Lentinan Alleviated PM2.5 Exposure-Induced Epithelial–Mesenchymal Transition in Pulmonary Epithelial Cells by Inhibiting the GARP/TGF-β/Smad Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and PM2.5 Sampling and Preparation
2.2. Cell Proliferation Assay
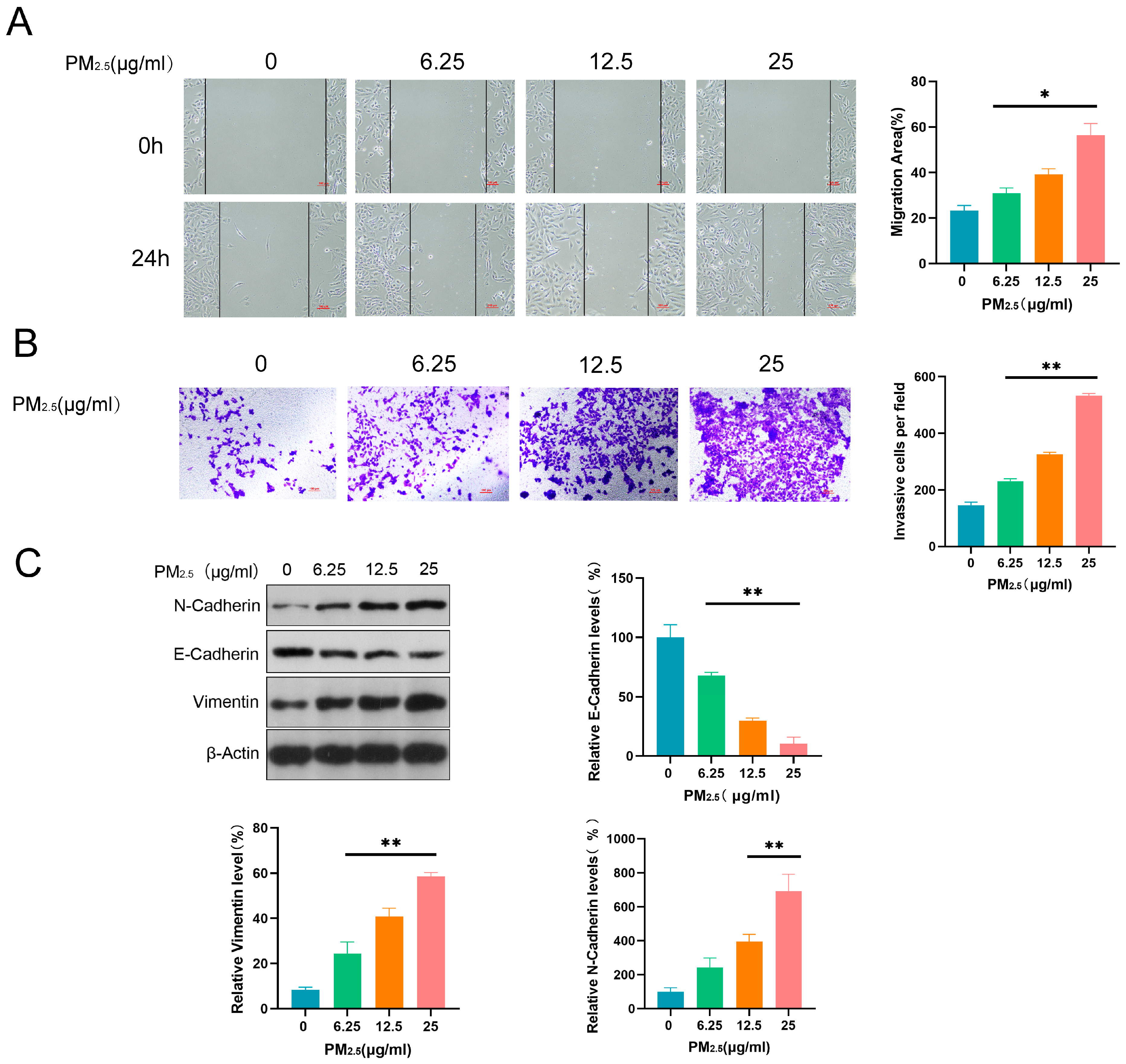
2.3. Wound Healing Assay (WHA)
2.4. Transwell Assay (TWA)
2.5. Real-Time Polymerase Chain Reaction (RT-PCR)
2.6. Western Blot (WB) Assay
2.7. RNA Interference
2.8. Statistical Analysis
3. Results
3.1. Lentinan Reversed the PM2.5-Induced Decrease in Cell Activity
3.2. PM2.5 Promoted Invasive, Migratory, and EMT Induction Abilities in Beas-2B Cells
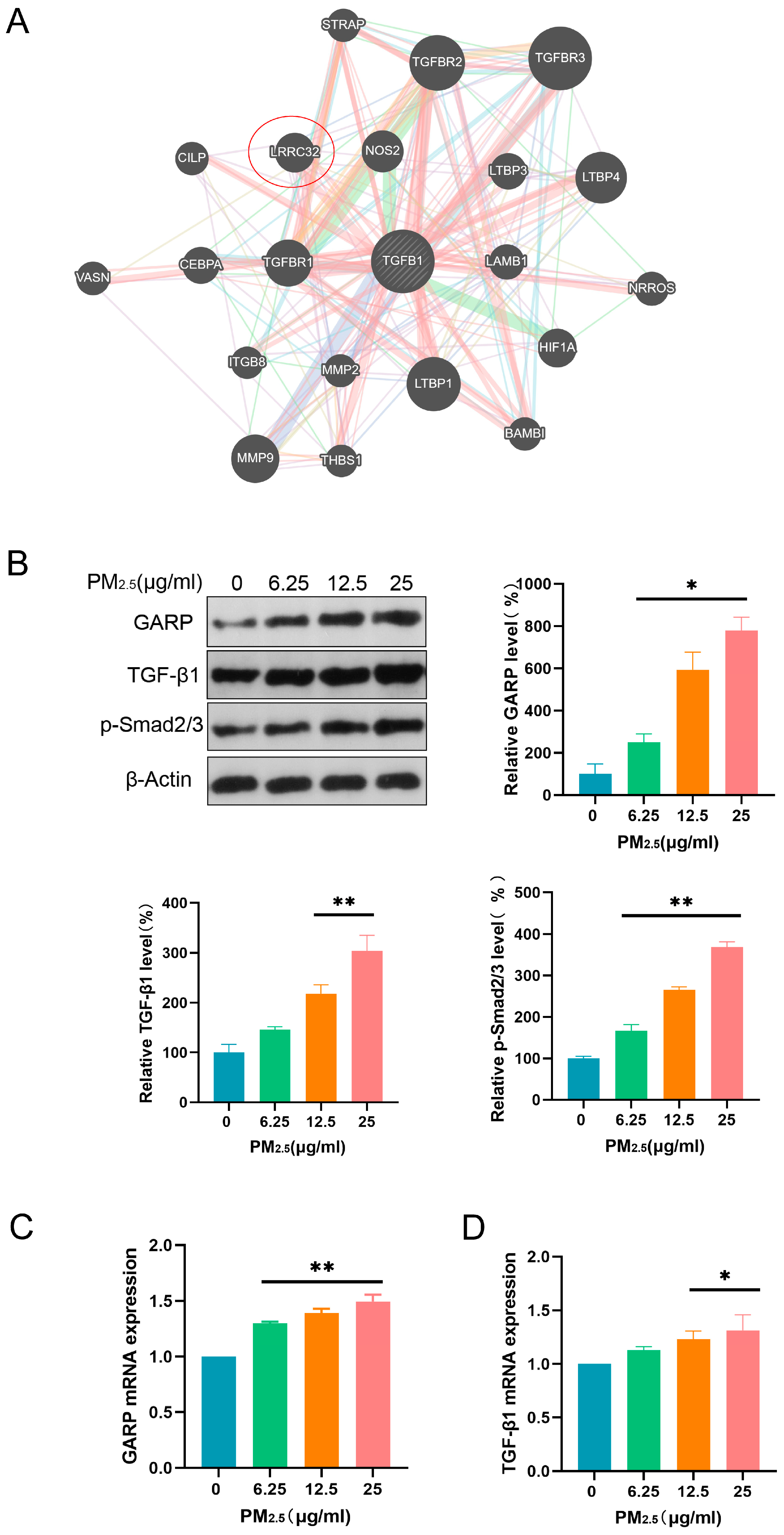
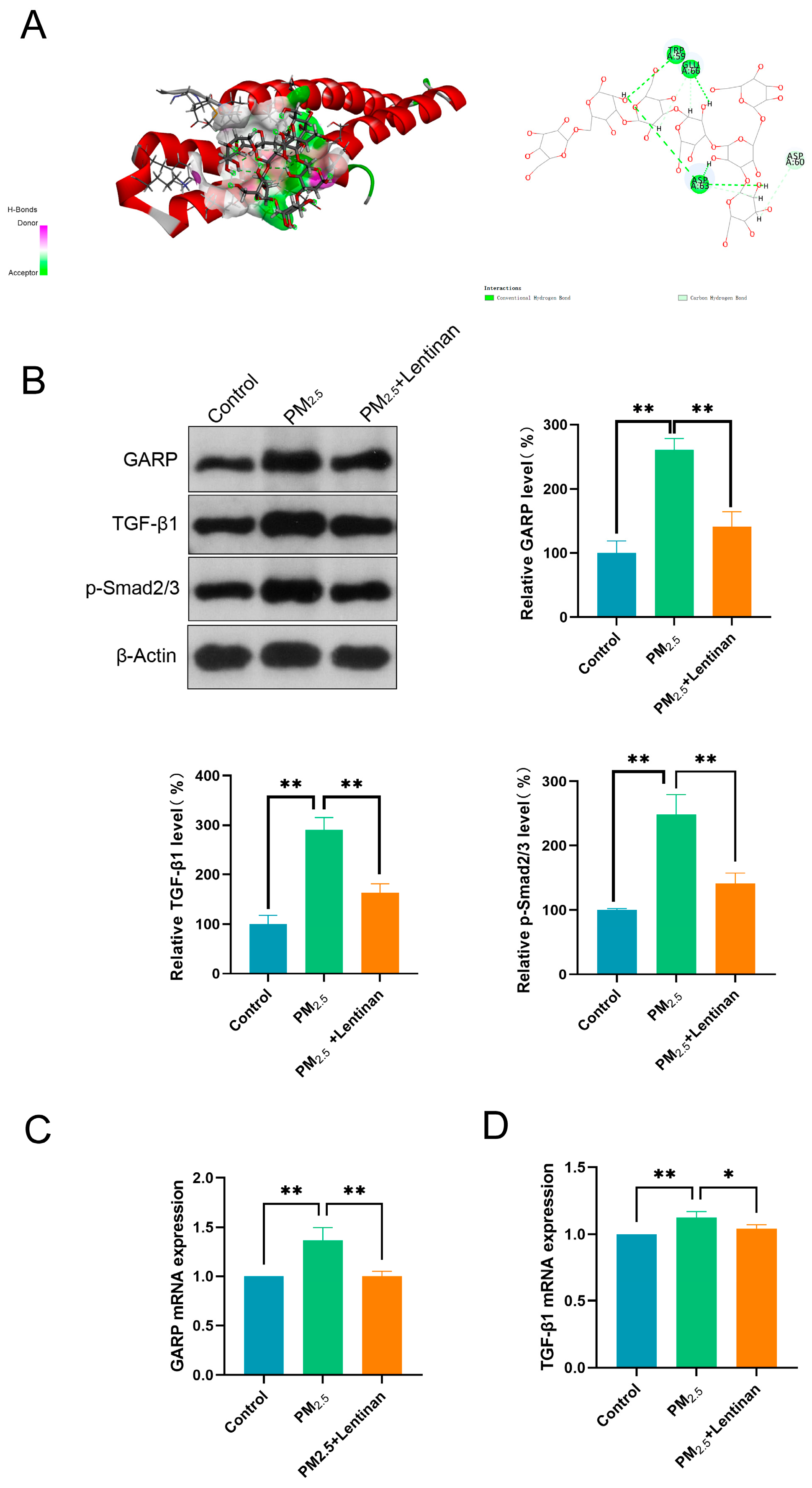
3.3. PM2.5 Activated the GARP/TGF-β/Smad Pathway
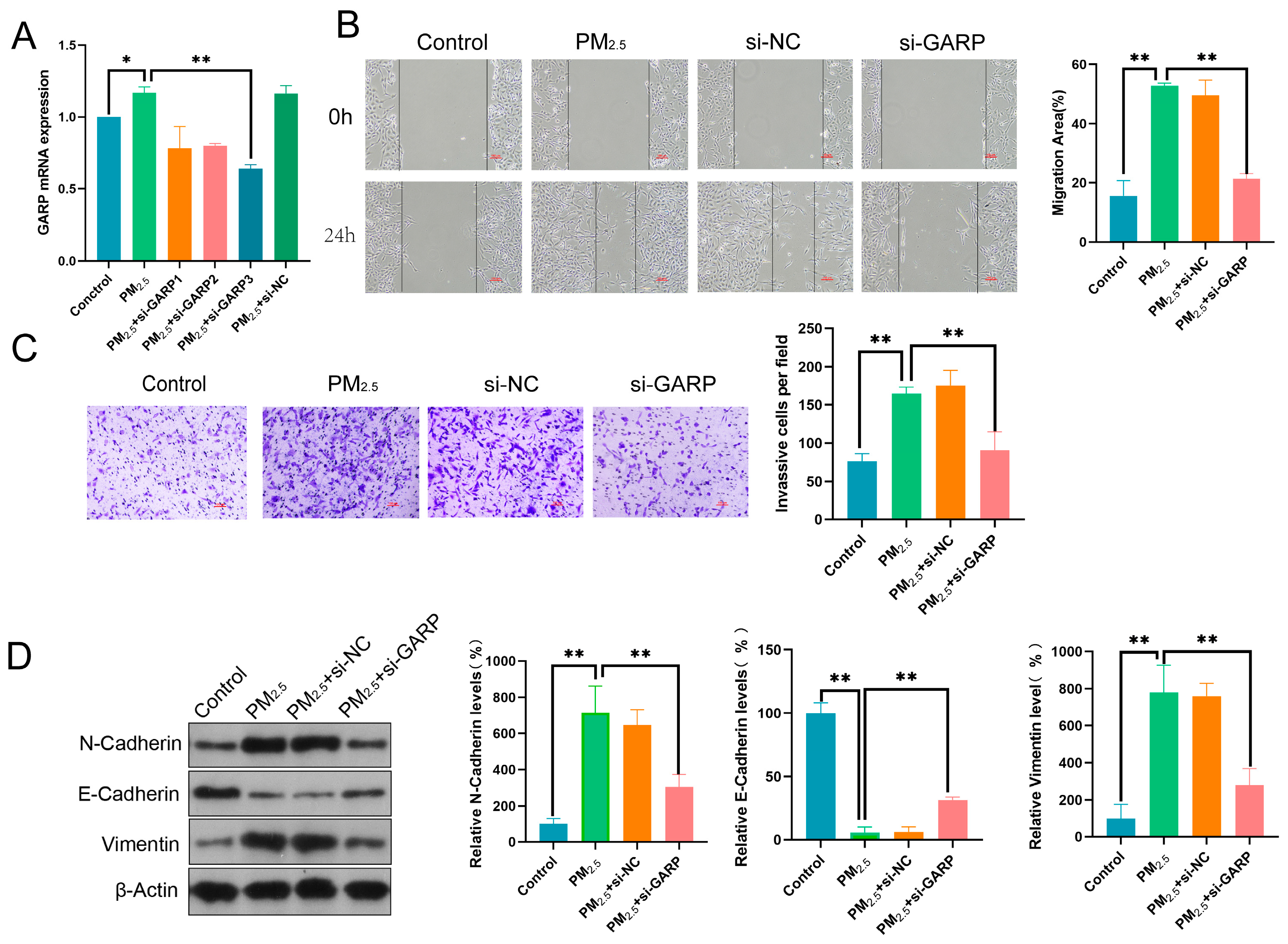
3.4. GARP Knockdown Reversed the Effects of PM2.5 on Beas-2B Cell Migratory, Invasive, and EMT Induction Abilities
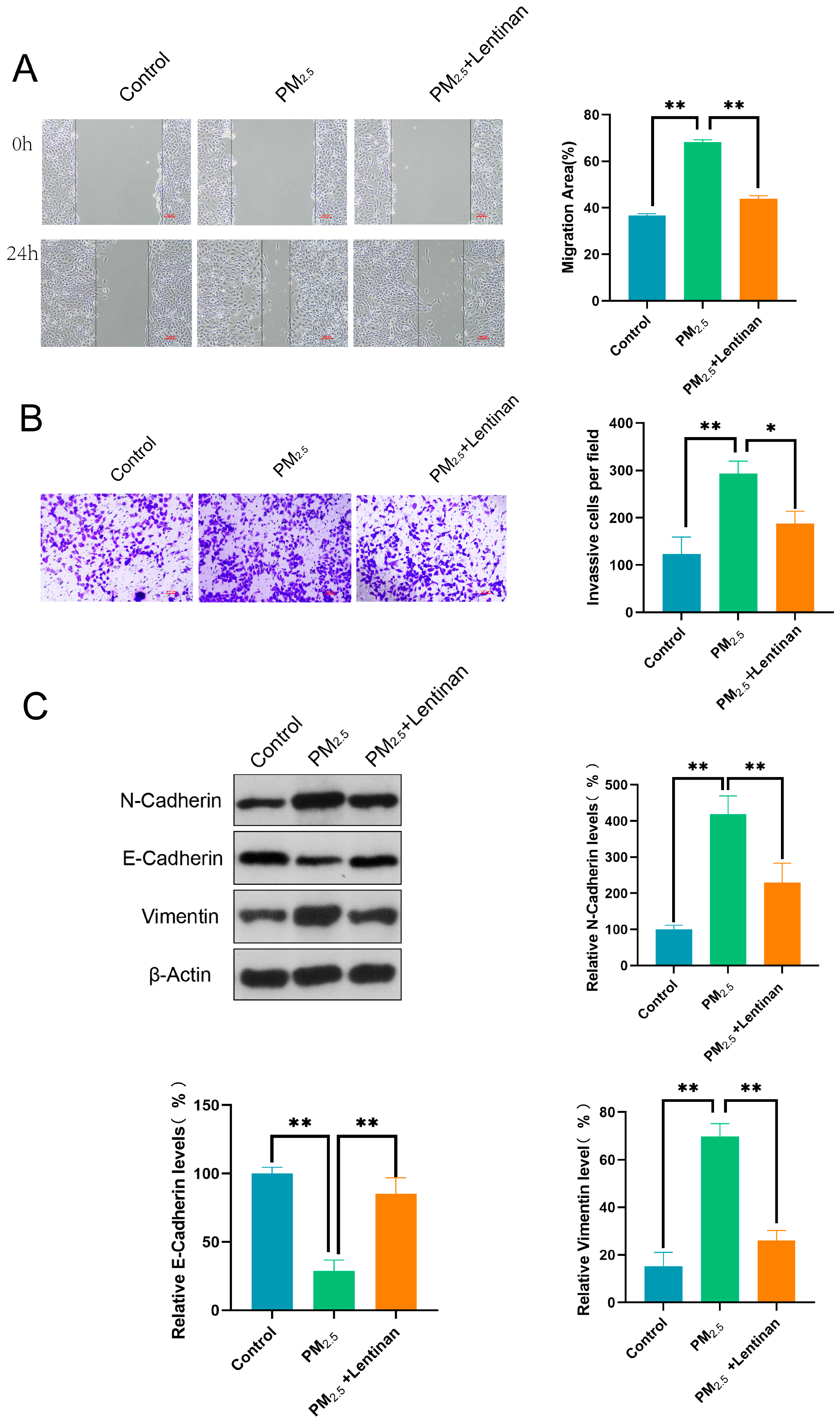
3.5. Lentinan Mitigated the Impact of PM2.5 on Beas-2B Cells
3.6. Lentinan Inhibited the PM2.5-Induced GARP/TGF-β/Smad Pathway Activation
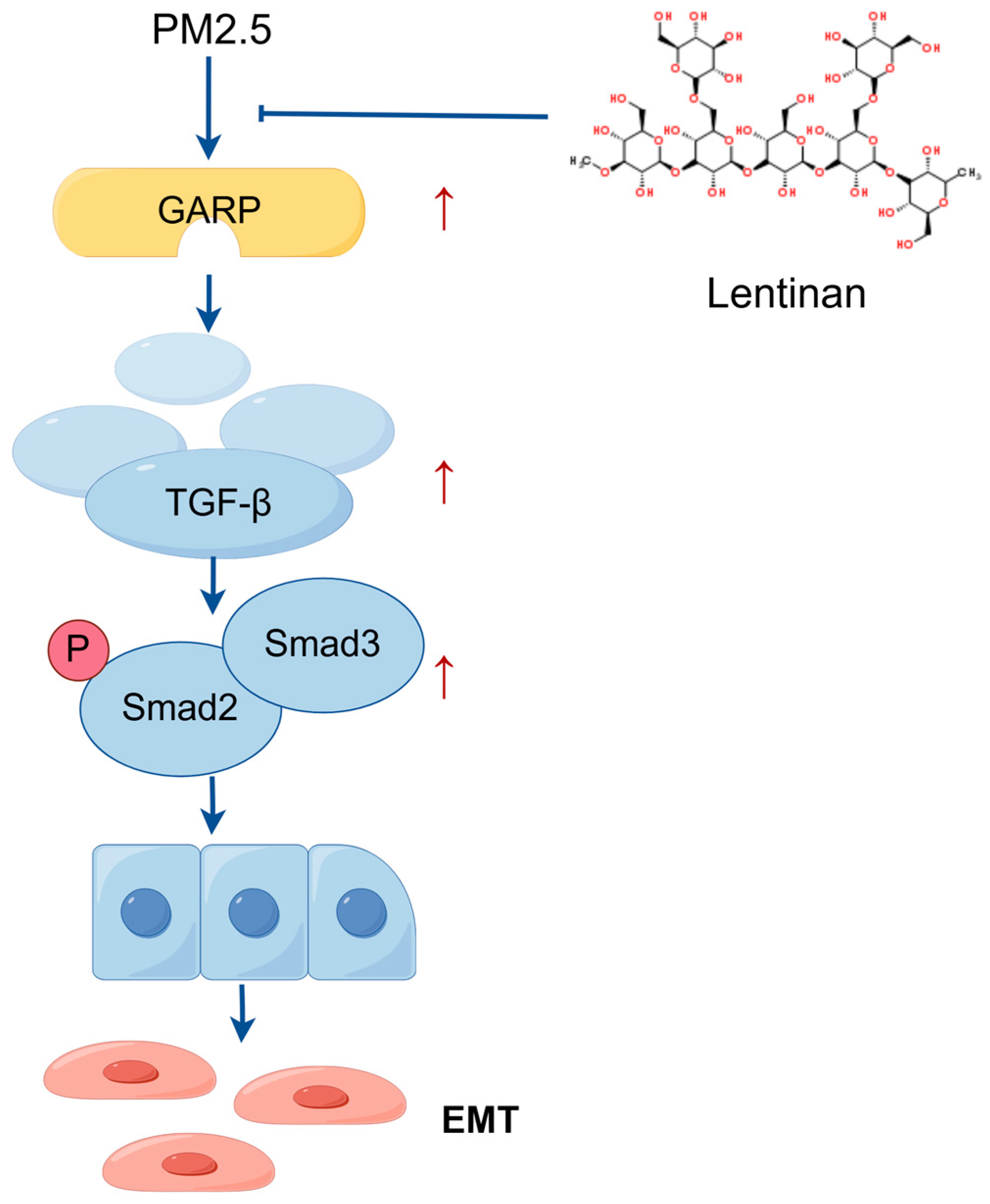
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Deng, Y.; Tin, M.S.; Lok, V.; Ngai, C.H.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; et al. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest 2022, 161, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Zaręba, Ł.; Piszczatowska, K.; Dżaman, K.; Soroczynska, K.; Motamedi, P.; Szczepański, M.J.; Ludwig, N. The Relationship between Fine Particle Matter (PM2.5) Exposure and Upper Respiratory Tract Diseases. J. Pers. Med. 2024, 14, 98. [Google Scholar] [CrossRef] [PubMed]
- Behinaein, P.; Hutchings, H.; Knapp, T.; Okereke, I.C. The Growing Impact of Air Quality on Lung-Related Illness: A Narrative Review. J. Thorac. Dis. 2023, 15, 5055–5063. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, A.; Guo, Y.; Bai, Q.; Wu, X.; Ji, S.-P.; Xia, R.-X. PM2.5 Exposure Induces Alveolar Epithelial Cell Apoptosis and Causes Emphysema through P53/Siva-1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3943–3950. [Google Scholar]
- Yue, D.; Zhang, Q.; Zhang, J.; Liu, W.; Chen, L.; Wang, M.; Li, R.; Qin, S.; Song, X.; Ji, Y. Diesel Exhaust PM2.5 Greatly Deteriorates Fibrosis Process in Pre-Existing Pulmonary Fibrosis via Ferroptosis. Environ. Int. 2023, 171, 107706. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Xiong, Y.; Zhang, L.; Xiong, A.; Wang, J.; He, X.; Li, G. Integrated Analysis of ATAC-Seq and RNA-Seq Unveils the Role of Ferroptosis in PM2.5-Induced Asthma Exacerbation. Int. Immunopharmacol. 2023, 125 Pt B, 111209. [Google Scholar] [CrossRef]
- Obeng, G.M.; Aram, S.A.; Agyei, D.; Saalidong, B.M. Exposure to Particulate Matter (PM2.5) and Volatile Organic Compounds (VOCs), and Self-Reported Health Symptoms among Fish Smokers: A Case Study in the Western Region of Ghana. PLoS ONE 2023, 18, e0283438. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Wei, J.; Zhao, Z.; Norbäck, D.; Zhang, X.; Lu, C.; Yu, W.; Wang, T.; Zheng, X.; et al. Associations of Early-Life Exposure to Submicron Particulate Matter With Childhood Asthma and Wheeze in China. JAMA Netw. Open 2022, 5, e2236003. [Google Scholar] [CrossRef]
- Zeng, X.; Xu, X.; Zheng, X.; Reponen, T.; Chen, A.; Huo, X. Heavy Metals in PM2.5 and in Blood, and Children’s Respiratory Symptoms and Asthma from an e-Waste Recycling Area. Environ. Pollut. 2016, 210, 346–353. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Parasin, N. Pathogenesis of PM2.5-Related Disorders in Different Age Groups: Children, Adults, and the Elderly. Epigenomes 2024, 8, 13. [Google Scholar] [CrossRef]
- Firoozi, Z.; Shahi, A.; Mohammadisoleimani, E.; Afzali, S.; Mansoori, B.; Bahmanyar, M.; Mohaghegh, P.; Dastsooz, H.; Pezeshki, B.; Nikfar, G.; et al. CircRNA-Associated ceRNA Networks (circCeNETs) in Chronic Obstructive Pulmonary Disease (COPD). Life Sci. 2024, 349, 122715. [Google Scholar] [CrossRef] [PubMed]
- Cochard, M.; Ledoux, F.; Landkocz, Y. Atmospheric Fine Particulate Matter and Epithelial Mesenchymal Transition in Pulmonary Cells: State of the Art and Critical Review of the in Vitro Studies. J. Toxicol. Environ. Health B Crit. Rev. 2020, 23, 293–318. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hu, S.; Li, C.; Ma, H.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J. Cigarette Smoke Induced Lung Barrier Dysfunction, EMT, and Tissue Remodeling: A Possible Link between COPD and Lung Cancer. BioMed Res. Int. 2019, 2019, 2025636. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, S.; Pan, Z.; Jiang, S.; Fan, J.; Yu, S.; Xue, L.; Yang, J.; Ma, S.; Liu, T.; et al. Hydrogen Sulfide Alleviates Particulate Matter-Induced Emphysema and Airway Inflammation by Suppressing Ferroptosis. Free Radic. Biol. Med. 2022, 186, 1–16. [Google Scholar] [CrossRef]
- Xu, Z.; Ding, W.; Deng, X. PM2.5, Fine Particulate Matter: A Novel Player in the Epithelial-Mesenchymal Transition? Front. Physiol. 2019, 10, 1404. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Liu, Z.; Li, Y.; Zhang, C.; Gong, Y.; Jiang, Y.; Xing, B. THBS1 Facilitates Colorectal Liver Metastasis through Enhancing Epithelial-Mesenchymal Transition. Clin. Transl. Oncol. 2020, 22, 1730–1740. [Google Scholar] [CrossRef]
- Hu, C.; Xin, Z.; Sun, X.; Hu, Y.; Zhang, C.; Yan, R.; Wang, Y.; Lu, M.; Huang, J.; Du, X.; et al. Activation of ACLY by SEC63 Deploys Metabolic Reprogramming to Facilitate Hepatocellular Carcinoma Metastasis upon Endoplasmic Reticulum Stress. J. Exp. Clin. Cancer Res. 2023, 42, 108. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Cai, H.; Zhu, G.; Zeng, Y.; Abuduxukuer, Z.; Chen, K.; Wang, J.; Ye, L.; Jin, M. Lentinan Attenuates Allergic Airway Inflammation and Epithelial Barrier Dysfunction in Asthma via Inhibition of the PI3K/AKT/NF-κB Pathway. Phytomedicine 2024, 134, 155965. [Google Scholar] [CrossRef]
- Zhang, Z.; Zha, Z.; Zhao, Z.; Liu, W.; Li, W. Lentinan Inhibits AGE-Induced Inflammation and the Expression of Matrix-Degrading Enzymes in Human Chondrocytes. Drug Des. Dev. Ther. 2020, 14, 2819–2829. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhao, Y.; Zong, S.; Tian, Y.; Chen, S.; Li, M.; Liu, H.; Zhang, Q.; Jing, X.; et al. Therapeutic Effects of Lentinan on Inflammatory Bowel Disease and Colitis-Associated Cancer. J. Cell. Mol. Med. 2019, 23, 750–760. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Y.; Wang, N.; Xiao, C. Lentinan Attenuated the PM2.5 Exposure-Induced Inflammatory Response, Epithelial-Mesenchymal Transition and Migration by Inhibiting the PVT1/miR-199a-5p/Caveolin1 Pathway in Lung Cancer. DNA Cell Biol. 2021, 40, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Metelli, A.; Salem, M.; Wallace, C.H.; Wu, B.X.; Li, A.; Li, X.; Li, Z. Immunoregulatory Functions and the Therapeutic Implications of GARP-TGF-β in Inflammation and Cancer. J. Hematol. Oncol. 2018, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and Extracellular TGF-β Signaling in Cancer: Some Recent Topics. Front. Med. 2018, 12, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Metelli, A.; Wu, B.X.; Riesenberg, B.; Guglietta, S.; Huck, J.D.; Mills, C.; Li, A.; Rachidi, S.; Krieg, C.; Rubinstein, M.P.; et al. Thrombin Contributes to Cancer Immune Evasion via Proteolysis of Platelet-Bound GARP to Activate LTGF-β. Sci. Transl. Med. 2020, 12, eaay4860. [Google Scholar] [CrossRef]
- Li, A.; Chang, Y.; Song, N.-J.; Wu, X.; Chung, D.; Riesenberg, B.P.; Velegraki, M.; Giuliani, G.D.; Das, K.; Okimoto, T.; et al. Selective Targeting of GARP-LTGFβ Axis in the Tumor Microenvironment Augments PD-1 Blockade via Enhancing CD8+ T Cell Antitumor Immunity. J. Immunother. Cancer 2022, 10, e005433. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Su, C.; Ma, W.; Zheng, X.; Ge, X.; Duan, X. Reduced Frequencies of Foxp3+ GARP+ Regulatory T Cells in COPD Patients Are Associated with Multi-Organ Loss of Tissue Phenotype. Respir. Res. 2022, 23, 176. [Google Scholar] [CrossRef]
- Nookala, S.; Mukundan, S.; Fife, A.; Alagarsamy, J.; Kotb, M. Heterogeneity in FoxP3- and GARP/LAP-Expressing T Regulatory Cells in an HLA Class II Transgenic Murine Model of Necrotizing Soft Tissue Infections by Group A Streptococcus. Infect. Immun. 2018, 86, e00432-18. [Google Scholar] [CrossRef]
- Yang, T.; Gao, Y.; Shi, X.; Zhang, Q.; Ren, Q.; Fu, Y.; Li, H.; Wang, Y.; He, T.; He, M.; et al. Analysis of Atmospheric PM2.5 Composition and Source in an Industrial City in Northern China. Chin. Rare Earths 2023, 44, 113–122. (In Chinese) [Google Scholar]
- Wang, Y.; Zhong, Y.; Hou, T.; Liao, J.; Zhang, C.; Sun, C.; Wang, G. PM2.5 Induces EMT and Promotes CSC Properties by Activating Notch Pathway in Vivo and Vitro. Ecotoxicol. Environ. Saf. 2019, 178, 159–167. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Y.; Zhang, C.; Liao, J.; Wang, G. PM2.5 Downregulates MicroRNA-139-5p and Induces EMT in Bronchiolar Epithelium Cells by Targeting Notch1. J. Cancer 2020, 11, 5758–5767. [Google Scholar] [CrossRef]
- Liu, K.; Hua, S.; Song, L. PM2.5 Exposure and Asthma Development: The Key Role of Oxidative Stress. Oxid. Med. Cell. Longev. 2022, 2022, 3618806. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Liu, S.; Ye, D.; Bai, G.; Guo, M.; Sun, R.; Ran, P. PM2.5 Induces Lung Inflammation and Fibrosis via Airway Smooth Muscle Cell Expression of the Wnt5a/JNK Pathway. J. Thorac. Dis. 2023, 15, 6094–6105. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, Y.; Zhong, Y.; Guo, X.; Lin, Y.; Yang, S.; Liu, J.; Xie, X.; Sun, Y.; Wang, D.; et al. Impaired AT2 to AT1 Cell Transition in PM2.5-Induced Mouse Model of Chronic Obstructive Pulmonary Disease. Respir. Res. 2022, 23, 70. [Google Scholar] [CrossRef] [PubMed]
- Leilei, L.; Xue, S.; Yan, L.; Yuyuan, L.; Ying, W.; Wenke, Q.; Xuesong, Y.; Ming, L. PM2.5-Exposed Hepatocytes Induce Hepatic Stellate Cells Activation by Releasing TGF-Β1. Biochem. Biophys. Res. Commun. 2021, 569, 125–131. [Google Scholar] [CrossRef]
- Lin, C.-H.; Liu, W.-S.; Wan, C.; Wang, H.-H. Pentraxin 3 Mediates Early Inflammatory Response and EMT Process in Human Tubule Epithelial Cells Induced by PM2.5. Int. Immunopharmacol. 2022, 112, 109258. [Google Scholar] [CrossRef]
- Zhao, C.; Pu, W.; Wazir, J.; Jin, X.; Wei, L.; Song, S.; Su, Z.; Li, J.; Deng, Y.; Wang, H. Long-Term Exposure to PM2.5 Aggravates Pulmonary Fibrosis and Acute Lung Injury by Disrupting Nrf2-Mediated Antioxidant Function. Environ. Pollut. 2022, 313, 120017. [Google Scholar] [CrossRef]
- Wang, H.; Wang, G.; Meng, Y.; Liu, Y.; Yao, X.; Feng, C. Modified Guo-Min Decoction Ameliorates PM2.5-Induced Lung Injury by Inhibition of PI3K-AKT and MAPK Signaling Pathways. Phytomedicine 2024, 123, 155211. [Google Scholar] [CrossRef]
- Chao, X.; Yi, L.; Lan, L.L.; Wei, H.Y.; Wei, D. Long-Term PM2.5 Exposure Increases the Risk of Non-Small Cell Lung Cancer (NSCLC) Progression by Enhancing Interleukin-17a (IL-17a)-Regulated Proliferation and Metastasis. Aging 2020, 12, 11579–11602. [Google Scholar] [CrossRef]
- Hao, Y.; Long, Z.; Gu, X. Farrerol Suppresses Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma via Suppression of TGF-Β1/Smad2/3 Signaling. Pathol. Res. Pract. 2024, 264, 155719. [Google Scholar] [CrossRef]
- Moreau, J.M.; Velegraki, M.; Bolyard, C.; Rosenblum, M.D.; Li, Z. Transforming Growth Factor-Β1 in Regulatory T Cell Biology. Sci. Immunol. 2022, 7, eabi4613. [Google Scholar] [CrossRef]
- Campbell, M.G.; Cormier, A.; Ito, S.; Seed, R.I.; Bondesson, A.J.; Lou, J.; Marks, J.D.; Baron, J.L.; Cheng, Y.; Nishimura, S.L. Cryo-EM Reveals Integrin-Mediated TGF-β Activation without Release from Latent TGF-β. Cell 2020, 180, 490–501.e16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Li, Y.; Kou, X.; Xue, Z. Mechanisms of Biochanin A Alleviating PM2.5 Organic Extracts-Induced EMT of A549 Cells through the PI3K/Akt Pathway. J. Nat. Prod. 2022, 85, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan Inhibits Oxidative Stress and Alleviates LPS-Induced Inflammation and Apoptosis of BMECs by Activating the Nrf2 Signaling Pathway. Int. J. Biol. Macromol. 2022, 222 Pt B, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, B.; Zhang, S.; Fan, M.; Ji, X.; Zhang, S.; Wang, Z.; Qiao, K. Lentinan Protects Caenorhabditis Elegans against Fluopyram-Induced Toxicity through DAF-16 and SKN-1 Pathways. Ecotoxicol. Environ. Saf. 2023, 265, 115510. [Google Scholar] [CrossRef]
- Yap, P.G.; Gan, C.Y. Optimized Extraction and Characterization of Ramie Leaf Polysaccharides Using Deep Eutectic Solvent and Microwave: Antioxidant, Metal Chelation, and UV Protection Properties. Int. J. Biol. Macromol. 2024, 282 Pt 3, 136927. [Google Scholar] [CrossRef]
- Yu, L.; Gao, Y.; Ye, Z.; Duan, H.; Zhao, J.; Zhang, H.; Narbad, A.; Tian, F.; Zhai, Q.; Chen, W. Interaction of Beta-Glucans with Gut Microbiota: Dietary Origins, Structures, Degradation, Metabolism, and Beneficial Function. Crit. Rev. Food Sci. Nutr. 2024, 64, 9884–9909. [Google Scholar] [CrossRef]
- Jiang, Y.; Chang, Z.; Xu, Y.; Zhan, X.; Wang, Y.; Gao, M. Advances in Molecular Enzymology of β-1,3-Glucanases: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 279 Pt 3, 135349. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xu, S.; Bian, B.; Hu, Z.; Wu, F.; Zhao, S.; Wang, X.; Wang, L.; Ma, T. Lentinan Alleviated PM2.5 Exposure-Induced Epithelial–Mesenchymal Transition in Pulmonary Epithelial Cells by Inhibiting the GARP/TGF-β/Smad Pathway. Toxics 2025, 13, 166. https://doi.org/10.3390/toxics13030166
Wang Z, Xu S, Bian B, Hu Z, Wu F, Zhao S, Wang X, Wang L, Ma T. Lentinan Alleviated PM2.5 Exposure-Induced Epithelial–Mesenchymal Transition in Pulmonary Epithelial Cells by Inhibiting the GARP/TGF-β/Smad Pathway. Toxics. 2025; 13(3):166. https://doi.org/10.3390/toxics13030166
Chicago/Turabian StyleWang, Zhi, Shiqing Xu, Bohao Bian, Zhida Hu, Feiyang Wu, Siqi Zhao, Xiaohui Wang, Li Wang, and Teng Ma. 2025. "Lentinan Alleviated PM2.5 Exposure-Induced Epithelial–Mesenchymal Transition in Pulmonary Epithelial Cells by Inhibiting the GARP/TGF-β/Smad Pathway" Toxics 13, no. 3: 166. https://doi.org/10.3390/toxics13030166
APA StyleWang, Z., Xu, S., Bian, B., Hu, Z., Wu, F., Zhao, S., Wang, X., Wang, L., & Ma, T. (2025). Lentinan Alleviated PM2.5 Exposure-Induced Epithelial–Mesenchymal Transition in Pulmonary Epithelial Cells by Inhibiting the GARP/TGF-β/Smad Pathway. Toxics, 13(3), 166. https://doi.org/10.3390/toxics13030166




