The Neurobehavioral Impact of Zinc Chloride Exposure in Zebrafish: Evaluating Cognitive Deficits and Probiotic Modulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
2.2. Animal Maintenance
2.3. Chemical Compounds Administration
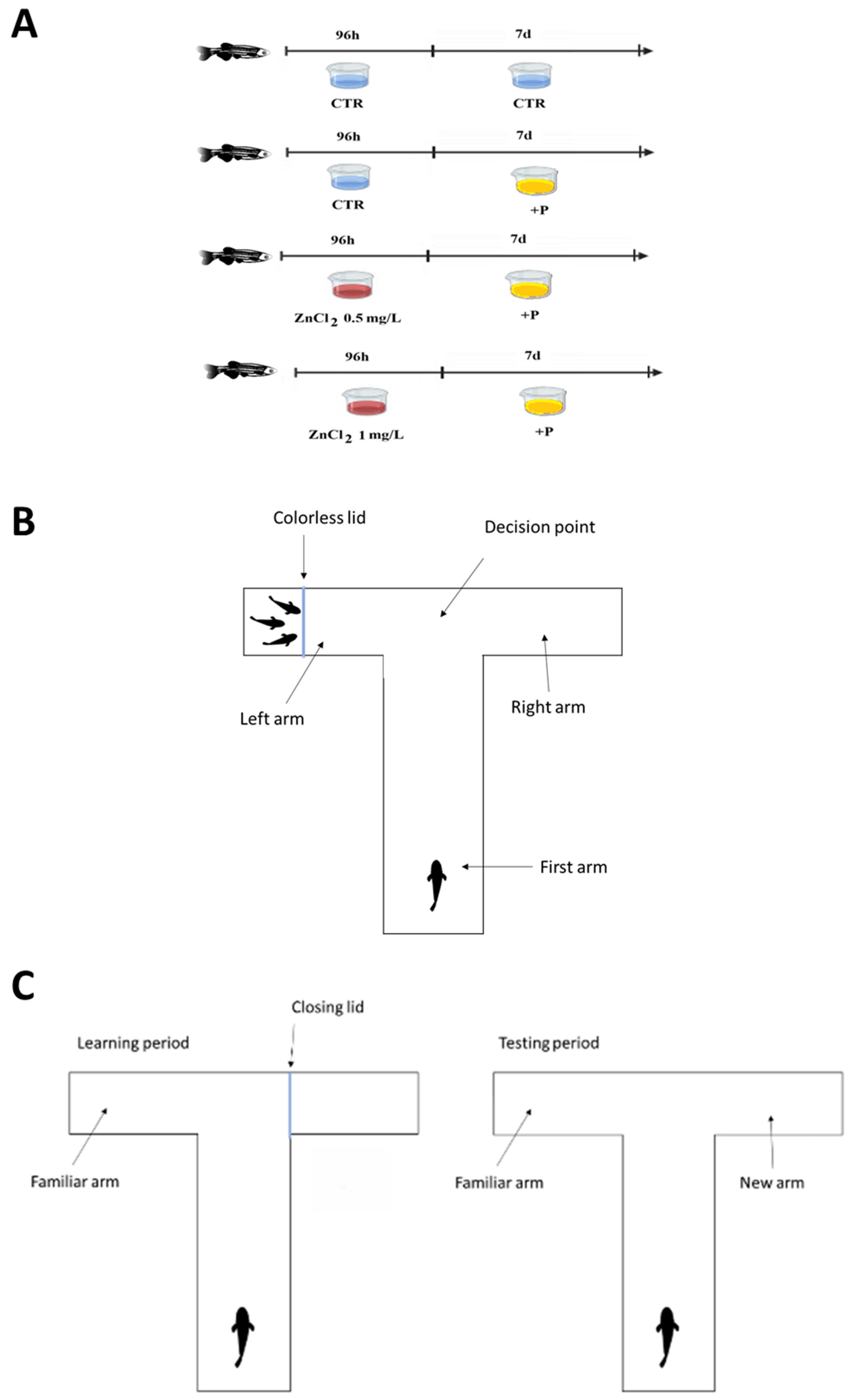
2.4. Experimental Design
2.4.1. Social Preference Test
2.4.2. Short-Term Memory Test
2.4.3. Long-Term Memory Test
2.4.4. Statistical Analysis
3. Results
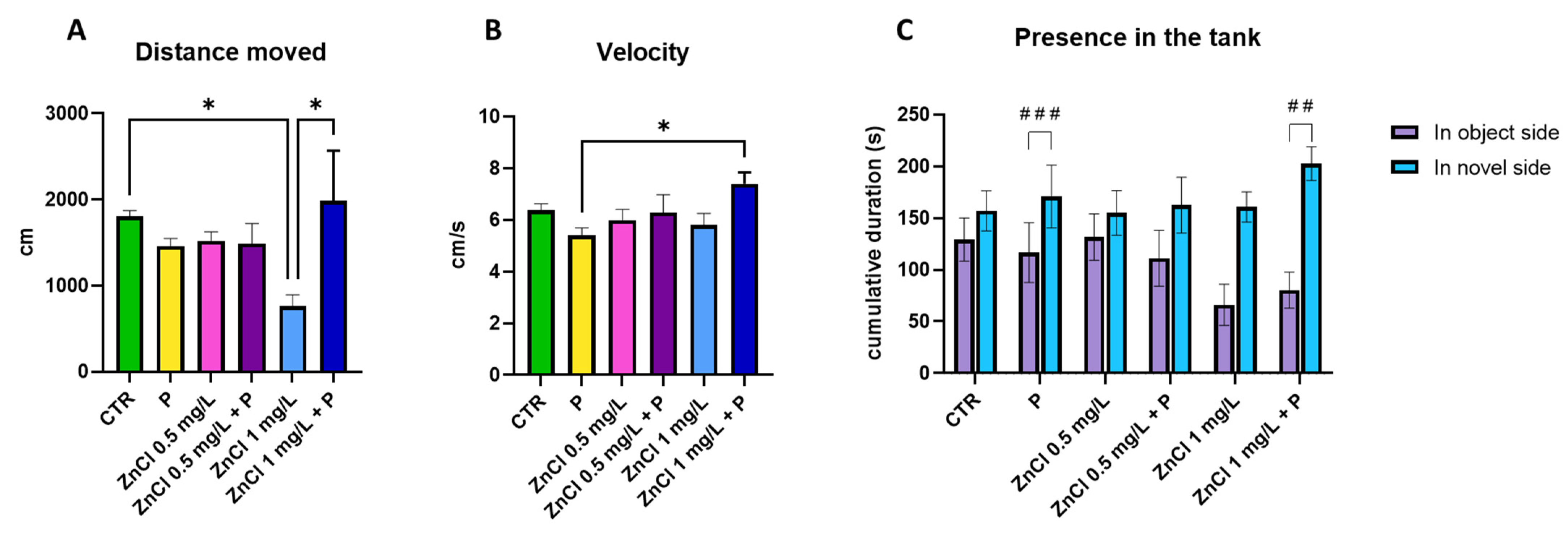
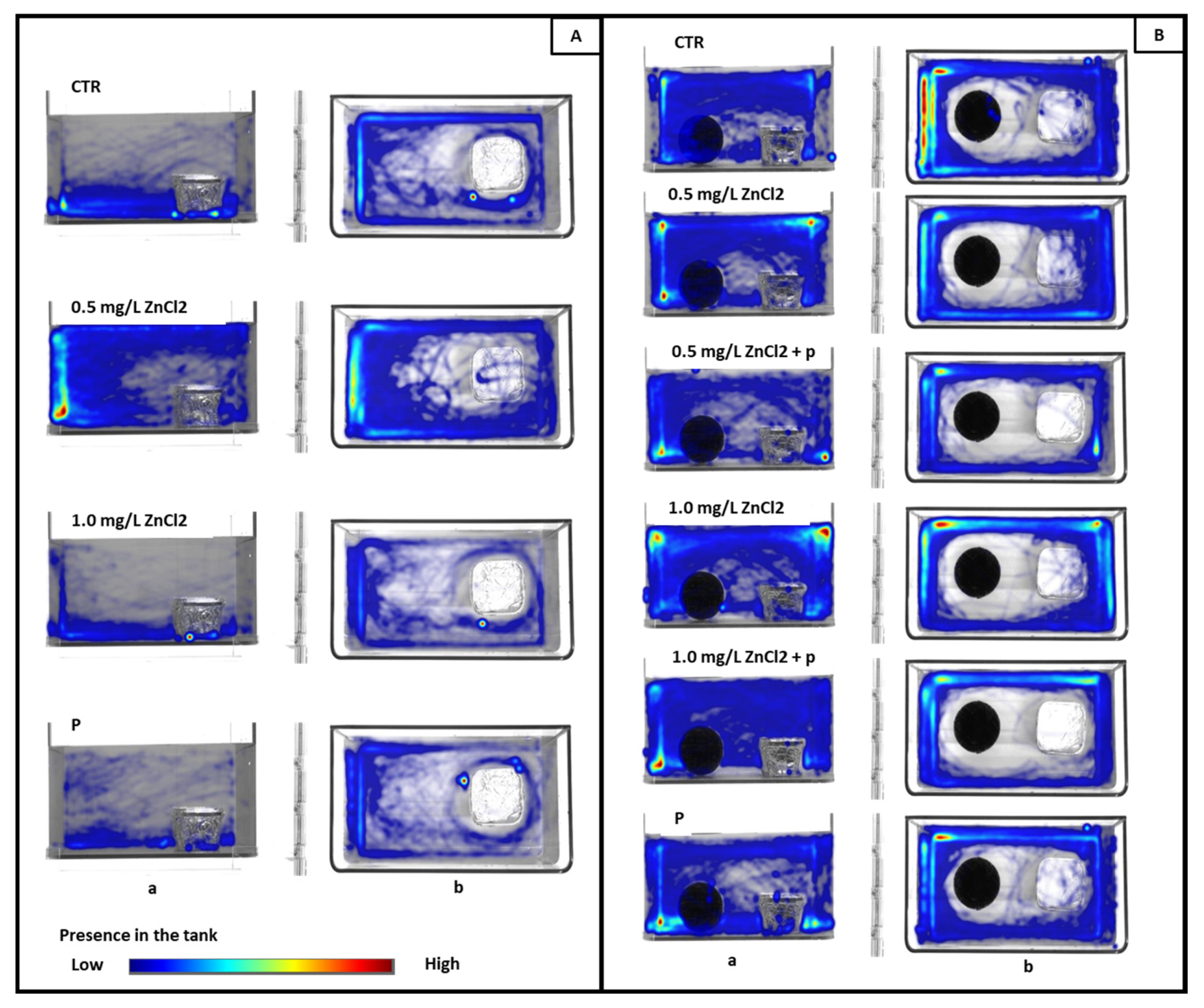
3.1. Social Preference Test
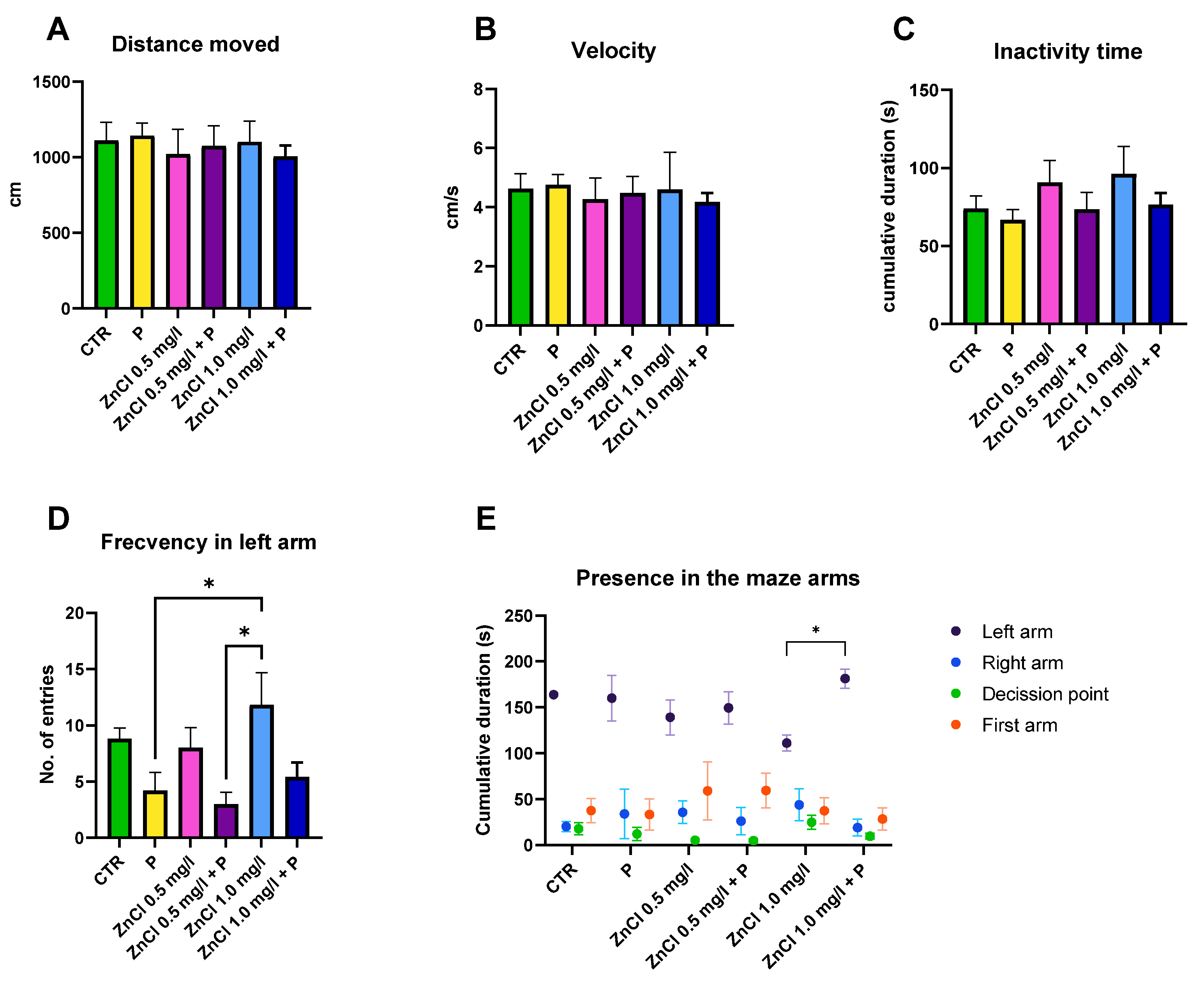
3.2. Short-Term Memory Test
3.3. Long-Term Memory Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.; Khan, K.; Younas, M.; Farooqi, A.; Cao, X.; Kavil, Y.N.; Alelyani, S.S.; Alkasbi, M.M.; Al-Sehemi, A.G. A review of heavy metals pollution in riverine sediment from various Asian and European countries: Distribution, sources, and environmental risk. Mar. Pollut. Bull. 2024, 206, 116775. [Google Scholar] [CrossRef] [PubMed]
- Iordache, A.M.; Nechita, C.; Voica, C.; Pluhacek, T.; Schug, K.A. Climate change extreme and seasonal toxic metal occurrence in Romanian freshwaters in the last two decades—Case study and critical review. NPJ Clean Water 2022, 5, 2. [Google Scholar] [CrossRef]
- Saravanan, P.; Saravanan, V.; Rajeshkannan, R.; Arnica, G.; Rajasimman, M.; Baskar, G.; Pugazhendhi, A. Comprehensive review on toxic heavy metals in the aquatic system: Sources, identification, treatment strategies, and health risk assessment. Environ. Res. 2024, 258, 119440. [Google Scholar] [CrossRef]
- DHAC: Australian Government Department of Health and Aged Care. Environmental Fate and Effects of Zinc Ions; Draft Evaluation Statement: EVA00143 [Internet]; Australian Government Department of Health and Aged Care: Canberra, Australia, 2024. Available online: https://www.industrialchemicals.gov.au/sites/default/files/2024-04/EVA00143%20-%20Draft%20Evaluation%20Statement%20-%2015%20April%202024.pdf (accessed on 7 January 2025).
- Pakulska, D.; Czerczak, S. Health hazards resulting from exposure to zinc and its inorganic compounds in industry. Med. Pr. 2017, 68, 779–794. [Google Scholar] [CrossRef]
- Seydi, E.; Soltani, M.; Ramazani, M.; Zarei, M.H.; Pourahmad, J. Occupational exposure in lead and zinc mines induces oxidative stress in miners’ lymphocytes: Role of mitochondrial/lysosomal damage. Main Group Met. Chem. 2020, 43, 154–163. [Google Scholar] [CrossRef]
- DCCEEW: Australian Government, Department of Climate Change, Energy, the Environment and Water. Zinc and Compounds. National Pollutant Inventory Fact Sheet. 2022. Available online: https://www.dcceew.gov.au/environment/protection/npi/substances/fact-sheets/zinc-and-compounds (accessed on 7 January 2025).
- Schoofs, H.; Schmit, J.; Rink, L. Zinc Toxicity: Understanding the Limits. Molecules 2024, 29, 3130. [Google Scholar] [CrossRef]
- El Idrissi, A.; van Berkel, L.; Bonekamp, N.E.; Dalemans, D.J.; van der Heyden, M.A. The toxicology of zinc chloride smoke producing bombs and screens. Clin. Toxicol. 2017, 55, 167–174. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Y.; Zhou, R.; Yang, Y.; Bu, Y. The effect of combined exposure of zinc and nickel on the development of zebrafish. J. Appl. Toxicol. 2021, 41, 1765–1778. [Google Scholar] [CrossRef]
- Sarasamma, S.; Audira, G.; Juniardi, S.; Sampurna, B.P.; Liang, S.T.; Hao, E.; Lai, Y.H.; Hsiao, C.D. Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish. Int. J. Mol. Sci. 2018, 19, 3195. [Google Scholar] [CrossRef]
- Horie, Y.; Yonekura, K.; Suzuki, A.; Takahashi, C. Zinc chloride influences embryonic development, growth, and Gh/Igf-1 gene expression during the early life stage in zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 230, 108684. [Google Scholar] [CrossRef] [PubMed]
- Zixi, Y.; Ruixuan, L.; Shuangshuang, L.; Denggao, Q.; Guanyi, L.; Chun, W.; Jiajia, N.; Yingxue, S.; Hongying, H. Oxidative stress, neurotoxicity, and intestinal microbial regulation after a chronic zinc exposure: An experimental study on adult zebrafish (Danio rerio). Water Reuse 2023, 13, 82–96. [Google Scholar] [CrossRef]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696–2704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riasatian, M.; Mazloomi, S.M.; Ahmadi, A.; Derakhshan, Z.; Rajabi, S. Benefits of fermented synbiotic soymilk containing Lactobacillus acidophilus, Bifidobacterium lactis, and inulin towards lead toxicity alleviation. Heliyon 2023, 9, e17518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daisley, B.A.; Monachese, M.; Trinder, M.; Bisanz, J.E.; Chmiel, J.A.; Burton, J.P.; Reid, G. Immobilization of cadmium and lead by Lactobacillus rhamnosus GR-1 mitigates apical-to-basolateral heavy metal translocation in a Caco-2 model of the intestinal epithelium. Gut Microbes 2019, 10, 321–333. [Google Scholar] [CrossRef] [PubMed Central]
- Cheng, J.; Laitila, A.; Ouwehand, A.C. Bifidobacterium animalis subsp. lactis HN019 Effects on Gut Health: A Review. Front. Nutr. 2021, 8, 790561. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, X.; Li, W.; Ding, M.; Liu, K.J.; Qi, Z.; Zhao, Y. Contribution of zinc accumulation to ischemic brain injury and its mechanisms about oxidative stress, inflammation, and autophagy: An update. Metallomics 2024, 16, mfae012. [Google Scholar] [CrossRef]
- Wang, B.; Fang, T.; Chen, H. Zinc and Central Nervous System Disorders. Nutrients 2023, 15, 2140. [Google Scholar] [CrossRef]
- Porru, S.; Esplugues, A.; Llop, S.; Delgado-Saborit, J.M. The effects of heavy metal exposure on brain and gut microbiota: A systematic review of animal studies. Environ. Pollut. 2024, 348, 123732. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Lin, K.; Peng, F.; He, K.; Qian, Z.; Mei, X.; Su, Z.; Wujimaiti, Y.; Xia, X.; Zhang, T. Research progress on intestinal microbiota regulating cognitive function through the gut-brain axis. Neurol. Sci. 2024, 45, 3711–3721. [Google Scholar] [CrossRef]
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Wlodarczyk, M.; Zielinska, A.; Poleszak, E.; Fichna, J.; Wlaz, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J.; Greff, B.; Varga, L. Action and immunomodulatory mechanisms, formulations, and safety concerns of probiotics. Biosci. Microbiota Food Health 2025, 44, 4–15. [Google Scholar] [CrossRef]
- Trisal, A.; Singh, I.; Garg, G.; Jorwal, K.; Singh, A.K. Gut-brain axis and brain health: Modulating neuroinflammation, cognitive decline, and neurodegeneration. 3 Biotech 2025, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Skalny, A.V.; Aschner, M.; Gritsenko, V.A.; Martins, A.C.; Tizabi, Y.; Korobeinikova, T.V.; Paoliello, M.M.B.; Tinkov, A.A. Modulation of gut microbiota with probiotics as a strategy to counteract endogenous and exogenous neurotoxicity. Adv. Neurotoxicol. 2024, 11, 133–176. [Google Scholar] [CrossRef]
- Kiran, N.S.; Yashaswini, C.; Chatterjee, A. Zebrafish: A trending model for gut-brain axis investigation. Aquat. Toxicol. 2024, 270, 106902. [Google Scholar] [CrossRef] [PubMed]
- Moradian, H.; Gabriel, T.; Barrau, M.; Roblin, X.; Paul, S. New methods to unveil host-microbe interaction mechanisms along the microbiota-gut-brain-axis. Gut Microbes 2024, 16, 2351520. [Google Scholar] [CrossRef]
- Zhong, X.; Li, J.; Lu, F.; Zhang, J.; Guo, L. Application of zebrafish in the study of the gut microbiome. Anim. Model. Exp. Med. 2022, 5, 323–336. [Google Scholar] [CrossRef]
- Firdous, S.M.; Pal, S.; Khanam, S.; Zakir, F. Behavioral neuroscience in zebrafish: Unravelling the complexity of brain-behavior relationships. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 9295–9313. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Newman, M.; Ebrahimie, E.; Lardelli, M. Using the zebrafish model for Alzheimer’s disease research. Front. Genet. 2014, 5, 189. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Rodríguez-Gómez, F.; Romero-Gil, V.; Benítez-Cabello, A.; Arroyo-López, F.N.; Garrido-Fernández, A. Reduction of the Bitter Taste in Packaged Natural Black Manzanilla Olives by Zinc Chloride. Front. Nutr. 2018, 5, 102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Zhao, C.; Yang, M.; Yin, D. ZnCl2 treatment improves nutrient quality and Zn accumulation in peanut seeds and sprouts. Sci. Rep. 2020, 10, 2364. [Google Scholar] [CrossRef] [PubMed]
- CFR-Code of Federal Regulations-Title 21. Available online: https://www.ecfr.gov/current/title-21 (accessed on 18 February 2025).
- Bauer, K.C.; Huus, K.E.; Finlay, B.B. Microbes and the mind: Emerging hallmarks of the gut microbiota–brain axis. Cell Microbiol. 2016, 18, 632–644. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Lou, P.; Dai, T.; Chen, Y.; Zhuge, A.; Yuan, Y.; Li, L. The Relationship Between the Gut Microbiome and Neurodegenerative Diseases. Neurosci. Bull. 2021, 37, 1510–1522. [Google Scholar] [CrossRef]
- Everard, A.; Cani, P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014, 15, 189–196. [Google Scholar] [CrossRef]
- Araujo, L.D.C.; Furlaneto, F.A.C.; da Silva, L.A.B.; Kapila, Y.L. Use of the Probiotic Bifidobacterium animalis subsp. lactis HN019 in Oral Diseases. Int. J. Mol. Sci. 2022, 23, 9334. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients With Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e8. [Google Scholar] [CrossRef]
- Capurso, L.M.D. Thirty Years of Lactobacillus rhamnosus GG: A Review. J. Clin. Gastroenterol. 2019, 53, S1–S41. [Google Scholar] [CrossRef]
- Shi, C.; Cheng, M.; Yang, X.; Lu, Y.; Yin, H.; Zeng, Y.; Wang, C. Probiotic Lactobacillus rhamnosus GG Promotes Mouse Gut Microbiota Diversity and T Cell Differentiation. Front. Microbiol. 2020, 11, 607735. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, R.; Anzalone, M.; Calabrese, F.; Milazzo, M.; Capuana, M.; Italia, A.; Occhipinti, S.; Marotta, F. The gut microbiota and its correlations with the central nervous system disorders. Panminerva Med. 2014, 57, 127–143. [Google Scholar]
- Luczynski, P.; McVey Neufeld, K.A.; Oriach, C.S.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Growing up in a bubble: Using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 2016, 19, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Grochowska, M.; Laskus, T.; Radkowski, M. Gut Microbiota in Neurological Disorders. Arch. Immunol. Ther. Exp. 2019, 67, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Dwivedi, R.; Bansal, M.; Tripathi, M.; Dada, R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J. Clin. Med. 2023, 12, 1650. [Google Scholar] [CrossRef]
- Yang, T.; Huang, C.; Guo, F.; Zhang, X.; Rostami, A.; Liu, C.; Yu, S.; Wang, L.; Zhang, H.; Hu, C.; et al. Probiotics and the Gut-Brain Axis: A Review. Nutrients 2022, 12, 1650. [Google Scholar] [CrossRef]
- Ilie, O.-D.; Paduraru, E.; Robea, M.-A.; Balmus, I.-M.; Jijie, R.; Nicoara, M.; Ciobica, A.S.; Nita, I.-B.; Dobrin, R.; Doroftei, B. The Possible Role of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 on Locomotor Activity and Oxidative Stress in a Rotenone-Induced Zebrafish Model of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2021, 2021, 9629102. [Google Scholar] [CrossRef]
- Yu, F.; Hou, Z.-S.; Luo, H.-R.; Cui, X.-F.; Xiao, J.; Kim, Y.-B.; Li, J.-L.; Feng, W.-R.; Tang, Y.-K.; Li, H.-X.; et al. Zinc alters behavioral phenotypes, neurotransmitter signatures, and immune homeostasis in male zebrafish (Danio rerio). Sci. Total Environ. 2022, 828, 154099. [Google Scholar] [CrossRef]
- Tan, J.K.; Nazar, F.H.; Makpol, S.; Teoh, S.L. Zebrafish: A Pharmacological Model for Learning and Memory Research. Molecules 2022, 27, 7374. [Google Scholar] [CrossRef]
- May, Z.; Morrill, A.; Holcombe, A.; Johnston, T.; Gallup, J.; Fouad, K.; Hamilton, T.J. Object recognition memory in zebrafish. Behav. Brain Res. 2016, 296, 199–210. [Google Scholar] [CrossRef]
- Oliveira, J.; Silveira, M.; Chacon, D.; Luchiari, A. The Zebrafish World of Colors and Shapes: Preference and Discrimination. Zebrafish 2015, 12, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini-Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Lucon-Xiccato, T.; Dadda, M. Assessing memory in zebrafish using the one-trial test. Behav. Process. 2014, 106, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Baralić, K.; Živančević, K.; Bozic, D.; Đukić-Ćosić, D. Probiotic cultures as a potential protective strategy against the toxicity of environmentally relevant chemicals: State-of-the-art knowledge. Food Chem. Toxicol. 2023, 172, 113582. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Liang, H.; Ye, L.; Lan, L.; Lu, F.; Wang, Q.; Lei, T.; Yang, X.; Cui, P.; et al. Differences in Alpha Diversity of Gut Microbiota in Neurological Diseases. Front. Neurosci. 2022, 16, 879318. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ene, M.; Savuca, A.; Ciobica, A.-S.; Jijie, R.; Gurzu, I.L.; Hritcu, L.D.; Chelaru, I.-A.; Plavan, G.-I.; Nicoara, M.N.; Gurzu, B. The Neurobehavioral Impact of Zinc Chloride Exposure in Zebrafish: Evaluating Cognitive Deficits and Probiotic Modulation. Toxics 2025, 13, 193. https://doi.org/10.3390/toxics13030193
Ene M, Savuca A, Ciobica A-S, Jijie R, Gurzu IL, Hritcu LD, Chelaru I-A, Plavan G-I, Nicoara MN, Gurzu B. The Neurobehavioral Impact of Zinc Chloride Exposure in Zebrafish: Evaluating Cognitive Deficits and Probiotic Modulation. Toxics. 2025; 13(3):193. https://doi.org/10.3390/toxics13030193
Chicago/Turabian StyleEne, Madalina, Alexandra Savuca, Alin-Stelian Ciobica, Roxana Jijie, Irina Luciana Gurzu, Luminita Diana Hritcu, Ionut-Alexandru Chelaru, Gabriel-Ionut Plavan, Mircea Nicusor Nicoara, and Bogdan Gurzu. 2025. "The Neurobehavioral Impact of Zinc Chloride Exposure in Zebrafish: Evaluating Cognitive Deficits and Probiotic Modulation" Toxics 13, no. 3: 193. https://doi.org/10.3390/toxics13030193
APA StyleEne, M., Savuca, A., Ciobica, A.-S., Jijie, R., Gurzu, I. L., Hritcu, L. D., Chelaru, I.-A., Plavan, G.-I., Nicoara, M. N., & Gurzu, B. (2025). The Neurobehavioral Impact of Zinc Chloride Exposure in Zebrafish: Evaluating Cognitive Deficits and Probiotic Modulation. Toxics, 13(3), 193. https://doi.org/10.3390/toxics13030193









