Reconstructing Historical Land Use and Anthropogenic Inputs in Lake Victoria Basin: Insights from PAH and n-Alkane Trends
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
| Core | GPS Location | Depth and Sampling | Site Description | Altitude (m Above Sea Level) | Rainfall | Temp (°C) | Population Density |
|---|---|---|---|---|---|---|---|
| KP1A | 0°05′26.0″ N, 35°09′07.0″ E | 400 cm, August 2014 (17 samples) | Kapsabet lies in the Kingwal Swamp, in the upper catchment of River Yala | 1863 ± 3.4 m asl | 1200–2000 mm/yr | 16 °C to 22 °C | 311 people/km2 [54,56] |
| SPK1 | 0°10′ N, 34°0′ E | 100 cm, June 2015 (10 samples) | Busia lies in the Sio River basin | 1188 m asl; 68 m above lake level | 1600 mm/yr | 22.5 °C | 526 people/km2 [11,56] |
| DK2 | 0°7′ S 34°44′ E | 100 cm, June 2015 (9 samples) | Kisumu lies in the Nyando River basin; the river is fed by precipitation from the Kericho Highlands | 1197 m asl, 63 m above lake level | 1250 mm/yr | 23 °C | 554 people/km2 [56] |
| HK2 | 0°7′ S 34°26′ E | 100 cm, June 2015 (11 samples) | Homa Bay is on the south shore of Winam Gulf | 1202 m asl; 68 m above lake level | 1600 mm/yr | 22.5 °C | 359 people/km2 [51,56] |
| KK1 | 0°4′ N, 34°8′ E | 100 cm, June 2015 (10 samples) | Siaya lies in the Nzoia River basin | 1152 m asl; 18 m above lake level | 2150 mm/yr | 21 °C | 393 people/km2 [11,56] |
| LV95-2P | 0°58′ S, 33°27′ E | 100 cm, April 1995 (13 samples) | This site, lying in the middle of Lake Victoria, Tanzania, is an oxygenated and stratified water column | 1133 m asl; depth—67 m | 1200 mm/year | 20 °C to 25 °C | [55] |
2.3. Organic Matter Extraction and Analyses
2.4. Statistics
2.5. Limitations of the Study
3. Results
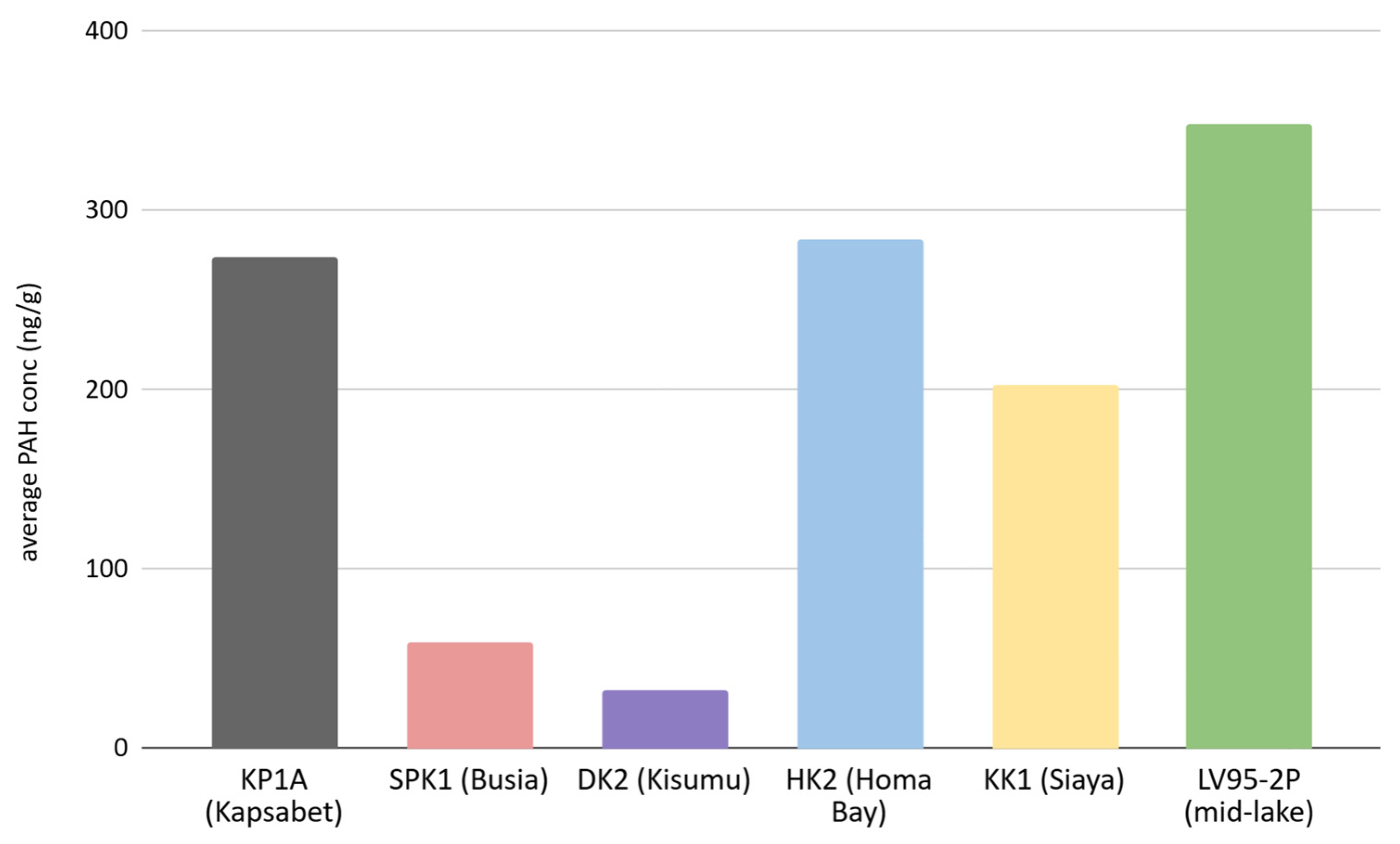
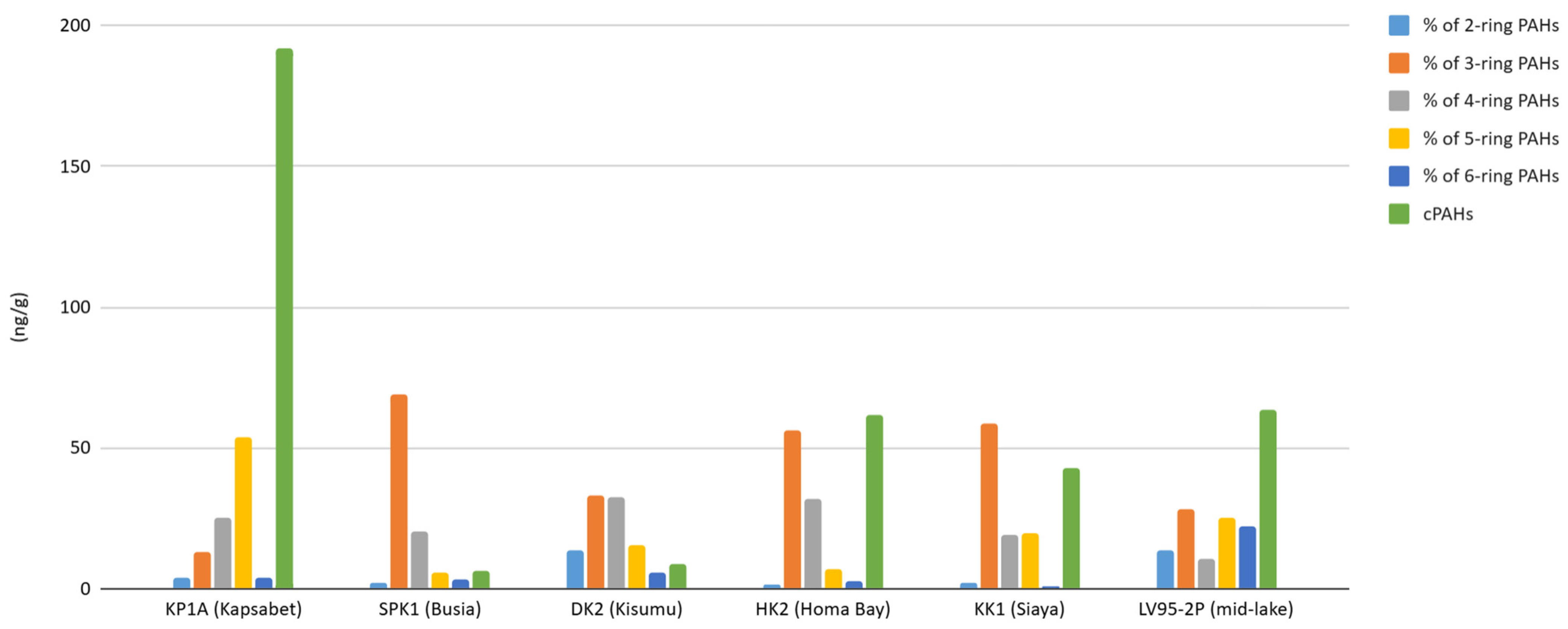
3.1. PAH Concentrations
3.2. PAH Ratios
3.2.1. ΣLow-Molecular-Weight PAH/Σ High-Molecular-Weight PAH Ratio
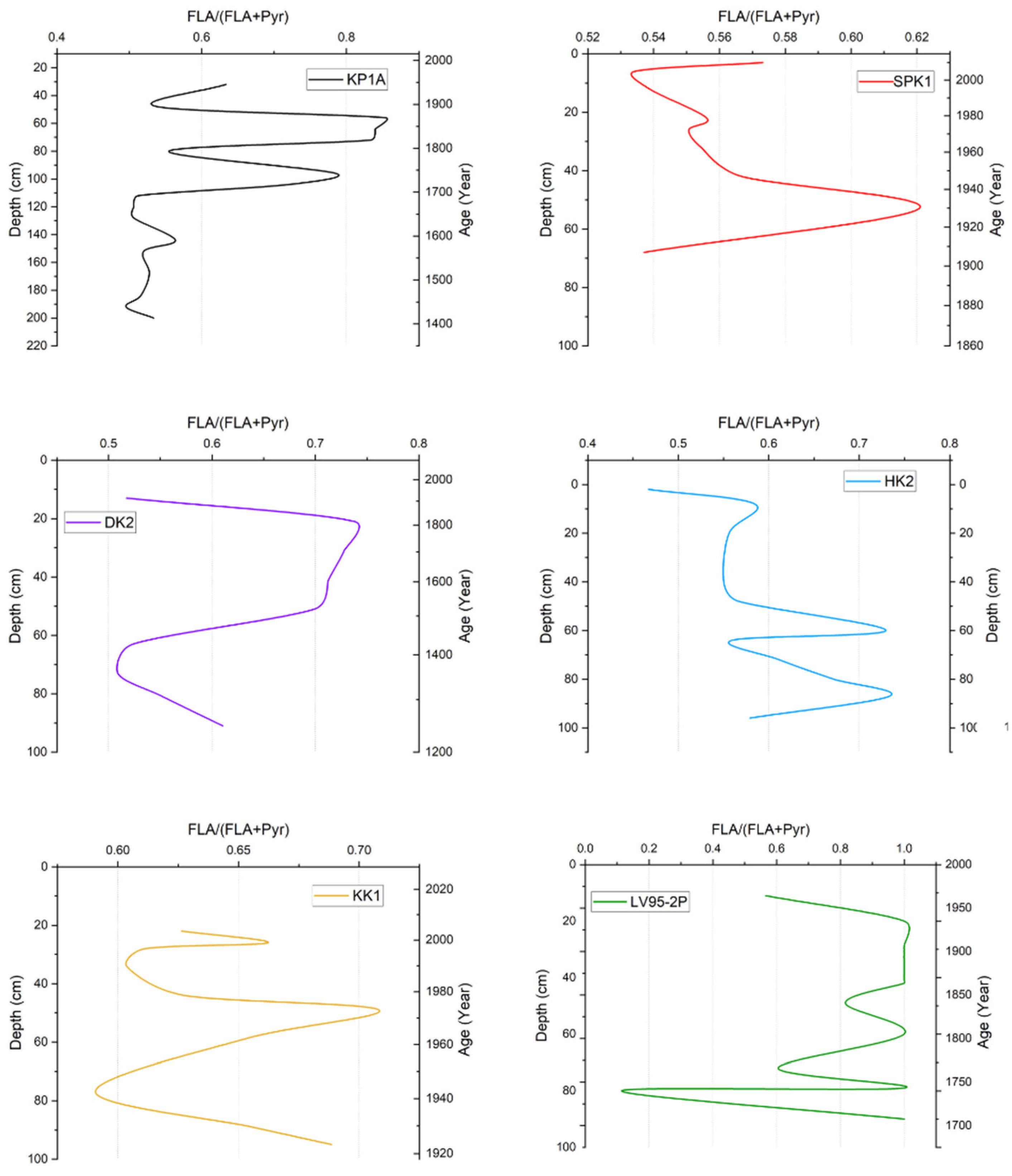
3.2.2. Fluoranthene/(Fluoranthene + Pyrene) Ratio
3.2.3. Indeno[1,2,3-cd]pyrene/Indeno[1,2,3-cd]pyrene + Benzo[g,h,i]perylene Ratio
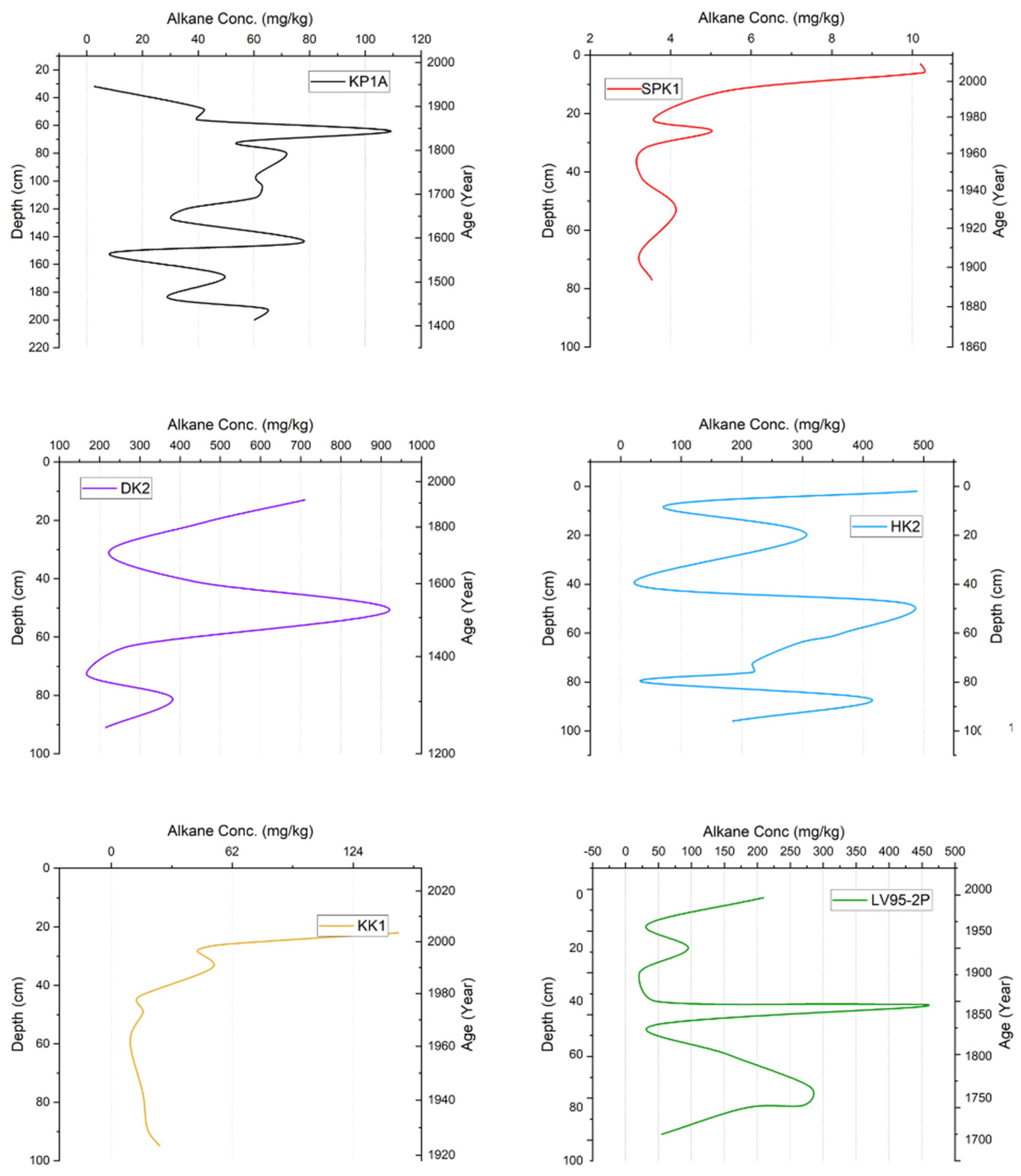
3.3. n-Alkane Concentrations
3.4. n-Alkane Ratios
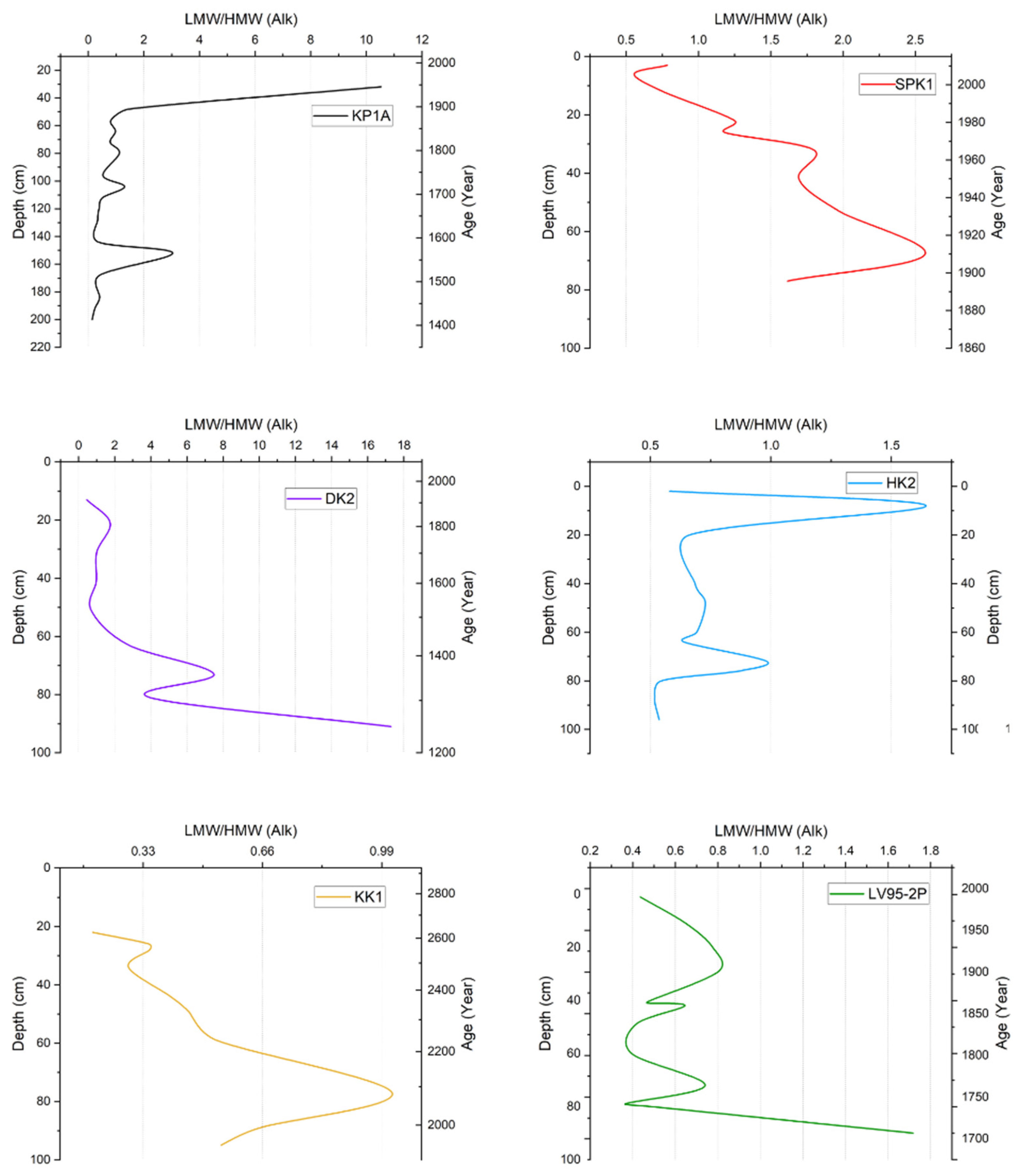
3.4.1. ΣLow-Molecular-Weight/ΣHigh-Molecular-Weight n-Alkane Ratio
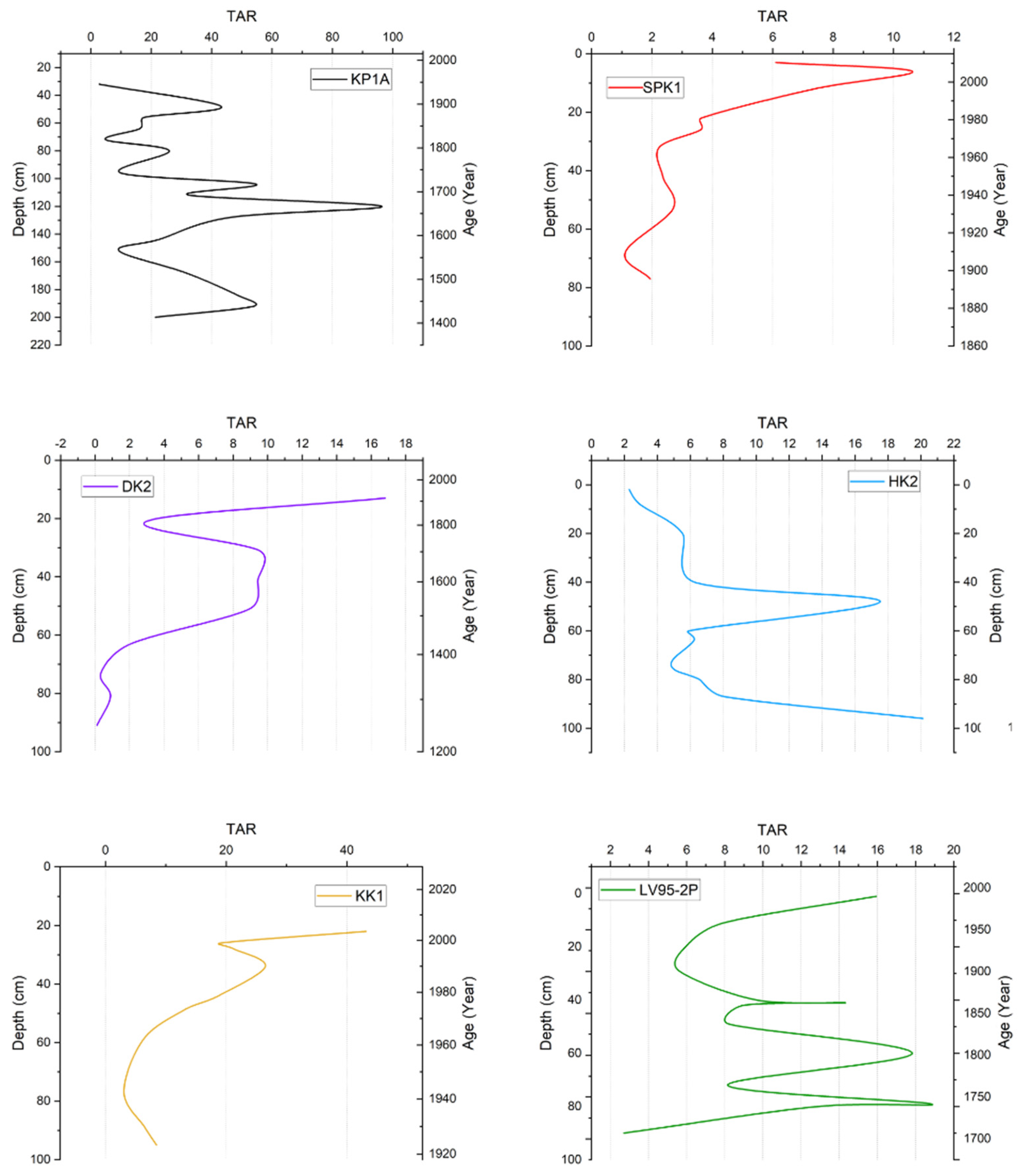
3.4.2. Terrigenous Aquatic Ratio
3.4.3. Carbon Preference Index
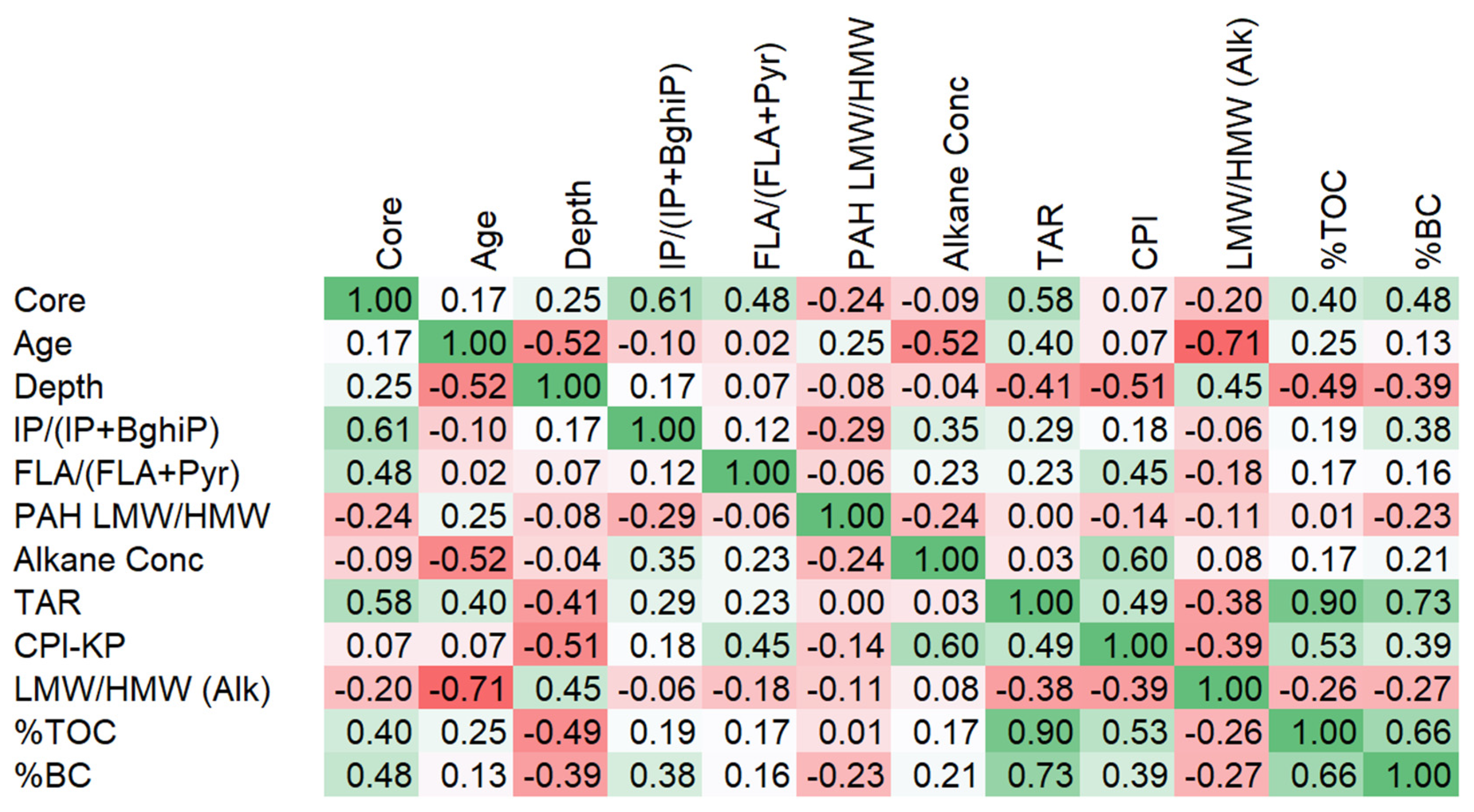
3.5. Statistical Trends
4. Discussion
4.1. Statistical Relevance
4.2. Geochemical Trends and Environmental Conditions
4.2.1. Kapsabet
4.2.2. Busia
4.2.3. Kisumu
4.2.4. Homa Bay
4.2.5. Siaya
4.2.6. Mid-Lake
4.3. PAH Characteristics and Risks
4.4. Land Use
4.5. Rural–Urban Influences and Timelines
- Natural/biogenic inputs of organic matter primarily influence rural areas. These regions are generally characterized by lower concentrations of PAHs and n-alkanes, a predominance of long-chain n-alkanes with high CPI values, and elevated TAR values, all indicating significant contributions from terrestrial sources. The sources include vegetation cover, agricultural practices, and the growing influence of industrialization. While organic matter inputs are primarily natural, the gradual rise in anthropogenic markers underscores the need for sustainable land use practices to protect both aquatic and terrestrial ecosystems.
- Urban areas reflect higher anthropogenic inputs. These are characterized by higher concentrations of BC, pyrolytic PAHs, and n-alkanes. The latter demonstrates elevated levels of short-chain n-alkanes with decreasing CPI and TAR values. The inputs comprise by-products from fossil fuel combustion, industrial discharge, urban runoff, and vehicular emissions. This is also evident in the mid-lake sediment core, which reveals a distinct shift in the accumulation of these compounds, mirroring trends observed in recent sediments in the catchment. Urban development and industrial activities drive increasing pollutant loads from diverse sources, posing significant ecological risks to the lake ecosystem.
- Pre-1960: PAH and n-alkane profiles consistent with biomass burning and traditional agricultural practices characterize this period. The dominance of long-chain n-alkanes with high CPI values indicates substantial inputs from terrestrial vegetation. The anthropogenic influence was minimal, and the organic matter inputs were largely biogenic.
- 1960s–1990s: Post-independence economic development and population growth drove a distinct shift in organic matter deposition trends, marked by rising pyrogenic PAH inputs that reflect increased fossil fuel combustion. There was also an increase in petroleum-derived n-alkanes from expanding transportation and industrial activities in the catchment. The decreasing CPI values and changes in n-alkane distributions indicate growing anthropogenic influence.
- Post-1990s: Urbanization has continued into the post-1990s period, as reflected in the most recent sediments obtained for this study. This period exhibits the highest concentrations of total PAHs and n-alkanes. The biomarker trends indicate evidence of mixed pyrogenic and petrogenic sources, with growing contributions from fossil fuel combustion. The variability in organic matter inputs reflects shifting energy and land use patterns.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kulk, G.; George, G.; Abdulaziz, A.; Menon, N.; Theenathayalan, V.; Jayaram, C.; Brewin, R.J.W.; Sathyendranath, S. Effect of Reduced Anthropogenic Activities on Water Quality in Lake Vembanad, India. Remote Sens. 2021, 13, 1631. [Google Scholar] [CrossRef]
- Walingo, M.K.; Liwenga, E.T.; Kangalawe, R.Y.M.; Madulu, N.F.; Kabumbuli, R. Perceived Impact of Land Use Changes and Livelihood Diversification Strategies of Communities in the Lake Victoria Basin of Kenya. J. Agric. Biotechnol. Sustain. Dev. 2009, 1, 69–78. [Google Scholar]
- Güereña, D.; Neufeldt, H.; Berazneva, J.; Duby, S. Water Hyacinth Control in Lake Victoria: Transforming an Ecological Catastrophe into Economic, Social, and Environmental Benefits. Sustain. Prod. Consum. 2015, 3, 59–69. [Google Scholar] [CrossRef]
- Wilson, D.C. Fisheries Management on Lake Victoria, Tanzania. In Proceedings of the Annual Meeting of the African Studies Association, New Brunswick, NJ, USA, 4–7 December 1993; pp. 4–7. [Google Scholar]
- SIDA Country Report 2007 Lake Victoria. 2008. Available online: https://www.sida.se/en/about-sida/publications-archive/lake-victoria (accessed on 1 February 2023).
- World Bank Reviving Lake Victoria by Restoring Livelihoods. Available online: https://www.worldbank.org/en/news/feature/2016/02/29/reviving-lake-victoria-by-restoring-livelihoods (accessed on 14 January 2025).
- Onyango, D.O.; Ikporukpo, C.O.; Taiwo, J.O.; Opiyo, S.B.; Otieno, K.O. Comparative Analysis of Land Use/Land Cover Change and Watershed Urbanization in the Lakeside Counties of the Kenyan Lake Victoria Basin Using Remote Sensing and GIS Techniques. Adv. J. 2021, 6, 671–688. [Google Scholar] [CrossRef]
- Battistel, D.; Argiriadis, E.; Kehrwald, N.; Spigariol, M.; Russell, J.M.; Barbante, C. Fire and Human Record at Lake Victoria, East Africa, during the Early Iron Age: Did Humans or Climate Cause Massive Ecosystem Changes? Holocene 2017, 27, 997–1007. [Google Scholar] [CrossRef]
- Kundu, R.; Aura, C.M.; Nyamweya, C.; Agembe, S.; Sitoki, L.; Lung’ayia, H.B.O.; Ongore, C.; Ogari, Z.; Werimo, K. Changes in Pollution Indicators in Lake Victoria, Kenya and Their Implications for Lake and Catchment Management. Lakes Reserv. Sci. Policy Manag. Sustain. Use 2017, 22, 199–214. [Google Scholar] [CrossRef]
- Muthoka, M.; Ogello, E.O.; Outa, N.O.; Ouko, K.O.; Obiero, K.O.; Mboya, J.B.; Mukaburu, B.O. Threats to Aquatic Biodiversity and Possible Management Strategies in Lake Victoria. Aquac. Fish Fish. 2024, 4, e143. [Google Scholar] [CrossRef]
- Njagi, D.M.; Routh, J.; Odhiambo, M.; Luo, C.; Basapuram, L.G.; Olago, D.; Klump, V.; Stager, C. A Century of Human-Induced Environmental Changes and the Combined Roles of Nutrients and Land Use in Lake Victoria Catchment on Eutrophication. Sci. Total Environ. 2022, 835, 155425. [Google Scholar] [CrossRef] [PubMed]
- Awange, J.L.; Ong’ang’a, O. Lake Victoria: Ecology, Resources, Environment; Springer Science & Business Media: Berlin, Germany, 2006; ISBN 978-3-540-32575-8. [Google Scholar]
- Hamilton, S. Lake Victoria Statistics from This Dataverse; Harvard Dataverse: Harvard, MA, USA, 2018. [Google Scholar]
- Opere, W.M.; John, M.; Ombori, O.; Kiulia, N.M. Identification of Enteroviruses along Lake Victoria Shoreline—A Potential Indicator of Sewage Pollution. Access Microbiol. 2022, 4, 334. [Google Scholar] [CrossRef] [PubMed]
- Scheren, P.A.G.M.; Zanting, H.A.; Lemmens, A.M.C. Estimation of Water Pollution Sources in Lake Victoria, East Africa: Application and Elaboration of the Rapid Assessment Methodology. J. Environ. Manag. 2000, 58, 235–248. [Google Scholar] [CrossRef]
- Nyamweya, C.S.; Natugonza, V.; Taabu-Munyaho, A.; Aura, C.M.; Njiru, J.M.; Ongore, C.; Mangeni-Sande, R.; Kashindye, B.B.; Odoli, C.O.; Ogari, Z.; et al. A Century of Drastic Change: Human-Induced Changes of Lake Victoria Fisheries and Ecology. Fish. Res. 2020, 230, 105564. [Google Scholar] [CrossRef]
- Verschuren, D.; Johnson, T.C.; Kling, H.J.; Edgington, D.N.; Leavitt, P.R.; Brown, E.T.; Talbot, M.R.; Hecky, R.E. History and Timing of Human Impact on Lake Victoria, East Africa. Proc. R. Soc. B Biol. Sci. 2002, 269, 289–294. [Google Scholar] [CrossRef]
- Gikuma-Njuru, P.; Rutagemwa, D.K.; Mugidde, R.; Hecky, R.E.; Mwebaza-Ndawula, L.; Mwirigi, P.M.; Abuodha, J.O.Z.; Waya, R.K.; Matovu, A.; Kinobe, J. Eutrophication of the Lake Victoria Ecosystem; South Eastern Kenya University: Kitui County, Kenya, 2005. [Google Scholar]
- Nyenje, P.M.; Foppen, J.W.; Uhlenbrook, S.; Kulabako, R.; Muwanga, A. Eutrophication and Nutrient Release in Urban Areas of Sub-Saharan Africa—A Review. Sci. Total Environ. 2010, 408, 447–455. [Google Scholar] [CrossRef]
- Stager, J.C.; Hecky, R.E.; Grzesik, D.; Cumming, B.F.; Kling, H. Diatom Evidence for the Timing and Causes of Eutrophication in Lake Victoria, East Africa. Hydrobiologia 2009, 636, 1–16. [Google Scholar] [CrossRef]
- Golterman, H.L.; de Oude, N.T. Eutrophication of Lakes, Rivers and Coastal Seas. In Water Pollution; Allard, B., Craun, G.F., de Oude, N.T., Falkenmark, M., Golterman, H.L., Lindstrom, T., Piver, W.T., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 79–124. ISBN 978-3-540-46685-7. [Google Scholar]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Gulf Professional Publishing: Houston, TX, USA, 2001. [Google Scholar]
- Boot, C.M.; Haddix, M.; Paustian, K.; Cotrufo, M.F. Distribution of Black Carbon in Ponderosa Pine Forest Floor and Soils Following the High Park Wildfire. Biogeosciences 2015, 12, 3029–3039. [Google Scholar] [CrossRef]
- Odhiambo, M.; Routh, J. Does Black Carbon Contribute to Eutrophication in Large Lakes? Curr Pollut. Rep 2016, 2, 236–238. [Google Scholar] [CrossRef]
- Roberts, G.; Wooster, M.J.; Lagoudakis, E. Annual and Diurnal African Biomass Burning Temporal Dynamics. Biogeosciences 2009, 6, 849–866. [Google Scholar] [CrossRef]
- Sinha, P.; Hobbs, P.V.; Yokelson, R.J.; Blake, D.R.; Gao, S.; Kirchstetter, T.W. Emissions from Miombo Woodland and Dambo Grassland Savanna Fires. J. Geophys. Res. 2004, 109, 2004JD004521. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, S.; Pant, P.; Dumka, U.C. Characteristics of Black Carbon over Delhi and Manora Peak-a Comparative Study. Atmosph. Sci. Lett. 2012, 13, 223–230. [Google Scholar] [CrossRef]
- Katz, S.D.; Kelly, R.P.; Robinson, R.S.; Pavia, F.J.; Pockalny, R.; Lohmann, R. Biomass Burning Is a Source of Modern Black Carbon to Equatorial Atlantic Ocean Sediments. Commun Earth Env. 2024, 5, 1–7. [Google Scholar] [CrossRef]
- Gustafsson, O.; Gschwend, P.M. The Flux of Black Carbon to Surface Sediments on the New England Continental Shelf. Geochim. Et Cosmochim. Acta 1998, 62, 465–472. [Google Scholar] [CrossRef]
- Hammes, K.; Schmidt, M.W.I.; Smernik, R.J.; Currie, L.A.; Ball, W.P.; Nguyen, T.H.; Louchouarn, P.; Houel, S.; Gustafsson, Ö.; Elmquist, M.; et al. Comparison of Quantification Methods to Measure Fire-Derived (Black-Elemental) Carbon in Soils and Sediments Using Reference Materials from Soil, Water, Sediment and the Atmosphere. Glob. Biogeochem. Cycles 2007, 21, 1–18. [Google Scholar] [CrossRef]
- Lipiatou, E.; Hecky, R.E.; Eisenreich, S.J.; Lockhart, L.; Muir, D.; Wilkinson, P. Recent Ecosystem Changes in Lake Victoria Reflected in Sedimentary Natural and Anthropogenic Organic Compounds. In Limnology, Climatology and Paleoclimatology of the East African Lakes; Routledge: London, UK, 1996; ISBN 978-0-203-74897-8. [Google Scholar]
- Soclo, H.H.; Garrigues, P.; Ewald, M. Origin of Polycyclic Aromatic Hydrocarbons (PAHs) in Coastal Marine Sediments: Case Studies in Cotonou (Benin) and Aquitaine (France) Areas. Mar. Pollut. Bull. 2000, 40, 387–396. [Google Scholar] [CrossRef]
- Balmer, J.E.; Hung, H.; Yu, Y.; Letcher, R.J.; Muir, D.C.G. Sources and Environmental Fate of Pyrogenic Polycyclic Aromatic Hydrocarbons (PAHs) in the Arctic. Emerg. Contam. 2019, 5, 128–142. [Google Scholar] [CrossRef]
- Pal, S.; Shrivastava, J.P.; Kalpana, M.S. Fire Activity across Cretaceous/Paleogene Transition: Evidence from Pyrogenic Biomarkers Preserved in the Mahadeo-Cherrapunji Section, Meghalaya, India. Geobiology 2023, 21, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.P.; Bong, C.-W.; Vaezzadeh, V. Fingerprinting of Petroleum Hydrocarbons in Malaysia Using Environmental Forensic Techniques. In Oil Spill Environmental Forensics Case Studies; Elsevier: London, UK, 2018; pp. 345–372. ISBN 978-0-12-804434-6. [Google Scholar]
- Meng, Y.; Liu, X.; Lu, S.; Zhang, T.; Jin, B.; Wang, Q.; Tang, Z.; Liu, Y.; Guo, X.; Zhou, J.; et al. A Review on Occurrence and Risk of Polycyclic Aromatic Hydrocarbons (PAHs) in Lakes of China. Sci. Total Environ. 2019, 651, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, R.; Li, P.; Wang, Y.; Tan, Y.; Ma, Y.; Yang, L.; Wu, L.; Du, Z.; Qi, X.; et al. Spatiotemporal Distribution, Sources Apportionment and Ecological Risks of PAHs: A Study in the Wuhan Section of the Yangtze River. Env. Geochem Health 2023, 45, 7405–7424. [Google Scholar] [CrossRef]
- Bianco, F.; Race, M.; Papirio, S.; Esposito, G. A Critical Review of the Remediation of PAH-Polluted Marine Sediments: Current Knowledge and Future Perspectives. Resour. Environ. Sustain. 2023, 11, 100101. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Lacustrine Organic Geochemistry—An Overview of Indicators of Organic Matter Sources and Diagenesis in Lake Sediments. Org. Geochem. 1993, 20, 867–900. [Google Scholar] [CrossRef]
- Bourbonniere, R.A.; Meyers, P.A. Sedimentary Geolipid Records of Historical Changes in the Watersheds and Productivities of Lakes Ontario and Erie. Limnol. Oceanogr. 1996, 41, 352–359. [Google Scholar] [CrossRef]
- Kuhn, T.K.; Krull, E.S.; Bowater, A.; Grice, K.; Gleixner, G. The Occurrence of Short Chain N-Alkanes with an Even over Odd Predominance in Higher Plants and Soils. Org. Geochem. 2010, 41, 88–95. [Google Scholar] [CrossRef]
- Liu, X.; Rendle-Bühring, R.; Henrich, R. High-and Low-Latitude Forcing of the East African Climate since the LGM: Inferred from the Elemental Composition of Marine Sediments off Tanzania. Quat. Sci. Rev. 2018, 196, 124–136. [Google Scholar] [CrossRef]
- Ranjan, R.K.; Routh, J.; Val Klump, J.; Ramanathan, A.L. Sediment Biomarker Profiles Trace Organic Matter Input in the Pichavaram Mangrove Complex, Southeastern India. Mar. Chem. 2015, 171, 44–57. [Google Scholar] [CrossRef]
- Wang, Z.; Stout, S.A.; Fingas, M. Forensic Fingerprinting of Biomarkers for Oil Spill Characterization and Source Identification. Environ. Forensics 2006, 7, 105–146. [Google Scholar] [CrossRef]
- Omuombo, C.; Williamson, D.; Olago, D. Biogeochemical Proxy Evidence of Gradual and Muted Geolimnological Response of Lake Nkunga, Mt. Kenya to Climate Changes and Human Influence during the Past Millennium. Sci. Afr. 2020, 8, e00416. [Google Scholar] [CrossRef]
- Ojany, F.F. The Physique of Kenya: A Contribution in Landscape Analysis. Ann. Assoc. Am. Geogr. 1966, 56, 183–196. [Google Scholar] [CrossRef]
- CGIAR. Women’s Empowerment and Climate Justice: Three Fish Preservation, Handling and Processing Interventions Improving Livelihoods in Kenya’s Lake Victoria Region; CGIAR: Montpellier, France, 2025. [Google Scholar]
- Odende, T.; Ogello, E.O.; Iteba, J.O.; Owori, H.; Outa, N.; Obiero, K.O.; Munguti, J.M.; Kyule, D.N.; Kimani, S.; Osia, M.M. Promoting Sustainable Smallholder Aquaculture Productivity Through Landscape and Seascape Aquapark Models: A Case Study of Busia County, Kenya. Front. Sustain. Food Syst. 2022, 6, 898044. [Google Scholar] [CrossRef]
- Okungu, J.O.; Njoka, S.; Abuodha, J.O.Z.; Hecky, R.E. An Introduction to Lake Victoria Catchment, Water Quality, Physical Limnology and Ecosystem Status (Kenyan Sector) [Chapter 1]. In Lake Victoria Environment Report Water Quality and Ecosystem Status: Kenya National Water Quality Synthesis Report. Inital Draft; Lake Victoria Environment Management Project (LVEMP): Kisumu, Kenya, 2005; pp. 1–27. [Google Scholar]
- Homabay County Homabay County | Agriculture & Agroprocessing. Available online: https://www.homabay.go.ke/investment-opportunities/agriculture-agroprocessing (accessed on 14 January 2025).
- Homa Bay Municipality. Available online: https://www.homabay.go.ke/department/homa-bay-municipality (accessed on 25 June 2024).
- MoALFC. Climate Risk Profile for Nandi County. Kenya County Climate Risk Profile Series; The Ministry of Agriculture, Livestock, Fisheries and Co-Operatives (MoALFC): Nairobi, Kenya, 2021; pp. 1–32. [Google Scholar]
- Hauser, S.; Norgrove, L. Slash-and-Burn Agriculture, Effects Of. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 551–562. ISBN 978-0-12-384720-1. [Google Scholar]
- Njagi, D.M.; Routh, J.; Olago, D.; Gayantha, K. A Multi-Proxy Reconstruction of the Late Holocene Climate Evolution in the Kapsabet Swamp, Kenya (East Africa). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 574, 110475. [Google Scholar] [CrossRef]
- Johnson, T.C.; Scholz, C.A.; Talbot, M.R.; Kelts, K.; Ricketts, R.D.; Ngobi, G.; Beuning, K.; Ssemmanda, I.; McGill, J.W. Late Pleistocene Desiccation of Lake Victoria and Rapid Evolution of Cichlid Fishes. Science 1996, 273, 1091–1093. [Google Scholar] [CrossRef]
- KNBS. Kenya Population and Housing Census Results–Kenya National Bureau of Statistics; KNBS: Nairobi, Kenya, 2019. [Google Scholar]
- Jansen, B.; Nierop, K.G.J.; Kotte, M.C.; de Voogt, P.; Verstraten, J.M. The Applicability of Accelerated Solvent Extraction (ASE) to Extract Lipid Biomarkers from Soils. Appl. Geochem. 2006, 21, 1006–1015. [Google Scholar] [CrossRef]
- Choudhary, P.; Routh, J. Distribution of Polycyclic Aromatic Hydrocarbons in Kumaun Himalayan Lakes, Northwest India. Org. Geochem. 2010, 41, 891–894. [Google Scholar] [CrossRef]
- Wolska, L.; Mechlińska, A.; Rogowska, J.; Namieśnik, J. Sources and Fate of PAHs and PCBs in the Marine Environment. Crit. Rev. Environ. Sci. Technol. 2012, 42, 1172–1189. [Google Scholar] [CrossRef]
- Davis, E.; Walker, T.R.; Adams, M.; Willis, R.; Norris, G.A.; Henry, R.C. Source Apportionment of Polycyclic Aromatic Hydrocarbons (PAHs) in Small Craft Harbor (SCH) Surficial Sediments in Nova Scotia, Canada. Sci. Total Environ. 2019, 691, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, J.; Mi, T.; Li, B. Occurrence of N-Alkanes and Polycyclic Aromatic Hydrocarbons in the Core Sediments of the Yellow Sea. Mar. Chem. 2001, 76, 1–15. [Google Scholar] [CrossRef]
- Meyers, P.A.; Ishiwatari, R. Organic Matter Accumulation Records in Lake Sediments. In Physics and Chemistry of Lakes; Lerman, A., Imboden, D.M., Gat, J.R., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; pp. 279–328. ISBN 978-3-642-85132-2. [Google Scholar]
- Hair, J.F. (Ed.) Multivariate Data Analysis, 7th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010; ISBN 978-0-13-813263-7. [Google Scholar]
- Chen, Y.; Wang, Y.; Yu, K.; Zhao, Z.; Lang, X. Occurrence Characteristics and Source Appointment of Polycyclic Aromatic Hydrocarbons and n-Alkanes over the Past 100 Years in Southwest China. Sci. Total Environ. 2022, 808, 151905. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Huang, C.; Chen, F.; Chen, C.; Zhang, H.; Deng, W.; Ye, F. Anthropogenic Influences on the Sources and Distribution of Organic Carbon, Black Carbon, and Heavy Metals in Daya Bay’s Surface Sediments. Mar. Pollut. Bull. 2023, 196, 115571. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Huang, Q.; Li, W.; Tang, Y.; Zhao, J. Source Apportionment of Polycyclic Aromatic Hydrocarbons (PAHs) in Surface Sediments of the Huangpu River, Shanghai, China. Sci. Total Environ. 2009, 407, 2931–2938. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Ross, P.S.; Johannessen, S.C.; Dangerfield, N. Alkane and PAH Provenance and Potential Bioavailability in Coastal Marine Sediments Subject to a Gradient of Anthropogenic Sources in British Columbia, Canada. Org. Geochem. 2015, 89, 80–116. [Google Scholar] [CrossRef]
- Henry, M.; Maingi, J. Characterizing Agricultural Burning in Kenya. Available online: https://storymaps.arcgis.com/stories/0dcc77fb4a0f429daa78dbd0fa682a1c (accessed on 22 July 2024).
- Mungai, T.M.; Wang, J. Heavy Metal Pollution in Suburban Topsoil of Nyeri, Kapsabet, Voi, Ngong and Juja Towns, in Kenya. SN Appl. Sci. 2019, 1, 1–11. [Google Scholar] [CrossRef]
- UNEP Toxic Blaze: The True Cost of Crop Burning. Available online: https://www.unep.org/news-and-stories/story/toxic-blaze-true-cost-crop-burning (accessed on 22 July 2024).
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front. Microbio.l 2020, 11, 562813. [Google Scholar] [CrossRef] [PubMed]
- Vaezzadeh, V.; Yi, X.; Rais, F.R.; Bong, C.W.; Thomes, M.W.; Lee, C.W.; Zakaria, M.P.; Wang, A.J.; Zhong, G.; Zhang, G. Distribution of Black Carbon and PAHs in Sediments of Peninsular Malaysia. Mar. Pollut. Bull. 2021, 172, 112871. [Google Scholar] [CrossRef] [PubMed]
- Olambo, E. KCC Factory to Open Soon in Kapsabet. Available online: https://nakala.fr/10.34847/nkl.87f4jz3g (accessed on 22 July 2024).
- Gao, Y.; Ling, Z.; Zhang, Z.; Lee, S. Characteristics of Fine Particulate Matter (PM2.5)-Bound n-Alkanes and Polycyclic Aromatic Hydrocarbons (PAHs) in a Hong Kong Suburban Area. Atmosphere 2022, 13, 980. [Google Scholar] [CrossRef]
- Berke, M.A. Reconstructing Terrestrial Paleoenvironments Using Sedimentary Organic Biomarkers. In Methods in Paleoecology: Reconstructing Cenozoic Terrestrial Environments and Ecological Communities; Croft, D.A., Su, D.F., Simpson, S.W., Eds.; Vertebrate Paleobiology and Paleoanthropology; Springer International Publishing: Cham, Switzerland, 2018; pp. 121–149. ISBN 978-3-319-94265-0. [Google Scholar]
- Keiluweit, M.; Kleber, M. Molecular-Level Interactions in Soils and Sediments: The Role of Aromatic π-Systems. Environ. Sci. Technol. 2009, 43, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Arellano, L.; Fernández, P.; van Drooge, B.L.; Rose, N.L.; Nickus, U.; Thies, H.; Stuchlík, E.; Camarero, L.; Catalan, J.; Grimalt, J.O. Drivers of Atmospheric Deposition of Polycyclic Aromatic Hydrocarbons at European High-Altitude Sites. Atmos. Chem. Phys. 2018, 18, 16081–16097. [Google Scholar] [CrossRef]
- Ayaa, D.D.; Kipterer, J.K. Effects of Changes in Use of Indigenous Knowledge Systems on Land Cover in Teso Busia County, Kenya. Afr. J. Environ. Sci. Technol. 2018, 12, 384–396. [Google Scholar] [CrossRef]
- Lorch, D. Where Uganda and Kenya Collide. The New York Times, 2 April 1994; 4. [Google Scholar]
- Ogutu, M.A. The Changing Role of Women in the Commercial History of Busia District in Kenya, 1900–1983. J. East. Afr. Res. Dev. 1985, 15, 74–90. [Google Scholar]
- Shilaro, P.M. A Failed Eldorado: Colonial Capitalism, Rural Industrialization, African Land Rights in Kenya, and the Kakamega Gold Rush, 1930–1952; University Press of America: Lanham, MD, USA, 2008; ISBN 978-0-7618-3606-3. [Google Scholar]
- Kumar, M.; Boski, T.; González-Vila, F.J.; de la Rosa, J.M.; González-Pérez, J.A. Discerning Natural and Anthropogenic Organic Matter Inputs to Salt Marsh Sediments of Ria Formosa Lagoon (South Portugal). Environ. Sci. Pollut. Res. 2020, 27, 28962–28985. [Google Scholar] [CrossRef]
- Wang, Z.; Fingas, M.; Page, D.S. Oil Spill Identification. J. Chromatogr. A 1999, 843, 369–411. [Google Scholar] [CrossRef]
- Yu, Q.; Gao, B.; Li, G.; Zhang, Y.; He, Q.; Deng, W.; Huang, Z.; Ding, X.; Hu, Q.; Huang, Z.; et al. Attributing Risk Burden of PM2.5-Bound Polycyclic Aromatic Hydrocarbons to Major Emission Sources: Case Study in Guangzhou, South China. Atmos. Environ. 2016, 142, 313–323. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Anthropogenic PAHs in Lake Sediments: A Literature Review (2002–2018). Environ. Sci. Process. Impacts 2018, 20, 1649–1666. [Google Scholar] [CrossRef] [PubMed]
- Kimutai, S.K.; Talai, S.M. Household Energy Utilization Trends in Kenya: Effects of Peri Urbanization. Eur. J. Energy Res. 2021, 1, 7–11. [Google Scholar] [CrossRef]
- Nassali, J.; Yongji, Z.; Fangninou, F.F. A Systematic Review of Threats to the Sustainable Utilization of Transboundary Fresh Water Lakes: A Case Study of Lake Victoria. Int. J. Sci. Res. Publ. 2020, 10, 657–668. [Google Scholar] [CrossRef]
- Nasrabadi, T.; Ruegner, H.; Schwientek, M.; Ghadiri, A.; Hashemi, S.H.; Grathwohl, P. Dilution of PAHs Loadings of Particulate Matter in Air, Dust and Rivers in Urban Areas: A Comparative Study (Tehran Megacity, Iran and City of Tübingen, SW-Germany). Sci. Total Environ. 2022, 806, 151268. [Google Scholar] [CrossRef] [PubMed]
- Akenga, T.; Sudoi, V.; Machuka, W.; Kerich, E. Heavy Metal Concentrations in Agricultural Farms in Homa Hills Homa Bay County, Kenya. Int. J. Sci. Res. 2016, 5, 1664–1669. [Google Scholar] [CrossRef]
- Kitheka, E.; Ogutu, C.; Oduor, N.; Ingutia, C.; Muga, M.; Githiomi, J. Piloting Biomass Energy Audit for Enery and Environmental Conservation in Homa Bay County Kenya. East Afr. Agric. For. J. 2021, 84(1), 136–146. [Google Scholar]
- Kogo, B.K.; Kumar, L.; Koech, R. Analysis of Spatio-Temporal Dynamics of Land Use and Cover Changes in Western Kenya. Geocarto Int. 2021, 36, 376–391. [Google Scholar] [CrossRef]
- Nyambati, R.O.; Ojung’a, S.O.; Gachie, P.; Okeyo, M.M. Kenya Forestry Research Institute, Kenya Status of Fruit Trees Farming among Small Scale Farmers: A Case Study of Busia, Bungoma and Siaya Counties of Kenya. J. Agrofor. Environ. 2022, 15, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lay, J.; Mahmoud, T.O.; M’Mukaria, G.M. Few Opportunities, Much Desperation: The Dichotomy of Non-Agricultural Activities and Inequality in Western Kenya. World Dev. 2008, 36, 2713–2732. [Google Scholar] [CrossRef]
- Odinga, E.O.; Mamboleo, D.; Nyantika, D. Assessing the Contributions and Quality of Various Food Crops Harvested by Household on Food Security in Ugenya Sub-County, Siaya County, Kenya. Res. J. Adv. Soc. Sci. 2022, 3, 66–77. [Google Scholar] [CrossRef]
- Abura, B.A. Impact of Artisanal and Small-Scale Gold Mining on Poverty Alleviation: A Case Study of Central-Sakwa Location in Siaya County. Master’s Thesis, University of Nairobi, Nairobi, Kenya, 2013. [Google Scholar]
- Kerebba, N.; Ssebugere, P.; Kwetegyeka, J.; Arinaitwe, K.; Wasswa, J. Concentrations and Sources Apportionment of Polycyclic Aromatic Hydrocarbons in Sediments from the Uganda Side of Lake Victoria. Env. Sci Process Impacts 2017, 19, 570–577. [Google Scholar] [CrossRef]
- Ngugi, C.C.; Oyoo-Okoth, E.; Gichuki, J.; Gatune, C.; Mwangi-Kinyanjui, J. Fingerprints of Upstream Catchment Land Use in Suspended Particulate Organic Matter (SPOM) at the River Discharge Sites in Lake Victoria (Kenya): Insights from Element, Stable Isotope and Lipid Biomarker Analysis. Aquat Sci 2017, 79, 73–87. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Johnson, T.C.; Kelts, K.; Odada, E. The Holocene History of Lake Victoria. Ambio 2000, 29, 2–11. [Google Scholar] [CrossRef]
- Mati, B.M.; Thomas, M.K. Overview of Sugar Industry in Kenya and Prospects for Production at the Coast. Agric. Sci. 2019, 10, 1477–1485. [Google Scholar] [CrossRef]
- Shilla, D.J.; Routh, J. Distribution, Behavior, and Sources of Polycyclic Aromatic Hydrocarbon in the Water Column, Sediments and Biota of the Rufiji Estuary, Tanzania. Front. Earth Sci. 2018, 6, 70. [Google Scholar] [CrossRef]






| Ratio | Background | Interpretations | Reference |
|---|---|---|---|
| ΣLow − Molecular Weight PAHs/ ΣHigh − Molecular Weight PAH | LMW PAHs with two or three fused aromatic rings HMW PAHs with five to six fused aromatic rings | <1 attributed to pyrogenic origin >1 attributed to petrogenic origin | [34,43,58,59] |
| FLA/(FLA + Pyr)[Flouranthene/(Fluoranthene + Pyrene)] | If PAHs are from petroleum or other combustion sources | Pyrolytic origin (>0.5) Petrogenic origin (<0.4) | [34,60,61] |
| IP/(IP + BghiP) [Indeno (1,2,3-cd) pyrene/Indeno[1,2,3-cd]pyrene + benzo[ghi]perylene)] | Identify if the PAH is from petroleum, coal combustion, or other combustion sources | <0.2—petroleum >0.5—wood, grass, and/or coal combustion Between 0.2 and 0.5—petroleum combustion | [60] |
| Ratio | Background | Interpretation | Reference |
|---|---|---|---|
| Low-Molecular-Weight n-Alkanes (C10–23)/High-Molecular-Weight n-Alkanes (C24–C36) | LMW alkanes are attributed to the abundance of natural or anthropogenic petroleum input HMW indicates an abundance of degraded oil or terrestrial plants | <1 indicates heavy and degraded oil and terrestrial plant origin >1 suggests a sign of fresh oil input, e.g., petrol, diesel | [35,44] |
| Terrigenous Aquatic Ratio (TAR) | TAR is used to identify the source of n-alkanes by calculating the abundance of C27, C29, and C31 n-alkanes (from terrestrial plants) in comparison to C17, C19, and C21 n-alkanes (from algal input) | >1 indicates land-based biogenic sources <1 indicates aquatic/biogenic source | [40] |
| Carbon Preference Index (CPI) | CPI is used to identify n-alkane sources, where C21-C25 alkanes are attributed to emergent plants and C27-C35 n-alkanes are attributed to terrestrial plants | Value from 5 to 10 indicates terrestrial plant origin Value close to 1 indicates petrogenic inputs | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enalbes, C.J.; Njagi, D.M.; Luo, C.; Olago, D.; Routh, J. Reconstructing Historical Land Use and Anthropogenic Inputs in Lake Victoria Basin: Insights from PAH and n-Alkane Trends. Toxics 2025, 13, 130. https://doi.org/10.3390/toxics13020130
Enalbes CJ, Njagi DM, Luo C, Olago D, Routh J. Reconstructing Historical Land Use and Anthropogenic Inputs in Lake Victoria Basin: Insights from PAH and n-Alkane Trends. Toxics. 2025; 13(2):130. https://doi.org/10.3390/toxics13020130
Chicago/Turabian StyleEnalbes, Camille Joy, Dennis M. Njagi, Chen Luo, Daniel Olago, and Joyanto Routh. 2025. "Reconstructing Historical Land Use and Anthropogenic Inputs in Lake Victoria Basin: Insights from PAH and n-Alkane Trends" Toxics 13, no. 2: 130. https://doi.org/10.3390/toxics13020130
APA StyleEnalbes, C. J., Njagi, D. M., Luo, C., Olago, D., & Routh, J. (2025). Reconstructing Historical Land Use and Anthropogenic Inputs in Lake Victoria Basin: Insights from PAH and n-Alkane Trends. Toxics, 13(2), 130. https://doi.org/10.3390/toxics13020130







