Association of Prenatal Ozone Exposure with Fetal Growth and Birth Outcomes: Roles of Maternal Inflammation and Metabolic Dysregulation
Highlights
- Prenatal O3 exposure significantly increased the risks of preterm birth (PTB) and low birth weight (LBW).
- Elevated maternal SAA and CRP levels were associated with higher risks of PTB and LBW.
- Higher maternal TNF-α levels were inversely related to PTB and LBW, suggesting a potential protective effect.
- The main findings indicate that prenatal O3 exposure and maternal immune–metabolic status are important factors in fetal growth and birth outcomes, highlighting the need for maternal risk assessment and intervention strategies during pregnancy.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. Air Pollutant Exposure Assessment
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Group Differences in Adverse Pregnancy Outcomes
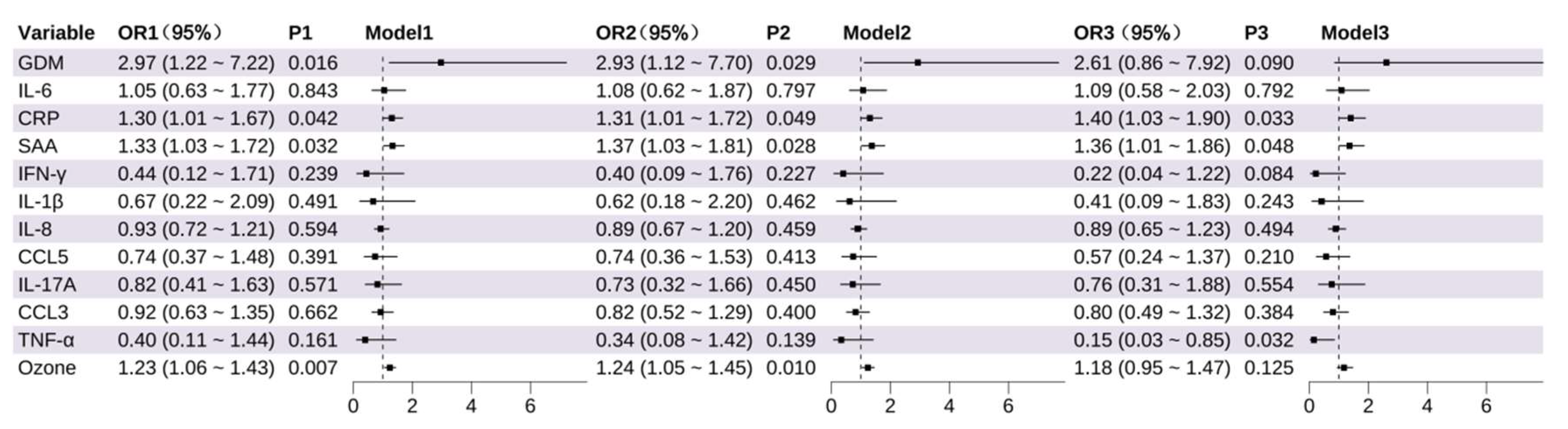
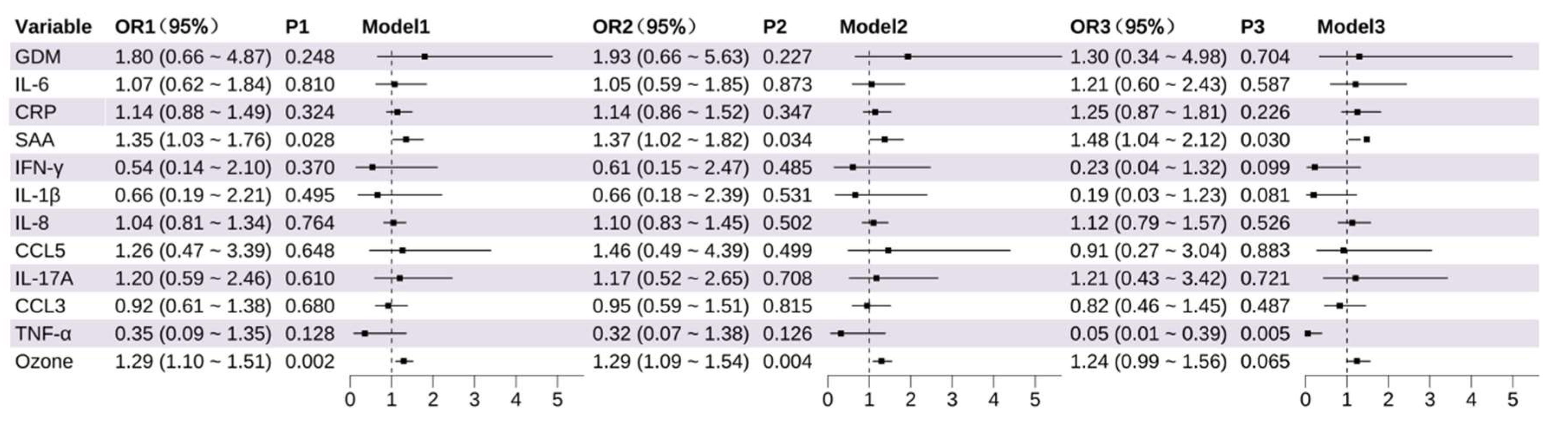
3.2. Identification of Key Variables Influencing PTB and LBW: Results from Logistic Regression
3.2.1. Identification of Factors Influencing Preterm Birth
3.2.2. Identification of Factors Influencing Low Birth Weight
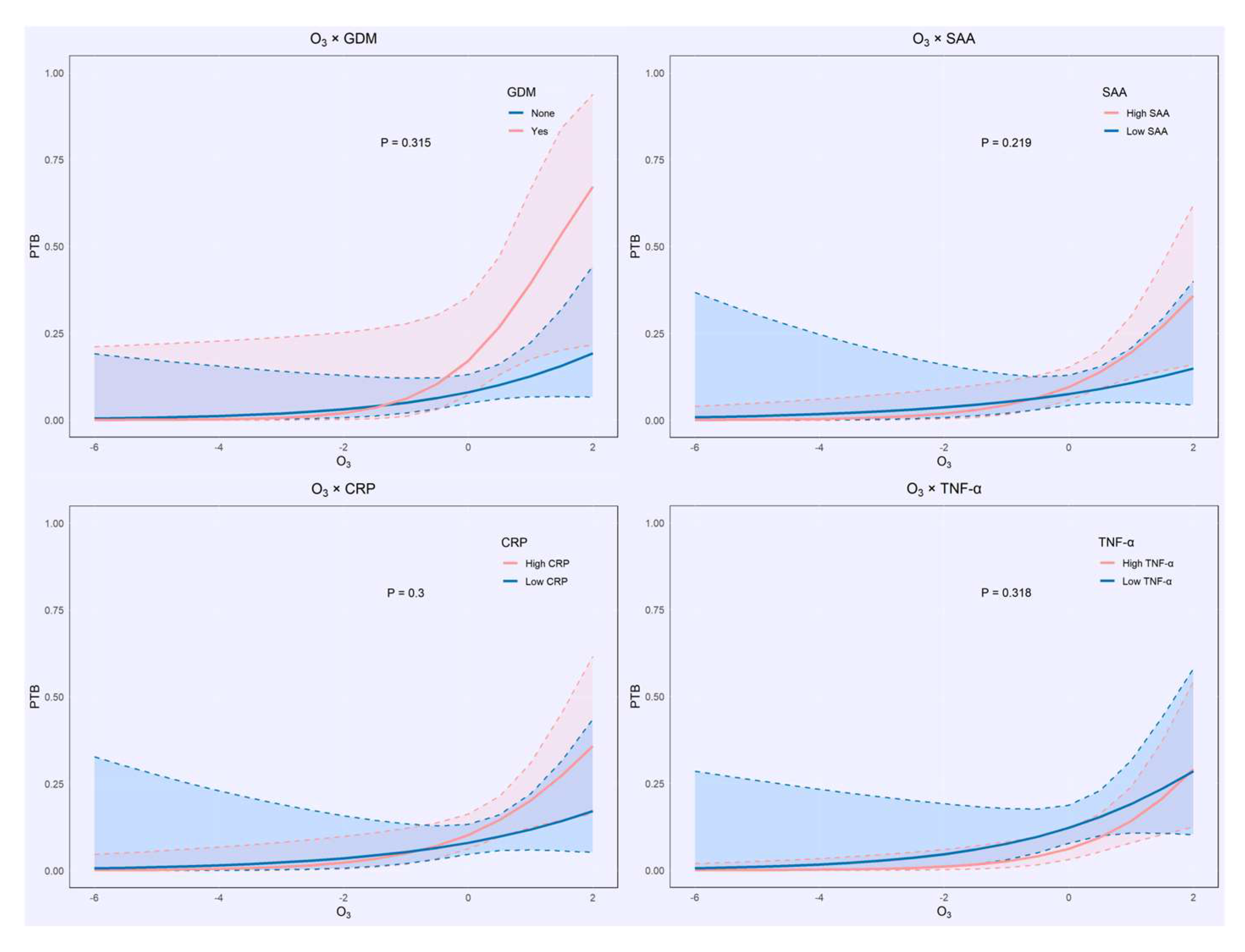
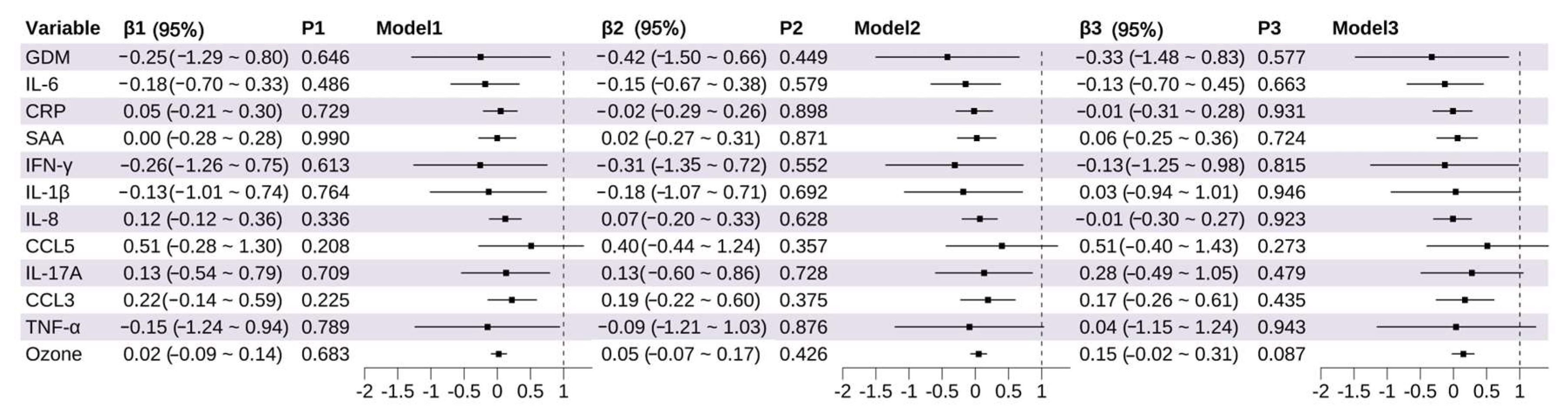
3.3. Mechanistic Exploration: Metabolic and Inflammatory Pathways Linking Prenatal O3 Exposure to Adverse Pregnancy Outcomes
3.3.1. Mediation Analysis
3.3.2. Interaction Analysis
3.4. Association Between Prenatal Exposure and Fetal Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Wang, P.; Zhang, L.; Shi, H.; Li, J.; Meng, X.; Xiao, X.; Dai, H.; Zhang, Y. Ozone Exposure During Pregnancy and Risk of Gestational Hypertension or Preeclampsia in China. JAMA Netw. Open 2023, 6, e236347. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.J.; Williams, A.; Ouidir, M.; Sherman, S.; Mendola, P. Differential Effect of Ambient Air Pollution Exposure on Risk of Gestational Hypertension and Preeclampsia. Hypertension 2019, 74, 384–390. [Google Scholar] [CrossRef]
- Hu, H.; Ha, S.; Henderson, B.H.; Warner, T.D.; Roth, J.; Kan, H.; Xu, X. Association of Atmospheric Particulate Matter and Ozone with Gestational Diabetes Mellitus. Environ. Health Perspect. 2015, 123, 853–859. [Google Scholar] [CrossRef]
- Ghosh, R.; Causey, K.; Burkart, K.; Wozniak, S.; Cohen, A.; Brauer, M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021, 18, e1003718. [Google Scholar] [CrossRef]
- Rappazzo, K.M.; Nichols, J.L.; Rice, R.B.; Luben, T.J. Ozone exposure during early pregnancy and preterm birth: A systematic review and meta-analysis. Environ. Res. 2021, 198, 111317. [Google Scholar] [CrossRef]
- Zhang, J.J.; Wei, Y.; Fang, Z. Ozone Pollution: A Major Health Hazard Worldwide. Front. Immunol. 2019, 10, 2518. [Google Scholar] [CrossRef]
- Li, Z.; Xu, R.; Wang, Z.; Qian, N.; Qian, Y.; Peng, J.; Zhu, X.; Guo, C.; Li, X.; Xu, Q.; et al. Ozone exposure induced risk of gestational diabetes mellitus. Chemosphere 2022, 308 Pt 1, 136241. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.; Dabelea, D.; Thomas, D.S.K.; Peel, J.L.; Adgate, J.L.; Magzamen, S.; Martenies, S.E.; Allshouse, W.B.; Starling, A.P. Exposure to ambient air pollution during pregnancy and inflammatory biomarkers in maternal and umbilical cord blood: The Healthy Start study. Environ. Res. 2021, 197, 111165. [Google Scholar] [CrossRef]
- Mozzoni, P.; Iodice, S.; Persico, N.; Ferrari, L.; Pinelli, S.; Corradi, M.; Rossi, S.; Miragoli, M.; Bergamaschi, E.; Bollati, V. Maternal air pollution exposure during the first trimester of pregnancy and markers of inflammation and endothelial dysfunction. Environ. Res. 2022, 212 Pt A, 113216. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Arancibia, S.; Guevara-Guzmán, R.; López-Vidal, Y.; Rodríguez-Martínez, E.; Zanardo-Gomes, M.; Angoa-Pérez, M.; Raisman-Vozari, R. Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol. Sci. 2010, 113, 187–197. [Google Scholar] [CrossRef]
- Assani, A.D.; Boldeanu, L.; Siloși, I.; Boldeanu, M.V.; Dijmărescu, A.L.; Assani, M.Z.; Manolea, M.M.; Văduva, C.C. Pregnancy Under Pressure: Oxidative Stress as a Common Thread in Maternal Disorders. Life 2025, 15, 1348. [Google Scholar] [CrossRef]
- Grevendonk, L.; Janssen, B.G.; Vanpoucke, C.; Lefebvre, W.; Hoxha, M.; Bollati, V.; Nawrot, T.S. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ. Health 2016, 15, 10. [Google Scholar] [CrossRef]
- Barrett, J.R. Connecting PM2.5 Exposure to Insulin Resistance: Oxidative Stress May Be an Intermediate Step. Environ. Health Perspect. 2016, 124, A236. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yue, P.; Deiuliis, J.A.; Lumeng, C.N.; Kampfrath, T.; Mikolaj, M.B.; Cai, Y.; Ostrowski, M.C.; Lu, B.; Parthasarathy, S.; et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009, 119, 538–546. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.J.; Li, Z.; Gow, A.; Chung, K.F.; Hu, M.; Sun, Z.; Zeng, L.; Zhu, T.; Jia, G.; et al. Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: Findings from a natural experiment in Beijing. FASEB J. 2016, 30, 2115–2122. [Google Scholar] [CrossRef]
- Yao, M.; Liu, Y.; Jin, D.; Yin, W.; Ma, S.; Tao, R.; Tao, F.; Zhu, P. Relationship betweentemporal distribution of air pollution exposure and glucose homeostasis during pregnancy. Environ. Res. 2020, 185, 109456. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Zhou, Y.; Cheng, Y.; Li, J.; Xiao, X.; Yin, C.; Li, J.; Meng, X.; Zhang, Y. Associations of ozone exposure with gestational diabetes mellitus and glucose homeostasis: Evidence from a birth cohort in Shanghai, China. Sci. Total Environ. 2023, 857 Pt 1, 159184. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Smilkstein, G. The family APGAR: A proposal for a family function test and its use by physicians. J. Fam. Pract. 1978, 6, 1231–1239. [Google Scholar] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva, A.; Hod, M.; Kitzmiler, J.L.; et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef]
- Villar, J.; Cheikh Ismail, L.; Victora, C.G.; Ohuma, E.O.; Bertino, E.; Altman, D.G.; Lambert, A.; Papageorghiou, A.T.; Carvalho, M.; Jaffer, Y.A.; et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014, 384, 857–868. [Google Scholar] [CrossRef]
- Ha, S.; Hu, H.; Roussos-Ross, D.; Haidong, K.; Roth, J.; Xu, X. The effects of air pollution on adverse birth outcomes. Environ. Res. 2014, 134, 198–204. [Google Scholar] [CrossRef]
- Lee, P.-C.; Roberts, J.M.; Catov, J.M.; Talbott, E.O.; Ritz, B. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Matern. Child Health J. 2013, 17, 545–555. [Google Scholar] [CrossRef]
- Wang, Q.; Benmarhnia, T.; Zhang, H.; Knibbs, L.D.; Sheridan, P.; Li, C.; Bao, J.; Ren, M.; Wang, S.; He, Y.; et al. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ. Int. 2018, 121 Pt 1, 317–324. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, J.; Wei, J.; Liu, Y.; Zhu, H.; Li, X.; Wang, J.; Chen, R. Individual ambient ozone exposure during pregnancy and adverse birth outcomes: Exploration of the potentially vulnerable windows. J. Hazard. Mater. 2024, 464, 132945. [Google Scholar] [CrossRef]
- Desye, B.; Berihun, G.; Geto, A.K.; Berhanu, L.; Daba, C. Exposure to ambient air pollutions and its association with adverse birth outcomes: A systematic review and meta-analysis of epidemiological studies. Front. Public. Health 2024, 12, 1488028. [Google Scholar] [CrossRef]
- Li, C.; Yang, M.; Zhu, Z.; Sun, S.; Zhang, Q.; Cao, J.; Ding, R. Maternal exposure to air pollution and the risk of low birth weight: A meta-analysis of cohort studies. Environ. Res. 2020, 190, 109970. [Google Scholar] [CrossRef]
- Sun, S.; Wang, J.; Cao, W.; Wu, L.; Tian, Y.; Sun, F.; Zhang, Z.; Ge, Y.; Du, J.; Li, X.; et al. A nationwide study of maternal exposure to ambient ozone and term birth weight in the United States. Environ. Int. 2022, 170, 107554. [Google Scholar] [CrossRef]
- Guo, P.; Chen, Y.; Wu, H.; Zeng, J.; Zeng, Z.; Li, W.; Zhang, Q.; Huo, X.; Feng, W.; Lin, J.; et al. Ambient air pollution and markers of fetal growth: A retrospective population-based cohort study of 2.57 million term singleton births in China. Environ. Int. 2020, 135, 105410. [Google Scholar] [CrossRef]
- Klepac, P.; Locatelli, I.; Korošec, S.; Künzli, N.; Kukec, A. Ambient air pollution and pregnancy outcomes: A comprehensive review and identification of environmental public health challenges. Environ. Res. 2018, 167, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Miao, H.; Warren, J.L.; Ren, M.; Benmarhnia, T.; Knibbs, L.D.; Zhang, H.; Zhao, Q.; Huang, C. Association of maternal ozone exposure with term low birth weight and susceptible window identification. Environ. Int. 2021, 146, 106208. [Google Scholar] [CrossRef]
- Sun, H.Z.; Chen, H.; Tian, Y.; Wang, Z.; Zhou, J.; Zhang, L.; Fang, J.; Gao, J.; van Daalen, K.R.; Shek, L.P.; et al. Synergistic risks of ambient air particulate matter and ozone exposure on low birth weight: An 11-year longitudinal Chinese maternity cohort study. Environ. Int. 2025, 202, 109640. [Google Scholar] [CrossRef]
- Siddika, N.; Rantala, A.K.; Antikainen, H.; Balogun, H.; Amegah, A.K.; Ryti, N.R.I.; Kukkonen, J.; Sofiev, M.; Jaakkola, M.S.; Jaakkola, J.J.K. Synergistic effects of prenatal exposure to fine particulate matter (PM2.5) and ozone (O3) on the risk of preterm birth: A population-based cohort study. Environ. Res. 2019, 176, 108549. [Google Scholar] [CrossRef] [PubMed]
- Vedika, R.; Sharma, P.; Reddy, A. Signature precursor and mature microRNAs in cervical ripening during gestational diabetes mellitus lead to pre-term labor and other impediments in future. J. Diabetes Metab. Disord. 2023, 22, 945–965. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Mo, M.; Muyiduli, X.; Wang, S.; Li, M.; Jiang, S.; Wu, Y.; Shao, B.; Shen, Y.; et al. The association of gestational diabetes mellitus with fetal birth weight. J. Diabetes Complicat. 2018, 32, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Olsson, D.; Ekström, M.; Forsberg, B. Temporal variation in air pollution concentrations and preterm birth-a population based epidemiological study. Int. J. Environ. Res. Public. Health 2012, 9, 272–285. [Google Scholar] [CrossRef]
- Gunnison, A.F.; Hatch, G.E. O3-induced inflammation in prepregnant, pregnant, and lactating rats correlates with O3 dose estimated by18O. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 276, L332–L340. [Google Scholar] [CrossRef]
- Chiriac, E.; Popa, Z.L.; Gorun, F.I.; Vilceanu, N.; Oros, R.; Buhas, L.C.; Dumitrescu, P.; Citu, C.; Tivadar, K.M.; Csep, A.; et al. The Predictive Role of Maternal Serum Amyloid A in Preterm Birth: An Observational Study in Romania. Cureus 2024, 16, e74996. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, I.E.; Vilceanu, N.; Maghiar, A.; Andrei, C.; Hanganu, B.; Daina, L.G.; Dunarintu, S.; Buhas, L.C. Maternal Serum Amyloid A as a Marker of Preterm Birth/PROM: A Systematic Review and Meta-Analysis. Medicina 2023, 59, 1025. [Google Scholar] [CrossRef]
- Grgic, G.; Skokic, F.; Bogdanovic, G. C-reactive protein as a biochemical marker of idiopathic preterm delivery. Med. Arch. 2010, 64, 132. [Google Scholar]
- Brogin Moreli, J.; Cirino Ruocco, A.M.; Vernini, J.M.; Rudge, M.V.C.; Calderon, I.M.P. Interleukin 10 and tumor necrosis factor-alpha in pregnancy: Aspects of interest in clinical obstetrics. Int. Sch. Res. Not. 2012, 2012, 230742. [Google Scholar] [CrossRef]
- Yockey, L.J.; Iwasaki, A. Interferons and Proinflammatory Cytokines in Pregnancy and Fetal Development. Immunity 2018, 49, 397–412. [Google Scholar] [CrossRef]
- Ângelo-Dias, M.; Martins, C.G.; Mata, M.; Barata, M.; Chung, A.; Sarzedas, S.; Fernandes, É.; Appleton, C.; Lima, J.; Borrego, L.M. Immunological reference intervals in pregnancy: Longitudinal analysis of adaptive lymphocyte subsets. Front. Immunol. 2025, 16, 1634176. [Google Scholar] [CrossRef]
- Uhlar, C.M.; Whitehead, A.S. Serum amyloid A, the major vertebrate acute-phase reactant. Eur. J. Biochem. 1999, 265, 501–523. [Google Scholar] [CrossRef]
- Ye, R.D.; Sun, L. Emerging functions of serum amyloid A in inflammation. J. Leukoc. Biol. 2015, 98, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.T.; Millstein, J.; Li, Y.F.; Lurmann, F.W.; Margolis, H.G.; Gilliland, F.D. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: Results from the Children’s Health Study. Environ. Health Perspect. 2005, 113, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; Zhu, P.; Wang, W.-S.; Sun, K. Serum amyloid A, a host-derived DAMP in pregnancy. Front. Immunol. 2022, 13, 978929. [Google Scholar] [CrossRef]
- Niu, Z.; Xie, C.; Wen, X.; Tian, F.; Ding, P.; He, Y.; Fan, L.; Yuan, S.; Jia, D.; Chen, W.-Q. Mediating role of maternal serum interleukin-1beta and tumor necrosis factor-alpha in the association between environmental tobacco smoke exposure in pregnancy and low birth weight at term. J. Matern. Fetal Neonatal Med. 2018, 31, 1251–1258. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, H. IL-17A Cytokine-Regulated Glut1 Expression in Placenta Cells. Curr. Issues Mol. Biol. 2024, 46, 7386–7394. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Mathad, J.S.; Naik, S.; Alexander, M.; Yadana, S.; Araujo-Pereira, M.; Kulkarni, V.; Deshpande, P.; Kumar, N.P.; Babu, S.; et al. Association of Maternal Inflammation During Pregnancy with Birth Outcomes and Infant Growth Among Women with or Without HIV in India. JAMA Netw. Open 2021, 4, e2140584. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Frequency (N) (Proportion, %) | |||
|---|---|---|---|---|
| Overall (N = 235) | Term Births [n = 208 (88.51%)] | Preterm Births [n = 27 (11.49%)] | p | |
| Age at Delivery, M (Q1, Q3) | 31.00 (29.00, 34.00) | 31.00 (29.00, 33.00) | 30.00 (28.00, 35.00) | 0.836 |
| Gestational Weight Gain, M (Q1, Q3) | 5.50 (3.00, 8.00) | 5.05 (3.00, 8.00) | 6.00 (3.00, 10.00) | 0.648 |
| Pre-pregnancy BMI, M (Q1, Q3) | 21.51 (19.72, 23.62) | 21.44 (19.55, 23.48) | 22.27 (21.12, 24.06) | 0.199 |
| O3, M (Q1, Q3) | 108.70 (106.47, 109.87) | 108.60 (106.37, 109.81) | 109.17 (107.37, 110.60) | 0.053 |
| Pre-pregnancy Health | 0.453 | |||
| Good | 151 (64.26) | 133 (63.94) | 18 (66.67) | |
| General | 80 (34.04) | 72 (34.62) | 8 (29.63) | |
| Bad | 4 (1.70) | 3 (1.44) | 1 (3.70) | |
| Primiparity | 0.833 | |||
| None | 135 (57.45) | 120 (57.69) | 15 (55.56) | |
| Yes | 100 (42.55) | 88 (42.31) | 12 (44.44) | |
| Mode of Conception | 0.666 | |||
| Natural Conception | 218 (92.77) | 194 (93.27) | 24 (88.89) | |
| Assisted Reproduction | 17 (7.23) | 14 (6.73) | 3 (11.11) | |
| Season of Conception | 0.900 | |||
| Spring | 34 (14.47) | 31 (14.90) | 3 (11.11) | |
| Summer | 1 (0.43) | 1 (0.48) | 0 (0.00) | |
| Autumn | 98 (41.70) | 87 (41.83) | 11 (40.74) | |
| Winter | 102 (43.40) | 89 (42.79) | 13 (48.15) | |
| Education Level | 0.878 | |||
| Junior High School or Below | 18 (7.66) | 16 (7.69) | 2 (7.41) | |
| High School | 38 (16.17) | 35 (16.83) | 3 (11.11) | |
| Bachelor’s Degree | 157 (66.81) | 138 (66.35) | 19 (70.37) | |
| Master’s Degree or Above | 22 (9.36) | 19 (9.13) | 3 (11.11) | |
| Careers | 0.303 | |||
| Enterprises and public institutions | 91 (38.72) | 83 (39.90) | 8 (29.63) | |
| Other careers | 144 (61.28) | 125 (60.10) | 19 (70.37) | |
| Per Capita Monthly Income (RMB) | 0.867 | |||
| Low | 14 (5.96) | 12 (5.77) | 2 (7.41) | |
| Medium | 124 (52.77) | 109 (52.40) | 15 (55.56) | |
| High | 97 (41.28) | 87 (41.83) | 10 (37.04) | |
| Address | 0.805 | |||
| City | 210 (89.36) | 185 (88.94) | 25 (92.59) | |
| Rural | 25 (10.64) | 23 (11.06) | 2 (7.41) | |
| Sleep Quality | 0.328 | |||
| Very Good | 48 (20.43) | 43 (20.67) | 5 (18.52) | |
| Fairly Good | 140 (59.57) | 126 (60.58) | 14 (51.85) | |
| Fairly Poor | 44 (18.72) | 37 (17.79) | 7 (25.93) | |
| Very Poor | 3 (1.28) | 2 (0.96) | 1 (3.70) | |
| Sleep Efficiency | 0.110 | |||
| >85% | 174 (74.04) | 156 (75.00) | 18 (66.67) | |
| 75~84% | 43 (18.30) | 39 (18.75) | 4 (14.81) | |
| 65~74% | 15 (6.38) | 11 (5.29) | 4 (14.81) | |
| <65% | 3 (1.28) | 2 (0.96) | 1 (3.70) | |
| Sleep Disturbance | 0.770 | |||
| None | 20 (8.51) | 19 (9.13) | 1 (3.70) | |
| Low | 171 (72.77) | 150 (72.12) | 21 (77.78) | |
| Medium | 43 (18.30) | 38 (18.27) | 5 (18.52) | |
| High | 1 (0.43) | 1 (0.48) | 0 (0.00) | |
| Path Structure | Effect | Boot SE | BootLLCI | BootULCI | p | |
|---|---|---|---|---|---|---|
| Indirect Effect | O3⇒GDM⇒PTB | 0.000 | 0.014 | −0.024 | 0.035 | 0.985 |
| O3⇒Log2SAA⇒PTB | 0.001 | 0.013 | −0.006 | 0.043 | 0.929 | |
| O3⇒GDM⇒Log2SAA⇒PTB | 0.000 | 0.001 | −0.003 | 0.003 | 0.990 | |
| Direct Effect | O3⇒PTB | 0.015 | 0.006 | 0.002 | 0.028 | 0.021 |
| Total Effect | O3⇒PTB | 0.016 | 0.007 | 0.004 | 0.029 | 0.012 |
| Indirect Effect | O3⇒GDM⇒PTB | 0.000 | 0.013 | −0.024 | 0.033 | 0.985 |
| O3⇒Log2CRP⇒PTB | 0.000 | 0.008 | −0.008 | 0.024 | 0.969 | |
| O3⇒GDM⇒Log2CRP⇒PTB | 0.000 | 0.002 | −0.003 | 0.005 | 0.989 | |
| Direct Effect | O3⇒PTB | 0.016 | 0.006 | 0.003 | 0.028 | 0.014 |
| Total Effect | O3⇒PTB | 0.016 | 0.007 | 0.004 | 0.029 | 0.012 |
| Indirect Effect | O3⇒GDM⇒PTB | 0.000 | 0.014 | −0.025 | 0.034 | 0.985 |
| O3⇒Log2TNF-α⇒PTB | −0.001 | 0.008 | −0.024 | 0.006 | 0.933 | |
| O3⇒GDM⇒Log2TNF-α⇒PTB | 0.000 | 0.001 | −0.002 | 0.002 | 0.989 | |
| Direct Effect | O3⇒PTB | 0.017 | 0.006 | 0.004 | 0.029 | 0.010 |
| Total Effect | O3⇒PTB | 0.016 | 0.007 | 0.004 | 0.029 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, C.; Lv, Y.; Chang, M.; Wang, H.; Du, Y.; Li, X.; Ji, J.; Guan, S. Association of Prenatal Ozone Exposure with Fetal Growth and Birth Outcomes: Roles of Maternal Inflammation and Metabolic Dysregulation. Toxics 2025, 13, 983. https://doi.org/10.3390/toxics13110983
Yu Z, Wang C, Lv Y, Chang M, Wang H, Du Y, Li X, Ji J, Guan S. Association of Prenatal Ozone Exposure with Fetal Growth and Birth Outcomes: Roles of Maternal Inflammation and Metabolic Dysregulation. Toxics. 2025; 13(11):983. https://doi.org/10.3390/toxics13110983
Chicago/Turabian StyleYu, Zexin, Chunyan Wang, Yueyi Lv, Mengjun Chang, Hao Wang, Yunyun Du, Xianjia Li, Jin Ji, and Suzhen Guan. 2025. "Association of Prenatal Ozone Exposure with Fetal Growth and Birth Outcomes: Roles of Maternal Inflammation and Metabolic Dysregulation" Toxics 13, no. 11: 983. https://doi.org/10.3390/toxics13110983
APA StyleYu, Z., Wang, C., Lv, Y., Chang, M., Wang, H., Du, Y., Li, X., Ji, J., & Guan, S. (2025). Association of Prenatal Ozone Exposure with Fetal Growth and Birth Outcomes: Roles of Maternal Inflammation and Metabolic Dysregulation. Toxics, 13(11), 983. https://doi.org/10.3390/toxics13110983





