Internal Exposure Levels and Health Risk Assessment of Melamine and Organophosphate Metabolites in Urine: Research Progress and Prospects
Abstract
1. Introduction
2. Methodology
3. Analytical Methods for Target Chemicals in Urine
3.1. Sample Preparation
3.1.1. Enzymatic Hydrolysis
3.1.2. Extraction and Enrichment
MEL and Its Derivatives
OPE Metabolites
3.2. Instrumental Analysis
3.2.1. MEL and Its Derivatives
3.2.2. OPE Metabolites
3.3. Method Validation
3.3.1. Recovery
(a) MEL and Its Derivatives
(b) OPE Metabolites
3.3.2. Limit of Detection (LOD) and Limit of Quantitation (LOQ)
(a) MEL and Its Derivatives
(b) OPE Metabolites
4. Exposure Characteristics and Health Risks of Multiple Classes of Contaminants in Urine
4.1. MEL and Its Derivatives
4.1.1. Exposed Levels
| Chemicals | Concentration (ng/mL) | EDI (ng/kg bw/day) | TDI (μg/kg bw/day) | Ref. |
|---|---|---|---|---|
MEL, CYA, AMN, AMD | Mean: MEL: 3.3; CYA: 16; AMD: 0.99; AMN: 0.62; : 20 | Mean: MEL: 65.5 CYA: 315 | 3.15 [60] 8.1 [61] 63 (US FDA) | [37] |
MEL, CYA, AMN, AMD | Mean: MEL: 10.19 ± 12.57 AMN: 1.83 ± 0.98 AMD: 4.80 ± 2.97 CYA: 510.53 ± 621.75 | 13.84–5973.49 | 3.150 [60] 8.1 [61] 63 (US FDA) | [27] |
MEL, CYA, AMN, AMD | Median: MEL: 11.41 AMN: nd AMD: 2.64 CYA: 15.30 ∑MEL: 35.02 | Median: MEL: 0.26 AMN: nd AMD: 0.06 CYA: 0.32 ∑MEL: 0.76 | 3.15 [60] 8.1 [61] 63 (US FDA) | [57] |
MEL, CYA | GM: MEL: 1.40 CYA: 5.34 | Median: MEL: 60 | 3.15 [60] 8.1 [61] 63 (US FDA) | [58] |
MEL, CYA, AMN, AMD | Median: MEL: 4.10; CYA: 25.6; AMD: 5.78; AMN: nd | Median: MEL: 327 CYA: 656 | MEL: 200 (WHO) CYA: 150 (WHO) | [47] |
MEL, CYA | Median: MEL: 4.7; CYA: 27.4 | MEL: 12–250 (children) CYA: 20–500 (children) | MEL: 3.15 CYA: 2.50 | [62] |
| MEL | Mean: Stone patients: 12.25 ± 25.42 MEL factory workers: 432.35 ± 451.26 Steel workers: 7.80 ± 11.28 | Median: Stone patients: 450 MEL factory workers: 23,360 Steel workers: 350 | 200 (WHO) 63 (FDA) | [59] |
4.1.2. Health Risks of MEL and Its Derivatives
4.2. OPE Metabolites
4.2.1. Exposed Levels
| Chemicals | Concentration (ng/mL) | EDI (ng/kg bw/day) | TDI (μg/kg bw/day) | Ref. |
|---|---|---|---|---|
| OPEs metabolites | ||||
BBOEP, BCEP, BCIPP, BDCIPP, DBP, DoCP, DpCP, DPHP | Mean: BBOEP: 0.09; BCEP: 2.83; BCIPP: 0.34 BDCIPP: 0.34; DBP: 0.24; DoCP: 0.09; DpCP: 0.09; DPHP: 0.43 | Mean: TBOEP: 40 TCEP: 1020 TCIPP: 80 TDCIPP: 70 TNBP: 60 TCP: 20 TPHP: 110 | TBOEP: 1.5 TCEP: 2.2 TCIPP: 5.0 TDCIPP: 1.5 TNBP: 2.4 TCP: 1.3 TPHP: 7.0 (the European Food Safety Authority) | [64] |
DNBP, BCIPP, DPHP, BBOEP, BDCIPP, EHPHP, BCIPHIPP, 4-OH-DPHP, BBOEHEP, 5-OH-EHDPHP, TCEP | Median: DNBP: nd; BCIPP: nd; DPHP: 0.33 BBOEP: nd; BDCIPP: 0.08; EHPHP: 0.57; BCIPHIPP: 0.68; 4-OH-DPHP: nd BBOEHEP: nd; 5-OH-EHDPHP: 0.004 TCEP: nd | Median: EHDPHP: 273 TCIPP: 146 TDCIPP: 21.5 TPHP: 199 TBOEP: 0.52 | TMP: 10 TNBP: 10 TCIPP: 10 TCEP: 7 TDCIPP: 20 TEHP: 100 TMPP: 20 DMMP: 60 (USEPA) | [53] |
BCEP, BCIPP, BDCIPP, BBOEP, DBP, DoCP, DpCP, DPHP | Median: BCEP: 0.24–0.27; BCIPP: 0.16–0.22; BDCIPP: 0.33–0.37; BBOEP: 0.026–0.028; DBP: 0.13–0.15; DoCP: 0.058–0.068; DpCP: 0.043–0.050; DPHP: 0.25–0.31 | Median: TBOEP: 6.07; TCEP: 53–57; TCIPP: 35–54; TDCIPP: 76–86; TNBP: 33–27; TCP: 27–23; TPHP: 68–51 | TBOEP: 1.5 TCEP: 2.2 TCIPP: 8.0 TDCIPP: 1.5 TNBP: 2.4 TCP: 1.3 TPHP: 7.0 | [68] |
BCEP, BCIPP, BDCIPP, BBOEP, DBP, DOCP, DPCP, DPHP | Median: BCEP: 0.85; BCIPP: 0.69; BDCIPP: 0.08; BBOEP: 0.04; DBP: 0.06; DOCP + DPCP: 0.004; DPHP: 0.27 | Median: TECP: 485; TCIPP: 80.5; TDCIPP: 8.11; TPHP: 119; TBOEP: 2.06 | TCEP: 2.2 TCIPP: 8.0 TDCIPP: 1.5 TPHP: 7.0 TBOEP: 1.5 | [71] |
BBOEP, BCEP, BCIPP, BDCIPP, DBP, DOCP, DPCP, DPHP | Median: BCEP: 1.04; DPHP: 0.28; BCIPP: 0.15; DBP: 0.12; BBOEP: 0.05; BDCIPP: 0.05; DOCP + DPCP: 0.02 | Median: TECP: 228; TPHP: 33.1; TCIPP: 22.6; TNBP: 19.2; TBOEP: 12.9; TDCIPP: 6.47; TCP: 2.78 | TBOEP: 1.5 TCEP: 2.2 TCIPP: 8 TDCIPP: 1.5 TNBP: 2.4 TCP: 1.3 TPHP: 7 | [72] |
DBP, BCEP, BCIPP, DPHP, BBOEP, BDCIPP, BCIPHIPP, OH-DPHP, BBOEHEP, TCEP | Median: BDCIPP: 3.89; DBP: 1.49; BCIPHIPP: 0.85; TCEP: 0.17; DPHP: 0.25; BCIPP: 0.11; BBOEP: 0.06; OH-DPHP: nd; BCEP: 0.63; BBOEHEP: nd | Median: TDCIPP: 110; TNBP: 43; TCIPP: 25; TCEP: 21 | TDCIPP: 1.5 TNBP: 2.4 TCIPP: 8.0 TCEP: 2.2 TPHP: 7.0 | [73] |
DPHP, BDCIPP, BCEP, DNBP | Median: DPHP: 0.64; BDCIPP: 0.68; BCEP: 0.32; DNBP: 0.18 | - | - | [12] |
DEP, DPRP, DNBP, DIBP, BBOEP, BEHP, BCEP, BCIPP, BDCIPP, DPHP, BMPP | DEP: 0.348; DPRP: 0.015; DNBP: 0.017; DIBP: 0.049; BBOEP: 0.033; BEHP: 0.013; BCEP: 0.035; BCIPP: 0.084; BDCIPP: 0.414; DPHP: 1.060; BMPP: 0.042 | TEP: 24; TNBP: 1.09; TIBP: 2.49 TBOEP: 2.10; TEHP: 1.01; TCEP: 5.97; TCIPP: 0.34; TDCIPP: 8.51; TPHP: 19.4 | TNBP: 80 TBOEP: 50 TCEP: 200 TDCIPP: 20 | [69] |
4.2.2. Health Risks
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oberg, T.; Iqbal, M.S. The chemical and environmental property space of REACH chemicals. Chemosphere 2012, 87, 975–981. [Google Scholar] [CrossRef]
- Li, W.L.; Liu, L.Y.; Zhang, Z.F.; Song, W.W.; Huo, C.Y.; Qiao, L.N.; Ma, W.L.; Li, Y.F. Brominated flame retardants in the surrounding soil of two manufacturing plants in China: Occurrence, composition profiles and spatial distribution. Environ. Pollut. 2016, 213, 1–7. [Google Scholar] [CrossRef]
- Zhang, M.; Li, S.; Yu, C.; Liu, G.; Jia, J.; Lu, C.; He, J.; Ma, Y.; Zhu, J.; Yu, C. Determination of melamine and cyanuric acid in human urine by a liquid chromatography tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2010, 878, 758–762. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, C.; Zeng, M.-X.; Wei, G.-L.; Zeng, L.-X.; Liu, L.-Y.; Zeng, E.Y. An overview of organophosphate esters and their metabolites in humans: Analytical methods, occurrence, and biomonitoring. Sci. Total Environ. 2022, 848, 157669. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; He, Y.; Wang, L.; Wang, Y.; Zeng, L. Elevated emissions of melamine and its derivatives in the indoor environments of typical e-waste recycling facilities and adjacent communities and implications for human exposure. J. Hazard. Mater. 2022, 432, 128652. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kannan, K. Melamine and cyanuric acid in foodstuffs from the United States and their implications for human exposure. Environ. Int. 2019, 130, 104950. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kannan, K. Determination of melamine and its derivatives in textiles and infant clothing purchased in the United States. Sci. Total Environ. 2020, 710, 136396. [Google Scholar] [CrossRef]
- Zhu, H.; Loganathan, B.G.; Kannan, K. Occurrence and Profiles of Melamine and Cyanuric Acid in Bovine Feed and Urine from China, India, and the United States. Environ. Sci. Technol. 2019, 53, 7029–7035. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Distribution Profiles of Melamine and Its Derivatives in Indoor Dust from 12 Countries and the Implications for Human Exposure. Environ. Sci. Technol. 2018, 52, 12801–12808. [Google Scholar] [CrossRef]
- Zheng, G.; Salamova, A. Are melamine and its derivatives the alternatives for per-and polyfluoroalkyl substance (PFAS) fabric treatments in infant clothes? Environ. Sci. Technol. 2020, 54, 10207–10216. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Occurrence and distribution of melamine and its derivatives in surface water, drinking water, precipitation, wastewater, and swimming pool water. Environ. Pollut. 2020, 258, 113743. [Google Scholar] [CrossRef]
- Kang, H.; Lee, J.; Lee, J.P.; Choi, K. Urinary metabolites of organophosphate esters (OPEs) are associated with chronic kidney disease in the general US population, NHANES 2013–2014. Environ. Int. 2019, 131, 105034. [Google Scholar] [CrossRef]
- Mendelsohn, E.; Hagopian, A.; Hoffman, K.; Butt, C.M.; Lorenzo, A.; Congleton, J.; Webster, T.F.; Stapleton, H.M. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int. 2016, 86, 45–51. [Google Scholar] [CrossRef]
- Du, J.; Li, H.; Xu, S.; Zhou, Q.; Jin, M.; Tang, J. A review of organophosphorus flame retardants (OPFRs): Occurrence, bioaccumulation, toxicity, and organism exposure. Environ. Sci. Pollut. Res. 2019, 26, 22126–22136. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Y.; Xiang, P.; Li, C.; Zhou, C.; Zhang, S.; Cui, X.; Ma, L.Q. Organophosphorus flame retardants and phthalate esters in indoor dust from different microenvironments: Bioaccessibility and risk assessment. Chemosphere 2016, 150, 528–535. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Q.; Yan, X.; Wang, Y.; Liao, C.; Jiang, G. A review of organophosphate flame retardants and plasticizers in the environment: Analysis, occurrence and risk assessment. Sci. Total Environ. 2020, 731, 139071. [Google Scholar] [CrossRef]
- He, C.-T.; Zheng, J.; Qiao, L.; Chen, S.-J.; Yang, J.-Z.; Yuan, J.-G.; Yang, Z.-Y.; Mai, B.-X. Occurrence of organophosphorus flame retardants in indoor dust in multiple microenvironments of southern China and implications for human exposure. Chemosphere 2015, 133, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, M.; Zhang, Q.; Wu, X.; Zhao, H.; Xie, Q.; Chen, J. Organophosphorus Flame Retardants and Plasticizers in Building and Decoration Materials and Their Potential Burdens in Newly Decorated Houses in China. Environ. Sci. Technol. 2017, 51, 10991–10999. [Google Scholar] [CrossRef]
- Ding, J.; Deng, T.; Xu, M.; Wang, S.; Yang, F. Residuals of organophosphate esters in foodstuffs and implication for human exposure. Environ. Pollut. 2018, 233, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.G.; Thomas, J.D.; Osterloh, J.D. Melamine toxicity. J. Med. Toxicol. 2010, 6, 50–55. [Google Scholar] [CrossRef]
- Chu, C.; Wang, C. Toxicity of melamine: The public health concern. J. Environ. Sci. Health Part C 2013, 31, 342–386. [Google Scholar] [CrossRef]
- Charron, I.; Le Magueresse-Battistoni, B.; Habert, R.; Canivenc-Lavier, M.C.; Mhaouty-Kodja, S.; Michel-Caillet, C. Melamine regulatory assessment for endocrine disruption. Environ. Int. 2024, 194, 9. [Google Scholar] [CrossRef]
- Chen, S.; Yang, W.; Zhang, X.; Jin, J.; Liang, C.; Wang, J.; Zhang, J. Melamine induces reproductive dysfunction via down-regulated the phosphorylation of p38 and downstream transcription factors Max and Sap1a in mice testes. Sci. Total Environ. 2021, 770, 144727. [Google Scholar] [CrossRef]
- Sun, W.; Li, X.; Tang, D.; Wu, Y.; An, L. Subacute melamine exposure disrupts task-based hippocampal information flow via inhibiting the subunits 2 and 3 of AMPA glutamate receptors expression. Hum. Exp. Toxicol. 2021, 40, 928–939. [Google Scholar] [CrossRef]
- Liu, X.; Cai, Y.; Wang, Y.; Xu, S.; Ji, K.; Choi, K. Effects of tris(1,3-dichloro-2-propyl) phosphate (TDCPP) and triphenyl phosphate (TPP) on sex-dependent alterations of thyroid hormones in adult zebrafish. Ecotoxicol. Environ. Saf. 2019, 170, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Bai, X.-Y.; Lu, S.-Y.; Zhang, B.; Xie, L.; Zheng, H.-C.; Jiang, Y.-C.; Zhou, M.-Z.; Zhou, Z.-Q.; Song, S.-M.; et al. Urinary metabolites of organophosphate flame retardants in China: Health risk from tris(2-chloroethyl) phosphate (TCEP) exposure. Environ. Int. 2018, 121, 1363–1371. [Google Scholar] [CrossRef]
- Liu, S.; Dong, R.; Wang, Y.; Yang, Z.; He, G.; Chen, B. Twenty-four-hour temporal trend of melamine and its derivatives in urine in association with meal consumption: A panel study in Shanghai, China. Environ. Sci. Pollut. Res. 2023, 30, 120225–120235. [Google Scholar] [CrossRef]
- Lin, M.; Tang, J.; Ma, S.; Yu, Y.; Li, G.; Mai, B.; Fan, R.; An, T. Simultaneous determination of polybrominated diphenyl ethers, polycyclic aromatic hydrocarbons and their hydroxylated metabolites in human hair: A potential methodology to distinguish external from internal exposure. Analyst 2019, 144, 7227–7235. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chinthakindi, S.; Kannan, K. A method for the analysis of 121 multi-class environmental chemicals in urine by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1646, 462146. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, C.; Li, Q.; Zhong, Y.; Ma, S.; Zhou, J.; Li, X.; Huang, R.; Yu, Y. Human health risks estimations from polycyclic aromatic hydrocarbons in serum and their hydroxylated metabolites in paired urine samples. Environ. Pollut. 2021, 290, 117975. [Google Scholar] [CrossRef]
- Zhang, Q.; da Costa, G.G.; Von Tungeln, L.S.; Jacob, C.C.; Brown, R.P.; Goering, P.L. Urinary Biomarker Detection of Melamine- and Cyanuric Acid-Induced Kidney Injury in Rats. Toxicol. Sci. 2012, 129, 1–8. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, S.; Xu, K.; Zhao, L.; Liu, Y.; Zou, Q.; Zhang, H.; Zhu, H.; Zhang, T.; Sun, H. Exposure to nitrogenous based flame retardants in Chinese population: Evidence from a national-scale study. J. Hazard. Mater. 2023, 445, 130653. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- Saillenfait, A.-M.; Ndaw, S.; Robert, A.; Sabate, J.-P. Recent biomonitoring reports on phosphate ester flame retardants: A short review. Arch. Toxicol. 2018, 92, 2749–2778. [Google Scholar] [CrossRef]
- Van den Eede, N.; Maho, W.; Erratico, C.; Neels, H.; Covaci, A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 2013, 223, 9–15. [Google Scholar] [CrossRef]
- Kang, H.-S.; Kyung, M.-S.; Ko, A.; Park, J.-H.; Hwang, M.-S.; Kwon, J.-E.; Suh, J.-H.; Lee, H.-S.; Moon, G.I.; Hong, J.-H.; et al. Urinary concentrations of parabens and their association with demographic factors: A population-based cross-sectional study. Environ. Res. 2016, 146, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kannan, K. Inter-day and inter-individual variability in urinary concentrations of melamine and cyanuric acid. Environ. Int. 2019, 123, 375–381. [Google Scholar] [CrossRef]
- Wang, L.; Kannan, K. Characteristic Profiles of Benzonphenone-3 and its Derivatives in Urine of Children and Adults from the United States and China. Environ. Sci. Technol. 2013, 47, 12532–12538. [Google Scholar] [CrossRef] [PubMed]
- Khosravipour, M.; Khosravipour, H. The association between urinary metabolites of polycyclic aromatic hydrocarbons and diabetes: A systematic review and meta-analysis study. Chemosphere 2020, 247, 125680. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Hwang, J.-S.; Sung, F.-C.; Lin, C.-Y.; Hsieh, C.-J.; Chen, P.-C.; Su, T.-C. Mono-2-ethylhexyl phthalate associated with insulin resistance and lower testosterone levels in a young population. Environ. Pollut. 2017, 225, 112–117. [Google Scholar] [CrossRef]
- Kaivosaari, S.; Finel, M.; Koskinen, M. N-glucuronidation of drugs and other xenobiotics by human and animal UDP-glucuronosyltransferases. Xenobiotica 2011, 41, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Jagani, R.; Pulivarthi, D.; Patel, D.; Wright, R.J.; Wright, R.O.; Arora, M.; Wolff, M.S.; Andra, S.S. Validated single urinary assay designed for exposomic multi-class biomarkers of common environmental exposures. Anal. Bioanal. Chem. 2022, 414, 5943–5966. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Romanoff, L.C.; Trinidad, D.A.; Hussain, N.; Jones, R.S.; Porter, E.N.; Patterson, D.G., Jr.; Sjodin, A. Measurement of urinary monohydroxy polycyclic aromatic hydrocarbons using automated liquid-liquid extraction and gas chromatography/isotope dilution high-resolution mass spectrometry. Anal. Chem. 2006, 78, 5744–5751. [Google Scholar] [CrossRef]
- Hou, R.; Xu, Y.; Wang, Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere 2016, 153, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Panesar, N.S.; Chan, K.W.; Lo, W.S.; Leung, V.H.; Ho, C.S. Co-contamination, but not mammalian cell conversion of melamine to cyanuric acid the likely cause of melamine-cyanurate nephrolithiasis. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 1830–1831. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Zhao, L.; Hua, L.; Xu, K.; Shi, Y.; Chen, S.; Zhao, H.; Zhu, H.; Wang, S. Unraveling the contribution of melamine tableware for human internal exposure to melamine and its derivatives: Insights from crossover and biomonitoring studies. Sci. Total Environ. 2024, 955, 176971. [Google Scholar] [CrossRef]
- Hoffman, K.; Lorenzo, A.; Butt, C.M.; Adair, L.; Herring, A.H.; Stapleton, H.M.; Daniels, J.L. Predictors of urinary flame retardant concentration among pregnant women. Environ. Int. 2017, 98, 96–101. [Google Scholar] [CrossRef]
- Hammel, S.C.; Zhang, S.; Lorenzo, A.M.; Eichner, B.; Stapleton, H.M.; Hoffman, K. Young infants’ exposure to organophosphate esters: Breast milk as a potential source of exposure. Environ. Int. 2020, 143, 106009. [Google Scholar] [CrossRef]
- Yang, W.; Braun, J.M.; Vuong, A.M.; Percy, Z.; Xu, Y.; Xie, C.; Deka, R.; Calafat, A.M.; Ospina, M.; Yolton, K.; et al. Patterns of urinary organophosphate ester metabolite trajectories in children: The HOME Study. J. Expo. Sci. Environ. Epidemiol. 2024, 34, 251–259. [Google Scholar] [CrossRef]
- Hernandez-Castro, I.; Eckel, S.P.; Howe, C.G.; Niu, Z.; Kannan, K.; Robinson, M.; Foley, H.B.; Grubbs, B.; Al-Marayati, L.; Lerner, D.; et al. Sex-specific effects of prenatal organophosphate ester (OPE) metabolite mixtures and adverse infant birth outcomes in the maternal and developmental risks from environmental and social stressors (MADRES) pregnancy cohort. Environ. Res. 2023, 226, 115703. [Google Scholar] [CrossRef]
- Wang, H.; Shi, S.; Liu, L.; Chen, D.; Lyu, Z.; Song, Z.; Youjie, W.; Song, L.; Mei, S. Internal exposure characteristics and health risk assessment of organophosphate esters in urban residents. Chin. J. Chromatogr. 2025, 43, 630–639. [Google Scholar] [CrossRef]
- Li, M.; Yao, Y.; Wang, Y.; Bastiaensen, M.; Covaci, A.; Sun, H. Organophosphate ester flame retardants and plasticizers in a Chinese population: Significance of hydroxylated metabolites and implication for human exposure. Environ. Pollut. 2020, 257, 113633. [Google Scholar] [CrossRef]
- Shi, X.; Dong, R.; Chen, J.; Yuan, Y.; Long, Q.; Guo, J.; Li, S.; Chen, B. An assessment of melamine exposure in Shanghai adults and its association with food consumption. Environ. Int. 2020, 135, 105363. [Google Scholar] [CrossRef] [PubMed]
- Adoamnei, E.; Mendiola, J.; Monino-Garcia, M.; Vela-Soria, F.; Iribarne-Duran, L.M.; Fernandez, M.F.; Olea, N.; Jorgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary concentrations of parabens and reproductive parameters in young men. Sci. Total Environ. 2018, 621, 201–209. [Google Scholar] [CrossRef]
- Melough, M.M.; Day, D.B.; Fretts, A.M.; Wang, S.; Flynn, J.T.; de Boer, I.H.; Zhu, H.; Kannan, K.; Sathyanarayana, S. Associations of Dietary Intake with Urinary Melamine and Derivative Concentrations among Children in the GAPPS Cohort. Int. J. Environ. Res. Public Health 2022, 19, 4964. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Q.; Huang, F.; Yang, Q.; Wang, Y.; Wang, H.; Sun, Y.; Yan, Y.; He, G.; Zhao, G.; et al. Exposure to melamine and its derivatives in Chinese adults: The cumulative risk assessment and the effect on routine blood parameters. Ecotoxicol. Environ. Saf. 2022, 241, 113714. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wu, C.; Zhang, J.; Chang, X.; Zhang, Y.; Cao, Y.; Zhou, Z. Associations of melamine and cyanuric acid exposure with markers of kidney function in adults: Results from NHANES 2003–2004. Environ. Int. 2020, 141, 105815. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liu, C.-C.; Wang, Y.-H.; Wu, C.-F.; Tsai, Y.-C.; Li, S.-S.; Hsieh, T.-J.; Wu, M.-T. Benchmark Dose of Melamine Exposure for a Renal Injury Marker Mediated by Oxidative Stress: Examples in Patients with Urolithiasis and Occupational Workers. Toxics 2024, 12, 584. [Google Scholar] [CrossRef]
- Choi, L.; Kwak, M.Y.; Kwak, E.H.; Kim, D.H.; Han, E.Y.; Roh, T.; Bae, J.Y.; Ahn, I.Y.; Jung, J.Y.; Kwon, M.J.; et al. Comparative Nephrotoxicitiy Induced by Melamine, Cyanuric Acid, or a Mixture of Both Chemicals in Either Sprague-Dawley Rats or Renal Cell Lines. J. Toxicol. Environ. Health-Part A-Curr. Issues 2010, 73, 1407–1419. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Flynn, J.T.; Messito, M.J.; Gross, R.; Whitlock, K.B.; Kannan, K.; Karthikraj, R.; Morrison, D.; Huie, M.; Christakis, D.; et al. Melamine and cyanuric acid exposure and kidney injury in US children. Environ. Res. 2019, 171, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.M.; Boor, B.E.; Schreder, E.; Salamova, A. Exposure to melamine and its derivatives in childcare facilities. Chemosphere 2020, 244, 125505. [Google Scholar] [CrossRef]
- Hsieh, D.P.H.; Chiang, C.F.; Chiang, P.H.; Wen, C.P. Toxicological analysis points to a lower tolerable daily intake of melamine in food. Regul. Toxicol. Pharmacol. 2009, 55, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, L.; Lu, S.; Kang, L.; Luo, X.; Liu, G.; Cui, X.; Yu, Y. Organophosphate ester and phthalate ester metabolites in urine from primiparas in Shenzhen, China: Implications for health risks. Environ. Pollut. 2019, 247, 944–952. [Google Scholar] [CrossRef]
- Fromme, H.; Lahrz, T.; Kraft, M.; Fembacher, L.; Mach, C.; Dietrich, S.; Burkardt, R.; Voelkel, W.; Goeen, T. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int. 2014, 71, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kosarac, I.; Kubwabo, C.; Foster, W.G. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS). J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2016, 1014, 24–30. [Google Scholar] [CrossRef]
- He, C.; Toms, L.-M.L.; Phong, T.; Van den Eede, N.; Wang, X.; Li, Y.; Baduel, C.; Harden, F.A.; Heffernan, A.L.; Hobson, P.; et al. Urinary metabolites of organophosphate esters: Concentrations and age trends in Australian children. Environ. Int. 2018, 111, 124–130. [Google Scholar] [CrossRef]
- Liao, G.; Weng, X.; Wang, F.; Yu, Y.H.K.; Arrandale, V.H.; Chan, A.H.-S.; Lu, S.; Tse, L.A. Estimated daily intake and cumulative risk assessment of organophosphate esters and associations with DNA damage among e-waste workers in Hong Kong. Chemosphere 2024, 360, 142406. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Martinez-Moral, M.P.; Sun, H.; Kannan, K. Metabolites of organophosphate esters in urine from the United States: Concentrations, temporal variability, and exposure assessment. Environ. Int. 2019, 122, 213–221. [Google Scholar] [CrossRef]
- Butt, C.M.; Hoffman, K.; Chen, A.; Lorenzo, A.; Congleton, J.; Stapleton, H.M. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ. Int. 2016, 94, 627–634. [Google Scholar] [CrossRef]
- Ingle, M.E.; Watkins, D.; Rosario, Z.; VelezVega, C.M.; Calafat, A.M.; Ospina, M.; Ferguson, K.K.; Cordero, J.F.; Alshawabkeh, A.; Meeker, J.D. An exploratory analysis of urinary organophosphate ester metabolites and oxidative stress among pregnant women in Puerto Rico. Sci. Total Environ. 2020, 703, 134798. [Google Scholar] [CrossRef]
- Ding, J.; Deng, T.; Ye, X.; Covaci, A.; Liu, J.; Yang, F. Urinary metabolites of organophosphate esters and implications for exposure pathways in adolescents from Eastern China. Sci. Total Environ. 2019, 695, 133894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lu, S.; Huang, M.; Zhou, M.; Zhou, Z.; Zheng, H.; Jiang, Y.; Bai, X.; Zhang, T. Urinary metabolites of organophosphate flame retardants in 0-5-year-old children: Potential exposure risk for inpatients and home stay infants. Environ. Pollut. 2018, 243, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fang, J.; Ren, L.; Fan, R.; Zhang, J.; Liu, G.; Zhou, L.; Chen, D.; Yu, Y.; Lu, S. Urinary metabolites of organophosphate esters in children in South China: Concentrations, profiles and estimated daily intake. Environ. Pollut. 2018, 235, 358–364. [Google Scholar] [CrossRef] [PubMed]
| Chemicals | Abb. | MF | CAS No. | Structural Formula |
|---|---|---|---|---|
| Melamine and Its Derivatives | ||||
| Melamine | MEL | C3H6N6 | 108-78-1 |  |
| Cyanuric Acid | CYA | C3N3(OH)3 | 108-80-5 | 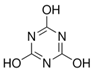 |
| Ammeline | AMN | C3H5N5O | 645-92-1 | 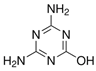 |
| Ammelide | AMD | C3H4N4O2 | 645-93-2 | 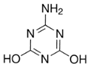 |
| OPEs | ||||
| Triethyl phosphate | TEP | C6H15O4P | 78-40-0 | 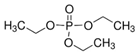 |
| Tributyl phosphate | TNBP | C12H27O4P | 126-73-8 |  |
| Tris(2-chloroethyl) phosphate | TCEP | C6H12Cl3O4P | 115-96-8 |  |
| Tripropyl phosphate | TPP | C9H21O4P | 513-08-6 |  |
| Triphenyl phosphate | TPHP | C18H15O4P | 115-86-6 | 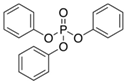 |
| Tris(1,3-dichloro-2-propyl) phosphate | TDCIPP | C9H15Cl6O4P | 13674-87-8 | 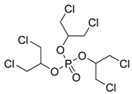 |
| OPE metabolites | ||||
| di-n-butyl phosphate | DNBP | C8H19O4P | 107-66-4 |  |
| di-iso-butyl phosphate | DIBP | C8H19O4P | 6303-30-6 |  |
| diphenyl phosphate | DPHP | C12H11O4P | 838-85-7 |  |
| bis(1,3-dichloro-2-propyl) phosphate | BDCIPP | C6H11Cl4O4P | 72236-72-7 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Jiang, Q.; Wang, X.-H.; Wang, L.; Tian, M.-H.; Chen, D.-Z.; Huo, C.-Y.; Li, W.-L. Internal Exposure Levels and Health Risk Assessment of Melamine and Organophosphate Metabolites in Urine: Research Progress and Prospects. Toxics 2025, 13, 950. https://doi.org/10.3390/toxics13110950
Zhang Q, Jiang Q, Wang X-H, Wang L, Tian M-H, Chen D-Z, Huo C-Y, Li W-L. Internal Exposure Levels and Health Risk Assessment of Melamine and Organophosphate Metabolites in Urine: Research Progress and Prospects. Toxics. 2025; 13(11):950. https://doi.org/10.3390/toxics13110950
Chicago/Turabian StyleZhang, Qu, Qi Jiang, Xin-Hong Wang, Liang Wang, Mei-Hua Tian, Da-Zhong Chen, Chun-Yan Huo, and Wen-Long Li. 2025. "Internal Exposure Levels and Health Risk Assessment of Melamine and Organophosphate Metabolites in Urine: Research Progress and Prospects" Toxics 13, no. 11: 950. https://doi.org/10.3390/toxics13110950
APA StyleZhang, Q., Jiang, Q., Wang, X.-H., Wang, L., Tian, M.-H., Chen, D.-Z., Huo, C.-Y., & Li, W.-L. (2025). Internal Exposure Levels and Health Risk Assessment of Melamine and Organophosphate Metabolites in Urine: Research Progress and Prospects. Toxics, 13(11), 950. https://doi.org/10.3390/toxics13110950










