1. Introduction
Iodine, as a ubiquitous trace element, has consistently been a research hotspot due to its significance in both biological and geochemical fields [
1,
2]. The forms of iodine in the environment are diverse. Currently, most research primarily focuses on inorganic iodine, mainly iodide ions (I
−) and iodate ions (IO
3−), with relatively limited attention paid to iodine-containing disinfection byproducts (I-DBPs) generated during drinking water disinfection [
3,
4,
5]. However, research on other organic iodides in water remains relatively scarce.
The distribution of iodine in the natural environment exhibits significant heterogeneity: apart from marine systems (where seawater contains relatively high iodine concentrations), iodine is also closely associated with natural gas extraction, with shale gas extraction as a typical example [
6]. This association leads to sustained elevated iodine levels in the surroundings of shale gas extraction sites. In recent years, halogenated organic compounds (HOCs) have been frequently detected in shale gas wastewater [
7,
8,
9,
10]. Notably, several studies have identified organic iodides as the dominant class of detected HOCs. For instance, Luek et al. reported that in flowback wastewater from the Marcellus Shale, organic compounds containing iodine, bromine, chlorine, and mixed halogens (two distinct halogens) accounted for 52%, 20%, 9%, and 19% of total HOCs, respectively [
10]. Furthermore, the concentration of organic iodides showed a gradual increase during wastewater discharge and remained relatively high even 10 months after gas well production began [
8]. In our previous study, 21 HOCs were identified from the discharged wastewater of the shale gas wastewater treatment plant in Chongqing, China [
11]. The composition included 5 chlorinated, 10 iodinated, 3 brominated, and 3 mixed-halogen (bromo/chloro or iodo/chloro) organic compounds. Consistent with prior findings, iodinated organic compounds constituted the most abundant HOC class detected. Quantitative analysis further revealed that diiodoalkanes (including 1,2-diiodoethane, 1,3-diiodopropane, and 1,4-diiodobutane) were present at relatively high concentrations. Among these, 1,2-diiodoethane reached a maximum concentration of 72.6 μg/L (0.26 μM) in surface water [
12]. The formation mechanism of organic iodides in shale gas extraction may resemble that of DBPs in drinking water: during hydraulic fracturing or wastewater treatment, dissolved inorganic iodine is oxidized by strong oxidants to generate reactive halogen species (e.g., molecular halogens and halogen-free radicals). These reactive species then react with natural organic matter (NOM) in geological formations to form “unknown-origin” organic iodides [
9,
11].
HOCs are typically persistent organic pollutants, characterized by chemical stability and low biodegradability, which pose severe threats to the ecological environment and human health. Consequently, HOCs remain a core focus in environmental science. As transformation products of inorganic iodine, organic iodides exhibit far more complex environmental behaviors and toxic effects than their inorganic counterparts. For example, organic iodides generated via atmospheric photochemical processes (e.g., iodomethane and iodoethane) have low intrinsic toxicity but trigger cascading environmental reactions: these volatile compounds undergo long-range atmospheric transport, decompose under ultraviolet (UV) radiation, and release reactive iodine atoms that deplete stratospheric ozone [
13]. Additionally, limited cellular and genetic studies on DBPs have demonstrated that brominated and iodinated DBPs exhibit significantly higher cytotoxicity and genotoxicity than their chlorinated congeners [
4,
14]. Based on these findings, it can be inferred that organic iodides formed during shale gas extraction may also possess substantial toxicity. However, the mechanisms underlying their toxic effects remain poorly understood, and relevant research is extremely limited. Given that shale gas has become a key focus of global energy development, and considering the “high complexity, strong toxicity, and persistence” of organic iodine pollutants derived from shale gas extraction, comprehensive ecological and human health risk assessments prior to shale gas development are of great significance.
Transcriptome analysis unravels the molecular mechanisms by which toxins disrupt cellular gene expression by systematically deciphering dynamic changes in cellular transcriptional states and gene regulatory networks. Within this framework, RNA sequencing (RNA-seq) stands as a core, foundational transcriptomic technology [
15]. By leveraging the advantages of high-throughput sequencing, this technique comprehensively captures cellular transcriptomic profiles and precisely identifies differentially expressed genes (DEGs) upon toxic exposure. Upon integration with bioinformatics analyses—such as gene functional annotation and pathway enrichment analysis—RNA-seq can further delineate the mechanisms by which toxic substances perturb core cellular pathways, including those governing metabolism, stress responses, and apoptotic regulation, thereby providing critical molecular insights into the toxic mechanisms of the target substances. Given the high detection frequency and potential high toxicity of iodoalkanes in shale gas wastewater, clarifying the key mechanisms underlying their biological damage is critical. In this study, we evaluated the toxic effects of three typical diiodoalkanes, namely 1,2-diiodoethane, 1,3-diiodopropane, and 1,4-diiodobutane, on HepG2 cells. Transcriptomic analysis was further performed on HepG2 cells exposed to these pollutants, with the aim of identifying key signaling pathways involved in the toxic effects of typical diiodoalkanes.
2. Materials and Methods
2.1. Chemicals and Reagents
1,2-diiodoethane (C2H4I2, CAS:624-73-7, 98% purity), 1,3-diiodopropan (C3H6I2, CAS:627-31-6, 98% purity), and 1,4-diiodobutane (C4H8I2, CAS:628-21-7, 98% purity) were purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). The Dulbecco’s modified Eagle medium (High Glucose DMEM) was bought from Datahill Biotechnology Co., Ltd. (Shanghai, China). The Fetal Bovine Serum (FBS) was obtained from Biological Industries Israel Beit Haemek Ltd (Beit Haemek, Israel). Serum-free animal protein-free cell freezing medium was derived from Nell Cell & Molecular Biotech Co., Ltd. (Jiangsu, China). 2′,7′-dichlorofluorescein diacetate (DCFH-DA) and 3-(4,5-dimethylthiazol-2)-2,5-diphenyltetrazolium bromide (MTT) were bought from Sigma-Aldrich (St. Louis, MO, USA). Other unspecified reagents were purchased from Beyotime Biotechnology (Shanghai, China).
2.2. Cell Culture
The human hepatocellular carcinoma (HepG2) cell line was purchased from the American Type Culture Collection (ATCC). Cells are routinely cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S), in a humidified incubator maintained at 37 °C with 5% carbon dioxide (CO2). Cells should be passaged at least twice. When cells exhibited stable morphology and reached the logarithmic growth phase (prior to confluence), they were seeded into 96-well or 6-well plates. Following 24 h of adherent culture to allow cell attachment, treatment with diiodoalkanes was initiated. Diiodoalkanes were dissolved in dimethyl sulfoxide (DMSO) for preparation. A solvent control group was included in the experiment, and the final concentration of DMSO in the medium across all groups was maintained at 0.1% to rule out potential solvent-induced interference with cell viability or function.
2.3. Cell Viability Assay
MTT assay was employed to evaluate the toxicity and cell viability of diiodoalkanes on HepG2 cells: HepG2 cells were seeded at a density of 4 × 103 cells/well in a 96-well plate. After 24 h of pre-incubation at 37 °C in a humidified incubator with 5% CO2 to allow cells to adhere and stabilize, cells were treated with 25 µM, 50 µM, 100 µM, and 200 µM concentrations of diiodoalkanes, and 0.1% DMSO (solvent control). After 24 h exposure, 100 µL of MTT reagent (final concentration ~5 mg/mL) was added to each well and incubated in the dark for 4 h to form MTT crystals. After removing the supernatant, 150 µL of DMSO was added to each well, and the mixture was incubated on a shaking platform in the dark for 3 min to completely dissolve the crystals. Finally, absorbance at 570 nm was measured using a Spark® 20M multimode microplate reader (Tecan, Switzerland). Absorbance values indirectly reflected live cell numbers and cell viability.
2.4. Cellular Morphology Analysis
Cells were gently rinsed 1–2 times with phosphate-buffered saline (PBS) to remove residual medium, and the sample was then mounted on the optical microscope stage. During observation, the cell distribution area was first located using the 10× low-power objective, after which the objective was switched to the 20× and 40× medium-to-high power ones. The overall cell morphology was examined and photographed using the microscope’s imaging system, with a focus on recording typical damage features such as cell shrinkage, membrane rupture, abnormal aggregation, or detachment. At least three representative fields per sample were selected to ensure the reliability of the analysis.
2.5. RNA-Sequencing and Bioinformatics Analysis
HepG2 cells were exposed to 50 µM 1,2-diiodoethane, 1,3-diiodopropane, and 1,4-diiodobutane for 12 h. Total cellular RNA was then extracted using TRIZOL reagent (Life Technologies, Carlsbad, CA, USA). RNA quality was assessed with an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA) to ensure suitability for subsequent experimental procedures. To guarantee the reliability of results, three biological replicates were included throughout the experiment. The quality assurance and quality control (QA/QC) information for the raw sequencing data and omics analysis of RNA-seq are provided in the
Supplementary Materials. cDNA library sequencing was performed by OE Biotech (Shanghai, China) using an Illumina HiSeqTM 2000 sequencer (Illumina, Inc., San Diego, CA, USA). Subsequently, DESeq software (Bioconductor 3.12) was utilized to standardize gene count data across all samples and calculate fold changes in gene expression. The significance of differential expression was evaluated using negative binomial distribution tests. Differentially expressed genes (DEGs) were identified based on the criteria of “absolute fold change (FC) ≥ 1.5 and
p < 0.05”. Finally, functional annotation and enrichment analysis of the identified DEGs were conducted using the Gene Ontology (GO; database link:
http://geneontology.org/ (accessed on 28 October 2025)) and the Kyoto Encyclopedia of Genes and Genomes (KEGG; database link:
https://www.kegg.jp/ (accessed on 28 October 2025), aiming to explore their biological functions and potential involvement in signaling pathways.
2.6. Detection of Reactive Oxygen Species
DCFH-DA fluorescent probe was used to detect intracellular ROS levels. HepG2 cell suspensions were seeded at a density of 1.5 × 105 cells per well in a 6-well plate. After the cells reached 70~80% confluence, they were exposed to different concentrations of diiodoalkanes (25, 50, 100, and 200 µM) and 0.1% dimethyl sulfoxide (DMSO, solvent control) for 4 h and 24 h, respectively. After exposure, remove the culture medium from the wells. Add 10 µM DCFH-DA probe solution to each well. Incubate the cells in a 37 °C dark incubator for 30 min to ensure complete probe loading. After incubation, discard unloaded probe solution and gently wash cells three times with phosphate-buffered saline (PBS) to remove residual probe and minimize background fluorescence interference. Subsequently, observe and capture cellular fluorescence images using the ZOE™ Fluorescence Cell Imager (BIO-RAD, Hercules, CA, USA). Analyze the fluorescence images with Image-Pro Plus software (Medical Cybernetics, Inc., Albemarle, NC, USA, Version 6.0) to quantitatively calculate intracellular fluorescence intensity—the level of fluorescence indirectly reflects changes in intracellular ROS levels.
2.7. Determination of Catalase, Glutathione, and Cysteine
Commercially available assay kits were used to measure the levels of catalase (CAT), glutathione (GSH), and cysteine (Cys) in HepG2 cells following exposure to the toxicant. As described above, HepG2 cells were first treated with different concentrations of diiodoalkanes (including a 50 µM iodinated alkane group and a 0.1% DMSO solvent control group) for 24 h. Following treatment, cells were collected via trypsin digestion and gently washed 2~3 times with phosphate-buffered saline (PBS) to remove residual medium and digestion solution. Subsequently, intracellular levels of the three antioxidant molecules were measured using the CAT assay kit, GSH assay kit, and Cys assay kit, respectively. All procedures strictly followed the manufacturers’ instructions for each kit. After detection, the absorbance values corresponding to each indicator were recorded using a Spark® 20M Multifunction Microplate Reader (Tecan, Switzerland). All assays were independently replicated three times to ensure the reliability of the experimental data.
2.8. Statistical Analysis
GraphPad Prism 9.0 software (San Diego, CA, USA) was used for graphing and statistical analysis. All experiments were independently replicated three times with at least three parallel samples. The data were presented as mean ± standard deviation (SD). Multiple comparisons between groups were analyzed by using one-way ANOVA and post hoc Tukey’s Test. The significance level of the data differences was determined by the p-values: p-value < 0.05 (*), p-value < 0.01 (**).
4. Discussions
Shale gas extraction not only releases pollutants into the surrounding environment—including fracturing additives and geogenic compounds—but also generates “unknown source” environmental transformation products, derived from both anticipated and unanticipated sources, in the process. Among these pollutants, organic iodides typically pose more significant health risks than chlorides or bromides; however, their presence and toxicological potential remain unclear. Previous studies have demonstrated that various di iodoalkanes exhibit cytotoxicity [
11]. Their toxic effects not only exhibit significant time- and dose-dependence, but also vary in the intensity of toxicity based on the number of iodine substitutions in the molecule. Building on this, the present study further showed that three diiodoalkanes exerted clear effects on HepG2 cells at concentrations ranging from 25 to 200 μM: They significantly inhibited cell proliferation, induced cell morphological abnormalities, and even caused extensive cell death. These results further suggested the dose-dependent characteristics of the diiodoalkanes’ toxicity. This difference indicates that the toxicity of diiodoalkanes is closely related to their molecular structure. Mechanistically, the strong electron-withdrawing property of iodine atoms increases the molecular polarity, thereby promoting their binding to the intracellular enzyme systems. Ultimately, differences in molecular structure and iodine atom substitution positions determine the toxicity intensity of these pollutants.
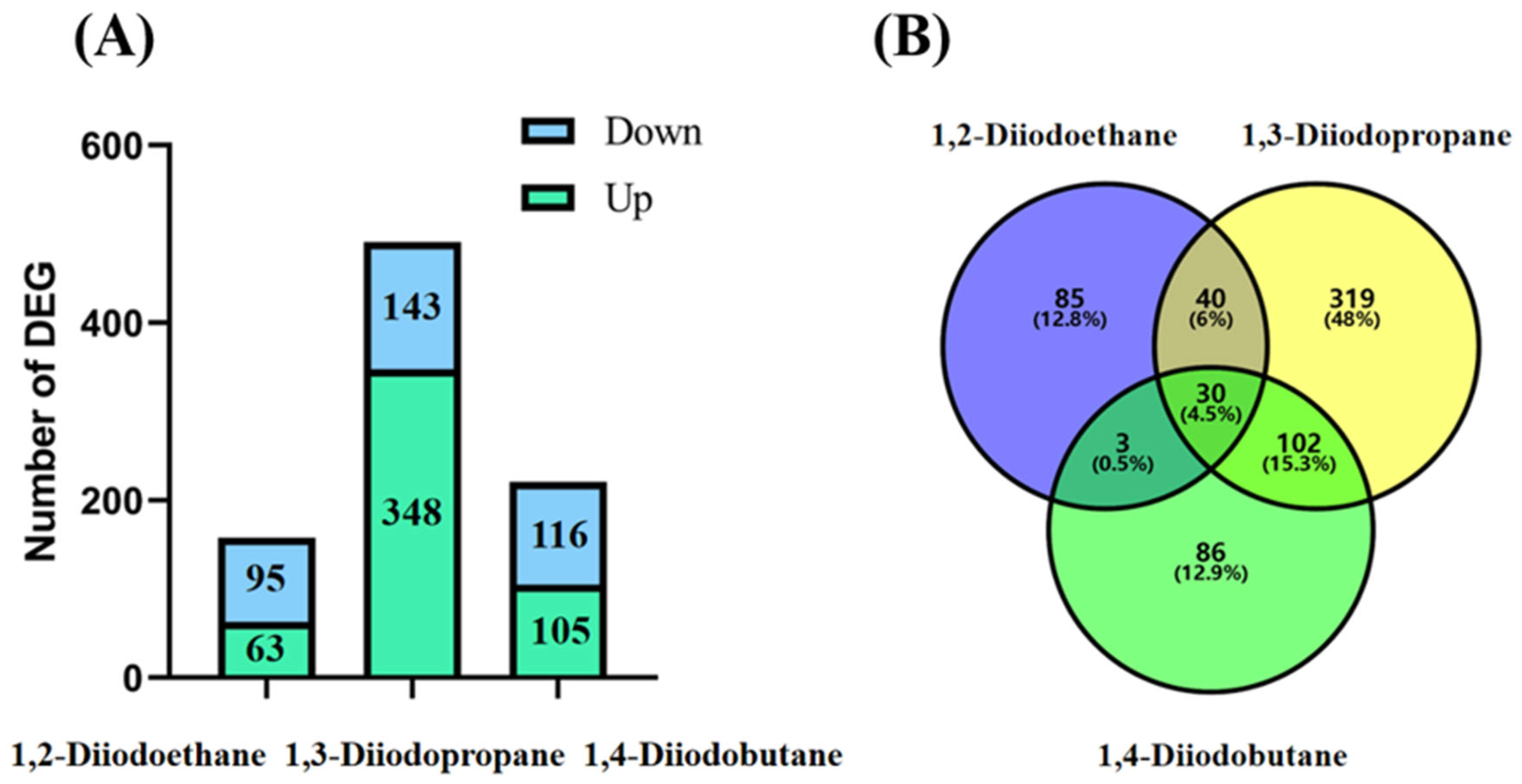
Transcriptomic sequencing results revealed that the number of differentially expressed genes (DEGs) in HepG2 cells exposed to 1,2-diiodopropane, 1,3-diiodobutane, and 1,4-diiodoethane was 158, 491, and 221, respectively (
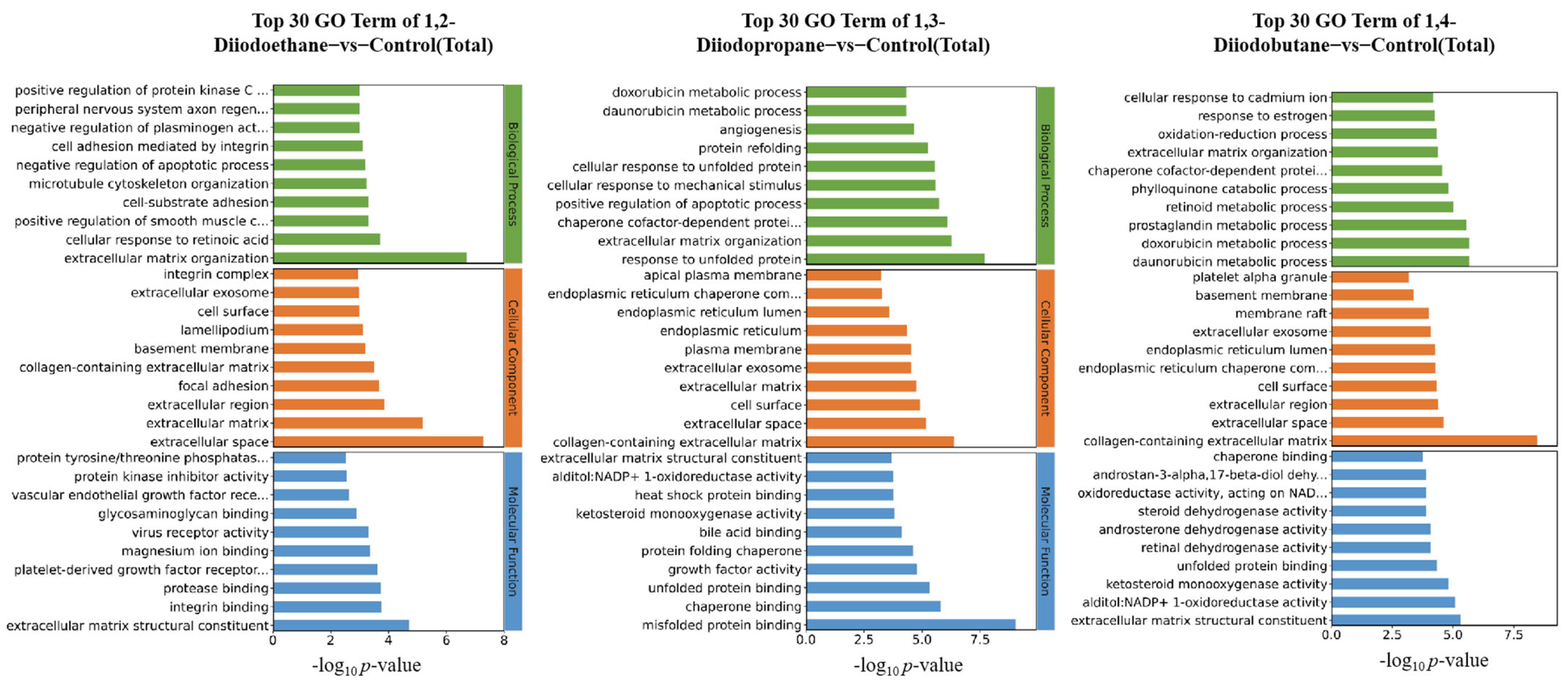
Figure 2). Notably, the 1,3-diiodopropane-exposed group exhibited significantly more DEGs than the 1,2-diiodoethane and 1,4-diiodobutane groups, suggesting that 1,3-diiodopropane may exert a stronger molecular perturbation effect on HepG2 cells than the other two diiodoalkanes. This finding aligns with the established toxicity hierarchy of the three diiodoalkanes, further validating the correlation between molecular structure and toxic intensity. To elucidate the biological functions of identified DEGs, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were conducted. The enriched GO terms were primarily associated with the extracellular space, extracellular matrix (ECM), and endoplasmic reticulum (ER). The extracellular space acts as an intermediary microenvironment for material exchange and signal transduction between cells and their surrounding environment. It is mainly composed of the ECM and extracellular fluids and also serves as a critical compartment for the diffusion and action of oxidative stress-related molecules, such as reactive oxygen species (ROS) and antioxidants. The ECM not only provides mechanical support and intercellular connectivity but also participates in intercellular signaling. It acts as a crucial binding site for cytokines and growth factors, and the maintenance of its structural and functional integrity is vital for sustaining cellular homeostasis [
24]. When cells are subjected to adverse stimuli, the impaired function of the antioxidant defense system results in the overproduction of ROS, which disrupts the oxidant-antioxidant balance and induces oxidative stress. Oxidative stress not only directly damages ECM components (e.g., collagen and elastin) and matrix metalloproteinases (MMPs) but also downregulates the expression levels of ECM-related regulatory factors [
25]. Notably, extracellular matrix remodeling is a key process jointly regulated by oxidative stress and DNA damage, exhibiting bidirectional interactions: oxidative stress/DNA damage induces ECM remodeling, while abnormal ECM remodeling conversely affects cellular oxidative stress status.
The ER serves as the primary site for protein folding within cells. The protein folding process mediated by it consumes ATP and involves redox reactions, consequently generating small amounts of ROS continuously. When cells are exposed to external toxins, the ER’s protein folding capacity becomes disrupted, leading to the accumulation of unfolded or misfolded proteins within its lumen. This induces endoplasmic reticulum stress (ERS), which further promotes the overproduction of ROS. Excessive ROS not only damages ER structure (e.g., by disrupting membrane integrity and oxidizing membrane phospholipids) but also oxidatively inactivates key functional molecules in the ER (e.g., molecular chaperones and foldases) [
19]. This ultimately results in the total collapse of ER homeostasis, potentially inducing apoptosis.
For the 1,2-diiodoethane, 1,3-diiodopropane, and 1,4-diiodobutane exposure groups, the GO enrichment results all included entries related to the ECM. It is well established that oxidative stress can alter the metabolic processes of ECM components and the expression of related regulatory factors, leading to excessive accumulation or degradation of specific ECM components and the disruption of ECM homeostasis. Consistent with this mechanism, the aforementioned GO enrichment results imply that 1,3-diiodopropane and 1,4-diiodobutane may impair cell viability in HepG2 cells by exacerbating oxidative stress-induced damage and disrupting antioxidant function. Furthermore, KEGG enrichment analysis identified multiple signaling pathways related to the ECM and ER, nearly all of which have direct or indirect associations with oxidative stress. This finding further supports the notion that exposure to the aforementioned diiodoalkanes may induce cytotoxicity in HepG2 cells by perturbing the balance between oxidative stress-induced damage and antioxidant defense.
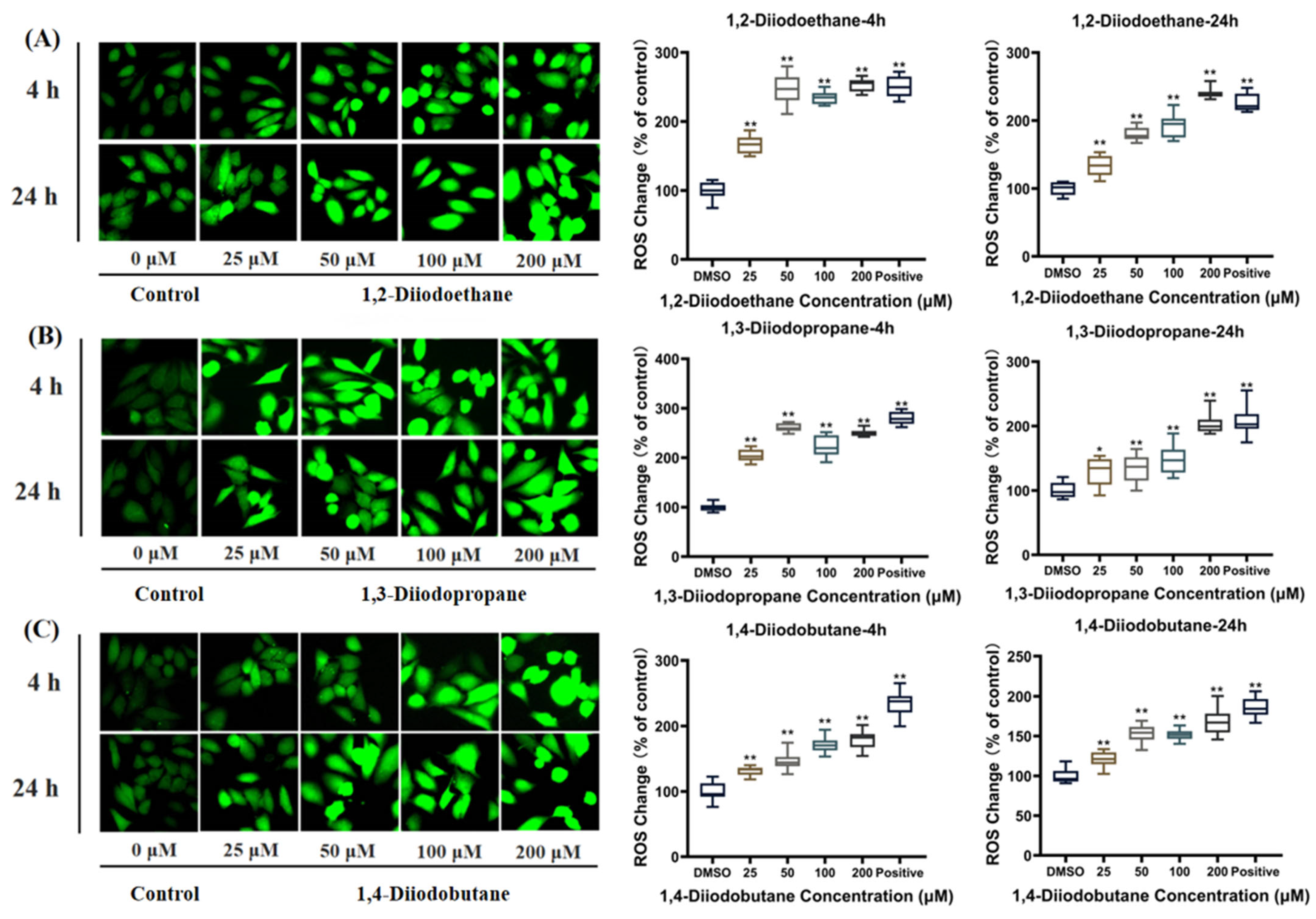
ROS are natural byproducts of normal cellular metabolic activity. Under physiological conditions, the production and clearance (quenching) of intracellular ROS maintain a dynamic equilibrium to ensure stable physiological functions. However, when cells are exposed to toxic stimuli, intracellular redox homeostasis is disrupted. The balance between ROS production and clearance shifts toward elevated ROS levels, leading to cellular dysfunction and the onset of oxidative stress [
26]. The results of this study indicated that diiodoalkanes significantly induce increased ROS levels in HepG2 cells across the experimental exposure concentrations (25~200 μM) and exposure time points (4 h and 24 h). This elevation exhibits clear dose-dependent effects (
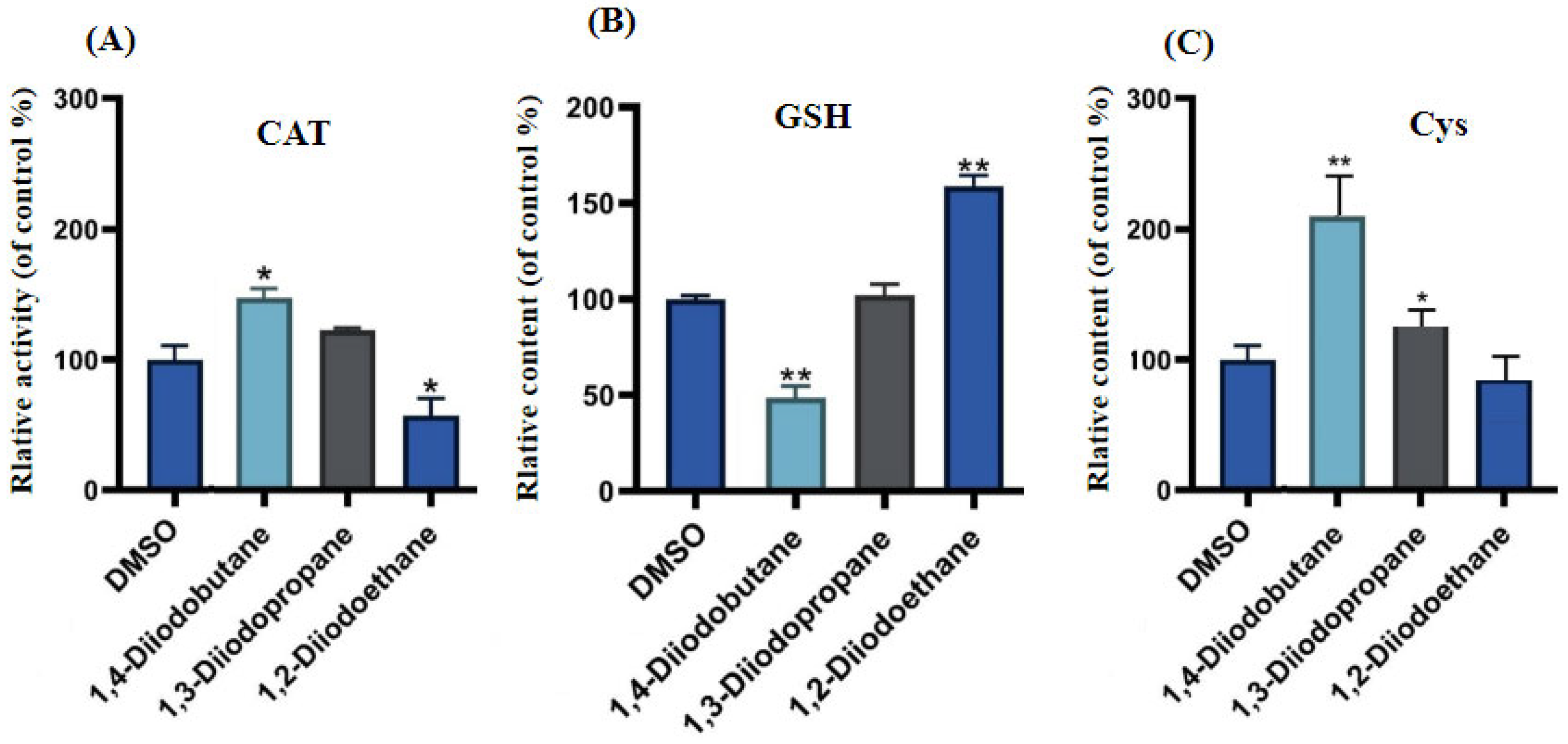
Figure 4). Excessive intracellular ROS can trigger multiple outcomes, including oxidative damage to proteins and lipids, DNA damage, apoptosis, and inflammatory responses, potentially exerting significant adverse effects on HepG2 cells. Cells typically rely on their antioxidant systems to maintain ROS homeostasis. Therefore, to further investigate the regulatory effects of diiodoalkanes on the cellular antioxidant system, this study examined the levels of key enzymes and molecules closely associated with antioxidant function and redox homeostasis in HepG2 cells following 24 h exposure to the three diiodoalkanes. Results showed that: Compared with the control group, the 1,2-diiodoethane-exposed group exhibited significantly elevated GSH levels and markedly reduced CAT activity, with no significant change in Cys levels. The 1,3-diiodopropane-exposed group caused only a significant increase in cellular Cys levels. The 1,4-diiodobutane-exposed group led to a significant decrease in both CAT and Cys levels, along with a significant reduction in GSH levels. These findings indicated that diiodoalkanes can modulate cellular antioxidant systems by regulating key enzymes and molecules to counteract ROS accumulation, yet their regulatory patterns exhibit compound-specific structural dependencies.
5. Conclusions
This study focuses on “unknown-source” organic iodides generated during shale gas extraction—pollutants for which their occurrence, toxic effects, and mechanisms of toxicity remain unclear—and systematically investigates the toxic effects and underlying mechanisms of three typical diiodoalkanes on human hepatocellular carcinoma (HepG2) cells. Results show that at concentrations ranging from 25 to 200 μM, all three diiodoalkanes significantly inhibit HepG2 cell proliferation, induce cellular morphological abnormalities, and trigger extensive cell death; their toxicity exhibits pronounced time- and dose-dependent patterns, and 1,3-diiodopropane demonstrates the strongest toxicity. Transcriptomic analysis revealed that the number of differentially expressed genes (DEGs) in HepG2 cells treated with 1,2-diiodoethane, 1,3-diiodopropane, and 1,4-diiodobutane was 158, 491, and 221, respectively—with the 1,3-diiodopropane group exhibiting the highest DEG count—and this DEG profile perfectly aligns with the aforementioned toxicity hierarchy. Further Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses showed that DEGs were primarily enriched in functional terms associated with the extracellular matrix (ECM) and endoplasmic reticulum (ER), suggesting that the toxic effects of diiodoalkanes may be linked to the disruption of cellular oxidant-antioxidant system homeostasis, and subsequent validation experiments suggested that diiodoalkanes exposure significantly increased reactive oxygen species (ROS) levels in HepG2 cells while markedly perturbing intracellular antioxidant molecules (glutathione [GSH], cysteine [Cys]) and the antioxidant enzyme catalase (CAT). However, this study has limitations, specifically as follows: First, the experimental model utilized HepG2 cells (a hepatocellular carcinoma cell line) rather than primary liver cells. Their physiological characteristics differ from those of normal liver cells, limiting the applicability of the findings for assessing health risks in real physiological scenarios. Second, the selected pollutants are volatile. Volatilization during experiments may have reduced the actual exposure concentration in the system, potentially leading to overestimated toxicity effect values that do not fully reflect the true toxicity profile under real environmental exposure conditions. Finally, due to technical limitations, we did not measure the actual concentration of pollutants taken up/utilized by the cells. Analysis was based solely on the initial exposure concentration. This hinders in-depth analysis of the metabolic pathways and mechanisms of action of pollutants within cells and also affects the precise interpretation of the dose–response relationship for toxic effects.