Sleep Deprivation and Subchronic Arsenite Exposure Synergistically Induced Skeletal Muscle Aging by Disrupting Melatonin and Cortisol Secretion in Mice
Abstract
1. Introduction
2. Materials and Methods
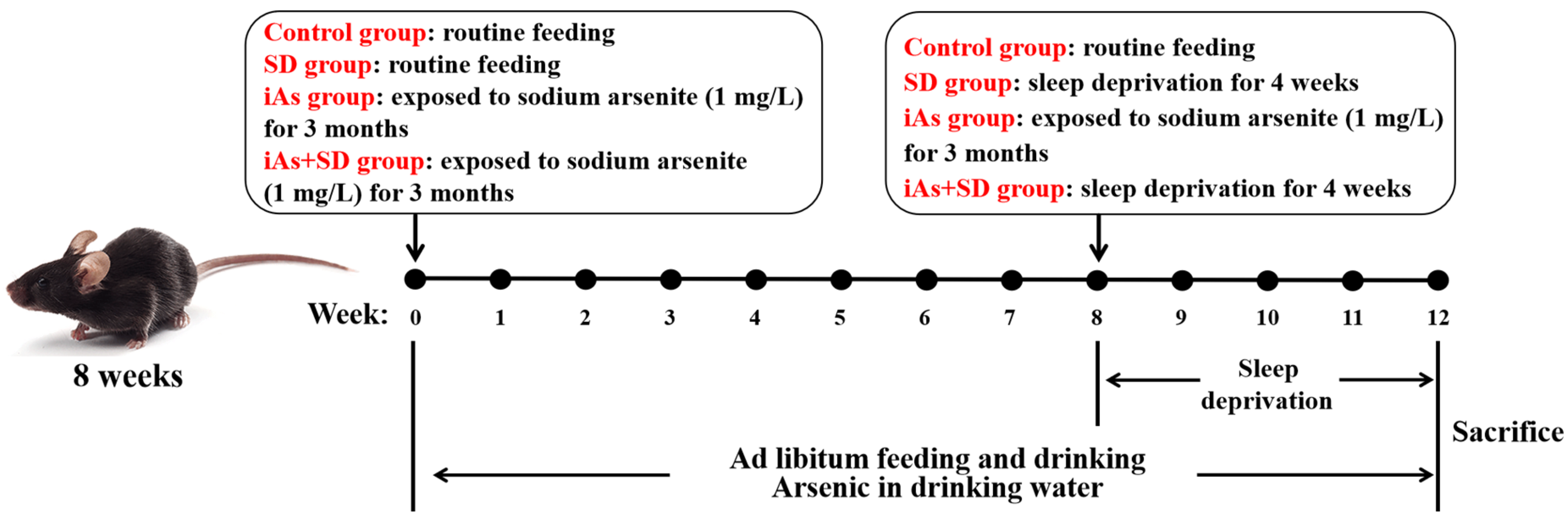
2.1. Animals and Treatments
2.2. Determination of Total Arsenic
2.3. Limb Grip Strength Testing
2.4. Calculation of the Muscle Index
2.5. Hematoxylin–Eosin (HE) Staining
2.6. Immunofluorescence Staining
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Quantitative Real-Time Polymerase Chain Reaction (QPCR)
2.9. Oxidative Stress Index Detection
2.10. Statistical Analyses
3. Results
3.1. The Effect of Sleep Deprivation and Arsenite Exposure on Limb Grip Strength in Mice
3.2. The Impact of Sleep Deprivation and Arsenite Exposure on Arsenic Concentration and Mass of Skeletal Muscle
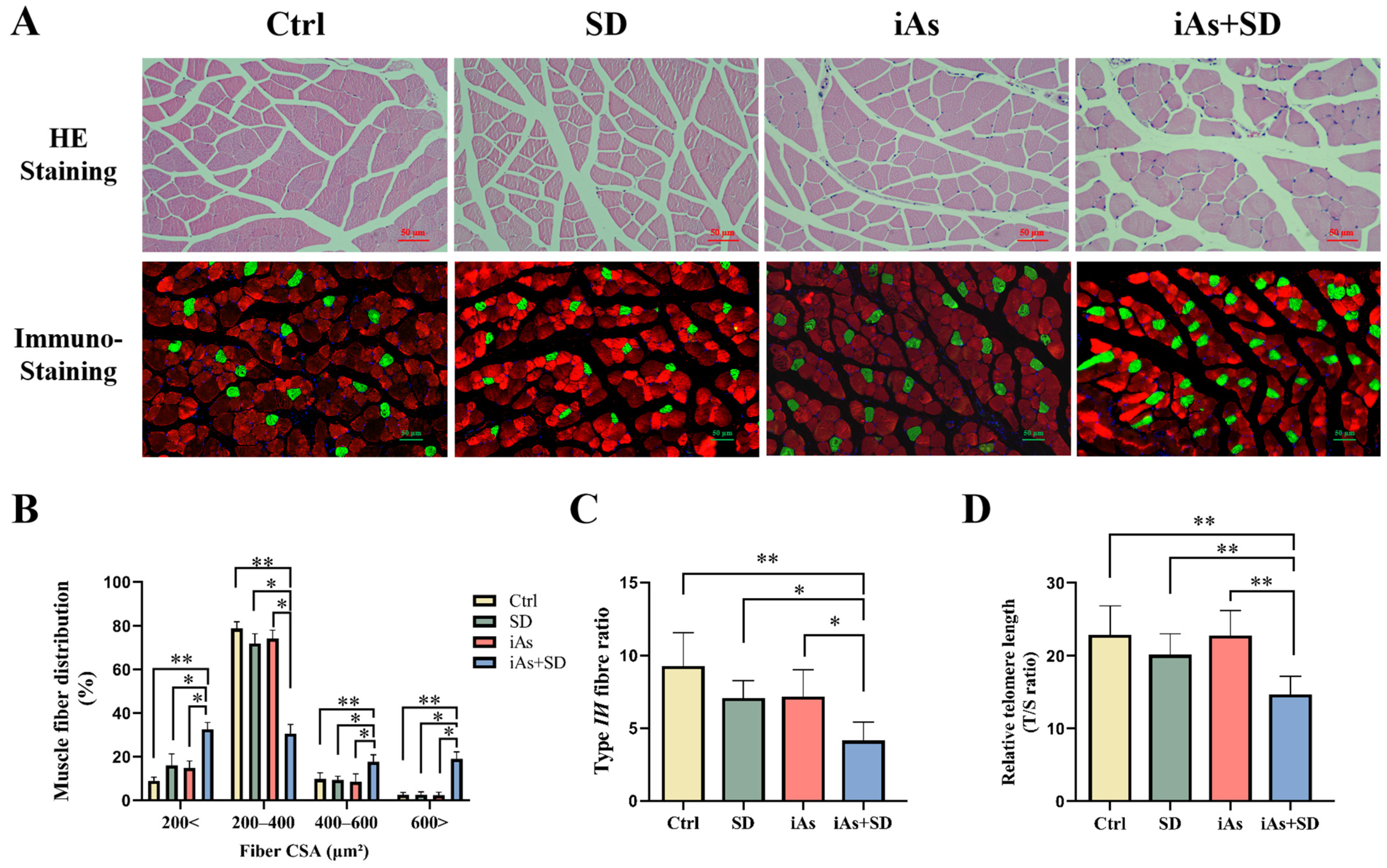
3.3. The Effect of Sleep Deprivation and Arsenite Exposure on Pathological Morphology and Telomere Length of Skeletal Muscle in Mice
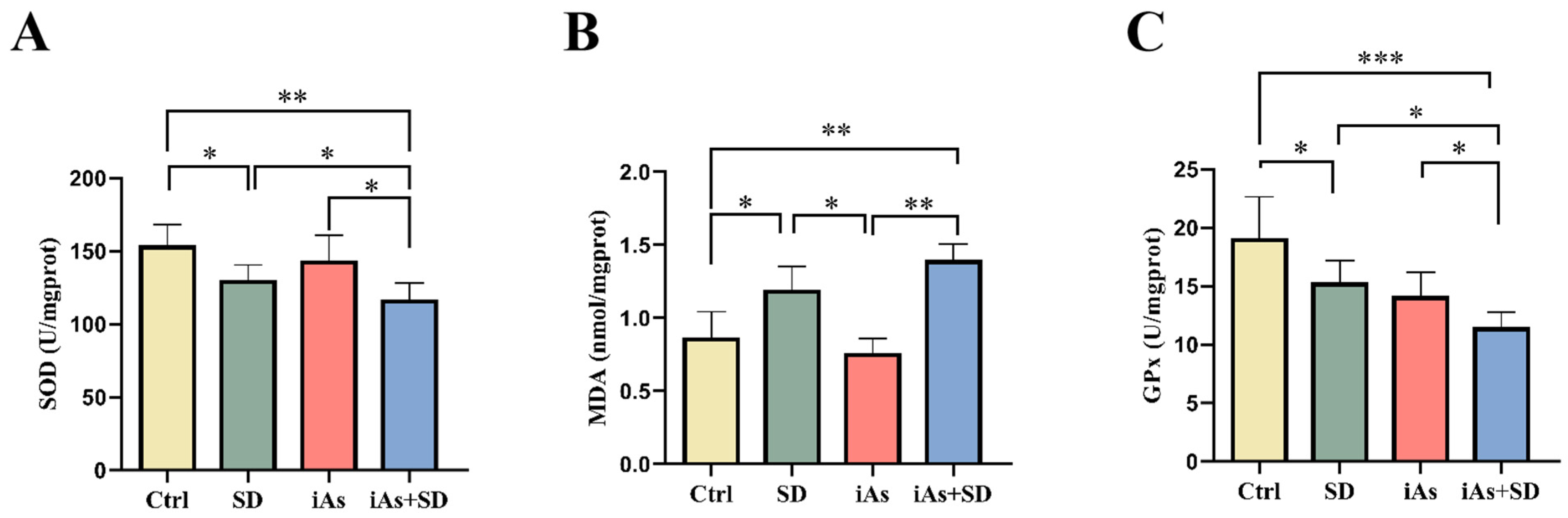
3.4. The Impact of Sleep Deprivation and Arsenite Exposure on Oxidative Damage in Mice
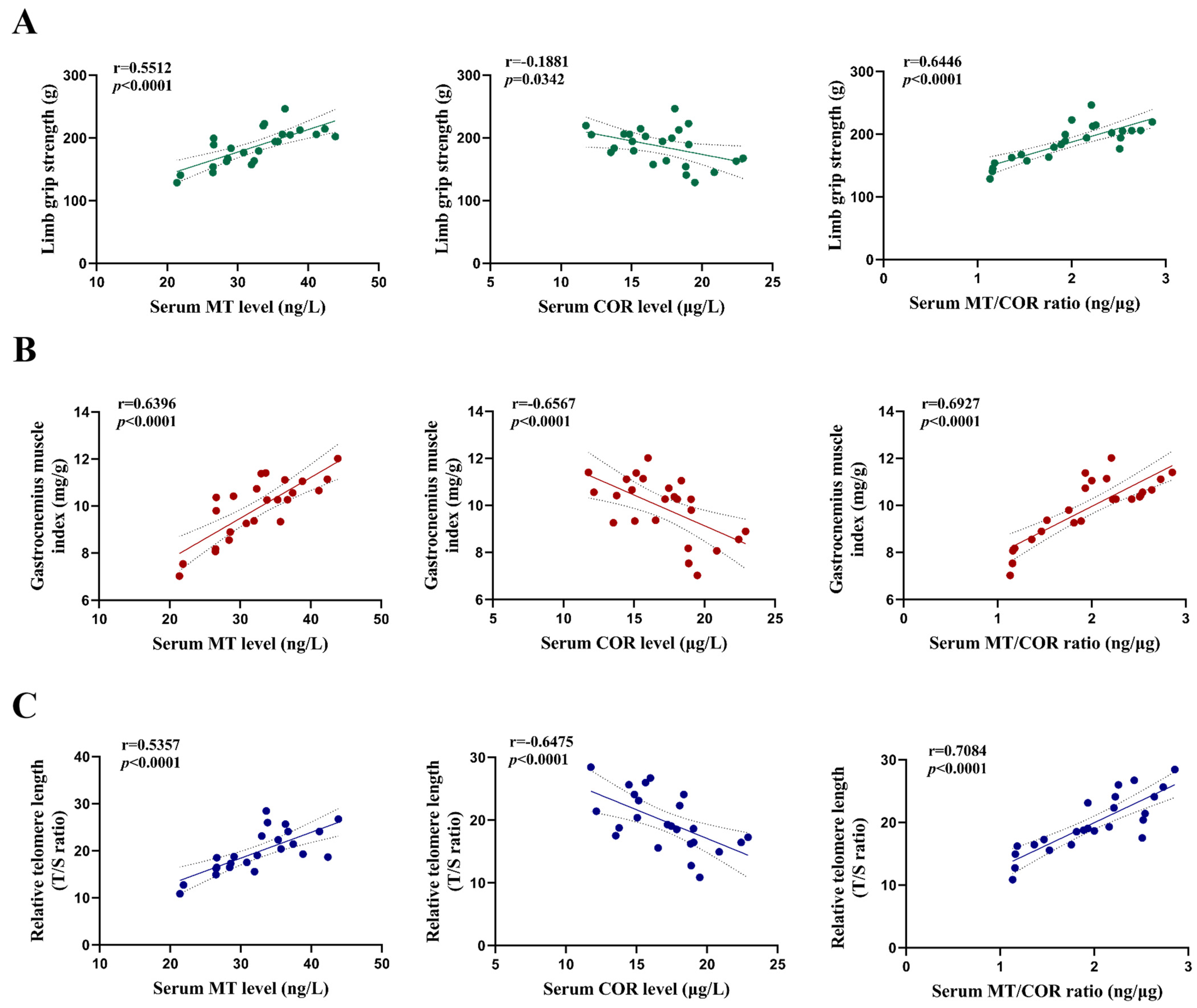
3.5. The Impact of Sleep Deprivation and Arsenite Exposure on Serum Levels of MT and COR in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef]
- Rastegari Mehr, M.; Keshavarzi, B.; Moore, F.; Hooda, P.S.; Busquets, R.; Ghorbani, Z. Arsenic in the rock-soil-plant system and related health risk in a magmatic-metamorphic belt, West of Iran. Environ. Geochem. Health 2020, 42, 3659–3673. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Rahman, M.M.; Mise, N.; Sikder, M.T.; Ichihara, G.; Uddin, M.K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Mondal, V.; Hosen, Z.; Hossen, F.; Siddique, A.E.; Tony, S.R.; Islam, Z.; Islam, M.S.; Hossain, S.; Islam, K.; Sarker, M.K.; et al. Arsenic exposure-related hyperglycemia is linked to insulin resistance with concomitant reduction of skeletal muscle mass. Environ. Int. 2020, 143, 105890. [Google Scholar] [CrossRef]
- Sarker, M.K.; Tony, S.R.; Siddique, A.E.; Karim, M.R.; Haque, N.; Islam, Z.; Islam, M.S.; Khatun, M.; Islam, J.; Hossain, S.; et al. Arsenic Secondary Methylation Capacity Is Inversely Associated with Arsenic Exposure-Related Muscle Mass Reduction. Int. J. Environ. Res. Public Health 2021, 18, 9730. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiu, T.; Yao, X.; Jiang, L.; Wei, S.; Pei, P.; Wang, Z.; Bai, J.; Liu, X.; Yang, G.; et al. Taurine protects against arsenic trioxide-induced insulin resistance via ROS-Autophagy pathway in skeletal muscle. Int. J. Biochem. Cell Biol. 2019, 112, 50–60. [Google Scholar] [CrossRef]
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Tobaldini, E.; Costantino, G.; Solbiati, M.; Cogliati, C.; Kara, T.; Nobili, L.; Montano, N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci. Biobehav. Rev. 2017, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Q.; Huang, X.; Li, Z. Association between sleep duration and possible sarcopenia in middle-aged and elderly Chinese individuals: Evidence from the China health and retirement longitudinal study. BMC Geriatr. 2024, 24, 594. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, N.; Spira, D.; Norman, K.; Demuth, I.; Eckardt, R.; Steinhagen-Thiessen, E. Sleep, Muscle Mass and Muscle Function in Older People. Dtsch. Arztebl. Int. 2016, 113, 253–260. [Google Scholar]
- Lamon, S.; Morabito, A.; Arentson-Lantz, E.; Knowles, O.; Vincent, G.E.; Condo, D.; Alexander, S.E.; Garnham, A.; Paddon-Jones, D.; Aisbett, B. The effect of acute sleep deprivation on skeletal muscle protein synthesis and the hormonal environment. Physiol. Rep. 2021, 9, e14660. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gan, L.; Luo, D.; Sun, C. Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J. Pineal Res. 2017, 62, e12383. [Google Scholar] [CrossRef]
- Zanuzzo, F.S.; Sabioni, R.E.; Marzocchi-Machado, C.M.; Urbinati, E.C. Modulation of stress and innate immune response by corticosteroids in pacu (Piaractus mesopotamicus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 231, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Salucci, S.; Taurone, S.; Burattini, S.; Gobbi, P.; Clausi, J.; Battistelli, M. Melatonin role in skeletal muscle disorders. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1024–1033. [Google Scholar] [PubMed]
- Lee, J.Y.; Kim, J.H.; Lee, D.C. Urine melatonin levels are inversely associated with sarcopenia in postmenopausal women. Menopause 2014, 21, 39–44. [Google Scholar] [CrossRef]
- Katsuhara, S.; Yokomoto-Umakoshi, M.; Umakoshi, H.; Matsuda, Y.; Iwahashi, N.; Kaneko, H.; Ogata, M.; Fukumoto, T.; Terada, E.; Sakamoto, R.; et al. Impact of Cortisol on Reduction in Muscle Strength and Mass: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e1477–e1487. [Google Scholar] [CrossRef]
- Valdés Salgado, M.A.; Schisterman, E.; Pino, P.; Bangdiwala, S.; Muñoz, M.P.; Iglesias, V. Is prenatal arsenic exposure associated with salivary cortisol in infants in Arica, Chile? An exploratory cohort study. Ann. Agric. Environ. Med. 2019, 26, 266–272. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Ma, L.; Wu, F.; Ren, Q.; Tao, J.; Chen, X.; Zhang, A. Chronic arsenite exposure induced skeletal muscle atrophy by disrupting angiotensin II-melatonin axis in rats. Environ. Toxicol. 2024, 39, 1350–1359. [Google Scholar] [CrossRef]
- Villafuerte, G.; Miguel-Puga, A.; Rodríguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrión, O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxid. Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin prevents the dysbiosis of intestinal microbiota in sleep-restricted mice by improving oxidative stress and inhibiting inflammation. Saudi J. Gastroenterol. 2022, 28, 209–217. [Google Scholar] [CrossRef]
- Chowdhury, U.K.; Biswas, B.K.; Chowdhury, T.R.; Samanta, G.; Mandal, B.K.; Basu, G.C.; Chanda, C.R.; Lodh, D.; Saha, K.C.; Mukherjee, S.K.; et al. Groundwater arsenic contamination in Bangladesh and West Bengal, India. Environ. Health Perspect. 2000, 108, 393–397. [Google Scholar] [CrossRef]
- Monteiro, D.; Oliveira, E.C.; Caixeta, E.S.; Santos, V.S.V.; Pereira, B.B. Arsenic exposure from groundwater: Environmental contamination, human health effects, and sustainable solutions. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 119–135. [Google Scholar] [CrossRef]
- Smith, S.; Turbill, C.; Penn, D.J. Chasing telomeres, not red herrings, in evolutionary ecology. Heredity 2011, 107, 372–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Han, R.; Zhu, W.; Xiu, J.; Shen, Y.; Xu, Q. Inverse changes in telomere length between the blood and brain in depressive-like mice. J. Affect. Disord. 2020, 273, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Englund, D.A.; Zhang, X.; Aversa, Z.; LeBrasseur, N.K. Skeletal muscle aging, cellular senescence, and senotherapeutics: Current knowledge and future directions. Mech. Ageing Dev. 2021, 200, 111595. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; He, J.; Yan, J.; Han, X.; Zhang, X.; Niu, Y.; Sha, W.; Li, J. DBC1 maintains skeletal muscle integrity by enhancing myogenesis and preventing myofibre wasting. J. Cachexia Sarcopenia Muscle 2024, 15, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, W.; Wang, D.; Ma, L.; Tao, J.; Zhang, A. Subchronic Arsenite Exposure Induced Atrophy and Erythropoietin Sensitivity Reduction in Skeletal Muscle Were Relevant to Declined Serum Melatonin Levels in Middle-Aged Rats. Toxics 2023, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xie, W.; Hu, P.; Tang, K.; Wang, X.; Wu, Y.; He, M.; Yu, D.; Li, Y. The role of melatonin in sarcopenia: Advances and application prospects. Exp. Gerontol. 2021, 149, 111319. [Google Scholar] [CrossRef]
- Vogel, F.; Braun, L.T.; Rubinstein, G.; Zopp, S.; Künzel, H.; Strasding, F.; Albani, A.; Riester, A.; Schmidmaier, R.; Bidlingmaier, M.; et al. Persisting Muscle Dysfunction in Cushing’s Syndrome Despite Biochemical Remission. J. Clin. Endocrinol. Metab. 2020, 105, e4490–e4498. [Google Scholar] [CrossRef]
- Berr, C.M.; Stieg, M.R.; Deutschbein, T.; Quinkler, M.; Schmidmaier, R.; Osswald, A.; Reisch, N.; Ritzel, K.; Dimopoulou, C.; Fazel, J.; et al. Persistence of myopathy in Cushing’s syndrome: Evaluation of the German Cushing’s Registry. Eur. J. Endocrinol. 2017, 176, 737–746. [Google Scholar] [CrossRef]
- Tyler, C.R.; Allan, A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014, 1, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.P., Jr.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Castaño, M.Y.; Garrido, M.; Rodríguez, A.B.; Gómez, M.Á. Melatonin Improves Mood Status and Quality of Life and Decreases Cortisol Levels in Fibromyalgia. Biol. Res. Nurs. 2019, 21, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Májovský, M.; Řezáčová, L.; Sumová, A.; Pospíšilová, L.; Netuka, D.; Bradáč, O.; Beneš, V. Melatonin and cortisol secretion profile in patients with pineal cyst before and after pineal cyst resection. J. Clin. Neurosci. 2017, 39, 155–163. [Google Scholar] [CrossRef]
- Soszyński, P.; Stowińska-Srzednicka, J.; Kasperlik-Zatuska, A.; Zgliczyński, S. Decreased melatonin concentration in Cushing’s syndrome. Horm. Metab. Res. 1989, 21, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Brismar, K.; Werner, S.; Thorén, M.; Wetterberg, L. Metyrapone: An agent for melatonin as well as ACTH and cortisol secretion. J. Endocrinol. Investig. 1985, 8, 91–95. [Google Scholar] [CrossRef]
- Corbalán-Tutau, D.; Madrid, J.A.; Nicolás, F.; Garaulet, M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol. Behav. 2014, 123, 231–235. [Google Scholar] [CrossRef]
- Wetterbuerg, L.; Beck-Friis, J.; Aperia, B.; Petterson, U. Melatonin/cortisol ratio in depression. Lancet 1979, 2, 1361. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Chen, X.; Yu, X.; Sun, B.; Tao, J.; Chen, X. Sleep Deprivation and Subchronic Arsenite Exposure Synergistically Induced Skeletal Muscle Aging by Disrupting Melatonin and Cortisol Secretion in Mice. Toxics 2025, 13, 97. https://doi.org/10.3390/toxics13020097
Yang H, Chen X, Yu X, Sun B, Tao J, Chen X. Sleep Deprivation and Subchronic Arsenite Exposure Synergistically Induced Skeletal Muscle Aging by Disrupting Melatonin and Cortisol Secretion in Mice. Toxics. 2025; 13(2):97. https://doi.org/10.3390/toxics13020097
Chicago/Turabian StyleYang, Hongyi, Xingyu Chen, Xuanfeng Yu, Baofei Sun, Junyan Tao, and Xiong Chen. 2025. "Sleep Deprivation and Subchronic Arsenite Exposure Synergistically Induced Skeletal Muscle Aging by Disrupting Melatonin and Cortisol Secretion in Mice" Toxics 13, no. 2: 97. https://doi.org/10.3390/toxics13020097
APA StyleYang, H., Chen, X., Yu, X., Sun, B., Tao, J., & Chen, X. (2025). Sleep Deprivation and Subchronic Arsenite Exposure Synergistically Induced Skeletal Muscle Aging by Disrupting Melatonin and Cortisol Secretion in Mice. Toxics, 13(2), 97. https://doi.org/10.3390/toxics13020097






