Cardiotoxic Effect Induced by F-53B via Nitric Oxide Signalling on Parkin−/− Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and F-53B Exposure
2.3. Masson Staining
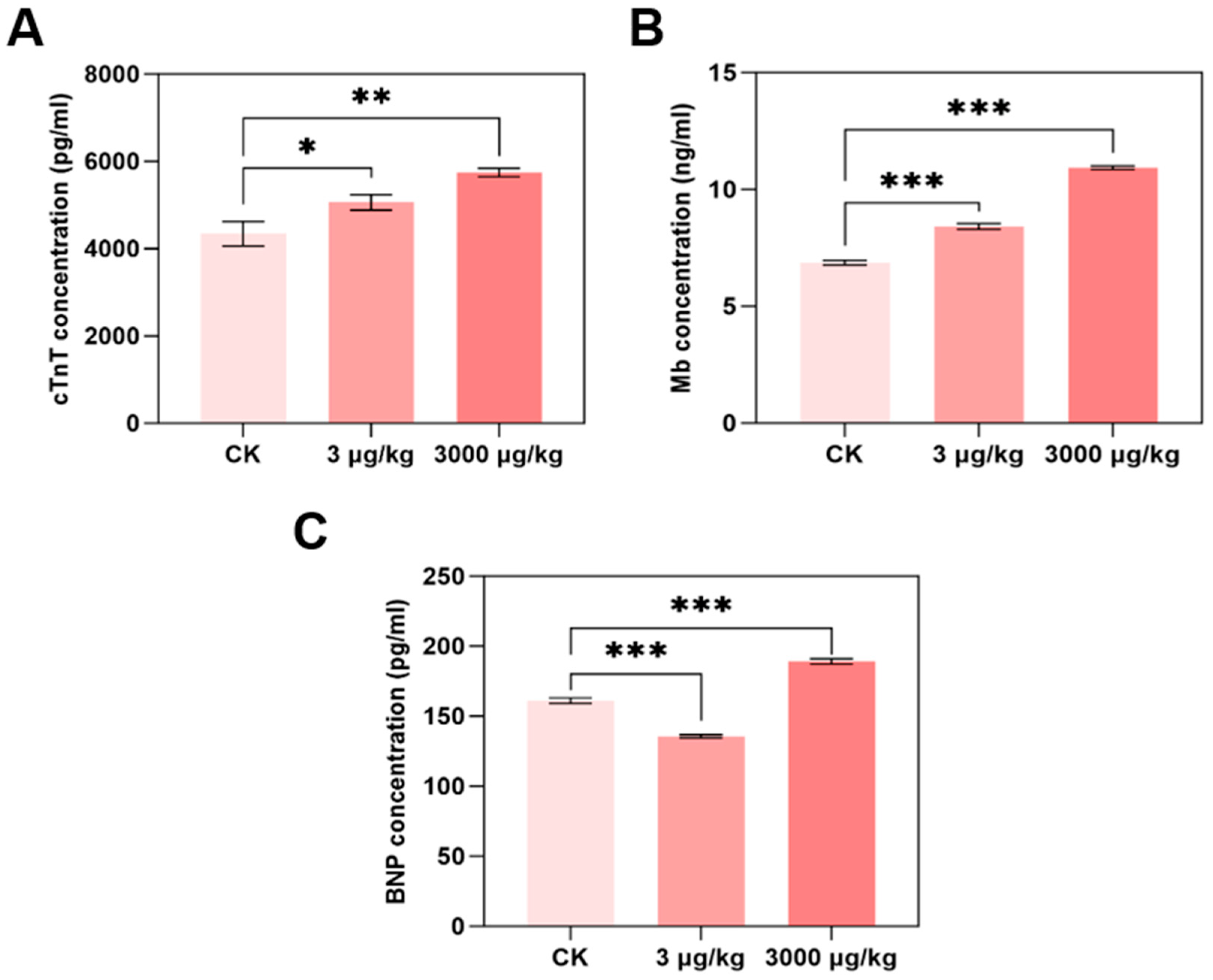
2.4. cTnT, BNP, and Mb Contents Determination
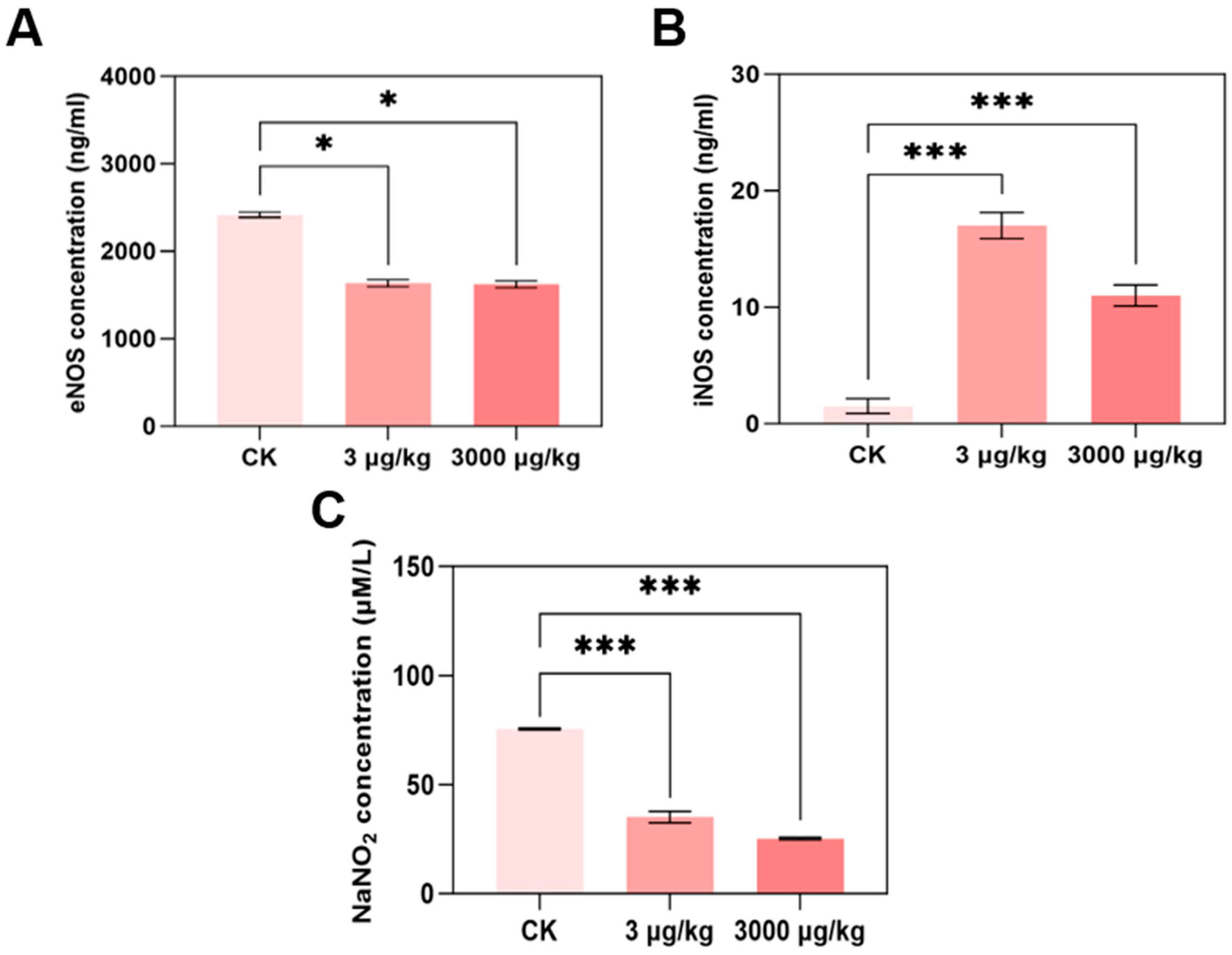
2.5. Nitric Oxide Determination
2.6. eNOS and iNOS Contents Determination
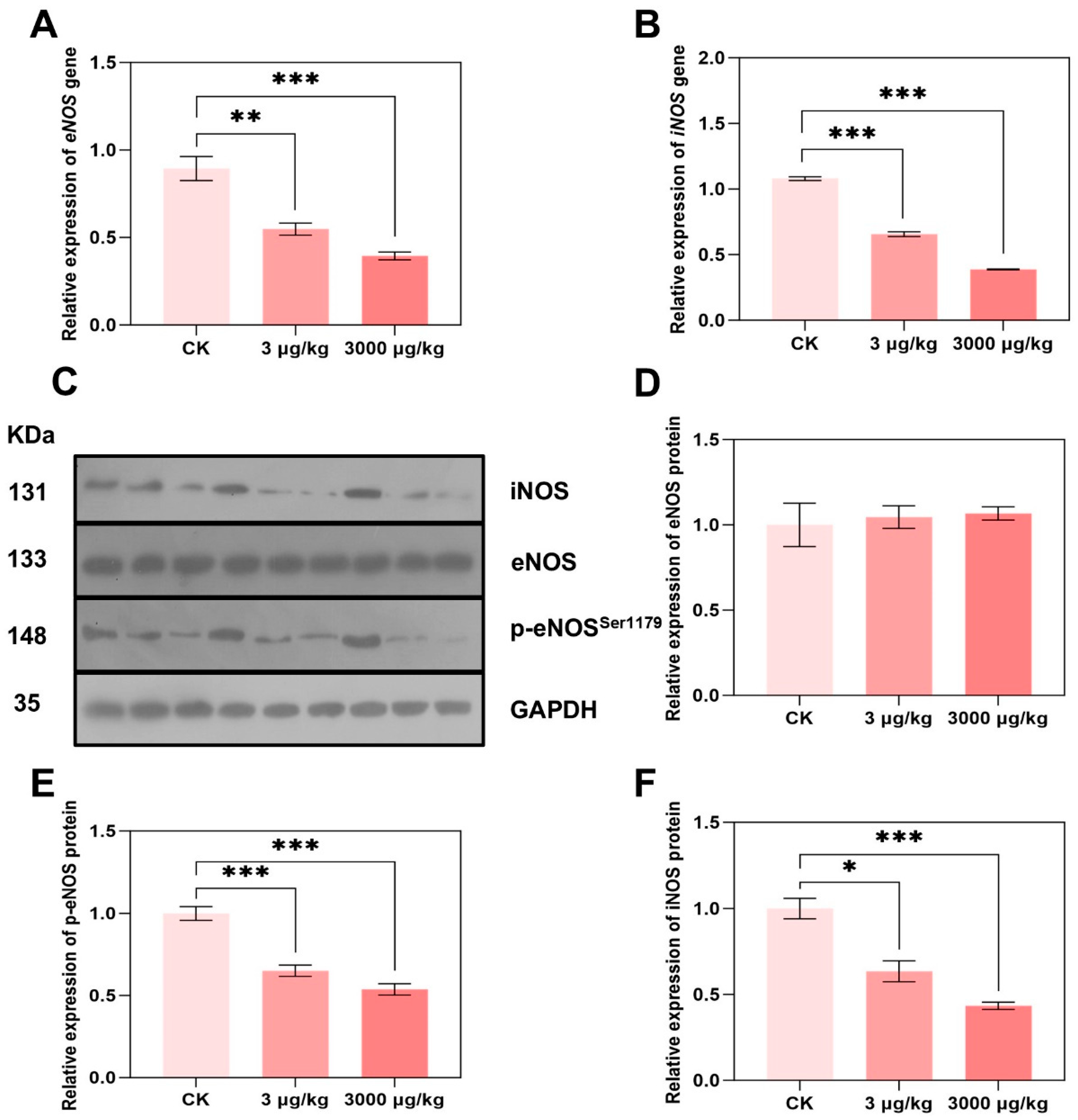
2.7. Quantitative Polymerase Chain Reaction (qPCR)
2.7.1. The Extraction Method of Total RNA
2.7.2. The Design of Primer Sequences
2.7.3. qPCR Experimental Materials and Methods
2.8. Western Blotting Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. F-53B Induces Cardiac Fibrosis in Parkin−/− Mice
3.2. F-53B Induces Cardiac Dysfunction in Parkin−/− Mice
3.3. F-53B Disrupts eNOS/iNOS/NO Signalling in Parkin−/− Mice
3.4. F-53B Alters eNOS and iNOS Expression and Phosphorylation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6:2 Cl-PFESA | 6:2 Chlorinated Polyfluorinated Ether Sulfonate Acid |
| PFAS | Per- and Polyfluoroalkyl Substance |
| PFOS | Perfluorooctane Sulfonic Acid |
| PFOA | Perfluorooctanoic Acid |
| PFOSA | Perfluorooctane Sulfonamide |
| CVD | Cardiovascular Disease |
| eNOS | Endothelial Nitric Oxide Synthase |
| iNOS | Inducible Nitric Oxide Synthase |
| NO | Nitric Oxide |
| cTnT | Cardiac Troponin T |
| BNP | Brain Natriuretic Peptide |
| Mb | Myoglobin |
| ROS | Reactive Oxygen Species |
| p-eNOS | Phosphorylation of eNOS |
| WT | Wild-Type |
References
- Wang, S.; Huang, J.; Yang, Y.; Hui, Y.; Ge, Y.; Larssen, T.; Yu, G.; Deng, S.; Wang, B.; Harman, C. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment. Environ. Sci. Technol. 2013, 47, 10163–10170. [Google Scholar] [CrossRef] [PubMed]
- He, Y.X.; Lv, D.; Li, C.H.; Liu, X.Q.; Liu, W.D.; Han, W.C. Human exposure to F-53B in China and the evaluation of its potential toxicity: An overview. Environ. Int. 2022, 161, 107108. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.P.; Li, Q.Q.; Zhang, Y.T.; Dong, G.H.; Canchola, A.; Zeng, X.W.; Chou, W.C. Development of a Physiologically Based Pharmacokinetic (PBPK) Model for F-53B in Pregnant Mice and Its Extrapolation to Humans. Environ. Sci. Technol. 2024, 58, 18928–18939. [Google Scholar] [CrossRef]
- Ji, J.J.; Song, L.Y.; Wang, J.; Yang, Z.Y.; Yan, H.T.; Li, T.; Yu, L.; Jian, L.Y.; Jiang, F.X.; Li, J.F.; et al. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ. Int. 2021, 153, 106524. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils—To mobilize or to immobilize or to degrade? J. Hazard. Mater. 2021, 401, 123892. [Google Scholar] [CrossRef]
- Xu, B.T.; Liu, S.; Zhou, J.L.; Zheng, C.M.; Jin, W.F.; Chen, B.; Zhang, T.; Qiu, W.H. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef]
- Feng, X.M.; Ye, M.Q.; Li, Y.; Zhou, J.; Sun, B.B.; Zhu, Y.M.; Zhu, L.Y. Potential sources and sediment-pore water partitioning behaviors of emerging per/polyfluoroalkyl substances in the South Yellow Sea. J. Hazard. Mater. 2020, 389, 122124. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.H.; Qian, J.; Hu, J.; Huang, Y.Y.; Wang, P.F.; Shen, J.W.; He, Y.X.; Tang, S.J.; Liu, Y.; Zhang, Y.H. Plant rhizosphere defense system respond differently to emerging polyfluoroalkyl substances F-53B and PFOS stress. J. Hazard. Mater. 2023, 443, 130119. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.T.; Zhang, H.X.; Cui, Q.Q.; Sheng, N.; Yeung, L.W.Y.; Sun, Y.; Guo, Y.; Dai, J.Y. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water. Environ. Sci. Technol. 2018, 52, 7621–7629. [Google Scholar] [CrossRef]
- Xu, C.; Song, X.; Liu, Z.; Ding, X.; Chen, H.; Ding, D. Occurrence, source apportionment, plant bioaccumulation and human exposure of legacy and emerging per- and polyfluoroalkyl substances in soil and plant leaves near a landfill in China. Sci. Total. Environ. 2021, 776, 145731. [Google Scholar] [CrossRef]
- Wu, L.Y.; Liang, L.X.; Zhou, Y.; Mohammed, Z.; Qian, Z.M.; Mcmillin, S.E.; Tabet, M.; Chu, C.; Fan, Y.Y.; Zhou, J.X.; et al. Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs) Are Associated with Eye Diseases in Humans and Eye Toxicity in Zebrafish. Environ. Health-Wash. 2024, 2, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.X.; Liang, Y.; Cai, Y.Q. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Sun, Y.; Duan, J.; Zhang, T.; Xiao, Y.; Zhu, Y.; Jia, Y.; Zhong, W.; Zhu, L. Impacts of Gestational F-53B Exposure on Fetal Neurodevelopment: Insights from Placental and Thyroid Hormone Disruption. Environ. Health 2025, 3, 308–320. [Google Scholar] [CrossRef]
- Liu, H.X.; Pan, Y.T.; Jin, S.N.; Sun, X.J.; Jiang, Y.Q.; Wang, Y.Y.; Ghassabian, A.; Li, Y.Y.; Xia, W.; Cui, Q.Q.; et al. Associations between six common per- and polyfluoroalkyl substances and estrogens in neonates of China. J. Hazard. Mater. 2021, 407, 124378. [Google Scholar] [CrossRef]
- Li, S.P.; Zhao, W.H.; Zhang, J.; Jiang, W.T.; Zhu, J.Y.; Luo, Y.X.; Xiang, P.; Bloom, M.; Jalava, P.; Dong, G.H.; et al. Effects of early-life F-53B exposure on thyroid function in juvenile rats: The role of the cAMP signaling pathway. J. Hazard. Mater. 2025, 489, 137751. [Google Scholar] [CrossRef]
- Liang, L.X.; Liang, J.J.; Li, Q.Q.; Zeeshan, M.; Zhang, Z.Q.; Jin, N.X.; Lin, L.Z.; Wu, L.Y.; Sun, M.K.; Tan, W.H.; et al. Early life exposure to F-53B induces neurobehavioral changes in developing children and disturbs dopamine-dependent synaptic signaling in weaning mice. Environ. Int. 2023, 181, 108272. [Google Scholar] [CrossRef]
- Gu, X.Y.; Yang, H.H.; Wu, L.; Fu, Z.L.; Zhou, S.B.; Zhang, Z.H.; Liu, Y.; Zhang, M.; Liu, S.; Lu, W.T.; et al. Contribution of gut microbiota to hepatic steatosis following F-53B exposure from the perspective of glucose and fatty acid metabolism. J. Hazard. Mater. 2024, 480, 136104. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, X.H.; Ma, C.X.; Zhang, S.W.; Li, K.X.; Yang, Y.Y.; Yang, Z.F. Nanoplastics Affect the Bioaccumulation and Gut Toxicity of Emerging Perfluoroalkyl Acid Alternatives to Aquatic Insects (Chironomus kiinensis): Importance of Plastic Surface Charge. ACS Nano 2024, 18, 5752–5765. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Gokce, N. Perfluoroalkyl/Polyfluoroalkyl Substances: Links to Cardiovascular Disease Risk. Circ. Res. 2024, 134, 1136–1159. [Google Scholar] [CrossRef]
- Dunder, L.; Salihovic, S.; Varotsis, G.; Lind, M.; Elmstahl, S.; Lind, L. Plasma levels of per- and polyfluoroalkyl substances (PFAS) and cardiovascular disease—Results from two independent population-based cohorts and a meta-analysis. Environ. Int. 2023, 181, 108250. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C.A.C.; Bafadhel, M.; Russell, R.E.K.; Sheikh, A.; Brindle, P.; Channon, K.M. Development and validation of a new algorithm for improved cardiovascular risk prediction. Nat. Med. 2024, 30, 1440–1447. [Google Scholar] [CrossRef]
- Fang, X.X.; Wang, H.; Han, D.; Xie, E.J.; Yang, X.; Wei, J.Y.; Gu, S.S.; Gao, F.; Zhu, N.L.; Yin, X.J.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Xiao, D.D.; Chang, W.G.; Ao, X.; Ye, L.; Wu, W.W.; Song, L.; Yuan, X.S.; Feng, L.X.; Wang, P.Y.; Wang, Y.; et al. Parkin inhibits iron overload-induced cardiomyocyte ferroptosis by ubiquitinating ACSL4 and modulating PUFA-phospholipids metabolism. Acta. Pharm. Sin. B 2025, 15, 1589–1607. [Google Scholar] [CrossRef]
- Xia, W.W.; Yin, J.; Zhang, S.P.; Guo, C.C.; Li, Y.Y.; Zhang, Y.; Zhang, A.H.; Jia, Z.J.; Chen, H.B. Parkin Modulates ERRα/eNOS Signaling Pathway in Endothelial Cells. Cell. Physiol. Biochem. 2018, 49, 2022–2034. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Xi, L. eNOS phosphorylation: A pivotal molecular switch in vasodilation and cardioprotection? J. Mol. Cell. Cardiol. 2007, 42, 280–282. [Google Scholar] [CrossRef]
- Dixon, L.J.; Morgan, D.R.; Hughes, S.M.; McGrath, L.T.; El-Sherbeeny, N.A.; Plumb, R.D.; Devine, A.; Leahey, W.; Johnston, G.D.; McVeigh, G.E. Functional consequences of endothelial nitric oxide synthase uncoupling in congestive cardiac failure. Circulation 2003, 107, 1725–1728. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lu, L.; Chen, J.; Zhang, C.; Liu, W.; Zhuang, S. Inhibited Nitric Oxide Production of Human Endothelial Nitric Oxide Synthase by Nitrated and Oxygenated Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 2020, 54, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Indolfi, C.; Bello, I.; Smimmo, M.; Vellecco, V.; Schettino, A.; Montanaro, R.; Morroni, F.; Sita, G.; Graziosi, A.; et al. The endocrine disruptor vinclozolin causes endothelial injury via eNOS/ Nox4/IRE1α α signaling. Eur. J. Pharmacol. 2024, 977, 176758. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, Z.; Yang, H.; Lu, L.; Chen, R.; Zhang, X.; Zhong, Y.; Zhang, H. Per- and Polyfluoroalkyl Substances (PFASs) Impair Lipid Metabolism in Rana nigromaculata: A Field Investigation and Laboratory Study. Environ. Sci. Technol. 2022, 56, 13222–13232. [Google Scholar] [CrossRef]
- Lu, L.; Shen, L.; Cui, S.; Huang, Y.; Gao, Y.; Zhu, X.; Lu, S.; Zhang, C.; Zhuang, S. Angiogenic Activity and Mechanism for Bisphenols on Endothelial Cell and Mouse: Evidence of a Structural-Selective Effect. Environ. Sci. Technol. 2023, 57, 11803–11813. [Google Scholar] [CrossRef]
- Hu, C.; Lu, L.P.; Guo, C.Y.; Zhan, T.J.; Zhang, X.F.; Zhang, H.J. Bisphenols and brominated bisphenols induced endothelial dysfunction via its disruption of endothelial nitric oxide synthase. Environ. Pollut. 2024, 346, 123600. [Google Scholar] [CrossRef] [PubMed]
- Fundere, A.; Rose, A.; Xiong, F.; Muthukumarasamy, K.M.; Altuntas, Y.; Dasari, H.; Villeneuve, L.; Sirois, M.G.; Tanguay, J.F.; Tardif, J.C.; et al. Daily exposure to chlordecone, an organochlorine pesticide, increases cardiac fibrosis and atrial fibrillation vulnerability. J. Hazard. Mater. 2024, 478, 135533. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, T.H.; Ho, C.H.; Chen, Y.C.; Hsu, C.C.; Lin, H.J.; Wang, J.J.; Chang, C.P.; Guo, H.R. Increased Risk of Congestive Heart Failure Following Carbon Monoxide Poisoning. Circ. Heart Fail. 2021, 14, e007267. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Tong, M.H.; Chu, A.A.; Wu, K.Y.; Niu, X.W.; Zhang, Z. PM2.5-Induced Programmed Myocardial Cell Death via mPTP Opening Results in Deteriorated Cardiac Function in HFpEF Mice. Cardiovasc. Toxicol. 2022, 22, 746–762. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef]
- Ezeani, M.; Noor, A.; Alt, K.; Lal, S.; Donnelly, P.S.; Hagemeyer, C.E.; Niego, B. Collagen-Targeted Peptides for Molecular Imaging of Diffuse Cardiac Fibrosis. J. Am. Heart. Assoc. 2021, 10, e022139. [Google Scholar] [CrossRef]
- Shi, G.; Cui, Q.; Pan, Y.; Sheng, N.; Sun, S.; Guo, Y.; Dai, J. 6:2 Chlorinated polyfluorinated ether sulfonate, a PFOS alternative, induces embryotoxicity and disrupts cardiac development in zebrafish embryos. Aquat. Toxicol. 2017, 185, 67–75. [Google Scholar] [CrossRef]
- Tan, Z.; Lv, J.; Li, H.; An, Z.; Li, L.; Ke, Y.; Liu, Y.; Liu, X.; Wang, L.; Li, A.; et al. Angiotoxic effects of chlorinated polyfluorinated ether sulfonate, a novel perfluorooctane sulfonate substitute, in vivo and in vitro. J. Hazard. Mater. 2024, 469, 133919. [Google Scholar] [CrossRef]
- de Couto, G.; Ouzounian, M.; Liu, P.P. Early detection of myocardial dysfunction and heart failure. Nat. Rev. Cardiol. 2010, 7, 334–344. [Google Scholar] [CrossRef]
- Li, S.A.; Wang, C.R.; Yang, C.; Chen, Y.Y.; Cheng, Q.H.; Liu, J.F.; Zhang, Y.L.; Jin, L.; Li, Z.W.; Ren, A.G.; et al. Prenatal exposure to poly/perfluoroalkyl substances and risk for congenital heart disease in offspring. J. Hazard. Mater. 2024, 469, 134008. [Google Scholar] [CrossRef]
- Chen, H.H.; Qiu, W.H.; Yang, X.J.; Chen, F.Y.; Chen, J.Y.; Tang, L.; Zhong, H.B.; Magnuson, J.T.; Zheng, C.M.; Xu, E.G. Perfluorooctane Sulfonamide (PFOSA) Induces Cardiotoxicity via Aryl Hydrocarbon Receptor Activation in Zebrafish. Environ. Sci. Technol. 2022, 56, 8438–8448. [Google Scholar] [CrossRef]
- Ni, H.; Yuan, J.H.; Ji, J.; Guo, Y.J.; Zhong, S.P.; Lin, Y.F.; Zheng, Y.X.; Jiang, Q.X. Long term toxicities following developmental exposure to perfluorooctanoic acid: Roles of peroxisome proliferation activated receptor alpha. Environ. Pollut. 2023, 317, 120722. [Google Scholar] [CrossRef]
- Yang, R.; Liu, S.; Liang, X.; Yin, N.; Ruan, T.; Jiang, L.; Faiola, F. F-53B and PFOS treatments skew human embryonic stem cell in vitro cardiac differentiation towards epicardial cells by partly disrupting the WNT signaling pathway. Environ. Pollut. 2020, 261, 114153. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Group, E.S.C.S.D. Fourth universal definition of myocardial infarction (2018). Eur. Heart. J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- Kuster, N.; Huet, F.; Dupuy, A.M.; Akodad, M.; Battistella, P.; Agullo, A.; Leclercq, F.; Kalmanovich, E.; Meilhac, A.; Aguilhon, S.; et al. Multimarker approach including CRP, sST2 and GDF-15 for prognostic stratification in stable heart failure. ESC Heart Fail. 2020, 7, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.P.; Chen, X.Y.; Jia, G.H.; Chen, G.J.; Gong, L.; Cheng, Y.X.; Li, Z.; Wang, H.; Chen, A.Y.; Zhang, G.H.; et al. WGX50 mitigates doxorubicin-induced cardiotoxicity through inhibition of mitochondrial ROS and ferroptosis. J. Transl. Med. 2023, 21, 823. [Google Scholar] [CrossRef]
- Kanatous, S.B.; Mammen, P.P.A. Regulation of myoglobin expression. J. Exp. Biol. 2010, 213, 2741–2747. [Google Scholar] [CrossRef]
- Koch, J.; Ludemann, J.; Spies, R.; Last, M.; Amemiya, C.T.; Burmester, T. Unusual Diversity of Myoglobin Genes in the Lungfish. Mol. Biol. Evol. 2016, 33, 3033–3041. [Google Scholar] [CrossRef]
- Elkholi, I.E.; Elsherbiny, M.E.; Emara, M. Myoglobin: From physiological roles to potential implications in cancer. Bba-Rev. Cancer 2022, 1877, 188706. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart. J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Wolsk, E.; Claggett, B.; Diaz, R.; Dickstein, K.; Gerstein, H.C.; Kober, L.; Lewis, E.F.; Maggioni, A.P.; McMurray, J.J.V.; Probstfield, J.L.; et al. Risk Estimates of Imminent Cardiovascular Death and Heart Failure Hospitalization Are Improved Using Serial Natriuretic Peptide Measurements in Patients With Coronary Artery Disease and Type 2 Diabetes. J. Am. Heart. Assoc. 2022, 11, e021327. [Google Scholar] [CrossRef]
- Law, Y.M.; Hoyer, A.W.; Reller, M.D.; Silberbach, M. Accuracy of plasma B-type natriuretic peptide to diagnose significant cardiovascular disease in children: The Better Not Pout Children! Study. J. Am. Coll. Cardiol. 2009, 54, 1467–1475. [Google Scholar] [CrossRef]
- Clerico, A.; Recchia, F.A.; Passino, C.; Emdin, M. Cardiac endocrine function is an essential component of the homeostatic regulation network: Physiological and clinical implications. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H17–H29. [Google Scholar]
- Yang, J.; Guo, M.; Wu, J.; Li, F.; Xu, S.; Wang, J.; Wu, F. Assessment of cardiotoxicity induced by PFOS exposure and mechanism research via untarget metabolomics. Drug Chem. Toxicol. 2025, 48, 303–313. [Google Scholar] [CrossRef]
- Li, H.Q.; Cheng, Z.J.; Wu, D.; Hu, Q.X. Nitric oxide and mitochondrial function in cardiovascular diseases. Nitric. Oxide 2025, 154, 42–50. [Google Scholar] [CrossRef]
- Xu, S.W.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.Y.; Luo, S.H.; Li, Z.M.; Liu, P.Q.; Han, J.H.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksass, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Back, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef]
- Heo, K.S.; Phan, L.P.; Le, N.T.T.; Jin, Y. Mechanistic insights and emerging therapeutic strategies targeting endothelial dysfunction in cardiovascular diseases. Arch. Pharm. Res. 2025, 48, 305–332. [Google Scholar] [CrossRef]
- Cortese-Krott, M.M.; Suvorava, T.; Leo, F.; Heuser, S.K.; LoBue, A.; Li, J.J.; Becher, S.; Schneckmann, R.; Srivrastava, T.; Erkens, R.; et al. Red blood cell eNOS is cardioprotective in acute myocardial infarction. Redox. Biol. 2022, 54, 102370. [Google Scholar] [CrossRef] [PubMed]
- Leo, F.; Suvorava, T.; Heuser, S.K.; Li, J.J.; LoBue, A.; Barbarino, F.; Piragine, E.; Schneckmann, R.; Hutzler, B.; Good, M.E.; et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation 2021, 144, 870–889. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Fadel, P.J. Nitric Oxide and Cardiovascular Regulation: Beyond the Endothelium. Hypertension 2017, 69, 778–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Hoang, T.H.; Jung, E.S.; Jung, S.J.; Han, S.K.; Chung, M.J.; Chae, S.W.; Chae, H.J. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 2020, 19, e13279. [Google Scholar] [CrossRef] [PubMed]
- Wilmes, V.; Scheiper, S.; Roehr, W.; Niess, C.; Kippenberger, S.; Steinhorst, K.; Verhoff, M.A.; Kauferstein, S. Increased inducible nitric oxide synthase (iNOS) expression in human myocardial infarction. Int. J. Legal. Med. 2020, 134, 575–581. [Google Scholar] [CrossRef]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Atochin, D.N.; Wang, A.; Liu, V.W.; Critchlow, J.D.; Dantas, A.P.; Looft-Wilson, R.; Murata, T.; Salomone, S.; Shin, H.K.; Ayata, C.; et al. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J. Clin. Investig. 2007, 117, 1961–1967. [Google Scholar] [CrossRef]
- Musial, A.; Eissa, N.T. Inducible nitric-oxide synthase is regulated by the proteasome degradation pathway. J. Biol. Chem. 2001, 276, 24268–24273. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, J.; Hu, C.; Huang, Y.; Ma, Y.; Lu, L. Cardiotoxic Effect Induced by F-53B via Nitric Oxide Signalling on Parkin−/− Mice. Toxics 2025, 13, 942. https://doi.org/10.3390/toxics13110942
Nie J, Hu C, Huang Y, Ma Y, Lu L. Cardiotoxic Effect Induced by F-53B via Nitric Oxide Signalling on Parkin−/− Mice. Toxics. 2025; 13(11):942. https://doi.org/10.3390/toxics13110942
Chicago/Turabian StyleNie, Jun, Chao Hu, Yuru Huang, Ying Ma, and Liping Lu. 2025. "Cardiotoxic Effect Induced by F-53B via Nitric Oxide Signalling on Parkin−/− Mice" Toxics 13, no. 11: 942. https://doi.org/10.3390/toxics13110942
APA StyleNie, J., Hu, C., Huang, Y., Ma, Y., & Lu, L. (2025). Cardiotoxic Effect Induced by F-53B via Nitric Oxide Signalling on Parkin−/− Mice. Toxics, 13(11), 942. https://doi.org/10.3390/toxics13110942






