Involvement of the Gut–Lung Axis in LMW-PAHs-Induced Pulmonary Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Chemicals
2.2. Histopathological Observation of Lung and Colon Tissues
2.3. Measurement of Oxidative Stress Levels in Lung Tissue
2.4. Detection of Inflammatory Factors in Lung Tissue
2.5. Western Blotting
2.6. Gut Microbiota and 16S rRNA Sequencing
2.7. Untargeted Metabolomics of Lung and Colonic Contents
2.8. Bioinformatics and Statistical Analysis
3. Results
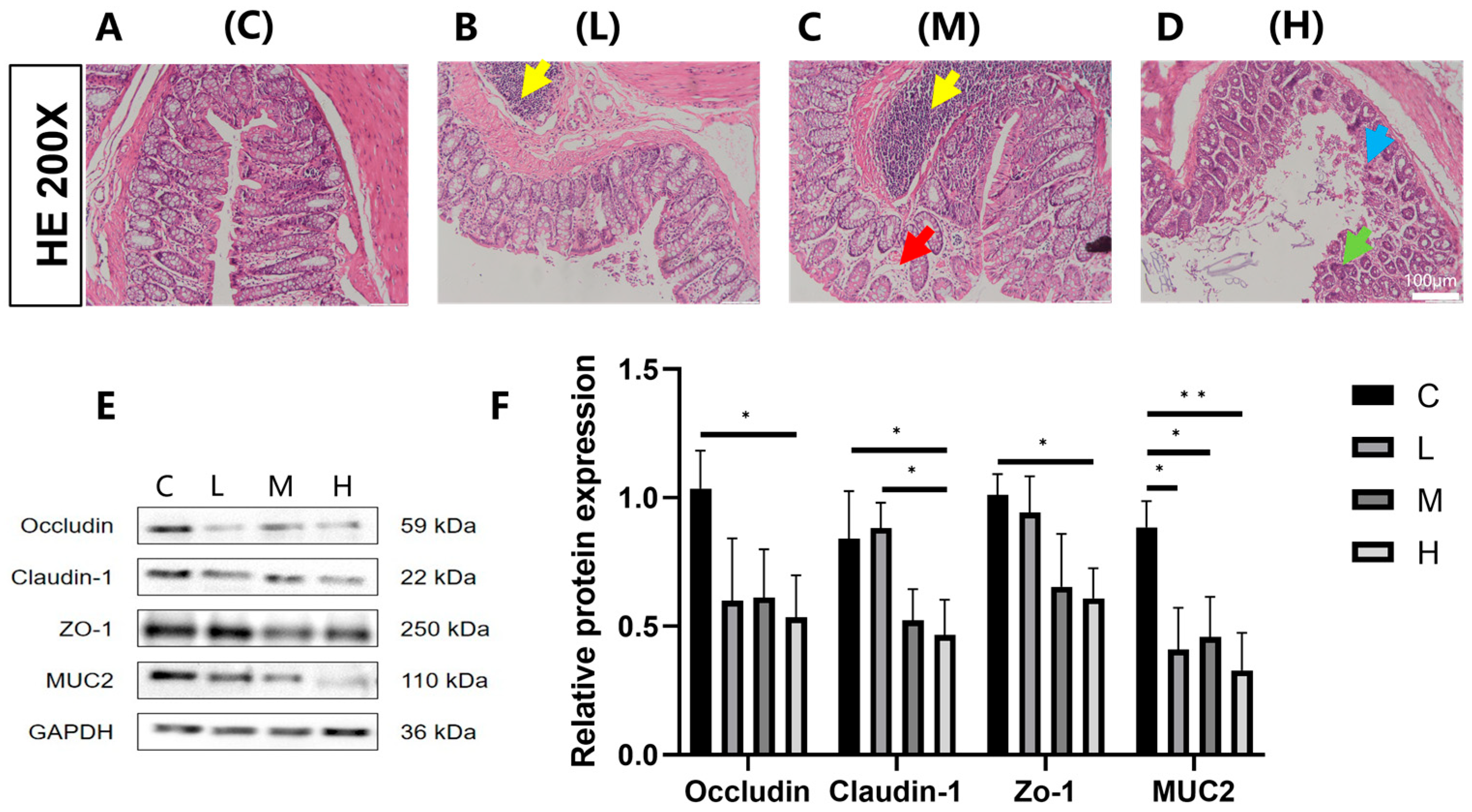
3.1. Effects of Phe and Flu Exposure on the Intestinal Barrier
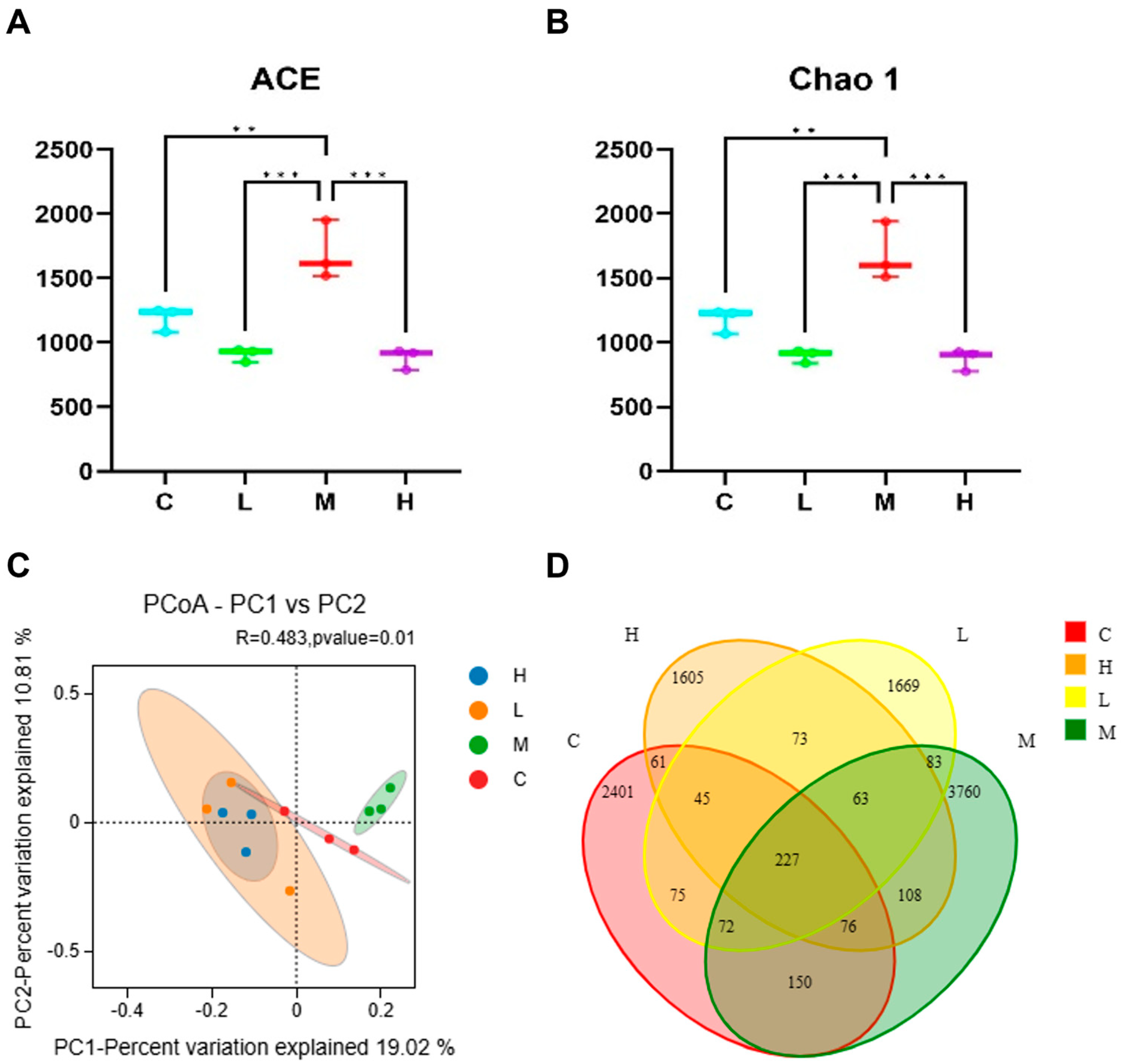
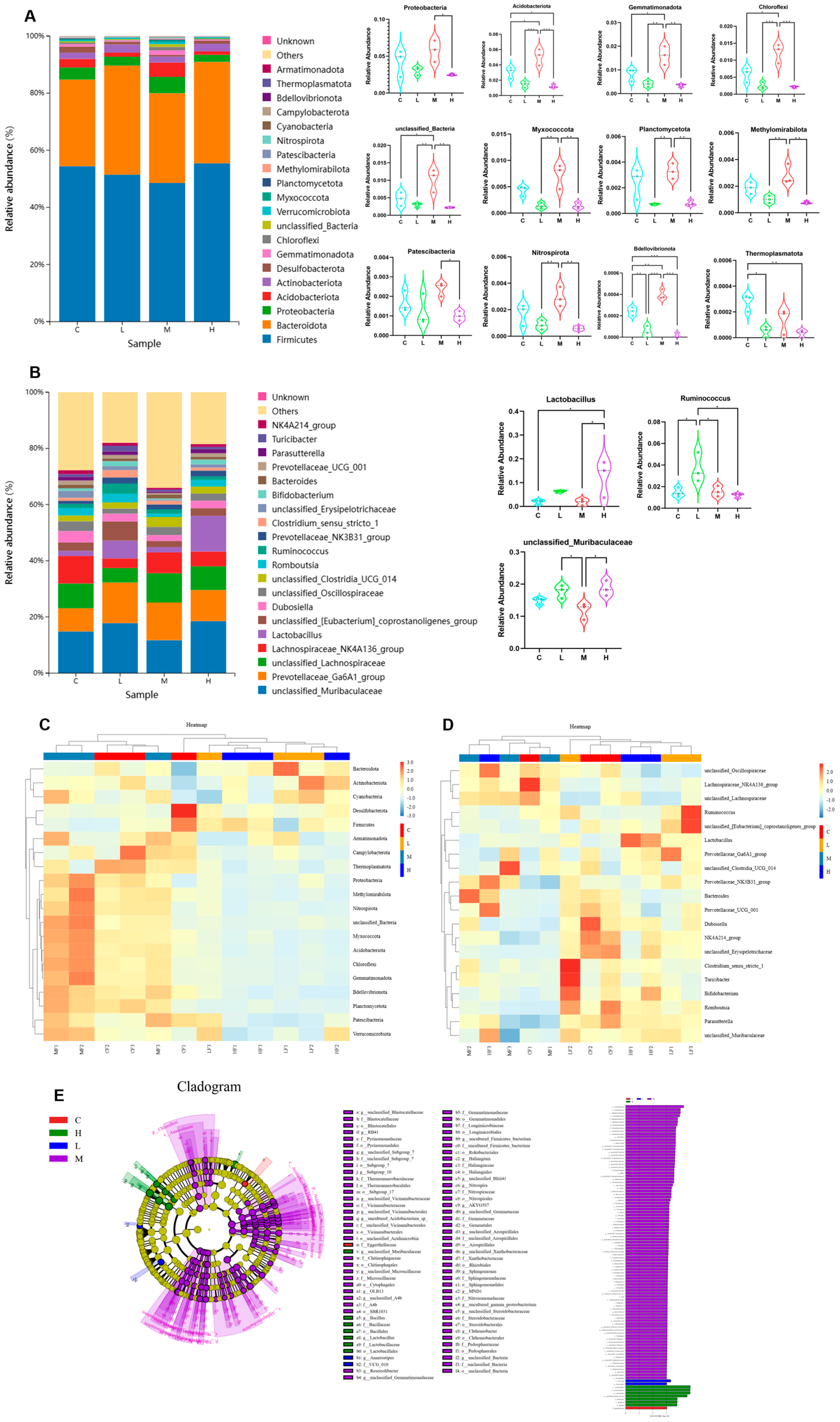
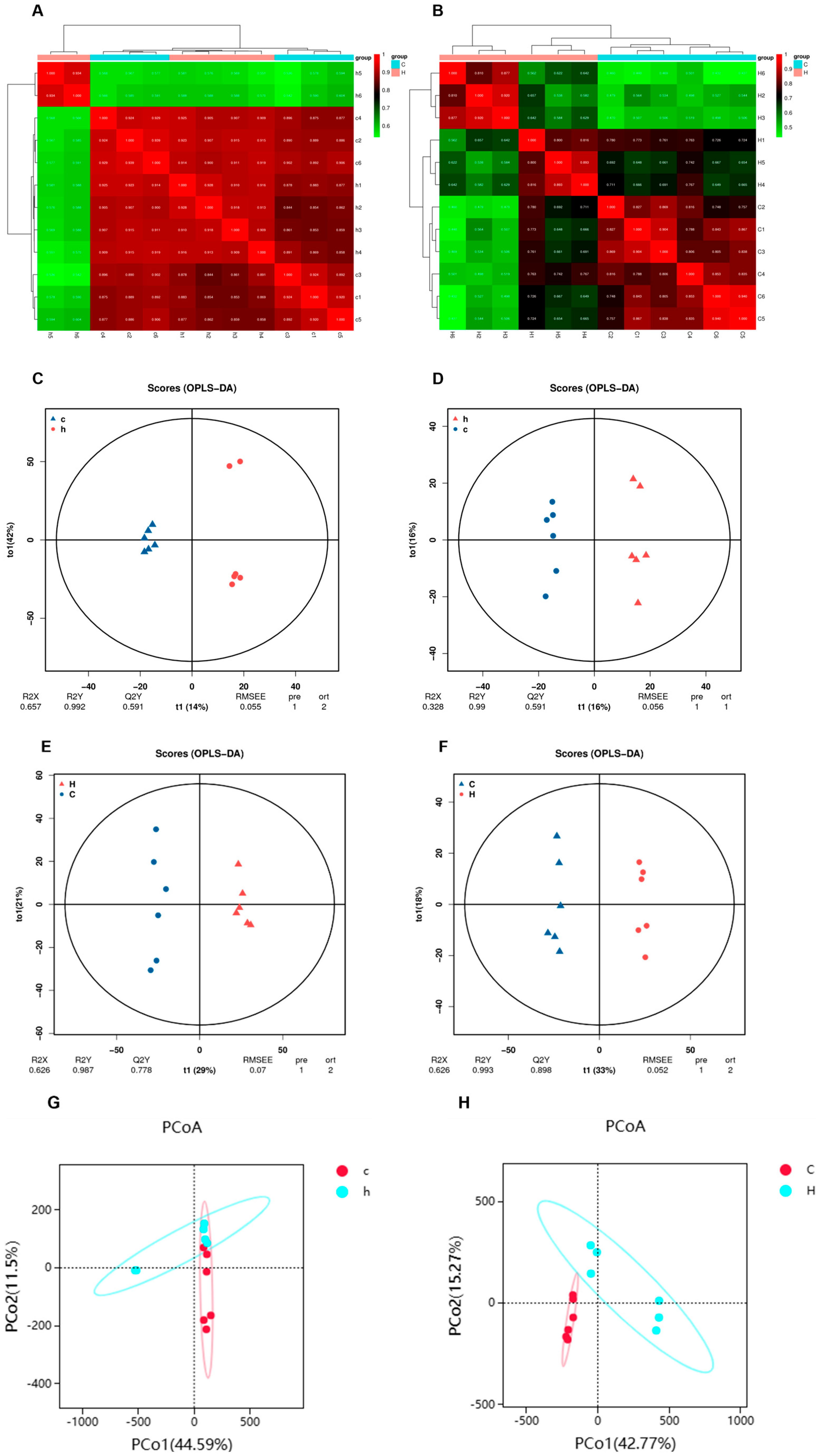
3.2. Effects of Phe and Flu Exposure on Gut Microbiota
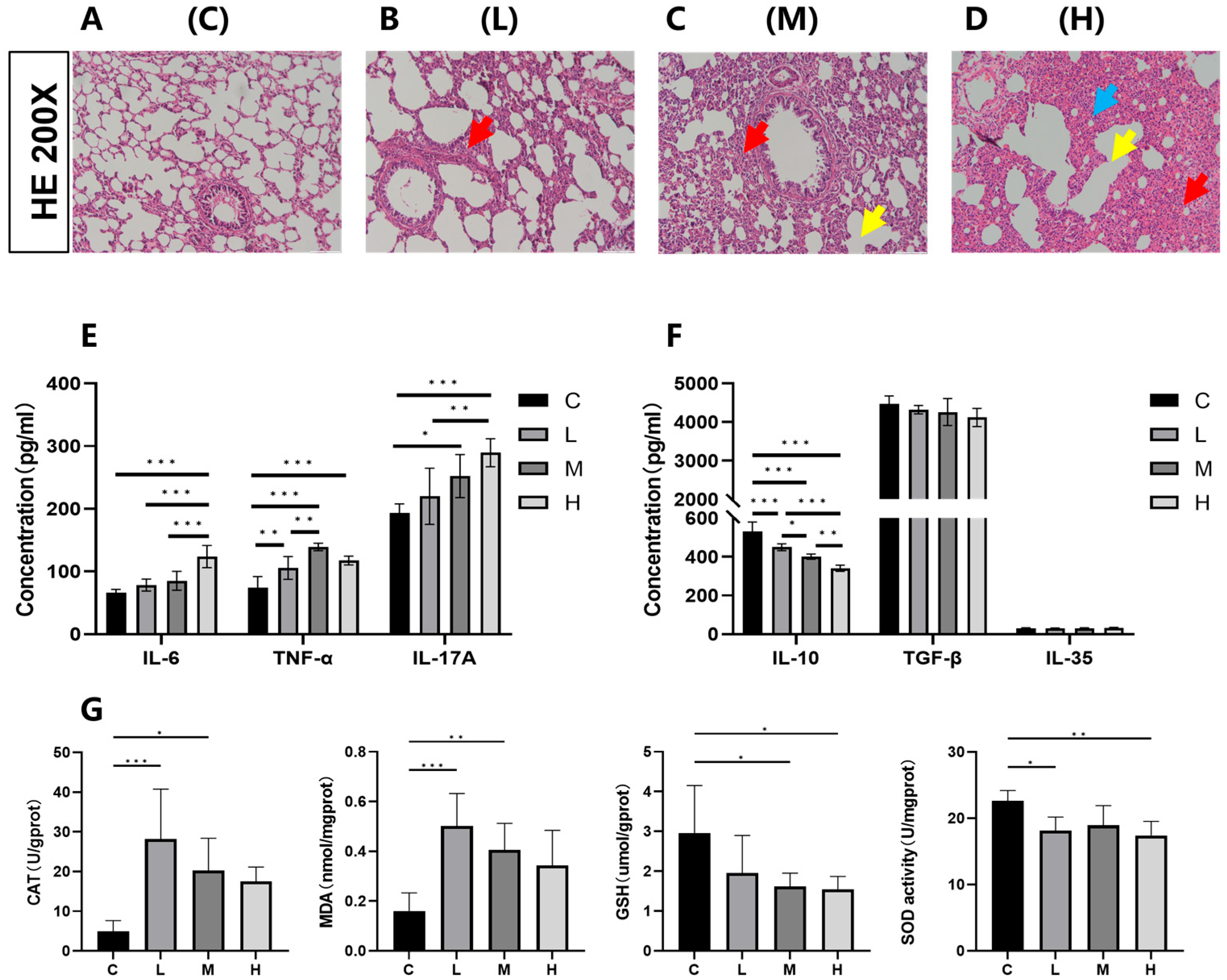
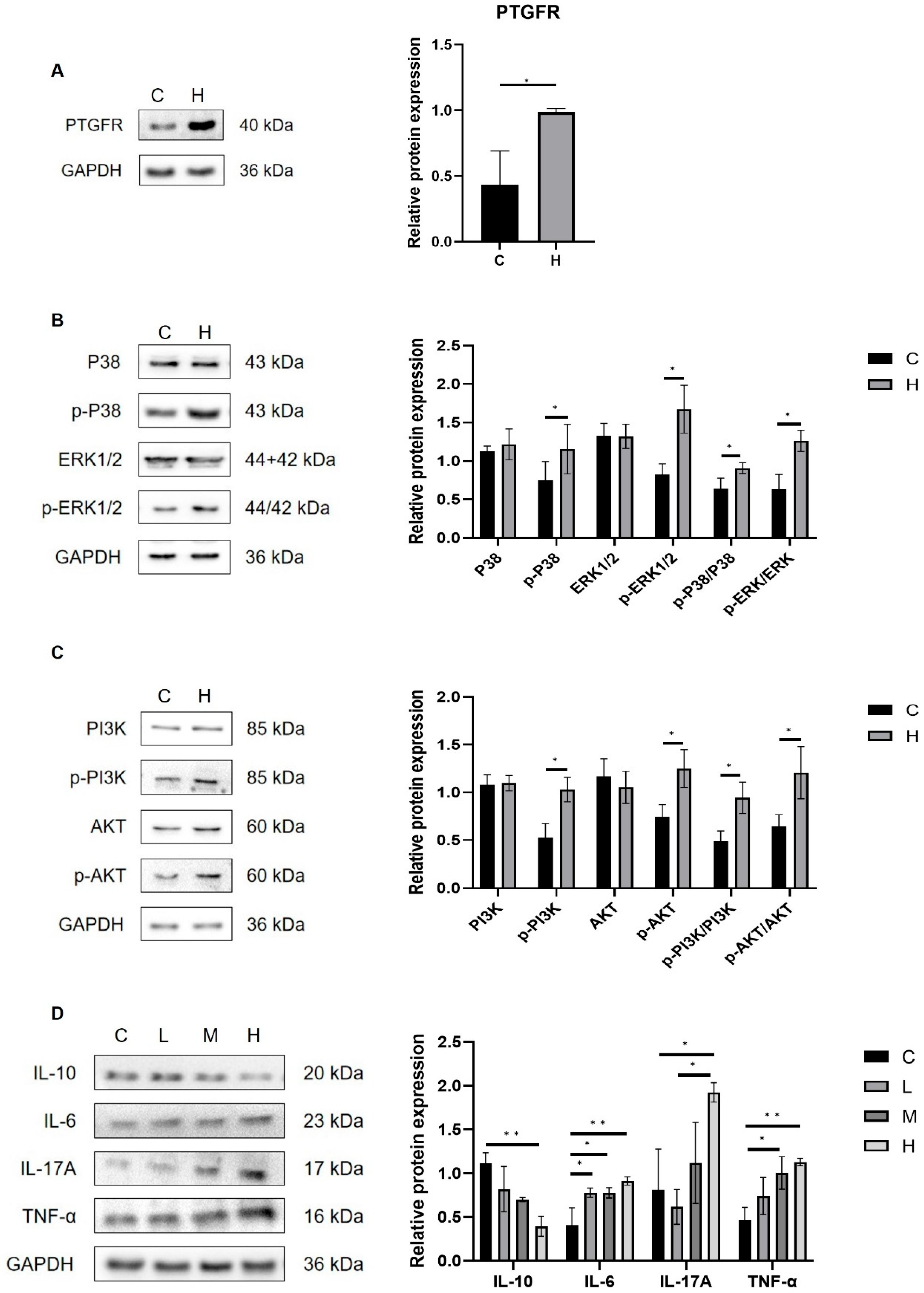
3.3. Effects of Phe and Flu Exposure on Pulmonary Inflammation and Oxidative Stress
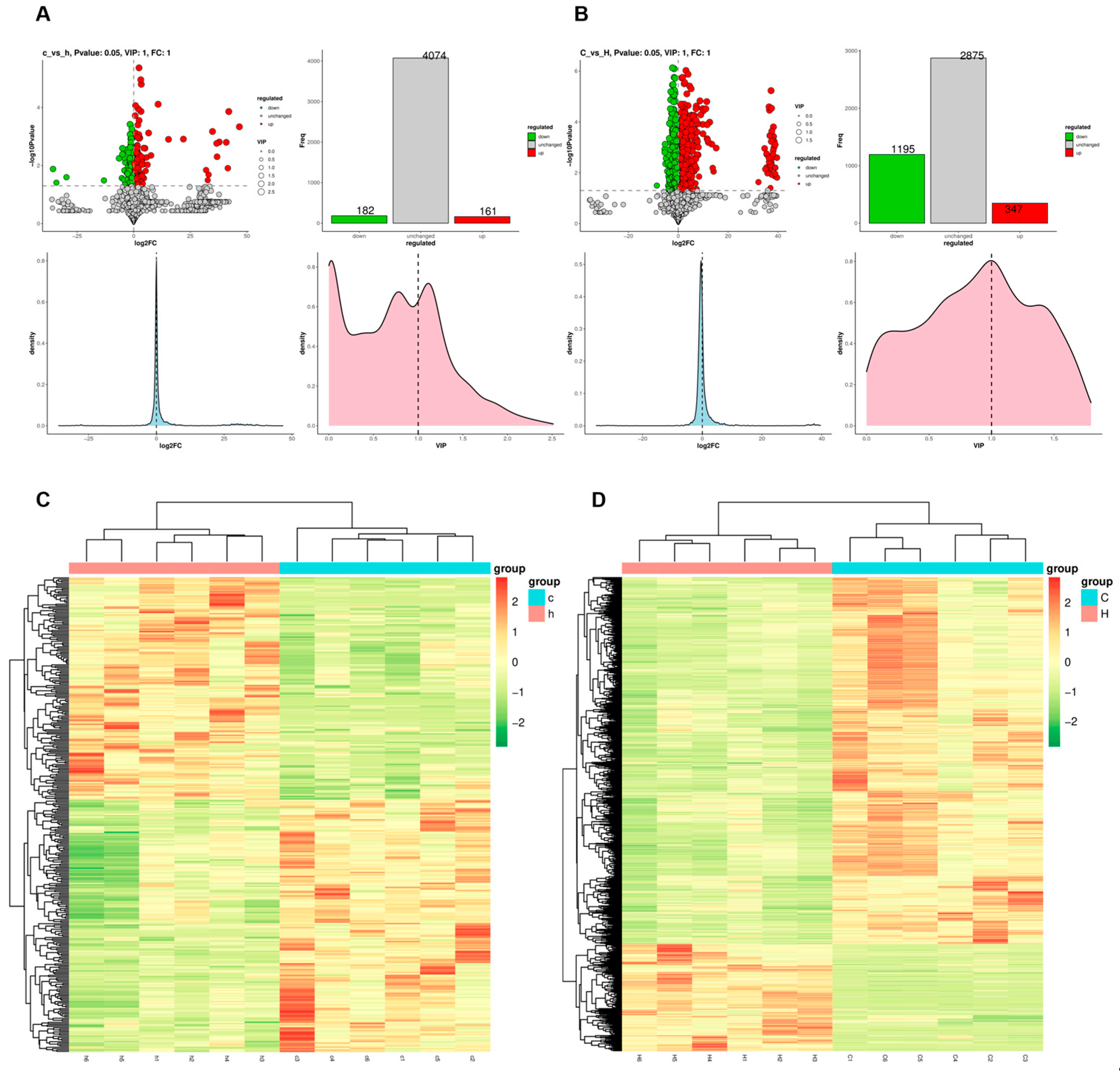
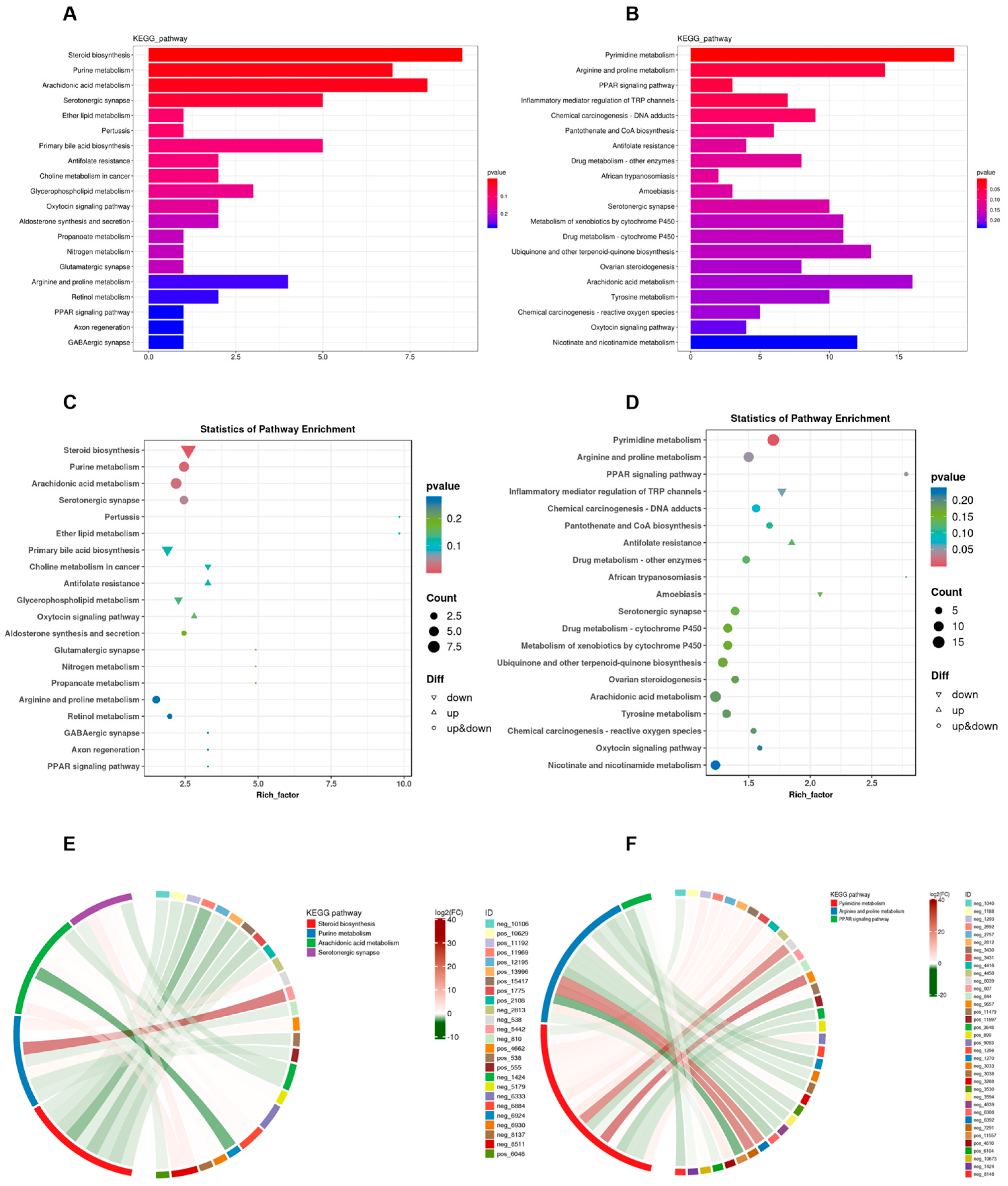
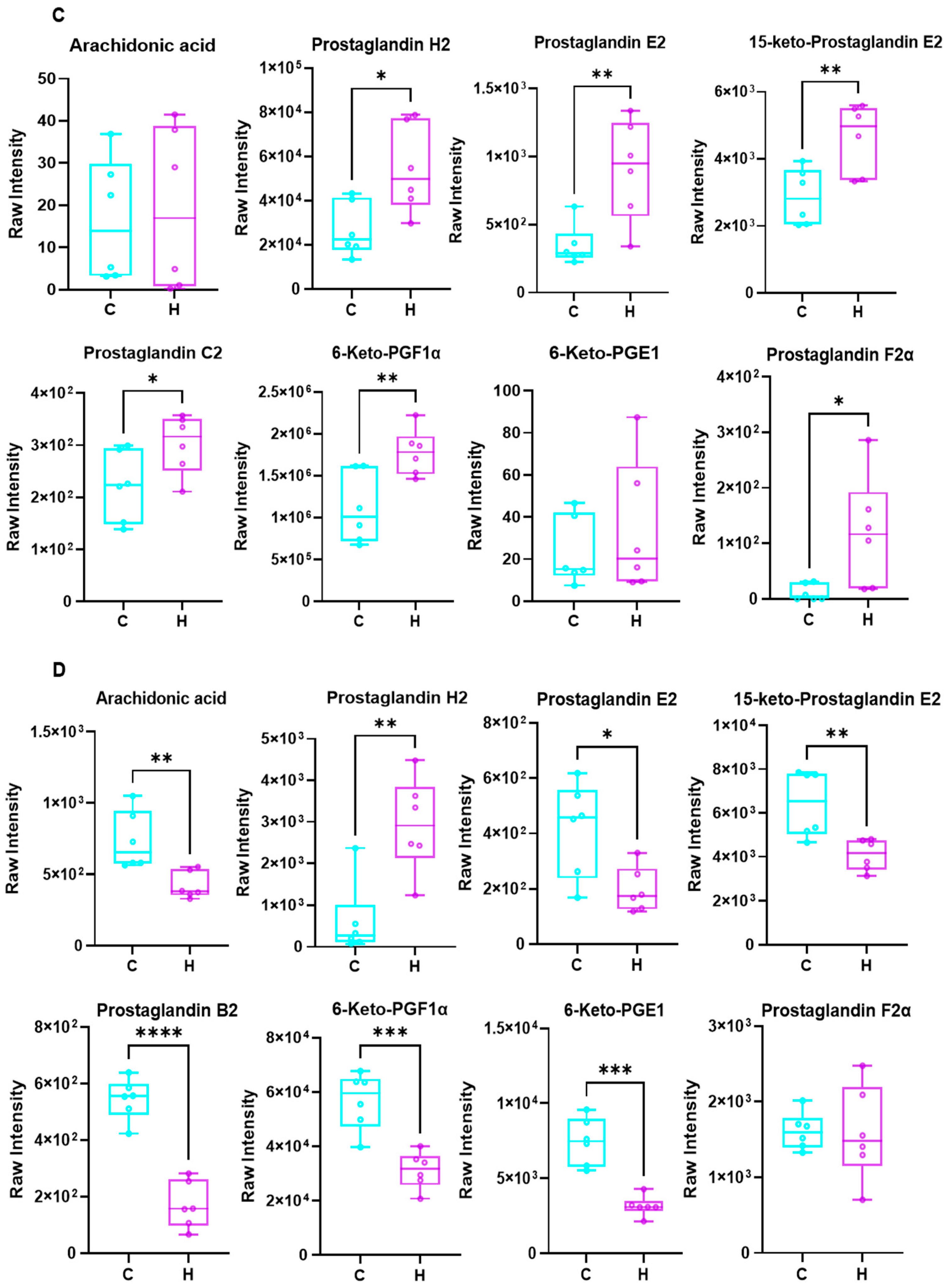
3.4. Effects of Phe and Flu on Lung and Colon Metabolism
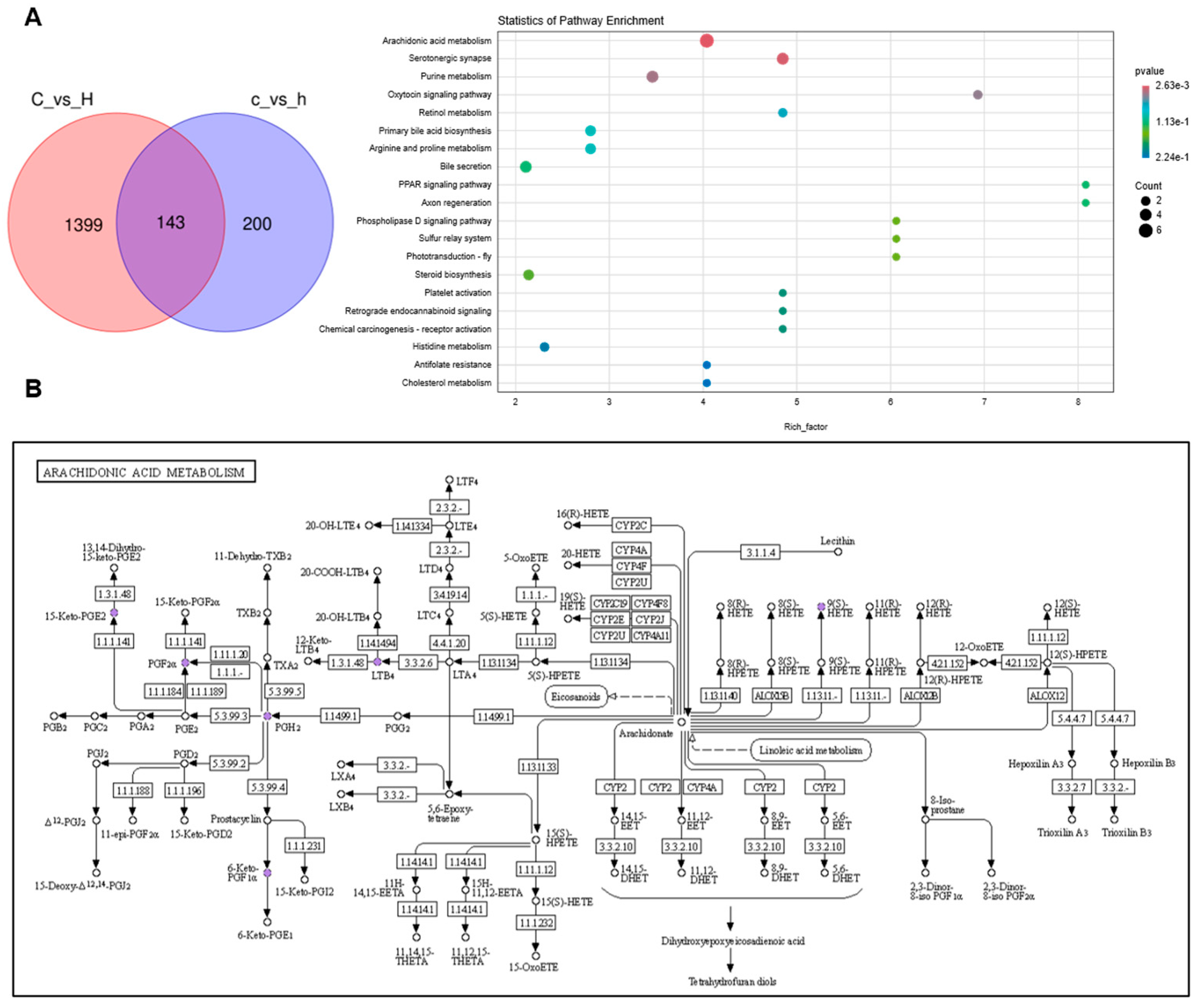
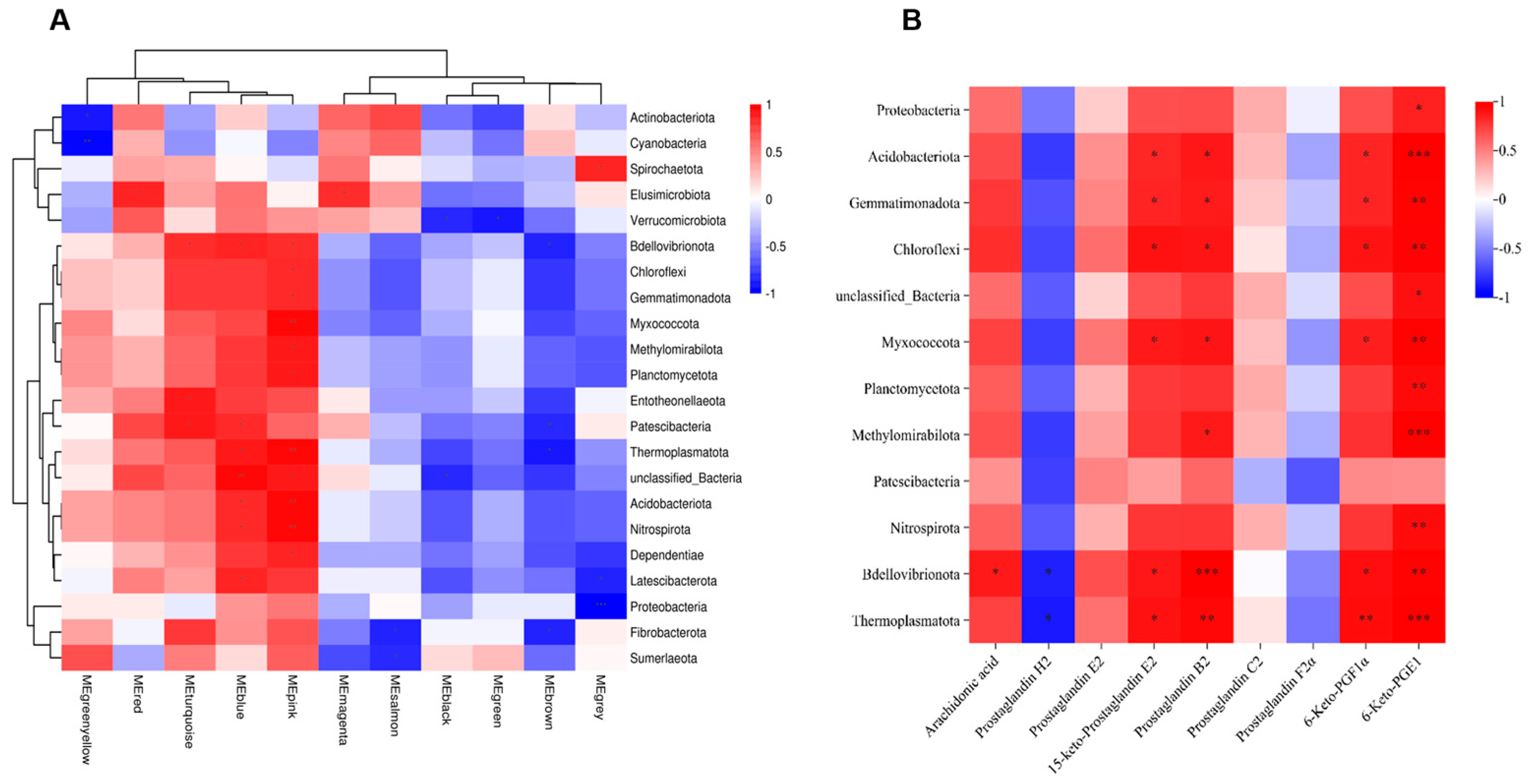
3.5. Joint Analysis Between the Gut Microbiota and Metabolic Profiles
3.6. Metabolites of AA Mediate Pulmonary Inflammation via the Gut–Lung Axis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, P.; Liu, B.; Chen, H.; Wang, H.; Guo, X.; Yuan, J. PAHs as environmental pollutants and their neurotoxic effects. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 283, 109975. [Google Scholar] [CrossRef]
- Ifegwu, O.C.; Anyakora, C. Polycyclic Aromatic Hydrocarbons: Part I. Exposure. Adv. Clin. Chem. 2015, 72, 277–304. [Google Scholar]
- Styszko, K.; Pamuła, J.; Pac, A.; Sochacka-Tatara, E. Biomarkers for polycyclic aromatic hydrocarbons in human excreta: Recent advances in analytical techniques-a review. Environ. Geochem. Health 2023, 45, 7099–7113. [Google Scholar] [CrossRef]
- Dai, X.; Ai, Y.; Wu, Y.; Li, Z.; Kang, N.; Zhang, T.; Tao, Y. Multiple exposure pathways and health risk assessment of PAHs in Lanzhou city, a semi-arid region in northwest China. Environ. Res. 2024, 252 Pt 4, 118867. [Google Scholar] [CrossRef]
- Colvin, V.C.; Bramer, L.M.; Rivera, B.N.; Pennington, J.M.; Waters, K.M.; Tilton, C.S. Modeling PAH Mixture Interactions in a Human In Vitro Organotypic Respiratory Model. Int. J. Mol. Sci. 2024, 25, 4326. [Google Scholar] [CrossRef] [PubMed]
- Yamini, V.; Rajeswari, V.D. Metabolic capacity to alter polycyclic aromatic hydrocarbons and its microbe-mediated remediation. Chemosphere 2023, 329, 138707. [Google Scholar] [CrossRef]
- Iwegbue, C.M. Polycyclic aromatic hydrocarbons profile of kitchen dusts. Bull. Environ. Contam. Toxicol. 2011, 86, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Gevao, B.; Al-Bahloul, M.; Zafar, J.; Al-Matrouk, K.; Helaleh, M. Polycyclic aromatic hydrocarbons in indoor air and dust in Kuwait: Implications for sources and nondietary human exposure. Arch. Environ. Contam. Toxicol. 2007, 53, 503–512. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhu, L.; Yan, L.; Lu, D.; Zhang, Q.; Zhao, M.; Li, Z. Never deem lightly the “less harmful” low-molecular-weight PAH, NPAH, and OPAH-Disturbance of the immune response at real environmental levels. Chemosphere 2017, 168, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, Y.; Wang, H.; Zhang, Z.; Li, C.; Hu, F.; Zhang, W.; Liu, Y.; Zeng, Y.; Wang, J. Low molecular weight-PAHs induced inflammation in A549 cells by activating PI3K/AKT and NF-κB signaling pathways. Toxicol. Res. 2021, 10, 150–157. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, Y.; Wang, H.; Zhang, H.; Shi, L.; Li, C.; Li, X.; Zeng, Y.; Liu, Y.; Wu, M.; et al. The effect of low molecular weight-polycyclic aromatic hydrocarbons responsive hsa_circ_0039929/hsa-miR-15b-3p_R-1/FGF2 circuit on inflammatory response of A549 cells via the PI3K/AKT pathway and epithelial-mesenchymal transition process. Environ. Toxicol. 2022, 37, 2005–2018. [Google Scholar] [CrossRef]
- Alhamdow, A.; Essig, Y.J.; Krais, A.M.; Gustavsson, P.; Tinnerberg, H.; Lindh, C.H.; Hagberg, J.; Graff, P.; Albin, M.; Broberg, K. Fluorene exposure among PAH-exposed workers is associated with epigenetic markers related to lung cancer. Occup. Environ. Med. 2020, 77, 488–495. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, D.; Ma, H.; Li, C.; Chang, X.; Low, P.; Hammond, S.K.; Turyk, M.E.; Wang, J.; Liu, S. The impact on T-regulatory cell related immune responses in rural women exposed to polycyclic aromatic hydrocarbons (PAHs) in household air pollution in Gansu, China: A pilot investigation. Environ. Res. 2019, 173, 306–317. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Guo, H.; Liu, Y.; Zeng, Y.; Hu, F.; Zhang, W.; Li, C.; Wang, J. A preliminary study on household air pollution exposure and health-related factors among rural housewives in Gansu province, northwest China. Arch. Environ. Occup. Health 2022, 77, 662–673. [Google Scholar] [CrossRef]
- Wang, L. The Atmospheric Pollution Characteristics and Health Risks of Polycyclic Aromatic Hydrocarbons in Lanzhou Valley, Western China. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2017. [Google Scholar]
- Liu, B.; Yu, X.; Lv, L.; Dong, W.; Chen, L.; Wu, W.; Yu, Y. A nationwide survey of polycyclic aromatic hydrocarbons (PAHs) in household dust in China: Spatial distribution, sources, and health risk assessment. Environ. Geochem. Health 2023, 45, 4979–4993. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, Z.; Wang, H.; Ma, H.; Hu, F.; Zhang, W.; Liu, Y.; Huang, Y.; Zeng, Y.; Li, C.; et al. Oxidative stress and inflammatory effects in human lung epithelial A549 cells induced by phenanthrene, fluorene, and their binary mixture. Environ. Toxicol. 2021, 36, 95–104. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Food Chem. Toxicol. 2015, 86, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, Y.; Zhou, H.; Cai, K.; Xu, B. A review of hazards in meat products: Multiple pathways, hazards and mitigation of polycyclic aromatic hydrocarbons. Food Chem. 2024, 445, 138718. [Google Scholar] [CrossRef] [PubMed]
- Mallah, M.A.; Changxing, L.; Mallah, M.A.; Noreen, S.; Liu, Y.; Saeed, M.; Xi, H.; Ahmed, B.; Feng, F.; Mirjat, A.A.; et al. Polycyclic aromatic hydrocarbon and its effects on human health: An overeview. Chemosphere 2022, 296, 133948. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.; Wang, H.; Wang, B.; Wang, X.; Ren, A.; Tao, S. Risk of human exposure to polycyclic aromatic hydrocarbons: A case study in Beijing, China. Environ. Pollut. 2015, 205, 70–77. [Google Scholar] [CrossRef]
- Ribière, C.; Peyret, P.; Parisot, N.; Darcha, C.; Déchelotte, P.G.; Barnich, N.; Peyretaillade, E.; Boucher, D. Oral exposure to environmental pollutant benzo[a]pyrene impacts the intestinal epithelium and induces gut microbial shifts in murine model. Sci. Rep. 2016, 6, 31027. [Google Scholar] [CrossRef]
- He, S.; Li, X.; Li, C.; Deng, H.; Shao, Y.; Yuan, L. Isoorientin attenuates benzo[a]pyrene-induced colonic injury and gut microbiota disorders in mice. Food Res. Int. 2019, 126, 108599. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, L.; Duan, H.; Zhao, R.; Xiao, Y.; Guo, M.; Zhao, J.; Zhang, H.; Chen, W.; Tian, F. The Protection of Lactiplantibacillus plantarum CCFM8661 Against Benzopyrene-Induced Toxicity via Regulation of the Gut Microbiota. Front. Immunol. 2021, 12, 736129. [Google Scholar] [CrossRef]
- Rayan, M.; Sayed, T.S.; Hussein, O.J.; Therachiyil, L.; Maayah, Z.H.; Maccalli, C.; Uddin, S.; Prehn, J.H.M.; Korashy, H.M. Unlocking the secrets: Exploring the influence of the aryl hydrocarbon receptor and microbiome on cancer development. Cell. Mol. Biol. Lett. 2024, 29, 33. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, J.; Zhou, X. Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct. Target. Ther. 2024, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; De Salis, L.V.V.; De Barros Cardoso, C.R. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut-lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef]
- Ma, H. Effects of Phenanthrene on Inflammationin Lung and Liver of Female Rats. Master’s Thesis, Lanzhou University, Lanzhou, China, 2020. [Google Scholar]
- Chinese Center for Disease Control and Prevention. Animal Acute, Subchronic and Chronic Toxicity Tests. Available online: https://www.phsciencedata.cn/Share/wiki/wikiView?id=e6c6bda3-0c2f-4119-b684-d6c079f0c4ac# (accessed on 12 December 2012).
- Yu, X.; Lv, K.; Guan, S.; Zhang, X.; Sun, L. Long-term exposure to phenanthrene at environmental-level induces intestinal dysbiosis and disrupted hepatic lipid metabolism in mice. Environ. Pollut. 2021, 268 Pt B, 115738. [Google Scholar] [CrossRef]
- Luis, A.S.; Hansson, G.C. Intestinal mucus and their glycans: A habitat for thriving microbiota. Cell Host Microbe 2023, 31, 1087–1100. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, Y.; Jin, R.; Yin, G.; Hou, L.; Liu, M.; Ju, F.; Han, P. Community pattern of potential phenanthrene (PHE) degrading bacteria in PHE contaminated soil revealed by (13)C-DNA stable isotope probing. Chemosphere 2023, 344, 140377. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Cao, Q.; Zhang, C.; Dong, Z.; Feng, D.; Ye, H.; Zuo, J. Dietary Catalase Supplementation Alleviates Deoxynivalenol-Induced Oxidative Stress and Gut Microbiota Dysbiosis in Broiler Chickens. Toxins 2022, 14, 830. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Watanabe, T.; Jia, Z.; Makoto, K.; Susumu, A. Molecular analyses reveal stability of bacterial communities in bulk soil of a Japanese paddy field: Estimation by denaturing gradient gel electrophoresis of 16S rRNA genes amplified from DNA accompanied with RNA. Soil Sci. Plant Nutr. 2007, 53, 448–458. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Q.; Song, Y.; Cheng, B.; Ai, X. Effect of Copper Sulphate Exposure on the Oxidative Stress, Gill Transcriptome and External Microbiota of Yellow Catfish, Pelteobagrus fulvidraco. Antioxidants 2023, 12, 1288. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.F.; Wei, Z.; Zhou, Y.; Li, Q.; Qi, Z.; Diao, X. Genomic Evidence for the Recycling of Complex Organic Carbon by Novel Thermoplasmatota Clades in Deep-Sea Sediments. mSystems 2022, 7, e0007722. [Google Scholar] [CrossRef]
- Liu, C.; Ruan, F.; Chen, Z.; Han, J.; Ding, X.; Han, C.; Ye, L.; Yang, C.; Yu, Y.; Zuo, Z.; et al. Phenanthrene-induced hyperuricemia with intestinal barrier damage and the protective role of theabrownin: Modulation by gut microbiota-mediated bile acid metabolism. Sci. Total Environ. 2024, 949, 174923. [Google Scholar] [CrossRef]
- Yu, X.; Li, X.; Yang, H. Unraveling intestinal microbiota’s dominance in polycystic ovary syndrome pathogenesis over vaginal microbiota. Front. Cell. Infect. Microbiol. 2024, 14, 1364097. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. Hormesis provides a generalized quantitative estimate of biological plasticity. J. Cell Commun. Signal. 2011, 5, 25–38. [Google Scholar] [CrossRef]
- Song, W.; Yang, X.; Wang, W.; Wang, Z.; Wu, J.; Huang, F. Sinomenine ameliorates septic acute lung injury in mice by modulating gut homeostasis via aryl hydrocarbon receptor/Nrf2 pathway. Eur. J. Pharmacol. 2021, 912, 174581. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Y.; Zhang, M.; Li, M.; Zhang, R.; Lu, X.; Gao, W.; Li, Q.; Xia, Y.; Pan, P.; et al. Etiology of Severe Community-Acquired Pneumonia in Adults Based on Metagenomic Next-Generation Sequencing: A Prospective Multicenter Study. Infect. Dis. Ther. 2020, 9, 1003–1015. [Google Scholar] [CrossRef]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, X.; Maruyama, D.; Arjomandi, M.; Prakash, A. Lung immune tone via gut-lung axis: Gut-derived LPS and short-chain fatty acids’ immunometabolic regulation of lung IL-1β, FFAR2, and FFAR3 expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L65–L78. [Google Scholar] [CrossRef] [PubMed]
- Özçam, M.; Lynch, S.V. The gut-airway microbiome axis in health and respiratory diseases. Nat. Rev. Microbiol. 2024, 22, 492–506. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.; Zhuge, A.; Li, L.; Ni, S. Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif. 2022, 55, e13194. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, C.; Guo, X.; Hu, G.; Li, G.; Zhuang, Y.; Cao, H.; Li, L.; Xing, C.; Zhang, C.; et al. Exposed to Mercury-Induced Oxidative Stress, Changes of Intestinal Microflora, and Association between them in Mice. Biol. Trace Elem. Res. 2021, 199, 1900–1907. [Google Scholar] [CrossRef]
- Hu, J.; Bao, Y.; Zhu, Y.; Osman, R.; Shen, M.; Zhang, Z.; Wang, L.; Cao, S.; Li, L.; Wu, Q. The Preliminary Study on the Association Between PAHs and Air Pollutants and Microbiota Diversity. Arch. Environ. Contam. Toxicol. 2020, 79, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, S.W. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef]
- Pinchaud, K.; Hafeez, Z.; Auger, S.; Chatel, J.M.; Chadi, S.; Langella, P.; Paoli, J.; Dary-Mourot, A.; Maguin-Gaté, K.; Olivier, J.L. Impact of Dietary Arachidonic Acid on Gut Microbiota Composition and Gut-Brain Axis in Male BALB/C Mice. Nutrients 2022, 14, 5338. [Google Scholar] [CrossRef]
- Xu, C.; Gu, L.; Hu, L.; Jiang, C.; Li, Q.; Sun, L.; Zhou, H.; Liu, Y.; Xue, H.; Li, J.; et al. FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer. Nat. Commun. 2023, 14, 2042. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Sharma-Walia, N. Curbing Lipids: Impacts ON Cancer and Viral Infection. Int. J. Mol. Sci. 2019, 20, 644. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, Z.; Chu, Z.; Li, W.; Qu, G.; Lu, H.; Tang, Y.; Sun, S.; Luo, Z.; Luo, F. An active peptide from yak inhibits hypoxia-induced lung injury via suppressing VEGF/MAPK/inflammatory signaling. Redox Biol. 2024, 75, 103252. [Google Scholar] [CrossRef]
- Goupil, E.; Tassy, D.; Bourguet, C.; Quiniou, C.; Wisehart, V.; Pétrin, D.; Gouill, C.L.; Devost, D.; Zingg, H.H.; Bouvier, M.; et al. A novel biased allosteric compound inhibitor of parturition selectively impedes the prostaglandin F2alpha-mediated Rho/ROCK signaling pathway. J. Biol. Chem. 2010, 285, 25624–25636. [Google Scholar] [CrossRef]
- Kondo, A.; Otsuka, T.; Kato, K.; Natsume, H.; Kuroyanagi, G.; Mizutani, J.; Ito, Y.; Matsushima-Nishiwaki, R.; Kozawa, O.; Tokuda, H. AMP-activated protein kinase inhibitor decreases prostaglandin F2α-stimulated interleukin-6 synthesis through p38 MAP kinase in osteoblasts. Int. J. Mol. Med. 2012, 30, 1487–1492. [Google Scholar] [CrossRef]
- Liagre, B.; Leger, D.Y.; Vergne-Salle, P.; Beneytout, J.L. MAP kinase subtypes and Akt regulate diosgenin-induced apoptosis of rheumatoid synovial cells in association with COX-2 expression and prostanoid production. Int. J. Mol. Med. 2007, 19, 113–122. [Google Scholar] [CrossRef]
- Maehara, T.; Higashitarumi, F.; Kondo, R.; Fujimori, K. Prostaglandin F(2α) receptor antagonist attenuates LPS-induced systemic inflammatory response in mice. FASEB J. 2020, 34, 15197–15207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Jiang, S.; Zhang, Z.; Wang, J.; Li, Y.; Li, Y.; Zhang, H.; Li, C.; Ma, H.; Wang, J. Involvement of the Gut–Lung Axis in LMW-PAHs-Induced Pulmonary Inflammation. Toxics 2025, 13, 1017. https://doi.org/10.3390/toxics13121017
Qin J, Jiang S, Zhang Z, Wang J, Li Y, Li Y, Zhang H, Li C, Ma H, Wang J. Involvement of the Gut–Lung Axis in LMW-PAHs-Induced Pulmonary Inflammation. Toxics. 2025; 13(12):1017. https://doi.org/10.3390/toxics13121017
Chicago/Turabian StyleQin, Jiali, Shiyao Jiang, Zhengyi Zhang, Jianding Wang, Yuanjie Li, Yunting Li, Haojun Zhang, Chengyun Li, Haitao Ma, and Junling Wang. 2025. "Involvement of the Gut–Lung Axis in LMW-PAHs-Induced Pulmonary Inflammation" Toxics 13, no. 12: 1017. https://doi.org/10.3390/toxics13121017
APA StyleQin, J., Jiang, S., Zhang, Z., Wang, J., Li, Y., Li, Y., Zhang, H., Li, C., Ma, H., & Wang, J. (2025). Involvement of the Gut–Lung Axis in LMW-PAHs-Induced Pulmonary Inflammation. Toxics, 13(12), 1017. https://doi.org/10.3390/toxics13121017






