Investigation of Oxidative DNA Damage Levels in Urine of Healthcare Workers Exposed to Ionizing Radiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups, Inclusion, and Exclusion Criteria, and Radiation Exposure Risk Assessments
2.2. Occupational Health and Safety Unit (OHSU) Risk Assessment
2.3. Workers’ Perception of Radiation Risk
2.4. National Legal Regulation on Radiation Regulation
2.5. Data Collection
2.6. Chemicals and Standards
2.7. Collection of Biological Samples and Measurement of Oxidative DNA Damage Parameters
2.8. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCWs | Healthcare workers |
| ILO | International Labour Organization |
| WHO | World Health Organization |
| ROS | Reactive Oxygen Species |
| GDP | Gross Domestic Product |
| OHS | Occupational Health and Safety |
| OHSU | Hospital Occupational Health and Safety Unit |
| CT | Computed Tomography |
| ALP | Alkaline Phosphatase |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| PPE | Personal Protective Equipment |
| MDA | Malondialdehyde |
| SOD | Superoxide Dismutase |
References
- Kim, E.A.; Lee, E.; Kang, S.K.; Jeong, M. Probability of causation for occupational cancer after exposure to ionizing radiation. Ann. Occup. Environ. Med. 2018, 30, 2–8. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.; Rosenberg, J. Melatonin as Protection Against Radiation Injury: A Systematic Review. Drug Res. 2016, 66, 281–296. [Google Scholar] [CrossRef]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA damage: A route to radiation-induced genomic instability and carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef]
- Osborn, S.B. Radiological Protection for Medical Exposure to Ionizing Radiation: Safety Guide; Safety Standards Series No RS-G-1.5; IAEA: Vienna, Austria, 2002; p. 76. ISBN 92-0-111-302-1. [Google Scholar]
- Stewart, F.A.; Akleyev, A.V.; Hauer-Jensen, M.; Hendry, J.H.; Kleiman, N.J.; Macvittie, T.J.; Aleman, B.M.; Edgar, A.B.; Mabuchi, K.; Muirhead, C.R.; et al. ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs: Threshold doses for tissue reactions in a radiation protection context. Ann. ICRP 2012, 41, 1–322. [Google Scholar] [CrossRef]
- Malekirad, A.A.; Ranjbar, A.; Rahzani, K.; Pilehvarian, A.A.; Rezaie, A.; Zamani, M.J.; Abdollahi, M. Oxidative stress in radiology staff. Environ. Toxicol. Pharmacol. 2005, 20, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Loft, S.; Fischer-Nielsen, A.; Jeding, I.B.; Vistisen, K.; Poulsen, H.E. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J. Toxicol. Environ. Health Part A Curr. Issues 1993, 40, 391–404. [Google Scholar] [CrossRef]
- Manisaligil, Y.A.; Gumustekin, M.; Micili, S.C.; Ural, C.; Cavdar, Z.; Sisman, G.; Yurt, A. The role of small GTPase Rac1 in ionizing radiation-induced testicular damage. Int. J. Radiat. Biol. 2022, 98, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Bolbol, S.A.; Zaitoun, M.F.; Abou El-Mag, S.A.; Mohammed, N.A. Healthcare workers’ exposure to ionizing radiation: Oxidative stress and antioxidant response. Indian J. Occup. Environ. 2021, 25, 72–77. [Google Scholar] [CrossRef]
- Ardıç, Z.; Şahin, T.K.; Uyar, M.; Küçükkendirci, H.; Kılınç, İ.; Öztürk, E.N.Y. Effects of occupational exposure to ionizing radiation on oxidative stress and inflammatory markers in healthcare workers of a university hospital in Konya, Turkey. J. Cell. Neurosci. Oxidative Stress 2021, 13, 994–1003. [Google Scholar] [CrossRef]
- Celik, H.; Koyuncu, I.; Karakilcik, A.Z.; Gonel, A.; Musa, D. Effects of ionizing and non-ionizing radiation on oxidative stress and the total antioxidant status in humans working in radiation environments. Bezmialem Sci. 2016, 4, 106–109. [Google Scholar] [CrossRef]
- Aneva, N.; Zaharieva, E.; Katsarska, O.; Savova, G.; Stankova, K.; Djounova, J.; Boteva, R. Inflammatory profile dysregulation in nuclear workers occupationally exposed to low-dose gamma radiation. J. Radiat. Res. 2019, 60, 768–779. [Google Scholar] [CrossRef]
- Gunduz, A.M.; Demir, C. Evaluation of oxidative stress in angiography workers. Ann. Med. Res. 2020, 27, 2382. [Google Scholar] [CrossRef]
- Chen, B.; Dai, Q.; Zhang, Q.; Yan, P.; Wang, A.; Qu, L.; Zhang, D. The relationship among occupational irradiation, DNA methylation status, and oxidative damage in interventional physicians. Medicine 2019, 98, e17373. [Google Scholar] [CrossRef] [PubMed]
- Hatzi, V.I.; Laskaratou, D.A.; Mavragani, I.V.; Nikitaki, Z.; Mangelis, A.; Panayiotidis, M.I.; Pantelias, G.E.; Terzoudi, G.I.; Georgakilas, A.G. Non-targeted radiation effects in vivo: A critical glance at the future in radiobiology. Cancer Lett. 2015, 356, 34–42. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Nunes, C.; Malta-Vacas, J.; Gomes, M.; Brito, M.; Mendonça, P.; Prista, J. Genotoxic effects in occupational exposure to formaldehyde: A study in anatomy and pathology laboratories and formaldehyde-resins production. J. Occup. Med. Toxicol. 2015, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Peeters, W.; Peng, Z. An approach towards global standardization of the risk matrix. J. Space Saf. Eng. 2015, 2, 31–38. [Google Scholar] [CrossRef]
- Dokuz Eylül University Risk Management Directive. 2014. Available online: https://strateji.deu.edu.tr/wp-content/uploads/2014/12/Dokuz-Eylül-Üniversitesi-Risk-Yönetimi-Yönergesi.pdf (accessed on 12 November 2025).
- Republic of Türkiye, Turkish Atomic Energy Authority, Radiation Safety Regulation, Official Gazette Date: 24.03.2000 Official Gazette Number: 23999. Available online: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=5272&MevzuatTur=7&MevzuatTertip=5 (accessed on 12 November 2025).
- Kant, M.; Akış, M.; Çalan, M.; Arkan, T.; Bayraktar, F.; Dizdaroglu, M.; İşlekel, H. Elevated urinary levels of 8-oxo-2′-deoxyguanosine, (5′R)- and (5′S)-8,5′-cyclo-2′-deoxyadenosines, and 8-iso-prsostaglandin F2α as potential biomarkers of oxidative stress in patients with prediabetes. DNA Repair 2016, 48, 1. [Google Scholar] [CrossRef]
- Tuna, G.; Bekar, N.E.D.; İşlekel, S.; İşlekel, G.H. Urinary 8-hydroxy-2′-deoxyguanosine levels are elevated in patients with IDH1-wildtype glioblastoma and are associated with tumor recurrence in gliomas. DNA Repair 2023, 124, 103463. [Google Scholar] [CrossRef]
- Dizdaroglu, M.; Coskun, E.; Jaruga, P. Measurement of oxidatively induced DNA damage and its repair, by mass spectrometric techniques. Free Radic. Res. 2015, 49, 525–548. [Google Scholar] [CrossRef]
- Beyan, A.C.; Emerce, E.; Tuna, G.; İşlekel, G.H. Chemical Risks, Genotoxicity, and Oxidative Stress in Healthcare Workers. Toxics 2025, 13, 189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- AbuArrah, M.; Yuli Setianto, B.; Faisal, A.; Hamim Sadewa, A. 8-Hydroxy-2-Deoxyguanosine as Oxidative DNA Damage Biomarker of Medical Ionizing Radiation: A Scoping Review. J. Biomed. Phys. Eng. 2021, 11, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Zabarmawi, Y.; Alqurashi, S.F.; Elzubier, M.E.; El-Readi, M.Z.; Alotaibi, K.S.; Bahwerth, F.S.; Almaimani, R.A.; Althubiti, M. Assessment of oxidative stress biomarkers in healthcare workers exposed to low dose of ionizing radiation in Saudi Arabia. J. Radiat. Res. Appl. Sci. 2025, 18, 101781. [Google Scholar] [CrossRef]
- Kumar, K.; Fornace, A.J.; Suman, S. 8-OxodG: A Potential Biomarker for Chronic Oxidative Stress Induced by High-LET Radiation. DNA 2024, 4, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.M.; Abdalla, M.Y.; Moore, T.A.; Bartenhagen, L.; Case, A.J.; Zimmerman, M.C. Healthcare workers occupationally exposed to ionizing radiation exhibit altered levels of inflammatory cytokines and redox parameters. Antioxidants 2019, 8, 12. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). UNSCEAR 2020/2021 Report: Volume III—Annex C: Biological Mechanisms Relevant for the Inference of Cancer Risks from Low-Dose and Low-Dose-Rate Radiation; United Nations: New York, NY, USA, 2021. [Google Scholar]
- Guéguen, Y.; Bontemps, A.; Ebrahimian, T.G. Adaptive responses to low doses of radiation or chemicals: Their cellular and molecular mechanisms. Cell. Mol. Life Sci. 2019, 76, 1255–1273. [Google Scholar] [CrossRef] [PubMed Central]
- Feinendegen, L.E. Evidence for beneficial low level radiation effects and radiation hormesis. Br. J. Radiol. 2005, 78, 3–7. [Google Scholar] [CrossRef]
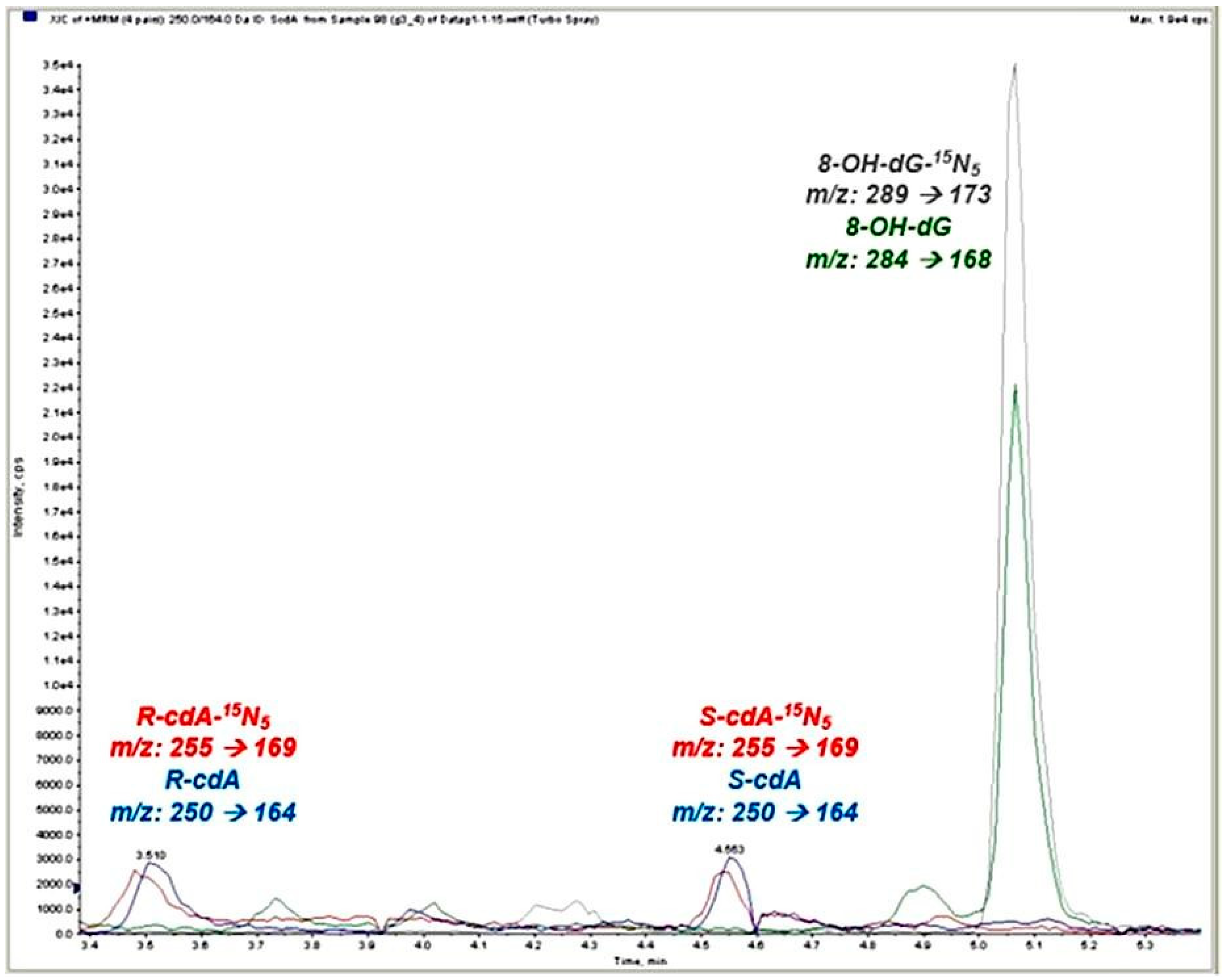
| Groups | Risk Category | Annual Average Whole-Body Dose Values (Mean + SD) | OHS Unit 5 × 5 Matrix Risk Assessment Results | Self-Assessment Thoughts on Radiation Exposure (%) | |
|---|---|---|---|---|---|
| Group 1 (n = 29) | High-risk HCWs (angiography, interventional radiology, nuclear medicine) | (0.645 ± 1.89 mSv) | Purple It is considered a “controlled area” according to national legislation [19]. | High Moderate Low None | 15 (51.7%) 7 (24.1%) 7 (24.1%) - |
| Group 2 (n = 23) | Moderate-risk HCWs (CT, mammography) | (0.068 ± 0.08 mSv) | Red It is considered a “monitored area” according to national legislation [19]. | High Moderate Low None | 3 (13.0%) 14 (60.9%) 6 (26.1%) - |
| Group 3 (n = 24) | Non-radiology HCWs with possible exposure (orthopedics, neurosurgery, OR staff, endoscopy) | (0.034 ± 0.05 mSv) | Green and Yellow It is considered a monitored area according to national legislation [19]. | High Moderate Low None | 7 (29.2%) 6 (25.0%) 10 (41.7%) 1 (4.2%) |
| Group 4 (n = 20) | Control group—no radiation exposure (other hospital units) | - | None | High Moderate Low None | - - 5 (25.0%) 15 (75.0%) |
| Characteristics | Exposed Groups (%) | Group 4 (Control Group) | p * | ||
|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | |||
| Age Mean ± SD (min–max) | 40.10 ± 10.48 (24–58) | 33.43 ± 10.53 (22–59) | 40.50 ± 9.27 (26–60) | 43.60 ± 9.17 (32–60) | 0.011 |
| Height (cm) Mean ± SD (min–max) | 168.97 ± 10.26 (152–194) | 164.04 ± 8.37 (150–180) | 171.54 ± 8.79 (154–185) | 166.30 ± 8.23 (150–183) | 0.038 |
| Weight (kg) Mean ± SD (min–max) | 72.86 ± 13.68 (45–98) | 61.96 ± 13.76 (43–101) | 76.92 ± 6.78 (47–120) | 67.85 ± 1.37 (48–90) | 0.003 |
| Biological Sex (%) Female Male | 62.1 37.9 | 73.9 26.1 | 45.8 54.2 | 80.0 20.0 | 0.081 |
| Smoking Status ** Non-smokers Smokers | 79.3 20.7 | 65.2 34.8 | 75.0 25.0 | 85.0 15.0 | 0.710 |
| Alcohol consumption more than 3 times a week (Yes) (%) | 3.4 | 0.0 | 0.0 | 5.0 | 0.730 |
| Chronic disease (Yes) (%) | 31.0 | 8.7 | 41.7 | 35.0 | 0.077 |
| Regular exercise (Yes) (%) | 6.9 | 4.3 | 16.7 | 4.3 | 0.240 |
| Group 1 Mean ± SD (Min–Max) | Group 2 Mean ± SD (Min–Max) | Group 3 Mean ± SD (Min–Max) | Group 4 Mean ± SD (Min–Max) | * p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Weekly work hours (h) | 36.79 ± 3.23 (35–45) | 35.74 ± 7.94 (5–48) | 45.21 ± 7.90 (35–70) | 40.75 ± 1.83 (40–45) | <0.001 | ||||
| Working year (year) | 9.10 ± 8.62 (1–31) | 10.41 ± 11.49 (0–40) | 13.50 ± 10.10 (0–34) | 11.80 ± 9.93 (1–34) | 0.249 | ||||
| Regularly Using (PPE) ** | 27 *** (93.1%) | 19 *** (82.6%) | 19 *** (79.1%) | - | |||||
| Personal Dosimeter Usage Yes (%) | 100.0 | 100.0 | 83.3 | 0.0 | <0.001 | ||||
| Night Shift Yes (%) | 31.0 | 34.8 | 41.7 | 0.0 | 0.014 | ||||
| Self-assessment thoughts on radiation exposure n (%) | High | 15 (51.7%) | High | 3 (13.0%) | High | 7 (29.2%) | High | - | |
| Moderate | 7 (4.1%) | Moderate | 14 (60.9%) | Moderate | 6 (25.0%) | Moderate | - | 0.021 | |
| Low | 7 (24.1%) | Low | 6 (26.1%) | Low | 10 (41.7%) | Low | 5 (25.0%) | ||
| None | - | None | - | None | 1 (4.2%) | None | 15 (75.0%) | ||
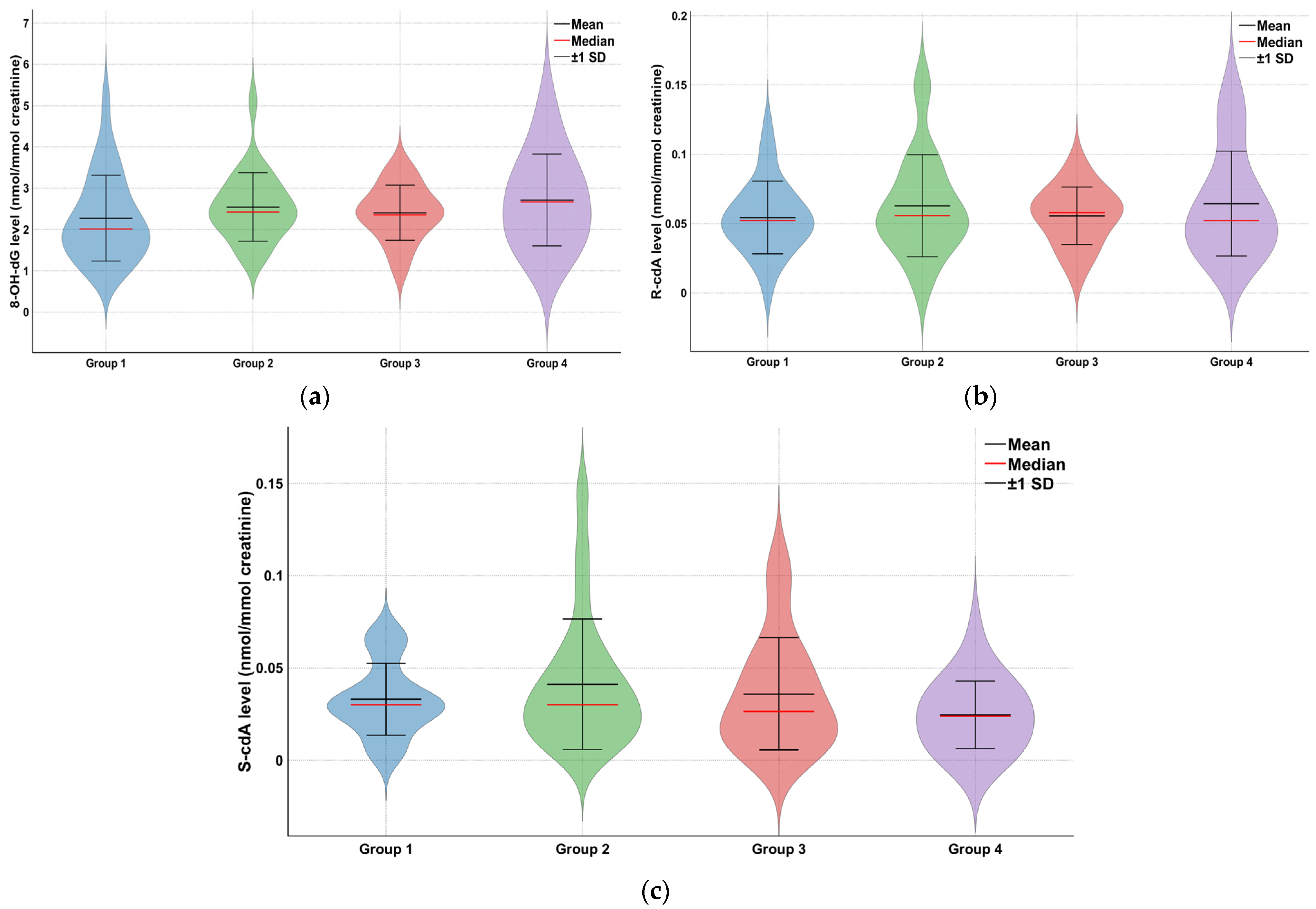
| Group | 8-OH-dG (nmol/mmol Creatinine) Mean ± SD (Min–Max) | S-cdA (nmol/mmol Creatinine) Mean ± SD (Min–Max) | R-cdA (nmol/mmol Creatinine) Mean ± SD (Min–Max) |
|---|---|---|---|
| Group 1 (n = 29) | 2.27 ± 1.04 (0.90–5.16) | 0.03 ± 0.02 (0.0005–0.07) | 0.05 ± 0.03 (0.00003–0.12) |
| Group 2 (n = 23) | 2.54 ± 0.83 (1.38–5.10) | 0.04 ± 0.04 (0.003–0.14) | 0.06 ± 0.04 (0.0014–0.15) |
| Group 3 (n = 24) | 2.41 ± 0.67 (0.98–3.58) | 0.04 ± 0.03 (0.001–0.11) | 0.06 ± 0.02 (0.013–0.09) |
| Group 4 (n = 20) | 2.71 ± 1.11 (1.13–5.20) | 0.02 ± 0.02 (0.002–0.07) | 0.06 ± 0.04 (0.025–0.14) |
| * p value | 0.132 | 0.179 | 0.611 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurt, A.; Beyan, A.C.; Tuna, G.; Manisalıgil, Y.A.; Özcan, S.; Oğuzhan, H. Investigation of Oxidative DNA Damage Levels in Urine of Healthcare Workers Exposed to Ionizing Radiation. Toxics 2025, 13, 990. https://doi.org/10.3390/toxics13110990
Yurt A, Beyan AC, Tuna G, Manisalıgil YA, Özcan S, Oğuzhan H. Investigation of Oxidative DNA Damage Levels in Urine of Healthcare Workers Exposed to Ionizing Radiation. Toxics. 2025; 13(11):990. https://doi.org/10.3390/toxics13110990
Chicago/Turabian StyleYurt, Ayşegül, Ayşe Coşkun Beyan, Gamze Tuna, Yaşar Aysun Manisalıgil, Sabriye Özcan, and Hande Oğuzhan. 2025. "Investigation of Oxidative DNA Damage Levels in Urine of Healthcare Workers Exposed to Ionizing Radiation" Toxics 13, no. 11: 990. https://doi.org/10.3390/toxics13110990
APA StyleYurt, A., Beyan, A. C., Tuna, G., Manisalıgil, Y. A., Özcan, S., & Oğuzhan, H. (2025). Investigation of Oxidative DNA Damage Levels in Urine of Healthcare Workers Exposed to Ionizing Radiation. Toxics, 13(11), 990. https://doi.org/10.3390/toxics13110990






