Is Fluoride the Culprit? Revisiting Evidence on Environmental Origins of Chronic Kidney Disease of Uncertain Etiology (CKDu): A Narrative Review
Abstract
1. Introduction
2. Material and Methods
3. Evidence Synthesis
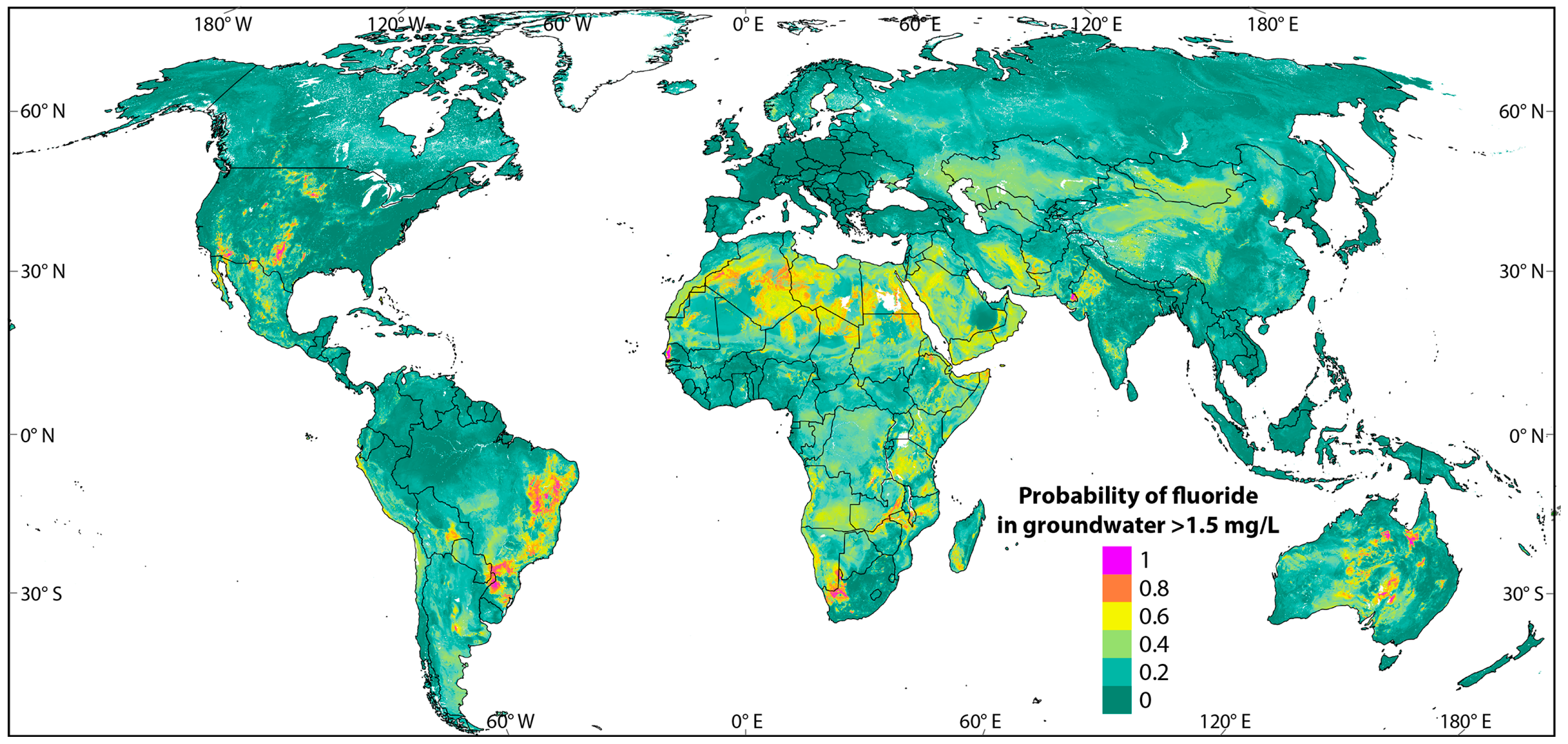
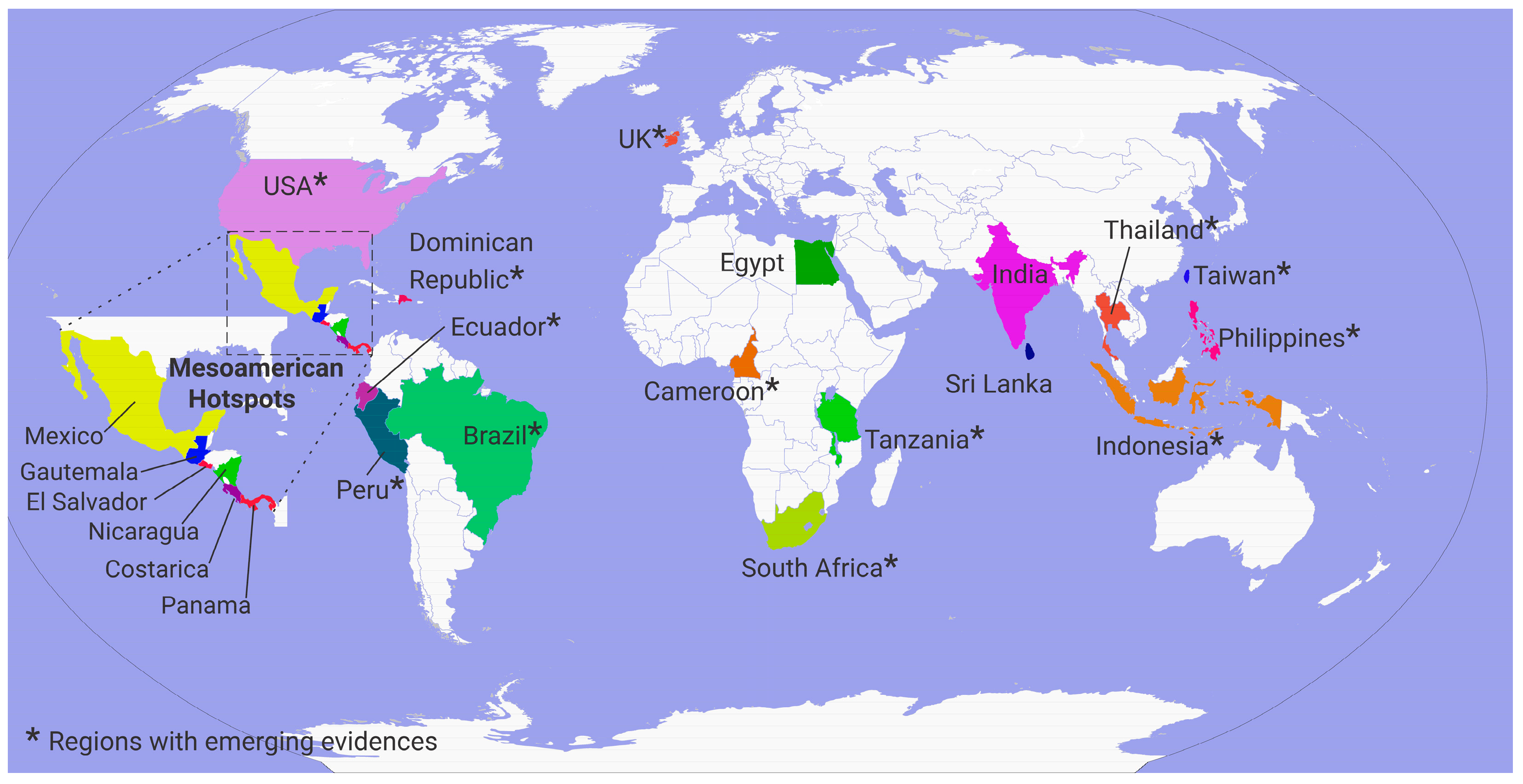
3.1. Fluoride Exposure Through Drinking Water: A Global Perspective
3.2. High-Fluoride Exposure and Associated Kidney Health Risks
3.2.1. Mechanisms of Fluoride-Mediated Kidney Injury
3.2.2. Evidence from Community Studies
3.2.3. Fluoride and CKDu
Evidence from Epidemiological and Hydrological Studies
Evidence from Autopsy and Renal Biopsy Studies
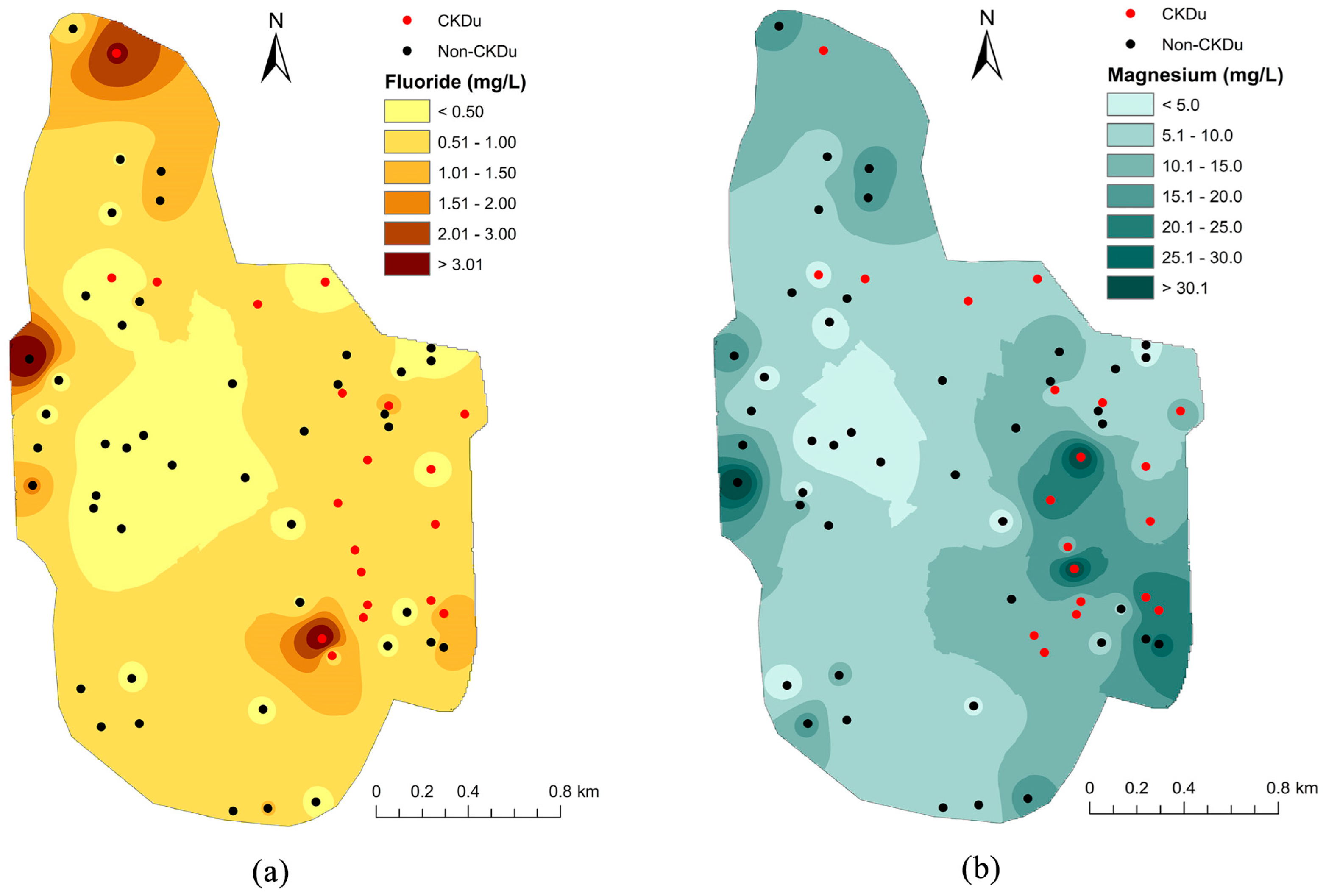
3.2.4. Spatial Distribution of Fluoride and CKDu Prevalence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACP | Acid phosphatase |
| AHR | Anti-hydroxyl radical |
| ASA | Anti-superoxide anion |
| BIS | Bureau of Indian Standards |
| BUN | Blood urea nitrogen |
| CAT | Catalase |
| CI | Confidence interval |
| cIMT | Carotid intima-media thickness |
| CKD | Chronic kidney disease |
| CKDu | Chronic kidney disease of uncertain etiology |
| CLU | Clusterin |
| eGFR | Estimated glomerular filtration rate |
| ET-1 | Endothelin-1 |
| FIN | Focal interstitial nephritis |
| GAP | Groundwater assessment platform |
| GND | Grama Niladhari division |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| ICAM-1 | Intercellular adhesion molecule 1 |
| KIM-1 | Kidney injury molecule |
| LDH | Lactate dehydrogenase |
| NAG | N-acetyl-beta-D-glucosaminidase |
| RBP | Retinol binding protein |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| UA | Uric acid |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| WHO | World Health Organization |
References
- Shaji, E.; Sarath, K.V.; Santosh, M.; Krishnaprasad, P.K.; Arya, B.K.; Babu, M.S. Fluoride Contamination in Groundwater: A Global Review of the Status, Processes, Challenges, and Remedial Measures. Geosci. Front. 2024, 15, 101734. [Google Scholar] [CrossRef]
- Mukherjee, I.; Singh, U.K. Groundwater Fluoride Contamination, Probable Release, and Containment Mechanisms: A Review on Indian Context. Environ. Geochem. Health 2018, 40, 2259–2301. [Google Scholar] [CrossRef] [PubMed]
- Choubisa, S.L.; Choubisa, D. Status of Industrial Fluoride Pollution and Its Diverse Adverse Health Effects in Man and Domestic Animals in India. Environ. Sci. Pollut Res. 2016, 23, 7244–7254. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gallagher, J.E.; Chestnutt, I.G.; Godson, J. Formulation and Fluoride Content of Dentifrices: A Review of Current Patterns. Br. Dent. J. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Samal, A.C.; Banerjee, S.; Pyne, J.; Santra, S.C. Assessment of Potential Health Risk of Fluoride Consumption through Rice, Pulses, and Vegetables in Addition to Consumption of Fluoride-Contaminated Drinking Water of West Bengal, India. Environ. Sci. Pollut Res. 2017, 24, 20300–20314. [Google Scholar] [CrossRef] [PubMed]
- Rizzu, M.; Tanda, A.; Cappai, C.; Roggero, P.P.; Seddaiu, G. Impacts of Soil and Water Fluoride Contamination on the Safety and Productivity of Food and Feed Crops: A Systematic Review. Sci. Total Environ. 2021, 787, 147650. [Google Scholar] [CrossRef]
- Krishnankutty, N.; Storgaard Jensen, T.; Kjær, J.; Jørgensen, J.S.; Nielsen, F.; Grandjean, P. Public-Health Risks from Tea Drinking: Fluoride Exposure. Scand. J. Public Health 2022, 50, 355–361. [Google Scholar] [CrossRef]
- Rocha, R.A.; de la Fuente, B.; Clemente, M.J.; Ruiz, A.; Vélez, D.; Devesa, V. Factors Affecting the Bioaccessibility of Fluoride from Seafood Products. Food Chem. Toxicol. 2013, 59, 104–110. [Google Scholar] [CrossRef]
- Wang, Q.; Song, H.; Wang, Q. Fluorine-Containing Agrochemicals in the Last Decade and Approaches for Fluorine Incorporation. Chin. Chem. Lett. 2022, 33, 626–642. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of Fluoride Removal from Drinking Water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Fluoride Removal from Water by Chitosan Derivatives and Composites: A Review. J. Fluor. Chem. 2011, 132, 231–240. [Google Scholar] [CrossRef]
- Kabir, H.; Gupta, A.K.; Tripathy, S. Fluoride and Human Health: Systematic Appraisal of Sources, Exposures, Metabolism, and Toxicity. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1116–1193. [Google Scholar] [CrossRef]
- Wijewickrama, E.S.; Gunawardena, N.; Jayasinghe, S.; Herath, C. CKD of Unknown Etiology (CKDu) in Sri Lanka: A Multilevel Clinical Case Definition for Surveillance and Epidemiological Studies. Kidney Int. Rep. 2019, 4, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Orantes, C.; Herrera, R.; Almaguer, M.; Lopez, L.; Silva, L.C.; Ordunez, P.; Siribaddana, S.; Gunatilake, S.; De Broe, M.E. Chronic Interstitial Nephritis in Agricultural Communities: A Worldwide Epidemic with Social, Occupational and Environmental Determinants. Nephrol. Dial. Transplant. 2017, 32, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Wijetunge, S.; Ratnatunga, N.V.I.; Abeysekera, T.D.J.; Wazil, A.W.M.; Selvarajah, M. Endemic Chronic Kidney Disease of Unknown Etiology in Sri Lanka: Correlation of Pathology with Clinical Stages. Indian J. Nephrol. 2015, 25, 274–280. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Komiya, T.; Ratnatunga, N.; Senevirathna, S.T.M.L.D.; Harada, K.H.; Hitomi, T.; Gobe, G.; Muso, E.; Abeysekera, T.; Koizumi, A. Tubulointerstitial Damage as the Major Pathological Lesion in Endemic Chronic Kidney Disease among Farmers in North Central Province of Sri Lanka. Environ. Health Prev. Med. 2012, 17, 213–221. [Google Scholar] [CrossRef]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Magara, Y. Fluoride in Drinking-Water; IWA Publishing: Cornwall, UK, 2006. [Google Scholar]
- Podgorski, J.; Berg, M. Global Analysis and Prediction of Fluoride in Groundwater. Nat. Commun. 2022, 13, 4232. [Google Scholar] [CrossRef]
- Rocha-Amador, D.O.; González-Martell, A.D.; Pérez-Vázquez, F.J.; Cilia López, V.G. Health Risk Assessment in Mexican Children Exposed to Fluoride from Sweetened Beverages. Biol. Trace Elem. Res. 2023, 201, 2250–2257. [Google Scholar] [CrossRef] [PubMed]
- Opydo-Szymaczek, J.; Opydo, J. Fluoride Content of Beverages Intended for Infants and Young Children in Poland. Food Chem. Toxicol. 2010, 48, 2702–2706. [Google Scholar] [CrossRef]
- Jedra, M.; Urbanek-Karłowska, B.; Gawarska, H.; Sawilska-Rautenstrauch, D. Fluoride content of soft drinks produced in Poland. Rocz. Panstw. Zakl. Hig. 2006, 57, 203–210. [Google Scholar]
- Malde, M.K.; Zerihun, L.; Julshamn, K.; Bjorvatn, K. Fluoride Intake in Children Living in a High-Fluoride Area in Ethiopia—Intake through Beverages. Int. J. Paediatr. Dent. 2003, 13, 27–34. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Q.; Xie, F.; Jiang, J. Brick Tea Consumption and Its Relationship with Fluorosis in Tibetan Areas. Front. Nutr. 2022, 9, 1030344. [Google Scholar] [CrossRef]
- Kebede, A.; Retta, N.; Abuye, C.; Whiting, S.J.; Kassaw, M.; Zeru, T.; Tessema, M.; Kjellevold, M. Dietary Fluoride Intake and Associated Skeletal and Dental Fluorosis in School Age Children in Rural Ethiopian Rift Valley. Int. J. Environ. Res. Public Health 2016, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Rango, T.; Vengosh, A.; Jeuland, M.; Tekle-Haimanot, R.; Weinthal, E.; Kravchenko, J.; Paul, C.; McCornick, P. Fluoride Exposure from Groundwater as Reflected by Urinary Fluoride and Children’s Dental Fluorosis in the Main Ethiopian Rift Valley. Sci. Total Environ. 2014, 496, 188–197. [Google Scholar] [CrossRef]
- Idowu, O.S.; Duckworth, R.M.; Valentine, R.A.; Zohoori, F.V. Biomarkers for the Assessment of Exposure to Fluoride in Adults. Caries Res. 2021, 55, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Upamalika, S.W.A.M.; Wannige, C.T.; Vidanagamachchi, S.M.; Gunasekara, S.C.; Kolli, R.T.; De Silva, P.M.C.S.; Kulasiri, D.; Jayasundara, N. A Review of Molecular Mechanisms Linked to Potential Renal Injury Agents in Tropical Rural Farming Communities. Environ. Toxicol. Pharmacol. 2022, 92, 103850. [Google Scholar] [CrossRef]
- Bharti, V.K.; Srivastava, R.S.; Kumar, H.; Bag, S.; Majumdar, A.C.; Singh, G.; Pandi-Perumal, S.R.; Brown, G.M. Effects of Melatonin and Epiphyseal Proteins on Fluoride-Induced Adverse Changes in Antioxidant Status of Heart, Liver, and Kidney of Rats. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 532969. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-González, M.C.; Del Razo, L.M.; Barrera-Chimal, J.; Jacobo-Estrada, T.; López-Bayghen, E.; Bobadilla, N.A.; Barbier, O. Proximal Renal Tubular Injury in Rats Sub-Chronically Exposed to Low Fluoride Concentrations. Toxicol. Appl. Pharmacol. 2013, 272, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Jha, N.K.; Pal, D.; Jha, S.K.; Anand, U.; Gopalakrishnan, A.V.; Dey, A.; Mukhopadhyay, P.K. Molecular Basis of Fluoride Toxicities: Beyond Benefits and Implications in Human Disorders. Genes Dis. 2023, 10, 1470–1493. [Google Scholar] [CrossRef]
- Yang, Y.-F.; Li, W.-G.; Wen, P.-P.; Jia, P.-P.; Li, Y.-Z.; Li, T.-Y.; Pei, D.-S. Exposure to Sri Lanka’s Local Groundwater in a CKDu Prevalent Area Causes Kidney Damage in Zebrafish. Aquat. Toxicol. 2022, 251, 106276. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Podder, S.; Agarwal, S.; Bhattacharya, S. Fluoride-Induced Histopathology and Synthesis of Stress Protein in Liver and Kidney of Mice. Arch. Toxicol. 2011, 85, 327–335. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yi, J.; Li, Y.; Yang, B.; Shang, P.; Mehmood, K.; Bilal, R.M.; Zhang, H.; Chang, Y.-F.; et al. The Potential Risks of Chronic Fluoride Exposure on Nephrotoxic via Altering Glucolipid Metabolism and Activating Autophagy and Apoptosis in Ducks. Toxicology 2021, 461, 152906. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gao, Y.; Zhang, W.; Liu, H.; Sun, D. Effect of High Fluoride and High Fat on Serum Lipid Levels and Oxidative Stress in Rabbits. Environ. Toxicol. Pharmacol. 2014, 38, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Samantha, A.; Bandyopadhyay, B.; Das, N. Fluoride Intoxication and Possible Changes in Mitochondrial Membrane Microviscosity and Organ Histology in Rats. Biotechnology 2016, 5, 42–45. [Google Scholar]
- Quadri, J.A.; Alam, M.M.; Sarwar, S.; Singh, S.; Shariff, A.; Das, T.K. Fluoride Induced Nephrotoxicity: Apoptosis, Ultrastructural Changes and Renal Tubular Injury in Experimental Animals. Int. J. Ayurveda Pharma Res. 2016, 4, 91–95. [Google Scholar]
- Dharmaratne, R.W. Exploring the Role of Excess Fluoride in Chronic Kidney Disease: A Review. Hum. Exp. Toxicol. 2019, 38, 269–279. [Google Scholar] [CrossRef]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular Mechanisms of Fluoride Toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, S.; Hema Shree, K.; Ramani, P. Fluoride Effect on Renal and Hepatic Functions: A Comprehensive Decade Review of In Vitro and In Vivo Studies. J. Oral Biol. Craniofac. Res. 2024, 14, 735–745. [Google Scholar] [CrossRef]
- Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Histopathological Findings of Renal Tissue Induced by Oxidative Stress Due to Different Concentrations of Fluoride. Oncotarget 2017, 8, 50430–50446. [Google Scholar] [CrossRef]
- Iano, F.G.; Ferreira, M.C.; Quaggio, G.B.; Fernandes, M.S.; Oliveira, R.C.; Ximenes, V.F.; Buzalaf, M.A.R. Effects of Chronic Fluoride Intake on the Antioxidant Systems of the Liver and Kidney in Rats. J. Fluor. Chem. 2014, 168, 212–217. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Moghaddam, A.H.; Eslami, S.; Nabavi, S.M. Protective Effects of Curcumin against Sodium Fluoride-Induced Toxicity in Rat Kidneys. Biol. Trace Elem. Res. 2012, 145, 369–374. [Google Scholar] [CrossRef]
- Validandi, V.; Dheeravath, S.; Kurella, S.; Jamalpur, R.P.; Mullapudi Venkata, S.; Gorain, S.; Sagubandi, Y.; Narayan Sinha, S. Effect of Fluoride Exposure on Kidney Damage in Rats: Biochemical and Histopathological Changes. Toxicol. Environ. Chem. 2025, 107, 363–382. [Google Scholar] [CrossRef]
- Perera, T.; Ranasinghe, S.; Alles, N.; Waduge, R. Effect of Fluoride on Major Organs with the Different Time of Exposure in Rats. Environ. Health Prev. Med. 2018, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Song, G.H.; Gao, J.P.; Wang, C.F.; Chen, C.Y.; Yan, X.Y.; Guo, M.; Wang, Y.; Huang, F.B. Sodium Fluoride Induces Apoptosis in the Kidney of Rats through Caspase-Mediated Pathways and DNA Damage. J. Physiol. Biochem. 2014, 70, 857–868. [Google Scholar] [CrossRef]
- Ulrich, J.C.; Hoffman, K.; Gunasekara, T.D.K.S.C.; Sandamini, P.M.M.A.; Jackson, B.P.; De Silva, P.M.C.S.; Jayasundara, N.; Ferguson, P.L. Glyphosate and Fluoride in High-Hardness Drinking Water Are Positively Associated with Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Environ. Sci. Technol. Lett. 2023, 10, 916–923. [Google Scholar] [CrossRef]
- Susheela, A.K.; Mondal, N.K.; Tripathi, N.; Gupta, R. Early Diagnosis and Complete Recovery from Fluorosis through Practice of Interventions. J. Assoc. Physicians India 2014, 62, 572–579. [Google Scholar] [PubMed]
- Quadri, J.A.; Alam, M.M.; Sarwar, S.; Ghanai, A.; Shariff, A.; Das, T.K. Multiple Myeloma-Like Spinal MRI Findings in Skeletal Fluorosis: An Unusual Presentation of Fluoride Toxicity in Human. Front. Oncol. 2016, 6, 245. [Google Scholar] [CrossRef]
- Khandare, A.L.; Gourineni, S.R.; Validandi, V. Dental Fluorosis, Nutritional Status, Kidney Damage, and Thyroid Function along with Bone Metabolic Indicators in School-Going Children Living in Fluoride-Affected Hilly Areas of Doda District, Jammu and Kashmir, India. Environ. Monit. Assess. 2017, 189, 579. [Google Scholar] [CrossRef]
- Quadri, J.; Sarwar, S.; Sinha, A.; Kalaivani, M.; Dinda, A.; Bagga, A.; Roy, T.; Das, T.; Shariff, A. Fluoride-Associated Ultrastructural Changes and Apoptosis in Human Renal Tubule: A Pilot Study. Hum. Exp. Toxicol. 2018, 37, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Lata, S.; Yadav, J.; Yadav, J.P. Relationship between Water, Urine and Serum Fluoride and Fluorosis in School Children of Jhajjar District, Haryana, India. Appl. Water Sci. 2017, 7, 3377–3384. [Google Scholar] [CrossRef]
- Majumdar, K.K. Dental Fluorosis and Fluoride in Urine in School Students Living in a Rural Area of West Bengal, India. Int. J. Res. Rev. 2019, 6, 60–66. [Google Scholar]
- Jiménez-Córdova, M.I.; Cárdenas-González, M.; Aguilar-Madrid, G.; Sanchez-Peña, L.C.; Barrera-Hernández, Á.; Domínguez-Guerrero, I.A.; González-Horta, C.; Barbier, O.C.; Del Razo, L.M. Evaluation of Kidney Injury Biomarkers in an Adult Mexican Population Environmentally Exposed to Fluoride and Low Arsenic Levels. Toxicol. Appl. Pharmacol. 2018, 352, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Córdova, M.I.; González-Horta, C.; Ayllón-Vergara, J.C.; Arreola-Mendoza, L.; Aguilar-Madrid, G.; Villareal-Vega, E.E.; Barrera-Hernández, Á.; Barbier, O.C.; Del Razo, L.M. Evaluation of Vascular and Kidney Injury Biomarkers in Mexican Children Exposed to Inorganic Fluoride. Environ. Res. 2019, 169, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Malin, A.J.; Lesseur, C.; Busgang, S.A.; Curtin, P.; Wright, R.O.; Sanders, A.P. Fluoride Exposure and Kidney and Liver Function among Adolescents in the United States: NHANES, 2013–2016. Environ. Int. 2019, 132, 105012. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fan, C.; Zhang, Z.; Zhang, X.; Lou, Q.; Guo, N.; Huang, W.; Zhang, M.; Yin, F.; Guan, Z.; et al. Association between Fluoride Exposure and Kidney Function in Adults: A Cross-Sectional Study Based on Endemic Fluorosis Area in China. Ecotoxicol. Environ. Saf. 2021, 225, 112735. [Google Scholar] [CrossRef]
- Lavanya, S.; Ramani, P.; Sureswar Reddy, M. Kidney Toxicity of Fluorides-A Biochemical Evaluation of Renal Function Markers in Adults Residing in Fluoride Endemic Areas of YSR Kadapa District. J. Oral Biol. Craniofac. Res. 2025, 15, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, T.D.K.S.C.; De Silva, P.M.C.S.; Herath, C.; Siribaddana, S.; Siribaddana, N.; Jayasumana, C.; Jayasinghe, S.; Cardenas-Gonzalez, M.; Jayasundara, N. The Utility of Novel Renal Biomarkers in Assessment of Chronic Kidney Disease of Unknown Etiology (CKDu): A Review. Int. J. Environ. Res. Public Health 2020, 17, 9522. [Google Scholar] [CrossRef]
- Chandrajith, R.; Dissanayake, C.B.; Ariyarathna, T.; Herath, H.M.J.M.K.; Padmasiri, J.P. Dose-Dependent Na and Ca in Fluoride-Rich Drinking Water—Another Major Cause of Chronic Renal Failure in Tropical Arid Regions. Sci. Total Environ. 2011, 409, 671–675. [Google Scholar] [CrossRef]
- Jayatilake, N.; Mendis, S.; Maheepala, P.; Mehta, F.R. Chronic Kidney Disease of Uncertain Aetiology: Prevalence and Causative Factors in a Developing Country. BMC Nephrol. 2013, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Jayasumana, C.; Gunatilake, S.; Senanayake, P. Glyphosate, Hard Water and Nephrotoxic Metals: Are They the Culprits Behind the Epidemic of Chronic Kidney Disease of Unknown Etiology in Sri Lanka? Int. J. Environ. Res. Public Health 2014, 11, 2125–2147. [Google Scholar] [CrossRef]
- Jayasumana, C.; Paranagama, P.; Agampodi, S.; Wijewardane, C.; Gunatilake, S.; Siribaddana, S. Drinking Well Water and Occupational Exposure to Herbicides Is Associated with Chronic Kidney Disease, in Padavi-Sripura, Sri Lanka. Environ. Health 2015, 14, 6. [Google Scholar] [CrossRef]
- Jayasumana, C.; Gunatilake, S.; Siribaddana, S. Simultaneous Exposure to Multiple Heavy Metals and Glyphosate May Contribute to Sri Lankan Agricultural Nephropathy. BMC Nephrol. 2015, 16, 103. [Google Scholar] [CrossRef]
- Chandrajith, R.; Nanayakkara, N.; Zwiener, C.; Daniel, C.; Amann, K.; Barth, J.A.C. Geochemical Characteristics of Groundwater Consumed by Patients with Chronic Kidney Disease with Unknown Aetiology in the Crystalline Dry Zone Terrain of Sri Lanka. Expo. Health 2024, 16, 183–195. [Google Scholar] [CrossRef]
- Dharma-wardana, M.W.C.; Amarasiri, S.L.; Dharmawardene, N.; Panabokke, C.R. Chronic Kidney Disease of Unknown Aetiology and Ground-Water Ionicity: Study Based on Sri Lanka. Environ. Geochem. Health 2015, 37, 221–231. [Google Scholar] [CrossRef]
- Wasana, H.M.S.; Aluthpatabendi, D.; Kularatne, W.M.T.D.; Wijekoon, P.; Weerasooriya, R.; Bandara, J. Drinking Water Quality and Chronic Kidney Disease of Unknown Etiology (CKDu): Synergic Effects of Fluoride, Cadmium and Hardness of Water. Environ. Geochem. Health 2016, 38, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Babich, R.; Ulrich, J.C.; Ekanayake, E.M.D.V.; Massarsky, A.; De Silva, P.M.C.S.; Manage, P.M.; Jackson, B.P.; Ferguson, P.L.; Di Giulio, R.T.; Drummond, I.A.; et al. Kidney Developmental Effects of Metal-Herbicide Mixtures: Implications for Chronic Kidney Disease of Unknown Etiology. Environ. Int. 2020, 144, 106019. [Google Scholar] [CrossRef]
- Herath, C.; Jayasumana, C.; De Silva, P.M.C.S.; De Silva, P.H.C.; Siribaddana, S.; De Broe, M.E. Kidney Diseases in Agricultural Communities: A Case Against Heat-Stress Nephropathy. Kidney Int. Rep. 2018, 3, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Jayasekara, K.B.; Kulasooriya, P.N.; Wijayasiri, K.N.; Rajapakse, E.D.; Dulshika, D.S.; Bandara, P.; Fried, L.F.; De Silva, A.; Albert, S.M. Relevance of Heat Stress and Dehydration to Chronic Kidney Disease (CKDu) in Sri Lanka. Prev. Med. Rep. 2019, 15, 100928. [Google Scholar] [CrossRef] [PubMed]
- Fernando, W.B.N.T.; Nanayakkara, N.; Gunarathne, L.; Chandrajith, R. Serum and Urine Fluoride Levels in Populations of High Environmental Fluoride Exposure with Endemic Ckdu: A Case–Control Study from Sri Lanka. Environ. Geochem. Health 2020, 42, 1497–1504. [Google Scholar] [CrossRef]
- Nanayakkara, S.; Senevirathna, S.T.M.L.D.; Harada, K.H.; Chandrajith, R.; Nanayakkara, N.; Koizumi, A. The Influence of Fluoride on Chronic Kidney Disease of Uncertain Aetiology (CKDu) in Sri Lanka. Chemosphere 2020, 257, 127186. [Google Scholar] [CrossRef]
- Perera, W.P.R.T.; Dayananda, M.D.N.R.; Dissanayake, D.M.U.C.; Rathnasekara, R.a.S.D.; Botheju, W.S.M.; Liyanage, J.A.; Weragoda, S.K.; Kularathne, K.a.M. Risk Assessment of Trace Element Contamination in Drinking Water and Agricultural Soil: A Study in Selected Chronic Kidney Disease of Unknown Etiology (CKDu) Endemic Areas in Sri Lanka. J. Chem. 2021, 2021, e6627254. [Google Scholar] [CrossRef]
- Liyanage, D.N.D.; Diyabalanage, S.; Dunuweera, S.P.; Rajapakse, S.; Rajapakse, R.M.G.; Chandrajith, R. Significance of Mg-Hardness and Fluoride in Drinking Water on Chronic Kidney Disease of Unknown Etiology in Monaragala, Sri Lanka. Environ. Res. 2022, 203, 111779. [Google Scholar] [CrossRef]
- Perera, W.P.R.T.; Dayananda, M.D.N.R.; Liyanage, J.A. Exploring the Root Cause for Chronic Kidney Disease of Unknown Etiology (CKDu) via Analysis of Metal Ion and Counterion Contaminants in Drinking Water: A Study in Sri Lanka. J. Chem. 2020, 2020, e8670974. [Google Scholar] [CrossRef]
- Balasooriya, S.; Munasinghe, H.; Herath, A.T.; Diyabalanage, S.; Ileperuma, O.A.; Manthrithilake, H.; Daniel, C.; Amann, K.; Zwiener, C.; Barth, J.A.C.; et al. Possible Links between Groundwater Geochemistry and Chronic Kidney Disease of Unknown Etiology (CKDu): An Investigation from the Ginnoruwa Region in Sri Lanka. Expo. Health 2020, 12, 823–834. [Google Scholar] [CrossRef]
- Botheju, W.S.M.; Liyanage, J.A.; Kannangara, S.D.P. The Groundwater Geochemistry and the Human Health Risk Assessment of Drinking Water in an Area with a High Prevalence of Chronic Kidney Disease of Unknown Etiology (CKDu), Sri Lanka. J. Chem. 2021, 2021, e1755140. [Google Scholar] [CrossRef]
- Gobalarajah, K.; Subramaniam, P.; Jayawardena, U.A.; Rasiah, G.; Rajendra, S.; Prabagar, J. Impact of Water Quality on Chronic Kidney Disease of Unknown Etiology (CKDu) in Thunukkai Division in Mullaitivu District, Sri Lanka. BMC Nephrol. 2020, 21, 507. [Google Scholar] [CrossRef] [PubMed]
- Imbulana, S.; Oguma, K.; Takizawa, S. Evaluation of Groundwater Quality and Reverse Osmosis Water Treatment Plants in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Sci. Total Environ. 2020, 745, 140716. [Google Scholar] [CrossRef] [PubMed]
- Imbulana, S.; Oguma, K.; Takizawa, S. Seasonal Variations in Groundwater Quality and Hydrogeochemistry in the Endemic Areas of Chronic Kidney Disease of Unknown Etiology (CKDu) in Sri Lanka. Water 2021, 13, 3356. [Google Scholar] [CrossRef]
- Piyathilake, I.D.U.H.; Udeshani, W.A.C.; Hapuarachchi, H.A.C.S.; Ranaweera, L.V.; Udayakumara, E.P.N.; Gunatilake, S.K.; Dissanayake, C.B. Geochemistry of Groundwater in the Uva Province, Sri Lanka—Implications for Chronic Kidney Disease of Uncertain Origin. Front. Water 2021, 3, 771501. [Google Scholar] [CrossRef]
- Herath, H.M.A.S.; Kubota, K.; Kawakami, T.; Nagasawa, S.; Motoyama, A.; Weragoda, S.K.; Chaminda, G.G.T.; Yatigammana, S.K. Potential Risk of Drinking Water to Human Health in Sri Lanka. Environ. Forensics 2017, 18, 241–250. [Google Scholar] [CrossRef]
- Fernando, T.D.; Mathota Arachchige, Y.L.N.; Sanjeewani, K.V.P.; Rajaguru, R.a.M.T.S. Comprehensive Groundwater Quality Analysis in Chronic Kidney Disease of Unknown Etiology (CKDu) Prevalence Areas of Sri Lanka to Investigate the Responsible Culprit. J. Chem. 2022, 2022, e1094427. [Google Scholar] [CrossRef]
- Sandanayake, S.; Diyabalanage, S.; Edirisinghe, E.A.N.V.; Guo, H.; Vithanage, M. Hydrogeochemical Characterization of Groundwater with a Focus on Hofmeister Ions and Water Quality Status in CKDu Endemic and CKDu Non–endemic Areas, Sri Lanka. Environ. Pollut. 2023, 328, 121596. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Gao, Z.; Guo, H.; Zeng, X.; Sandanayake, S.; Vithanage, M. Hydrogeochemical Factors Controlling the Occurrence of Chronic Kidney Disease of Unknown Etiology (CKDu). Environ. Geochem. Health 2023, 45, 2611–2627. [Google Scholar] [CrossRef] [PubMed]
- Lal, K.; Sehgal, M.; Gupta, V.; Sharma, A.; John, O.; Gummidi, B.; Jha, V.; Kumari, A. Assessment of Groundwater Quality of CKDu Affected Uddanam Region in Srikakulam District and across Andhra Pradesh, India. Groundw. Sustain. Dev. 2020, 11, 100432. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, R.K.; Jena, B.; Padhan, K.; Dash, P.K.; Sahoo, S.K.; Das, J. Potential Health Risk of Fluoride in Groundwater of CKDu Endemic Areas in Cuttack District of Odisha, India. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Jayalal, T.B.A.; Mahawithanage, S.T.C.; Senanayaka, S.M.H.M.K.; Dassanayaka, P.B. Evidence of Selected Nephrotoxic Elements in Sri Lankan Human Autopsy Bone Samples of Patients with CKDu and Controls. BMC Nephrol. 2020, 21, 384. [Google Scholar] [CrossRef]
- Gulegoda, C.R.; Dissanayake, C.B.; Amarasekara, D.S.; Wijeratne, S.; Premadasa, J.K.; Chandrajith, R.; Udagama, P.V. Impact of Fluoride Exposure on Male Reproductive Parameters: A Pilot Case–Control Study in Sri Lanka. Expo. Health 2022, 14, 447–457. [Google Scholar] [CrossRef]
- Ranasinghe, A.V.; Kumara, G.W.G.P.; Karunarathna, R.H.; De Silva, A.P.; Sachintani, K.G.D.; Gunawardena, J.M.C.N.; Kumari, S.K.C.R.; Sarjana, M.S.F.; Chandraguptha, J.S.; De Silva, M.V.C. The Incidence, Prevalence and Trends of Chronic Kidney Disease and Chronic Kidney Disease of Uncertain Aetiology (CKDu) in the North Central Province of Sri Lanka: An Analysis of 30,566 Patients. BMC Nephrol. 2019, 20, 338. [Google Scholar] [CrossRef]
- John, O.; Gummudi, B.; Jha, A.; Gopalakrishnan, N.; Kalra, O.P.; Kaur, P.; Kher, V.; Kumar, V.; Machiraju, R.S.; Osborne, N.; et al. Chronic Kidney Disease of Unknown Etiology in India: What Do We Know and Where We Need to Go. Kidney Int. Rep. 2021, 6, 2743–2751. [Google Scholar] [CrossRef]
- Parameswaran, S.; Rinu, P.K.; Kar, S.S.; Harichandrakumar, K.T.; James, T.D.; Priyamvada, P.S.P.; Haridasan, S.; Mohan, S.; Radhakrishnan, J. A Newly Recognized Endemic Region of CKD of Undetermined Etiology (CKDu) in South India—“Tondaimandalam Nephropathy”. Kidney Int. Rep. 2020, 5, 2066–2073. [Google Scholar] [CrossRef]
- Demelash, H.; Beyene, A.; Abebe, Z.; Melese, A. Fluoride Concentration in Ground Water and Prevalence of Dental Fluorosis in Ethiopian Rift Valley: Systematic Review and Meta-Analysis. BMC Public Health 2019, 19, 1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Tang, D.; Zhao, J.; Yang, X.; Liu, Y.; Peng, F.; Shu, L.; Wang, J.; He, Z.; et al. Effects of Water Improvement and Defluoridation on Fluorosis-Endemic Areas in China: A Meta-Analysis. Environ. Pollut. 2021, 270, 116227. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, N.; Kruger, E.; Tennant, M. Spatial Distribution of Groundwater Fluoride Levels and Population at Risk for Dental Caries and Dental Fluorosis in Sri Lanka. Int. Dent. J. 2019, 69, 295–302. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunasekara, T.D.K.S.C.; De Silva, P.M.C.S.; Wijesundara, W.M.P.A.; Chaminda, G.G.T.; Herath, C.; Siribaddana, S.; Bravo, M.A.; Jayasundara, N. Is Fluoride the Culprit? Revisiting Evidence on Environmental Origins of Chronic Kidney Disease of Uncertain Etiology (CKDu): A Narrative Review. Toxics 2025, 13, 966. https://doi.org/10.3390/toxics13110966
Gunasekara TDKSC, De Silva PMCS, Wijesundara WMPA, Chaminda GGT, Herath C, Siribaddana S, Bravo MA, Jayasundara N. Is Fluoride the Culprit? Revisiting Evidence on Environmental Origins of Chronic Kidney Disease of Uncertain Etiology (CKDu): A Narrative Review. Toxics. 2025; 13(11):966. https://doi.org/10.3390/toxics13110966
Chicago/Turabian StyleGunasekara, T. D. K. S. C., P. Mangala C. S. De Silva, W. M. P. A. Wijesundara, G. G. T. Chaminda, Chula Herath, Sisira Siribaddana, Mercedes A. Bravo, and Nishad Jayasundara. 2025. "Is Fluoride the Culprit? Revisiting Evidence on Environmental Origins of Chronic Kidney Disease of Uncertain Etiology (CKDu): A Narrative Review" Toxics 13, no. 11: 966. https://doi.org/10.3390/toxics13110966
APA StyleGunasekara, T. D. K. S. C., De Silva, P. M. C. S., Wijesundara, W. M. P. A., Chaminda, G. G. T., Herath, C., Siribaddana, S., Bravo, M. A., & Jayasundara, N. (2025). Is Fluoride the Culprit? Revisiting Evidence on Environmental Origins of Chronic Kidney Disease of Uncertain Etiology (CKDu): A Narrative Review. Toxics, 13(11), 966. https://doi.org/10.3390/toxics13110966







