Comparative Assessment of Short- and Long-Term Effects of Triadimenol Fungicide on Danio rerio Erythrocytes Using the Micronucleus and Erythrocyte Nuclear Abnormality Assays
Abstract
1. Introduction
2. Materials and Methods
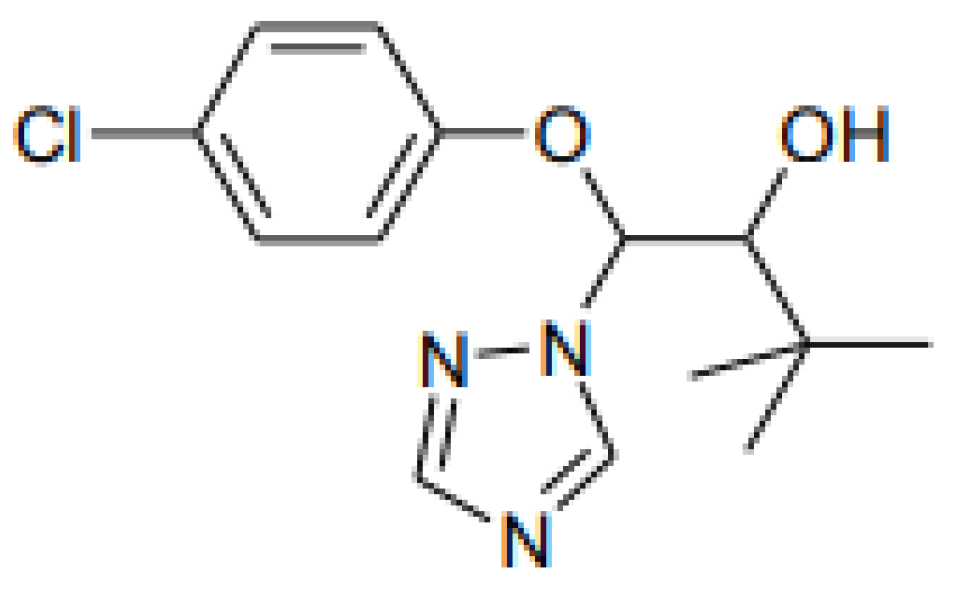
2.1. Test Chemicals
2.2. Concentrations and Treatment Times of Triadimenol Fungicide
2.3. Fish Acclimation and Conditioning Process
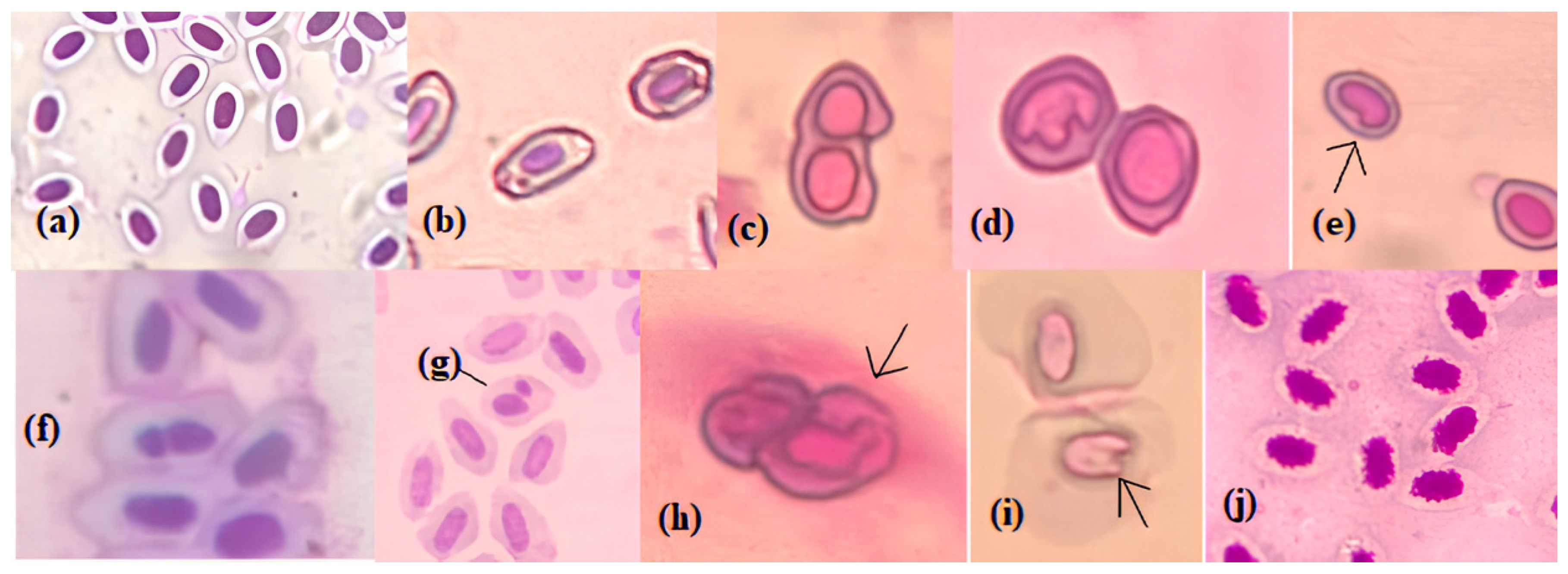
2.4. Micronucleus (MN) Assay
2.5. Erythrocyte Nuclear Abnormality (ENA) Assay
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT, 2020. Pesticides Use. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 18 February 2025).
- Marciano, L.P.A.; Costa, L.F.; Cardoso, N.S.; Freire, J.; Feltrim, F.; Oliveira, G.S.; Paula, F.B.A.; Silvério, A.C.P.; Martins, I. Biomonitoring and risk assessment of human exposure to triazole fungicides. Regul. Toxicol. Pharmacol. 2024, 147, 105565. [Google Scholar] [CrossRef] [PubMed]
- How, C.M.; Li, S.W.; Liao, V.H. Chronic exposure to triadimenol at environmentally relevant concentration adversely affects aging biomarkers in Caenorhabditis elegans associated with insulin/IGF-1 signaling pathway. Sci. Total Environ. 2018, 640–641, 485–492. [Google Scholar] [CrossRef]
- Ascoli-Morrete, T.; Bandeira, N.M.G.; Signor, E.; Gazola, H.A.; Homrich, I.S.; Biondo, R.; Rossato-Grando, L.G.; Zanella, N. Bioaccumulation of pesticides and genotoxicity in anurans from southern Brazil. Environ. Sci. Pollut. Res. Int. 2022, 29, 45549–45559. [Google Scholar] [CrossRef]
- Unal, F.; Demir, H.; Yuzbasioglu, D. Genotoxic effects of environmental contaminant methidathion and triadimenol pesticides. In Proceedings of the 3rd International Symposium on EuroAsian Biodiversity, Minsk, Belarus, 5–8 July 2017. [Google Scholar]
- Huang, T.; Jiang, H.; Zhao, Y.; He, J.; Cheng, H.; Martyniuk, C.J. A comprehensive review of 1,2,4-triazole fungicide toxicity in zebrafish (Danio rerio): A mitochondrial and metabolic perspective. Sci. Total Environ. 2022, 809, 151177. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, X.; Dong, S.; Zhao, X.; Li, X.; Zhang, M.; Shi, Y.; Ding, G. Developmental toxicity and cardiotoxicity induced by PFOS and its novel alternative OBS in early life stage of zebrafsh (Danio rerio). Water Air Soil Pollut. 2023, 234, 481. [Google Scholar] [CrossRef]
- Tagkalidou, N.; Multisanti, C.R.; Bleda, M.J.; Bedrossiantz, J.; Prats, E.; Faggio, C.; Barata, C.; Raldúa, D. Analyzing the effects of age, time of day, and experiment on the basal locomotor activity and light-off visual motor response assays in zebrafish larvae. Toxics 2024, 12, 349. [Google Scholar] [CrossRef]
- Rasgele, P.G.; Demir, F.; Kirankaya, S.G. Determination of micronuclei frequency in Danio rerio for assessing genotoxicity induced by propineb. Drug Chem. Toxicol. 2024, 47, 848–853. [Google Scholar] [CrossRef]
- Canedo, A.; Rocha, T.L. Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: Historical review, current status and trends. Sci. Total Environ. 2021, 762, 144084. [Google Scholar] [CrossRef]
- Gusenleitner, D.; Auerbach, S.S.; Melia, T.; Gómez, H.F.; Sherr, D.H.; Monti, S. Genomic models of short-term exposure accurately predict long-term chemical carcinogenicity and identify putative mechanisms of action. PLoS ONE 2014, 9, e102579. [Google Scholar] [CrossRef]
- Rasgele, P.G.; Ozer, H.; Kirankaya, S.G. In Vivo Genotoxicity testing of bentazone herbicide in Danio rerio erythrocytes using the micronucleus and nuclear abnormality assays. Water Air Soil Pollut. 2024, 235, 29. [Google Scholar] [CrossRef]
- Parvez, S.; Raisuddin, S. Protein carbonyls: Novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish. Environ. Toxicol. Pharmacol. 2005, 20, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jin, X.; Liu, N.; Luo, Y.; Yan, Z.; Chen, M.; Liu, Y.; Xie, H.; Giesy, J.P.; Wu, F.; et al. Triadimefon in aquatic environments: Occurrence, fate, toxicity, and ecological risk. Environ. Sci. Eur. 2022, 34, 12. [Google Scholar] [CrossRef]
- Dikilitas, M.; Kocyigit, A.; Bilinc, H.; Taskin, A.; Karakas, S. Evaluation of genotoxicity of environmentally friendly pesticides on higher cells using the single cell gel electrophoresis (SCGE) assay. Fresenius Environ. Bul. 2015, 24, 39–47. [Google Scholar]
- FAO. FAO Specifications and Evaluations for Agricultural Pesticides Triadimenol. 2019. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/79fa4a25-d326-4bd9-a876-4a497fb3e00e/content (accessed on 18 February 2025).
- Qiao, K.; Fu, W.; Jiang, Y.; Chen, L.; Li, S.; Ye, Q.; Gui, W. QSAR models for the acute toxicity of 1,2,4-triazole fungicides to zebrafish (Danio rerio) embryos. Environ. Pollut. 2020, 265 Pt B, 114837. [Google Scholar] [CrossRef]
- Dogan, I.; Dogan, N. Estimation of sample size with resource equation method in experimental animal studies. Türkiye Klinikleri J. Biostat. 2020, 12, 211–217. [Google Scholar] [CrossRef]
- Arifin, W.N.; Zahiruddin, W.M. Sample size calculation in animal studies using resource equation approach. Malays. J. Med. Sci. 2017, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Grisolia, C.K.; Cordeiro, C.M.T. Variability in micronucleus induction with different mutagens applied to several species of fish. Genet. Mol. Biol. 2000, 23, 235–239. [Google Scholar] [CrossRef]
- Zang, L.; Shimada, Y.; Nishimura, Y.; Tanaka, T.; Nishimura, N. Repeated blood collection for blood tests in adult zebrafish. J. Vis. Exp. 2015, 102, e53272. [Google Scholar] [CrossRef]
- Al-Sabti, K.; Metcalfe, C.D. Fish micronuclei for assessing genotoxicity in water. Mutat. Res. 1995, 343, 121–135. [Google Scholar] [CrossRef]
- Tsarpali, V.; Kassara, C.; Barboutis, C. Assessing the seasonal and intrinsic variability of neurotoxic and cytogenotoxic biomarkers in blood of free-living Eleonoras’ falcons. Sci. Total Environ. 2020, 711, 135101. [Google Scholar] [CrossRef]
- Carrola, J.; Santos, N.; Rocha, M.J.; Fontainhas-Fernandes, A.; Pardal, M.A.; Monteiro, R.A.F.; Rocha, E. Frequency of micronuclei and of other nuclear abnormalities in erythrocytes of the grey mullet from the Mondego, Douro and Ave estuaries—Portugal. Environ. Sci. Pollut. Res. 2014, 21, 6057–6068. [Google Scholar] [CrossRef]
- EPA, United States Environmental Protection Agency Washington, D.C. 20460, Office of Chemical Safety and Pollution Prevention, Chemicals Evaluated for Carcinogenic Potential by the Office of Pesticide Programs. 2022. Available online: https://npic.orst.edu/chemicals_evaluated.pdf (accessed on 18 February 2025).
- Castro, T.F.D.; da Silva Souza, J.G.; de Carvalho, A.F.S.; de Lima Assis, I.; Palmieri, M.J.; Vieira, L.F.A.; Marcussi, S.; Machado, M.R.F.; Murgas, L.D.S. Anxiety-associated behavior and genotoxicity found in adult Danio rerio exposed to tebuconazole-based commercial product. Environ. Toxicol. Pharmacol. 2018, 62, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Martili, A. Cytogenetic Effects of Fungicide Triadimenol in Root Tip Cells of Allium cepa L. Master’s Thesis, Gazi University Institute of Science and Technology, Ankara, Türkiye, 2004. [Google Scholar]
- Sharma, R.; Jindal, R. In vivo genotoxic effects of commercial grade cypermethrin on fish peripheral erythrocytes. Environ. Mol. Mutagen. 2022, 63, 204–214. [Google Scholar] [CrossRef] [PubMed]
- EFSA, European Food Safety Authority. Conclusion on the peer review of triadimenol. EFSA Sci. Rep. 2008, 177, 1–134. [Google Scholar] [CrossRef][Green Version]
- Knasmueller, S.; Fenech, M. The Micronucleus Assay in Toxicology; Royal Society of Chemistry: London, UK, 2019; 657p. [Google Scholar] [CrossRef]
- Singh, S.; Gautam, K.; Mir, S.S.; Anbumani, S. Genotoxicity and cytotoxicity assessment of ‘forever chemicals’ in zebrafish (Danio rerio). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 897, 503788. [Google Scholar] [CrossRef]
- Rivero-Wendt, C.L.G.; Miranda-Vilela, A.L.; Garcia-Fernandes, L.; Negreli-Santos, A.; Leal, I.; Jaques, J.; Fernandes, C.E. Cytogenotoxic potential and toxicity in adult Danio rerio (zebrafish) exposed to chloramine T. PeerJ 2023, 11, e16452. [Google Scholar] [CrossRef]
- Schwarzbacherová, V.; Šiviková, K.; Drážovská, M.; Dianovský, J. Evaluation of DNA damage and cytotoxicity induced by triazole fungicide in cultured bovine lymphocytes. Caryologia Inter. J. Cyt. Cytosystem. Cytogenet. 2015, 68, 233–238. [Google Scholar] [CrossRef]
- Walia, G.K.; Handa, D.; Kaur, H. Erythrocyte abnormalities in a freshwater fish, Labeo rohita exposed to tannery industry effluent. Int. J. Pharm. Bio. Sci. 2013, 3, 287–295. [Google Scholar]

| Group No. | Short-Term Treatment | Concentrations | Group No. | Long-Term Treatment | Concentrations |
|---|---|---|---|---|---|
| 1 | 24 h | Negative control | 17 | 10 days | Negative control |
| 2 | 1.5 mg/L | 18 | 1.5 mg/L | ||
| 3 | 3 mg/L | 19 | 3 mg/L | ||
| 4 | 6 mg/L | 20 | 6 mg/L | ||
| 5 | 48 h | Negative control | 21 | 20 days | Negative control |
| 6 | 1.5 mg/L | 22 | 1.5 mg/L | ||
| 7 | 3 mg/L | 23 | 3 mg/L | ||
| 8 | 6 mg/L | 24 | 6 mg/L | ||
| 9 | 72 h | Negative control | 25 | 30 days | Negative control |
| 10 | 1.5 mg/L | 26 | 1.5 mg/L | ||
| 11 | 3 mg/L | 27 | 3 mg/L | ||
| 12 | 6 mg/L | 28 | 6 mg/L | ||
| 13 | 96 h | Negative control | |||
| 14 | 1.5 mg/L | ||||
| 15 | 3 mg/L | ||||
| 16 | 6 mg/L |
| Micronucleated Erythrocyte | Erythrocyte Nuclear Morphological Abnormalities | TAC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | Conc. | MN | PMN | BNC | MNC | PMCN | SN | KS | BN | LN | NN | Ech | VN | NND | |
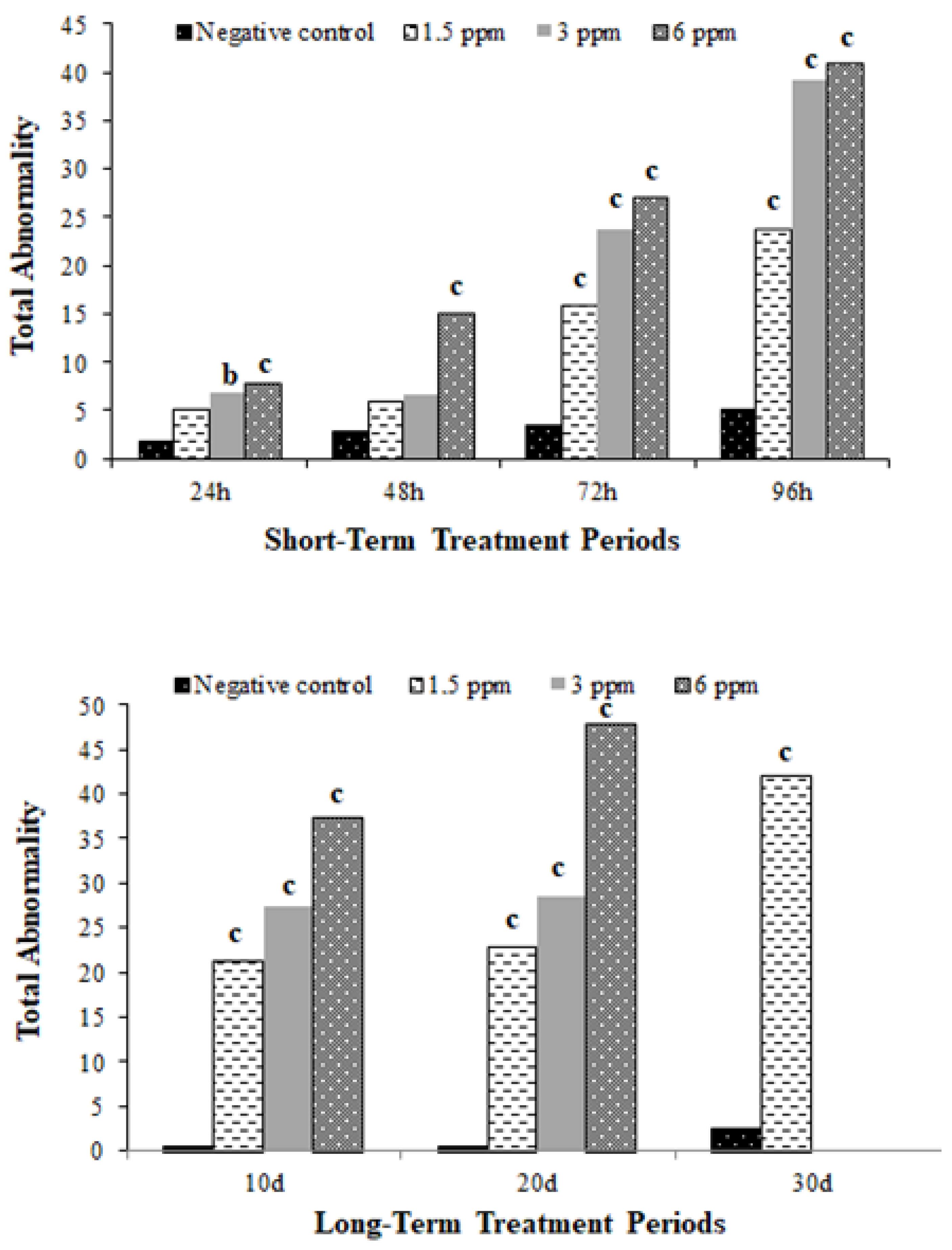
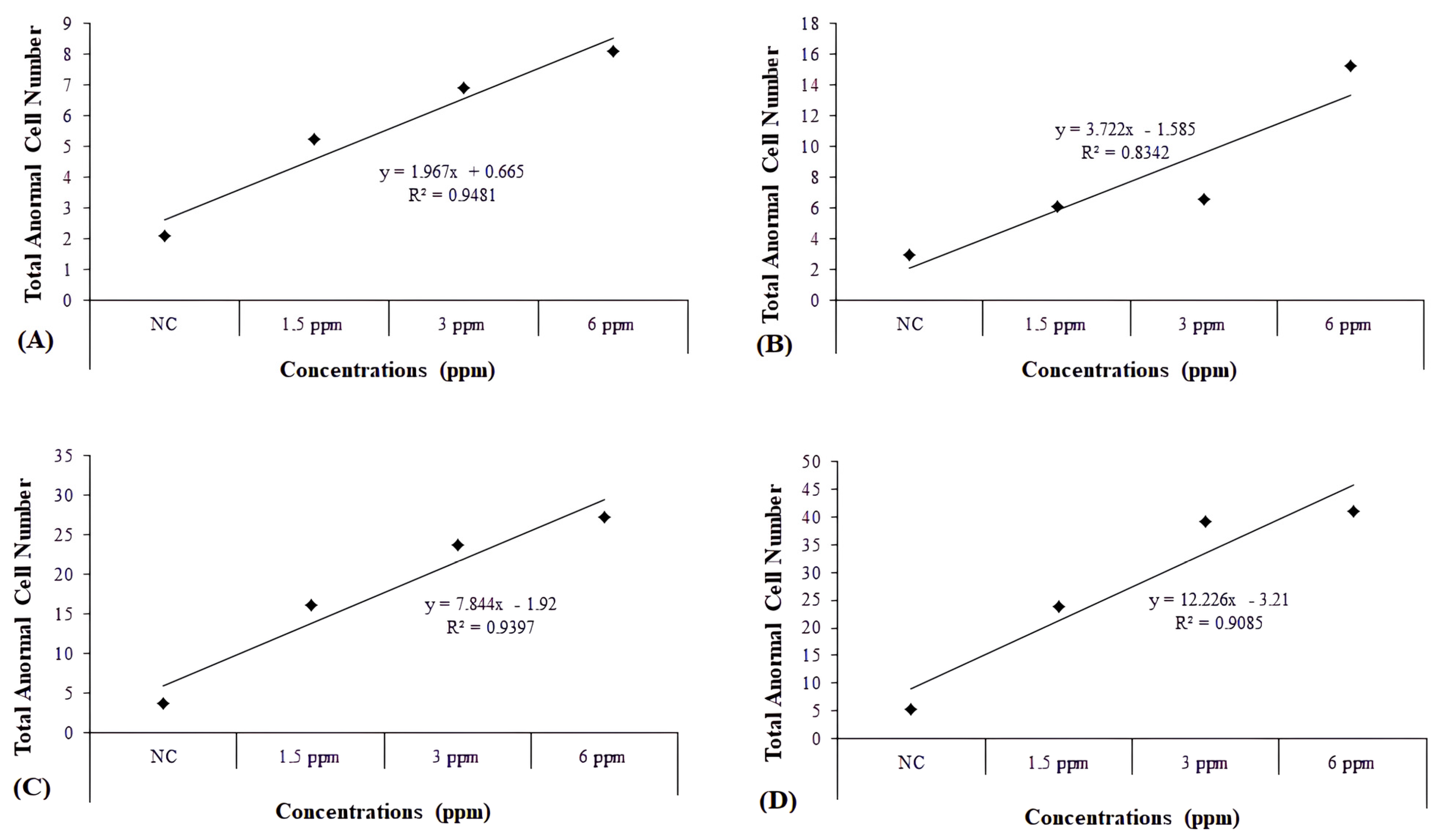
| 24 h | NC | 1.67 ± 0.33 | 0.00 ± 0.00 | 0.42 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.08 ± 0.38 |
| 1.5 mg/L | 4.00 ± 0.73 a | 0.00 ± 0.00 | 0.75 ± 0.39 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.25 ± 0.18 | 0.08 ± 0.08 | 0.08 ± 0.08 | 0.08 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.25 ± 0.79 | |
| 3 mg/L | 4.08 ± 0.68 a | 0.00 ± 0.00 | 1.42 ± 0.47 | 0.08 ± 0.08 | 0.00 ± 0.00 | 0.50 ± 0.34 | 0.00 ± 0.00 | 0.67 ± 0.36 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.17 ± 0.17 | 0.00 ± 0.00 | 6.92 ± 0.95 b | |
| 6 mg/L | 3.92 ± 0.70 a | 0.00 ± 0.00 | 0.42 ± 0.19 | 0.08 ± 0.08 | 0.00 ± 0.00 | 2.55 ± 1.11 b | 0.83 ± 0.41 a | 0.00 ± 0.00 | 0.50 ± 0.29 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.08 ± 1.77 c | |
| 48 h | NC | 1.00 ± 0.25 | 0.00 ± 0.00 | 0.67 ± 0.19 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.33 ± 0.22 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.00 ± 0.44 |
| 1.5 mg/L | 2.08 ± 0.36 | 0.00 ± 0.00 | 0.83 ± 0.41 | 0.08 ± 0.08 | 0.00 ± 0.00 | 2.83 ± 0.58 | 0.25 ± 0.18 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.08 ± 0.93 | |
| 3 mg/L | 2.91 ± 0.56 | 0.00 ± 0.00 | 0.55 ± 0.28 | 0.00 ± 0.00 | 0.09 ± 0.09 | 2.18 ± 0.50 | 0.45 ± 0.25 | 0.18 ± 0.12 | 0.18 ± 0.12 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.55 ± 0.65 | |
| 6 mg/L | 7.83 ± 0.94 c | 0.00 ± 0.00 | 2.00 ± 0.89 | 0.08 ± 0.08 | 0.00 ± 0.00 | 4.92 ± 1.43 b | 0.42 ± 0.19 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 15.25 ± 2.96 c | |
| 72 h | NC | 0.58 ± 0.19 | 0.00 ± 0.00 | 0.50 ± 0.23 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.17 ± 0.44 | 0.08 ± 0.08 | 0.00 ± 0.00 | 0.42 ± 0.19 | 0.08 ± 0.08 | 0.58 ± 0.23 | 0.00 ± 0.00 | 0.25 ± 0.13 | 3.67 ± 0.47 |
| 1.5 mg/L | 3.91 ± 0.71 c | 0.00 ± 0.00 | 0.36 ± 0.24 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.45 ± 0.87 | 0.00 ± 0.00 | 0.36 ± 0.28 | 0.36 ± 0.24 | 0.00 ± 0.00 | 5.91 ± 2.18 | 0.00 ± 0.00 | 2.73 ± 1.32 | 16.09 ± 2.68 c | |
| 3 mg/L | 3.64 ± 0.70 b | 0.00 ± 0.00 | 0.64 ± 0.39 | 0.00 ± 0.00 | 0.91 ± 0.91 | 0.55 ± 0.45 | 0.00 ± 0.00 | 1.09 ± 0.58 | 1.64 ± 0.74 | 0.00 ± 0.00 | 12.45 ± 2.47 c | 0.00 ± 0.00 | 2.82 ± 2.08 | 23.73 ± 2.39 c | |
| 6 mg/L | 2.55 ± 0.73 | 0.00 ± 0.00 | 0.91 ± 0.58 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.64 ± 1.17 | 0.64 ± 0.43 | 0.00 ± 0.00 | 0.55 ± 0.39 | 0.09 ± 0.09 | 16.09 ± 2.26 c | 0.09 ± 0.09 | 2.73 ± 0.99 | 27.27 ± 2.90 c | |
| 96 h | NC | 0.67 ± 0.19 | 0.17 ± 0.11 | 0.50 ± 0.15 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.00 ± 0.25 | 1.00 ± 0.17 | 0.42 ± 0.19 | 0.67 ± 0.14 | 0.17 ± 0.11 | 0.50 ± 0.23 | 0.00 ± 0.00 | 0.25 ± 0.13 | 5.33 ± 0.48 |
| 1.5 mg/L | 1.00 ± 0.33 | 0.08 ± 0.08 | 0.42 ± 0.29 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.92 ± 0.29 | 0.91 ± 0.37 | 2.75 ± 0.77 c | 1.17 ± 0.42 | 0.00 ± 0.00 | 7.58 ± 1.45 c | 6.92 ± 1.76 c | 2.25 ± 1.39 | 23.92 ± 2.44 c | |
| 3 mg/L | 4.33 ± 0.99 a | 0.00 ± 0.00 | 1.17 ± 0.42 | 0.17 ± 0.11 | 0.42 ± 0.29 | 1.33 ± 0.48 | 2.67 ± 0.73 | 0.08 ± 0.08 | 0.83 ± 0.41 | 0.00 ± 0.00 | 27.08 ± 1.76 c | 0.58 ± 0.40 | 0.50 ± 0.50 | 39.17 ± 2.10 c | |
| 6 mg/L | 2.55 ± 0.47 | 0.00 ± 0.00 | 2.09 ± 0.58 a | 0.00 ± 0.00 | 0.09 ± 0.09 | 0.91 ± 0.37 | 3.09 ± 0.92 | 0.18 ± 0.18 | 0.55 ± 0.31 | 0.00 ± 0.00 | 30.91 ± 4.87 c | 0.64 ± 0.43 | 0.00 ± 0.00 | 41.00 ± 4.80 c | |
| Micronucleated Erythrocyte | Erythrocyte Nuclear Morphological Abnormalities | TAC | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | Conc. | MN | PMN | BNC | MNC | PMCN | SN | KS | BN | LN | NN | Ech | VN | NND | |
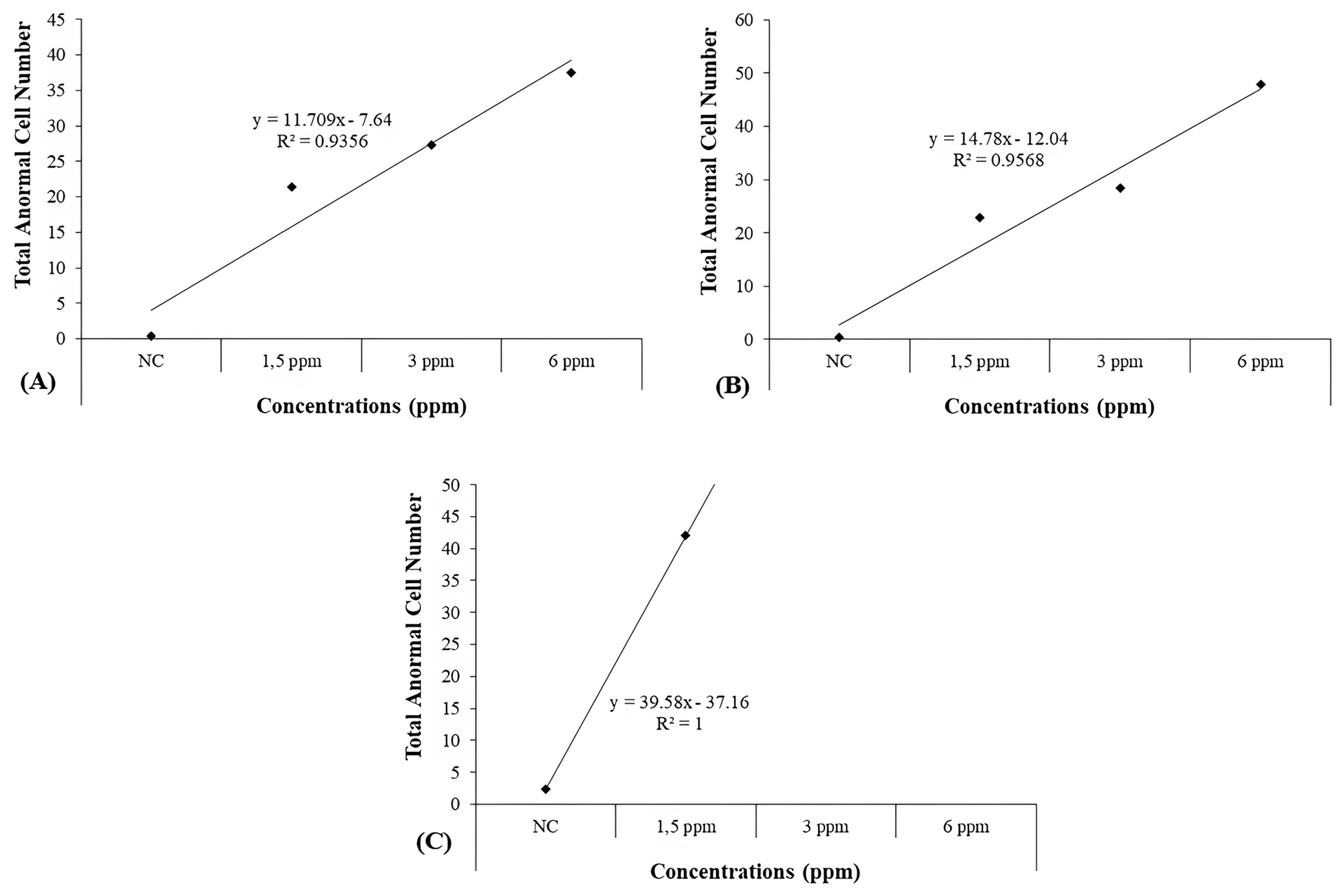
| 10 d | NC | 0.42 ± 0.29 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.42 ± 0.29 |
| 1.5mg/L | 6.00 ± 0.41 c | 1.25 ± 0.58 | 0.58 ± 0.23 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.33 ± 0.26 | 0.50 ± 0.15 | 0.25 ± 0.13 | 0.33 ± 0.14 a | 0.08 ± 0.08 | 2.92 ± 1.14 | 3.00 ± 1.44 | 5.08 ± 1.70 | 21.33 ± 2.54 c | |
| 3 mg/L | 5.67 ± 0.80 c | 0.92 ± 0.34 | 1.00 ± 0.41 | 0.17 ± 0.11 | 0.00 ± 0.00 | 1.92 ± 0.67 a | 0.67 ± 0.28 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.17 ± 0.11 | 8.50 ± 1.21 c | 3.83 ± 0.87 | 4.50 ± 1.80 | 27.33 ± 2.99 c | |
| 6 mg/L | 8.73 ± 0.85 c | 0.91 ± 0.41 | 1.00 ± 0.45 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.18 ± 0.71 b | 0.73 ± 0.24 a | 0.36 ± 0.20 | 0.09 ± 0.09 | 0.00 ± 0.00 | 9.55 ± 1.83 c | 7.00 ± 2.11 a | 6.91 ± 1.84 b | 37.45 ± 3.43 c | |
| 20 d | NC | 0.33 ± 0.26 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.42 ± 0.26 |
| 1.5 mg/L | 5.00 ± 0.71 c | 1.56 ± 0.38 c | 1.30 ± 0.37 c | 0.00 ± 0.00 | 0.60 ± 0.40 | 2.20 ± 0.36 c | 1.10 ± 0.28 b | 1.60 ± 0.96 | 0.50 ± 0.27 | 0.20 ± 0.13 | 1.70 ± 1.70 | 6.80 ± 3.33 | 0.50 ± 0.50 | 22.90 ± 4.03 c | |
| 3 mg/L | 2.50 ± 0.56 a | 0.67 ± 0.28 | 0.25 ± 0.18 | 0.00 ± 0.00 | 0.08 ± 0.08 | 1.25 ± 0.49 a | 0.67 ± 0.22 | 1.50 ± 0.50 | 0.83 ± 0.34 | 0.42 ± 0.34 | 6.92 ± 2.21 a | 1.08 ± 0.56 | 12.33 ± 2.89 b | 28.50 ± 2.77 c | |
| 6 mg/L | 2.00 ± 0.57 | 0.36 ± 0.15 | 0.27 ± 0.19 | 0.00 ± 0.00 | 3.91 ± 1.18 c | 2.09 ± 0.41 c | 0.73 ± 0.30 | 1.27 ± 0.66 | 0.82 ± 0.33 | 0.09 ± 0.09 | 6.27 ± 2.45 | 5.64 ± 2.57 | 24.36 ± 4.29 c | 47.82 ± 3.87 c | |
| 30 d | NC | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.42 ± 0.15 | 0.00 ± 0.00 | 0.17 ± 0.11 | 0.33 ± 0.14 | 0.50 ± 0.15 | 0.25 ± 0.13 | 0.33 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.17 ± 0.11 | 0.25 ± 0.13 | 2.42 ± 0.34 |
| 1.5 mg/L | 1.33 ± 0.49 | 0.00 ± 0.00 | 0.17 ± 0.17 | 0.00 ± 0.00 | 0.67 ± 0.67 | 2.67 ± 1.02 | 0.00 ± 0.00 | 0.33 ± 0.14 | 0.00 ± 0.00 | 0.00 ± 0.00 | 21.33 ± 1.89 c | 4.33 ± 1.48 | 11.17 ± 2.59 c | 42.00 ± 3.46 c | |
| 3 mg/L | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| 6 mg/L | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasgele, P.G. Comparative Assessment of Short- and Long-Term Effects of Triadimenol Fungicide on Danio rerio Erythrocytes Using the Micronucleus and Erythrocyte Nuclear Abnormality Assays. Toxics 2025, 13, 199. https://doi.org/10.3390/toxics13030199
Rasgele PG. Comparative Assessment of Short- and Long-Term Effects of Triadimenol Fungicide on Danio rerio Erythrocytes Using the Micronucleus and Erythrocyte Nuclear Abnormality Assays. Toxics. 2025; 13(3):199. https://doi.org/10.3390/toxics13030199
Chicago/Turabian StyleRasgele, Pinar Goc. 2025. "Comparative Assessment of Short- and Long-Term Effects of Triadimenol Fungicide on Danio rerio Erythrocytes Using the Micronucleus and Erythrocyte Nuclear Abnormality Assays" Toxics 13, no. 3: 199. https://doi.org/10.3390/toxics13030199
APA StyleRasgele, P. G. (2025). Comparative Assessment of Short- and Long-Term Effects of Triadimenol Fungicide on Danio rerio Erythrocytes Using the Micronucleus and Erythrocyte Nuclear Abnormality Assays. Toxics, 13(3), 199. https://doi.org/10.3390/toxics13030199






