10.93 | 7.05 | 37.47 |
| Butyl paraben | 9013 | 0.18% | 0.25 | 5.14 | 0.33 |
| Methyl paraben | 9013 | 2.49% | 59.09 | 6.03 | 213.80 |
| Propyl paraben (ng/mL) | 9013 | 0.18% | 7.33 | 9.78 | 43.70 |
| PAHs (ng/L) | |||||
| One hydroxynaphthalene | 7194 | 0.06% | 2235.54 | 4.68 | 5480.00 |
| Two hydroxynaphthalene | 7221 | 0.00% | 4474.30 | 3.20 | 8318.10 |
| Three hydroxyfluorene | 7256 | 1.86% | 104.31 | 3.97 | 208.15 |
| Two hydroxyfluorene | 7267 | 0.00% | 261.61 | 3.43 | 470.50 |
| Three hydroxyphenanthrene | 5439 | 1.84% | 79.04 | 2.80 | 118.80 |
| One hydroxyphenanthrene | 7280 | 0.38% | 123.95 | 2.56 | 164.73 |
| Two hydroxyphenanthrene | 5417 | 1.14% | 68.29 | 2.55 | 90.00 |
| One hydroxypyrene | 7253 | 7.33% | 118.89 | 2.84 | 176.30 |
| Nine hydroxyfluorene | 5448 | 0.00% | 304.70 | 3.00 | 490.23 |
| Cotinine (ng/mL) | 27,646 | 27.41% | 0.31 | 47.50 | 8.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascari, M.; Reeves, K.; Balasubramanian, R.; Liu, Z.; Laouali, N.; Oulhote, Y. Associations of Environmental Pollutant Mixtures and Red Blood Cell Folate Concentrations: A Mixture Analysis of the U.S. Adult Population Based on NHANES Data, 2007–2016. Toxics 2025, 13, 200. https://doi.org/10.3390/toxics13030200
Mascari M, Reeves K, Balasubramanian R, Liu Z, Laouali N, Oulhote Y. Associations of Environmental Pollutant Mixtures and Red Blood Cell Folate Concentrations: A Mixture Analysis of the U.S. Adult Population Based on NHANES Data, 2007–2016. Toxics. 2025; 13(3):200. https://doi.org/10.3390/toxics13030200
Chicago/Turabian StyleMascari, Michael, Katherine Reeves, Raji Balasubramanian, Zhenhua Liu, Nasser Laouali, and Youssef Oulhote. 2025. "Associations of Environmental Pollutant Mixtures and Red Blood Cell Folate Concentrations: A Mixture Analysis of the U.S. Adult Population Based on NHANES Data, 2007–2016" Toxics 13, no. 3: 200. https://doi.org/10.3390/toxics13030200
APA StyleMascari, M., Reeves, K., Balasubramanian, R., Liu, Z., Laouali, N., & Oulhote, Y. (2025). Associations of Environmental Pollutant Mixtures and Red Blood Cell Folate Concentrations: A Mixture Analysis of the U.S. Adult Population Based on NHANES Data, 2007–2016. Toxics, 13(3), 200. https://doi.org/10.3390/toxics13030200








