Effects of Decabromodiphenyl Ether (BDE209) Exposure on Toxicity and Oxidative Stress of Beas-2B Cells
Abstract
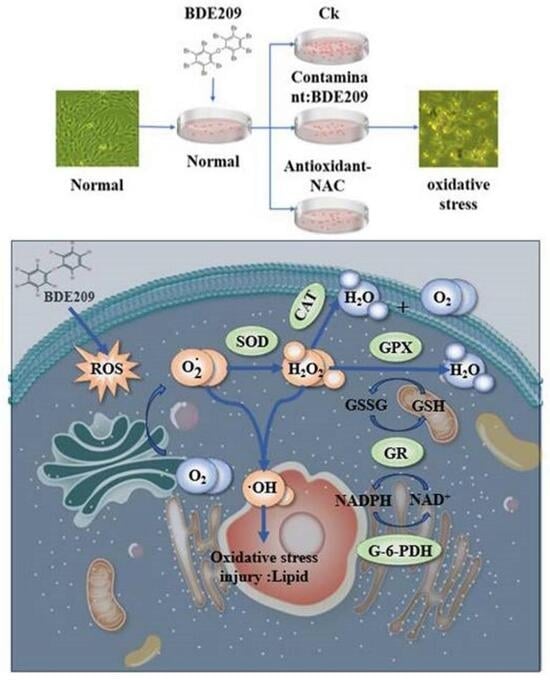
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Determining IC50 Values Using RTCA
2.4. Cytotoxicity Exposure
2.5. Cell Morphology Observation
2.6. Determination of Intracellular GSH, SOD, CAT, MDA
2.7. Determination of Intracellular ROS
2.8. Statistical Analysis
3. Results and Discussion
3.1. Cell Proliferation Toxicity
3.2. ROS Level
3.3. Antioxidant Enzyme Activity Levels and Lipid Oxidation Damage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, M.; Yu, Z.; Yang, F.; Zhao, Z.; Zhou, M.; Wang, C.; Zhang, B.; Liang, G.; Liu, X.; Shao, J. BDE209-Promoted Dio2 Degradation in H4 Glioma Cells through the Autophagy Pathway, Resulting in Hypothyroidism and Leading to Neurotoxicity. Toxicology 2023, 494, 153581. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, L.; Lin, Q.; Ma, S.; Yu, Y. Polybrominated Diphenyl Ethers in the Environment and Human External and Internal Exposure in China: A Review. Sci. Total Environ. 2019, 696, 133902. [Google Scholar] [CrossRef]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallah, M.A.-E.; Fernie, K.; Toms, L.-M.L.; Takigami, H. Levels and Trends of PBDEs and HBCDs in the Global Environment: Status at the End of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Zhang, H.; Huo, Y.; Liu, X.; Che, Y.; Wang, J.; Sun, G.; Zhang, H. The Phytotoxicity of Exposure to Two Polybrominated Diphenyl Ethers (BDE47 and BDE209) on Photosynthesis and the Response of the Hormone Signaling and ROS Scavenging System in Tobacco Leaves. J. Hazard. Mater. 2022, 426, 128012. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, Y.; Zhu, X.; Rao, Q.; Zhao, Z.; Yang, J. Hepatotoxicity Evaluation and Possible Mechanisms of Decabrominated Diphenyl Ethers (BDE-209) in Broilers: Oxidative Stress, Inflammatory, and Transcriptomics. Ecotoxicol. Environ. Saf. 2023, 264, 115460. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Cheng, Y.; Shang, X.; Wang, R.; Wang, R.; Hao, X.; Li, S.; Wang, Y.; Li, Y.; Liu, X.; et al. Disturbance of the Dlk1-Dio3 Imprinted Domain May Underlie Placental Dio3 Suppression and Extracellular Thyroid Hormone Disturbance in Placenta-Derived JEG-3 Cells Following Decabromodiphenyl Ether (BDE209) Exposure. Toxicology 2021, 458, 152837. [Google Scholar] [CrossRef]
- Aran, K.R. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease—A Step towards Mitochondria Based Therapeutic Strategies. Aging Health Res. 2023, 13, 100169. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Shen, Y.; Xiao, H. Resveratrol Attenuates Rotenone-Induced Inflammation and Oxidative Stress via STAT1 and Nrf2/Keap1/SLC7A11 Pathway in a Microglia Cell Line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Gao, S.; Yue, M.; Zhu, S.; Liu, Q.; Zhao, X.-E. Recent Advances in Analytical Methods of Oxidative Stress Biomarkers Induced by Environmental Pollutant Exposure. TrAC Trends Anal. Chem. 2023, 160, 116978. [Google Scholar] [CrossRef]
- Kaur, S.; Rubal; Kaur, S.; Kaur, A.; Kaur, S.; Gupta, S.; Mittal, S.; Dhiman, M. A Cross-Sectional Study to Correlate Antioxidant Enzymes, Oxidative Stress and Inflammation with Prevalence of Hypertension. Life Sci. 2023, 313, 121134. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Li, J.; Hu, J.; Yang, K.; Tao, L. Oxidative Stress: A Common Pathological State in a High-Risk Population for Osteoporosis. Biomed. Pharmacother. 2023, 163, 114834. [Google Scholar] [CrossRef]
- Hussain, A.; Ashique, S.; Afzal, O.; Altamimi, M.A.; Malik, A.; Kumar, S.; Garg, A.; Sharma, N.; Farid, A.; Khan, T.; et al. A Correlation between Oxidative Stress and Diabetic Retinopathy: An Updated Review. Exp. Eye Res. 2023, 236, 109650. [Google Scholar] [CrossRef]
- Zilberter, Y.; Tabuena, D.R.; Zilberter, M. NOX-Induced Oxidative Stress Is a Primary Trigger of Major Neurodegenerative Disorders. Prog. Neurobiol. 2023, 231, 102539. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Zhang, R.; Han, Z.; Huang, Y.; Deng, C.; Dong, W.; Zhuang, G. Effects of N-Acetylcysteine on Oxidative Stress and Inflammation Reactions in a Rat Model of Allergic Rhinitis after PM2.5 Exposure. Biochem. Biophys. Res. Commun. 2020, 533, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Shieh, P.; Jan, C.-R.; Liang, W.-Z. The Protective Effects of the Antioxidant N-Acetylcysteine (NAC) against Oxidative Stress-Associated Apoptosis Evoked by the Organophosphorus Insecticide Malathion in Normal Human Astrocytes. Toxicology 2019, 417, 1–14. [Google Scholar] [CrossRef]
- Zhao, K.; Han, D.; He, S.-R.; Wu, L.-Y.; Liu, W.-Y.; Zhong, Z.-M. N-Acetyl-L-Cysteine Attenuates Oxidative Stress-Induced Bone Marrow Endothelial Cells Apoptosis by Inhibiting BAX/Caspase 3 Pathway. Biochem. Biophys. Res. Commun. 2023, 656, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Kacar, S.; Sahinturk, V.; Kutlu, H.M. Effect of Acrylamide on BEAS-2B Normal Human Lung Cells: Cytotoxic, Oxidative, Apoptotic and Morphometric Analysis. Acta Histochem. 2019, 121, 595–603. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Song, Z.-M.; Zeng, M.-N.; Wang, Y.-Z.; Cheng, Y.-X.; Qiao, L.-Q.; Peng, R.; Feng, W.-S. Diterpenes from Pinus Kesiya Var. Langbianensis (A. Chev.) Gaussen Ex Bui (Pinaceae) and Their Protective Effects in LPS-Treated BEAS-2B Cells. Phytochemistry 2022, 203, 113360. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Gao, H.; Zhang, X.; Liu, X.; Chen, J.; Liao, X.; Zhang, H.; Huang, Q. Aryl Hydrocarbon Receptor Mediates Benzo[a]Pyrene-Induced Metabolic Reprogramming in Human Lung Epithelial BEAS-2B Cells. Sci. Total Environ. 2021, 756, 144130. [Google Scholar] [CrossRef]
- Özkay, Y.; Yurttaş, L.; Dikmen, M.; Engür, S. Synthesis and Antiproliferative Activity Evaluation of New Thiazole–Benzimidazole Derivatives Using Real-Time Cell Analysis (RTCA DP). Med. Chem. Res. 2016, 25, 482–493. [Google Scholar] [CrossRef]
- Tang, J.; Hu, B.; Zheng, H.; Qian, X.; Zhang, Y.; Zhu, J.; Xu, G.; Chen, D.; Jin, X.; Li, W.; et al. 2,2′,4,4′-Tetrabromodiphenyl Ether (BDE-47) Activates Aryl Hydrocarbon Receptor (AhR) Mediated ROS and NLRP3 Inflammasome/P38 MAPK Pathway Inducing Necrosis in Cochlear Hair Cells. Ecotoxicol. Environ. Saf. 2021, 221, 112423. [Google Scholar] [CrossRef]
- Lenart, J.; Zieminska, E.; Diamandakis, D.; Lazarewicz, J.W. Altered Expression of Genes Involved in Programmed Cell Death in Primary Cultured Rat Cerebellar Granule Cells Acutely Challenged with Tetrabromobisphenol A. NeuroToxicology 2017, 63, 126–136. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Li, X.; Cheng, Y.; Zhang, H.; Chang, L.; Sun, M.; Zhang, Z.; Wang, Z.; Niu, Q.; et al. Oxidative and Nitrosative Stress in the Neurotoxicity of Polybrominated Diphenyl Ether-153: Possible Mechanism and Potential Targeted Intervention. Chemosphere 2020, 238, 124602. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Xue, J.; Gao, L.; Li, X.; Zhao, M.; Zhao, D.; Zhou, X. Ferroptosis Mediates Decabromodiphenyl Ether-Induced Liver Damage and Inflammation. Ecotoxicol. Environ. Saf. 2023, 255, 114771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Lu, Y.; Qi, X.; Huang, Y.; Meng, H.; Yin, D.; Sun, T.; Deng, C.; Wu, D. Effects of Decabrominated Diphenylether-209 on Cell Proliferation and Cell Apoptosis in Human Liver L-02 Cells. Acta Sci. Nat. Univ. Pekin. 2020, 56, 966. [Google Scholar] [CrossRef]
- Zhi, H.; Yuan, N.; Wu, J.-P.; Lu, L.-M.; Chen, X.-Y.; Wu, S.-K.; Mai, B.-X. MicroRNA–21 Attenuates BDE-209-Induced Lipid Accumulation in THP-1 Macrophages by Downregulating Toll-like Receptor 4 Expression. Food Chem. Toxicol. 2019, 125, 71–77. [Google Scholar] [CrossRef]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular Mechanisms of N-Acetylcysteine Actions. Cell. Mol. Life Sci. (CMLS) 2003, 60, 6–20. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, W.; Ito, J.; Henkelmann, B.; Xu, C.; Mishima, E.; Conrad, M. N-Acetyl-l-Cysteine Averts Ferroptosis by Fostering Glutathione Peroxidase 4. Cell Chem. Biol. 2025, 32, 767–775.e5. [Google Scholar] [CrossRef]
- Espinosa Ruiz, C.; Manuguerra, S.; Cuesta, A.; Santulli, A.; Messina, C.M. Oxidative Stress, Induced by Sub-Lethal Doses of BDE 209, Promotes Energy Management and Cell Cycle Modulation in the Marine Fish Cell Line SAF-1. Int. J. Environ. Res. Public Health 2019, 16, 474. [Google Scholar] [CrossRef]
- Lee, M.-C.; Puthumana, J.; Lee, S.-H.; Kang, H.-M.; Park, J.C.; Jeong, C.-B.; Han, J.; Hwang, D.-S.; Seo, J.S.; Park, H.G.; et al. BDE-47 Induces Oxidative Stress, Activates MAPK Signaling Pathway, and Elevates de Novo Lipogenesis in the Copepod Paracyclopina Nana. Aquat. Toxicol. 2016, 181, 104–112. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Chen, H.; Tang, X.; Wang, Y. Reactive Oxygen Species (ROS) and the Calcium-(Ca2+) Mediated Extrinsic and Intrinsic Pathways Underlying BDE-47-Induced Apoptosis in Rainbow Trout (Oncorhynchus mykiss) Gonadal Cells. Sci. Total Environ. 2019, 656, 778–788. [Google Scholar] [CrossRef]
- Costa, L.G.; Pellacani, C.; Dao, K.; Kavanagh, T.J.; Roque, P.J. The Brominated Flame Retardant BDE-47 Causes Oxidative Stress and Apoptotic Cell Death in Vitro and in Vivo in Mice. Neurotoxicology 2015, 48, 68–76. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, Y.; Tang, Z.; Mei, X.; Cao, X.; Liu, J. Toxicity Mechanism of Acrolein on DNA Damage and Apoptosis in BEAS-2B Cells: Insights from Cell Biology and Molecular Docking Analyses. Toxicology 2022, 466, 153083. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Zhou, B.; Zhou, Z.; Xu, N.; Wang, Y. A ROS-Mediated Mitochondrial Pathway and Nrf2 Pathway Activation Are Involved in BDE-47 Induced Apoptosis in Neuro-2a Cells. Chemosphere 2017, 184, 679–686. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Serov, D.A.; Baimler, I.V.; Gritsaeva, A.V.; Chapala, P.; Simakin, A.V.; Astashev, M.E.; Karmanova, E.E.; Dubinin, M.V.; Nizameeva, G.R.; et al. Polymethyl Methacrylate-like Photopolymer Resin with Titanium Metal Nanoparticles Is a Promising Material for Biomedical Applications. Polymers 2025, 17, 1830. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, H.; Liang, J.; Li, Y.; Yang, J.; Luo, C.; Tang, Y.; Ding, Y.; Liu, X.; Yuan, Q.; et al. Dual Role of Reactive Oxygen Species and Their Application in Cancer Therapy. J. Cancer 2021, 12, 5543–5561. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Tang, X.; Zhao, Y. Toxicity of 2, 2′, 4, 4′-Tetrabromodiphenyl Ether (BDE-47) on the Green microalgae chlorella Sp. and the Role of Cellular Oxidative Stress. Mar. Pollut. Bull. 2022, 180, 113810. [Google Scholar] [CrossRef] [PubMed]
- Atamanalp, M.; Parlak, V.; Özgeriş, F.B.; Çilingir Yeltekin, A.; Ucar, A.; Keleş, M.S.; Alak, G. Treatment of Oxidative Stress, Apoptosis, and DNA Injury with N-Acetylcysteine at Simulative Pesticide Toxicity in Fish. Toxicol. Mech. Methods 2021, 31, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.-Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Roberts, K.N.; Scheff, S.W. Oxidative Stress and Modification of Synaptic Proteins in Hippocampus after Traumatic Brain Injury. Free Radic. Biol. Med. 2008, 45, 443–452. [Google Scholar] [CrossRef]
- Jian, X.; Tang, X.; Xu, N.; Sha, J.; Wang, Y. Responses of the Rotifer Brachionus Plicatilis to Flame Retardant (BDE-47) Stress. Mar. Pollut. Bull. 2017, 116, 298–306. [Google Scholar] [CrossRef]
- He, F.; Liu, Q.; Jing, M.; Wan, J.; Huo, C.; Zong, W.; Tang, J.; Liu, R. Toxic Mechanism on Phenanthrene-Induced Cytotoxicity, Oxidative Stress and Activity Changes of Superoxide Dismutase and Catalase in Earthworm (Eisenia foetida): A Combined Molecular and Cellular Study. J. Hazard. Mater. 2021, 418, 126302. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, J.; Wang, C.; Wang, Q.; Li, X.; Zhang, D.; Wang, J.; Zhu, L.; Wang, J. Toxicity of Dibutyl Phthalate to Pakchoi (Brassica campestris L.): Evaluation through Different Levels of Biological Organization. Sci. Total Environ. 2022, 849, 157943. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, M.; Jiang, N.; Yao, X.; Wang, Q.; Lv, H.; Wang, C.; Wang, J. Evaluation of Soil Ecological Health after Exposure to Environmentally Relevant Doses of Di (2-Ethylhexyl) Phthalate: Insights from Toxicological Studies of Earthworms at Different Ecological Niches. Environ. Pollut. 2023, 322, 121204. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, A.; Egea-Guerrero, J.J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative Stress in Traumatic Brain Injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef]
- Mahmoud, S.M.; Abdel Moneim, A.E.; Qayed, M.M.; El-Yamany, N.A. Potential Role of N-Acetylcysteine on Chlorpyrifos-Induced Neurotoxicity in Rats. Env. Sci. Pollut. Res. 2019, 26, 20731–20741. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Han, J.; Feng, J.; Guo, T.; Li, Z.; Min, F.; Jin, R.; Peng, X. N-Acetylcysteine Inhibits Patulin-Induced Apoptosis by Affecting ROS-Mediated Oxidative Damage Pathway. Toxins 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Diplock, A.T. Free Radicals in Biology and Medicine. Biochem. Soc. Trans. 1985, 13, 976. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, B.; Chen, H.; Lu, K.; Wang, Y. Oxidative Stress Activates the Nrf2-Mediated Antioxidant Response and P38 MAPK Pathway: A Possible Apoptotic Mechanism Induced by BDE-47 in Rainbow Trout (Oncorhynchus Mykiss) Gonadal RTG-2 Cells. Environ. Pollut. 2021, 287, 117341. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, Z.; Miao, J.; Tian, Y.; Pan, L. Effects of Benzo[a]Pyrene Exposure on Oxidative Stress and Apoptosis of Gill Cells of Chlamys Farreri in Vitro. Environ. Toxicol. Pharmacol. 2022, 93, 103867. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T.; Samini, F. Anti-Oxidative Effects of Curcumin on Immobilization-Induced Oxidative Stress in Rat Brain, Liver and Kidney. Biomed. Pharmacother. 2017, 87, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Gao, J.; Li, X.; Zhang, C.; Zhu, L.; Wang, J.; Wang, J. Phthalate Induced Oxidative Stress and DNA Damage in Earthworms (Eisenia fetida). Environ. Int. 2019, 129, 10–17. [Google Scholar] [CrossRef]
- Fu, M.; Liu, F.; Abbas, G.; Zhou, S.; Ling, S.; Zhang, W.; Peng, C.; Yang, J.; Zhou, B. Cytotoxicity Profiling of Decabromodiphenyl Ethane to Earthworm (Eisenia fetida): Abnormity-Recovery-Dysregulation Physiological Pattern Reflects the Coping Mechanism. Sci. Total Environ. 2022, 813, 152607. [Google Scholar] [CrossRef]
- Yao, X.; Wang, C.; Li, M.; Jiao, Y.; Wang, Q.; Li, X.; Liu, K.; Liu, G.; Wang, J.; Zhu, L.; et al. Extreme Environmental Doses of Diisobutyl Phthalate Exposure Induce Oxidative Stress and DNA Damage in Earthworms (Eisenia fetida): Evidence at the Biochemical and Molecular Levels. J. Environ. Manag. 2023, 331, 117321. [Google Scholar] [CrossRef] [PubMed]




| 1 | 2 | 3 | 4 | 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BDE209 (µM) | NAC (mM) | BDE209 (µM) | NAC (mM) | BDE209 (µM) | NAC (mM) | BDE209 (µM) | NAC (mM) | BDE209 (µM) | NAC (mM) | |
| Control | - | - | - | - | - | - | - | - | - | - |
| BDE209 | 5 | - | 20 | - | 35 | - | 50 | - | 65 | - |
| NAC + BDE209 | 5 | 1 | 20 | 1 | 35 | 1 | 50 | 1 | 65 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xiao, Z.; Mao, P.; Yang, F.; Ma, Y.; Xian, B.; Fu, M.; Li, G. Effects of Decabromodiphenyl Ether (BDE209) Exposure on Toxicity and Oxidative Stress of Beas-2B Cells. Toxics 2025, 13, 987. https://doi.org/10.3390/toxics13110987
Zhang Y, Xiao Z, Mao P, Yang F, Ma Y, Xian B, Fu M, Li G. Effects of Decabromodiphenyl Ether (BDE209) Exposure on Toxicity and Oxidative Stress of Beas-2B Cells. Toxics. 2025; 13(11):987. https://doi.org/10.3390/toxics13110987
Chicago/Turabian StyleZhang, Yanan, Ziyu Xiao, Pu Mao, Fengrui Yang, Yingdi Ma, Bensen Xian, Mingming Fu, and Guiying Li. 2025. "Effects of Decabromodiphenyl Ether (BDE209) Exposure on Toxicity and Oxidative Stress of Beas-2B Cells" Toxics 13, no. 11: 987. https://doi.org/10.3390/toxics13110987
APA StyleZhang, Y., Xiao, Z., Mao, P., Yang, F., Ma, Y., Xian, B., Fu, M., & Li, G. (2025). Effects of Decabromodiphenyl Ether (BDE209) Exposure on Toxicity and Oxidative Stress of Beas-2B Cells. Toxics, 13(11), 987. https://doi.org/10.3390/toxics13110987