Etoxazole Exposure Triggers Sublethal Metabolic Responses in Earthworms (Eisenia fetida): An NMR-Based Metabolomics Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Earthworm Exposure
2.2. Sample Extraction
2.3. NMR Analysis
2.4. Data Process
3. Results
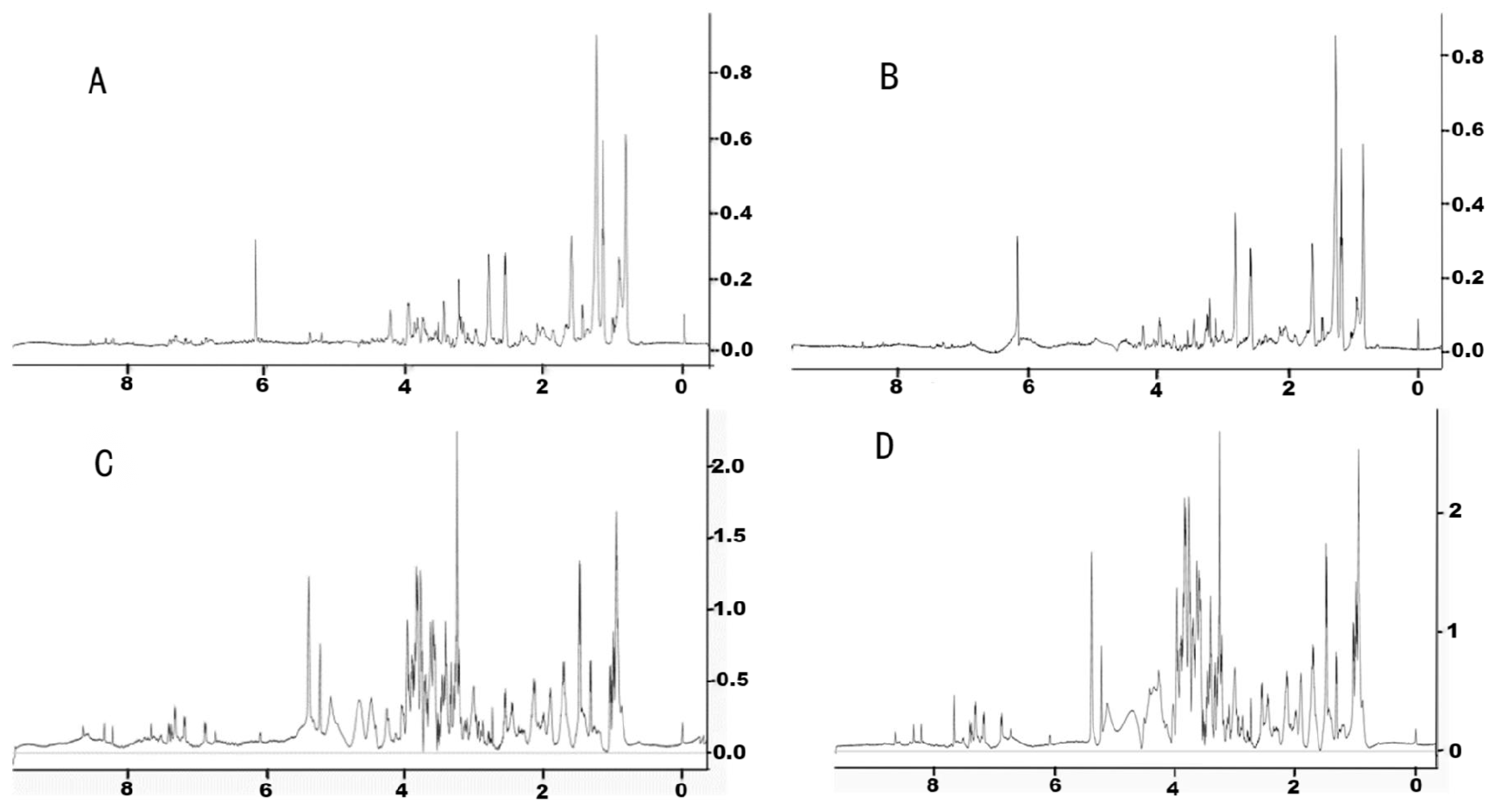
3.1. NMR Method Development
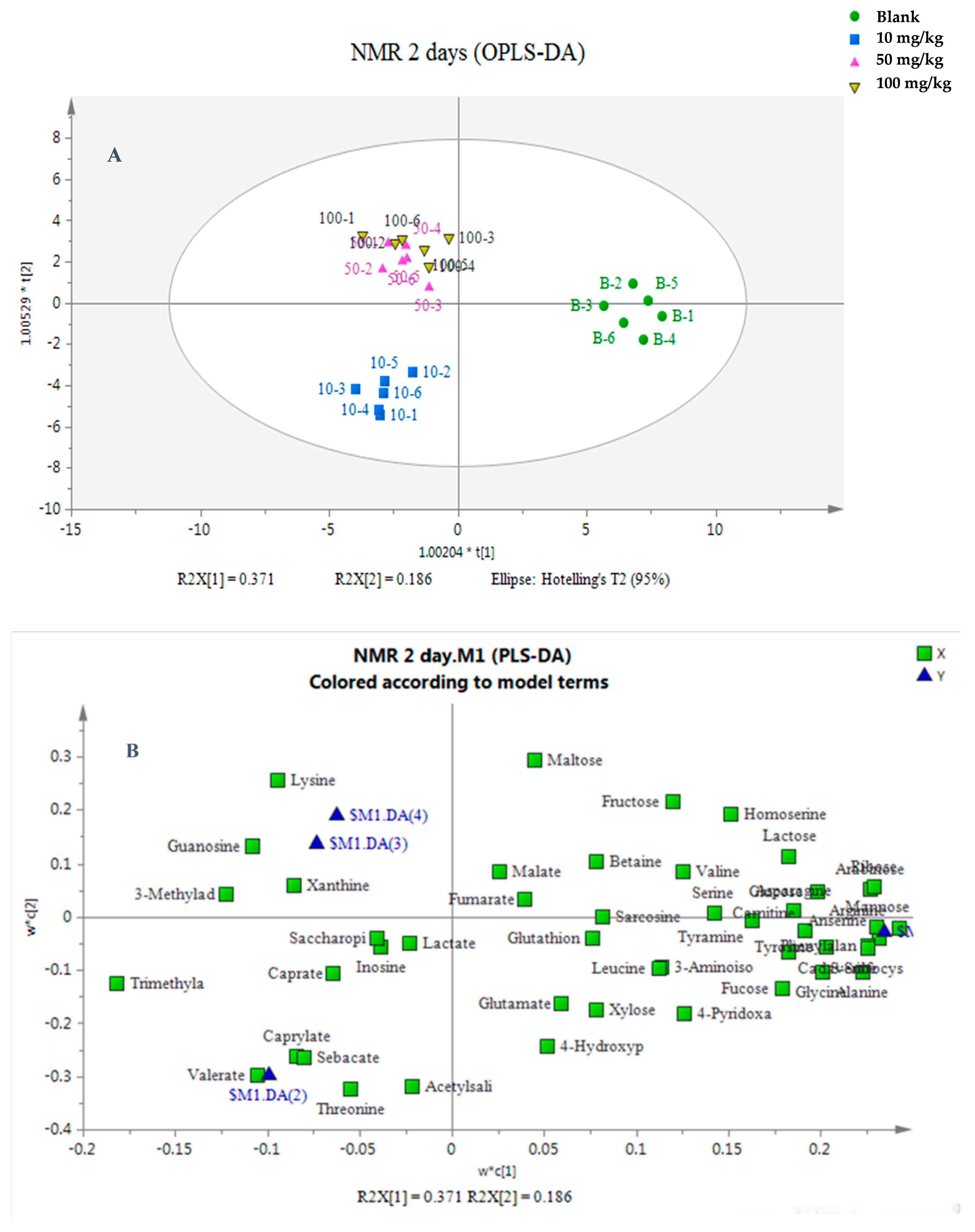
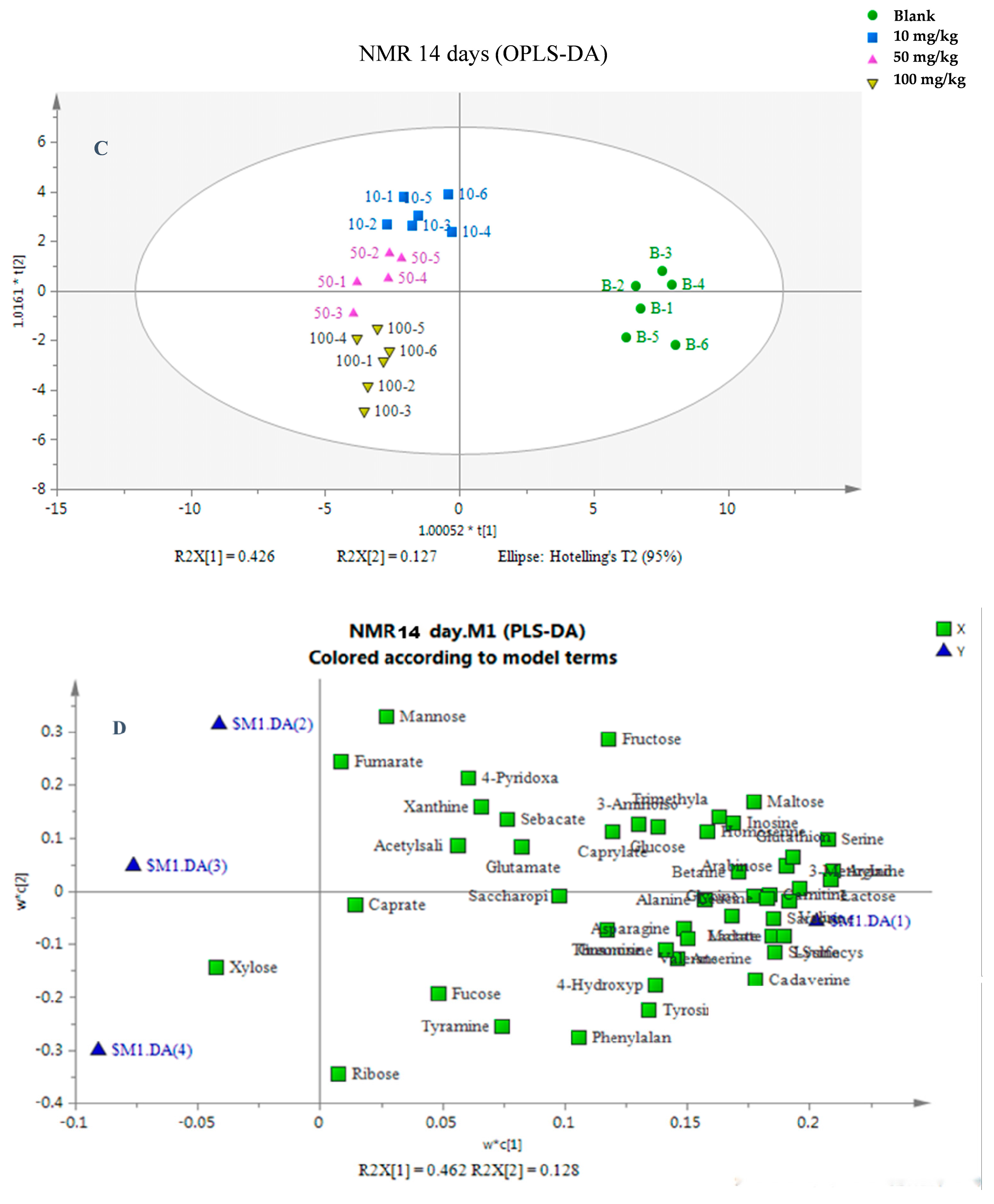
3.2. Multivariate Analysis of the Metabolic Response of Earthworms to Etoxazole
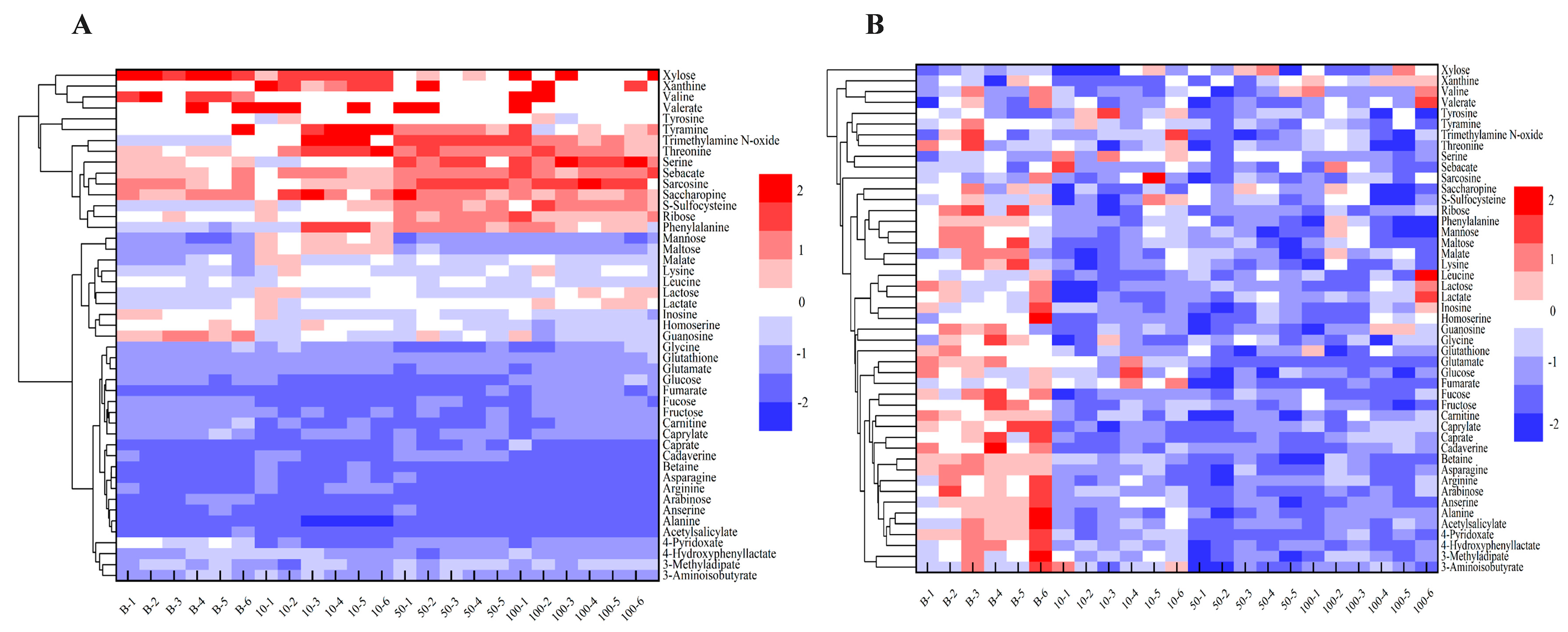
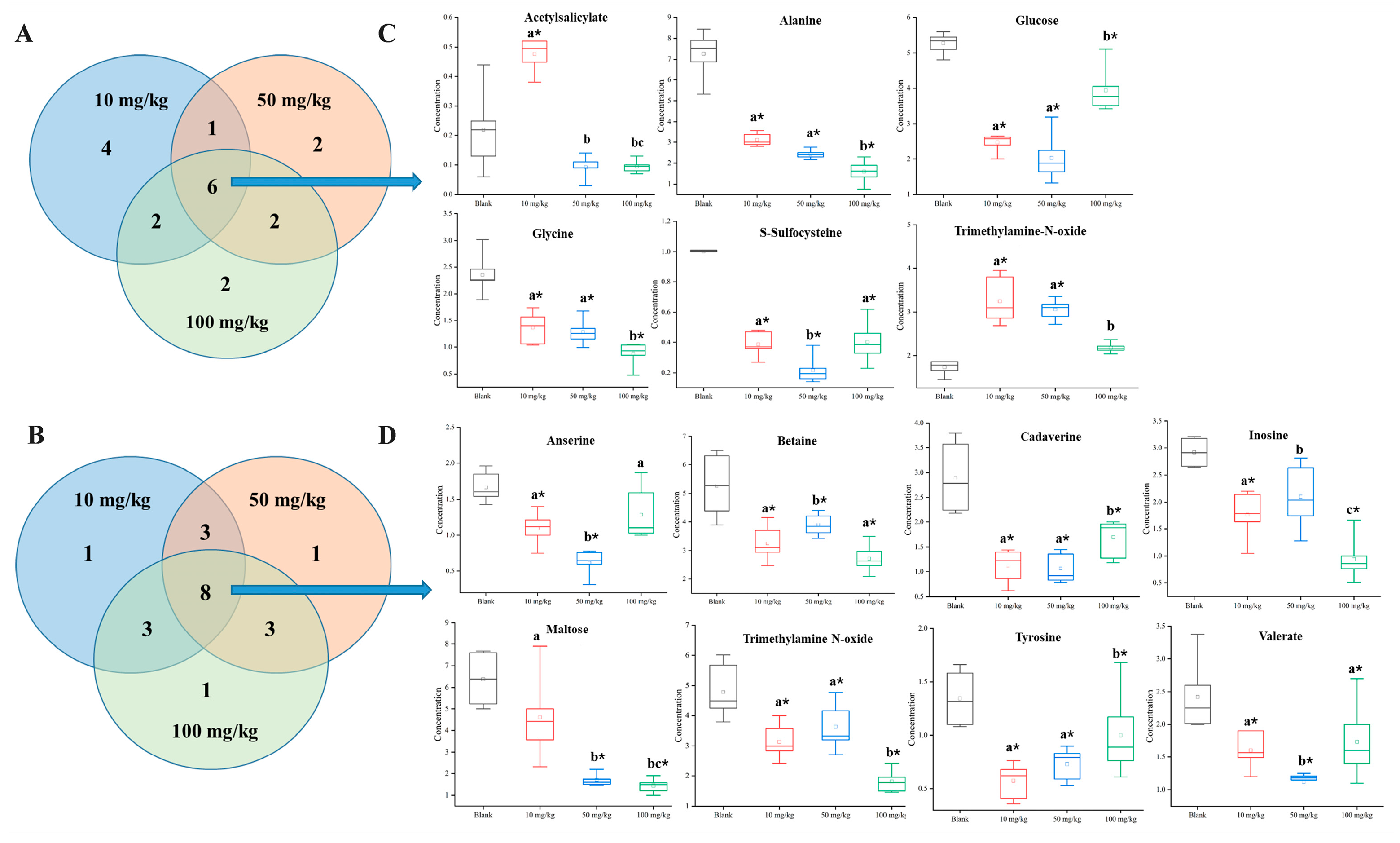
3.3. Changes in Endogenous Metabolites in Earthworm After Etoxazole Treatment
3.4. Possible Metabolic Pathways in Earthworms Affected by Etoxazole
4. Discussion
4.1. Organic Acids and Their Derivatives
4.2. Organic Oxygen Compounds
4.3. Lipids and Lipid-like Molecules
4.4. Other Compounds
4.5. Possible Metabolic Pathways
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMR | Nuclear magnetic resonance |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares-discriminant analysis |
| VIP | Variable importance in projection |
| TMAO | Trimethylamine N-oxide |
| HPLC | High-performance liquid Chromatography |
| GC-MS | Gas chromatography-mass spectrometry |
| LC-MS/MS | Liquid chromatography-tandem mass spectrometry |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malonaldehyde |
| LDH | Lactate dehydrogenase |
| LC50 | Lethal concentration 50 |
| NOEC | No observed effect concentration |
| GABA | γ-aminobutyric acid |
| CPMG | Carr–Purcell–Meiboom–Gill |
| ATP | Adenosine triphosphate |
| 4-HPL | 4-hydroxyphenyllactic acid |
| OECD | Organisation for economic co-operation and development |
| SCs | Suspension concentrates |
| DSS | Disuccinimidyl suberate |
| ANOVA | Analysis of variance. |
Appendix A
| Organic Acids and Derivatives | Organo-Oxygen Compounds (mainly Sugars) | Amino Acids, Peptides, and Analogs | Amines and Nitrogen-Containing Compounds | Lipids and Lipid-like Molecules | Nucleosides, Nucleotides, and Metabolites | Disaccharides |
|---|---|---|---|---|---|---|
| 3-Aminoisobutyrate | Arabinose | Alanine | Betaine | Caprate | Guanosine | Lactose |
| 3-Methyladipate | Fructose | Arginine | Cadaverine | Caprylate | Inosine | Maltose |
| 4-Hydroxyphenyllactate | Fucose | Asparagine | Carnitine | Sebacate | Xanthine | None |
| 4-Pyridoxate | Glucose | Glutamate | S-Sulfocysteine | None | None | None |
| Acetylsalicylate | Mannose | Glycine | Trimethylamine N-oxide | None | None | None |
| Fumarate | Ribose | Homoserine | Tyramine | None | None | None |
| Lactate | Xylose | Leucine | Saccharopine | None | None | None |
| Malate | None | Lysine | None | None | None | None |
| Valerate | None | Phenylalanine | None | None | None | None |
| None | None | Sarcosine | None | None | None | None |
| None | None | Serine | None | None | None | None |
| None | None | Threonine | None | None | None | None |
| None | None | Tyrosine | None | None | None | None |
| None | None | Valine | None | None | None | None |
| None | None | Glutathione | None | None | None | None |
| None | None | Anserine | None | None | None | None |
References
- Dekeyser, M.A. Acaricide mode of action. Pest Manag. Sci. Former. Pestic. Sci. 2005, 61, 103–110. [Google Scholar] [CrossRef]
- Suzuki, J.; Ishida, T.; Kikuchi, Y.; Ito, Y.; Morikawa, C.; Tsukidate, Y.; Tanji, I.; Ota, Y.; Toda, K. Synthesis and activity of novel acaricidal/insecticidal 2, 4-diphenyl-1, 3-oxazolines. J. Pestic. Sci. 2002, 27, 1–8. [Google Scholar] [CrossRef]
- Nauen, R.; Smagghe, G. Mode of action of etoxazole. Pest Manag. Sci. Former. Pestic. Sci. 2006, 62, 379–382. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Reasoned opinion on the review of the existing maximum residue levels (MRLs) for etoxazole according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2012, 10, 2931. [Google Scholar] [CrossRef]
- Xu, M.; Luo, X.; Yan, X.; Cai, X.; Wang, L.; Ge, F.; Wang, H. A new strategy for removing insecticide etoxazole from soil using a combination of a novel Paracoccus versutus Y4 and a fungal mycelium carrier. J. Hazard. Mater. 2025, 494, 138448. [Google Scholar] [CrossRef]
- Sun, D.; Wang, Y.; Zhang, Q.; Pang, J. Investigation of etoxazole metabolites in citrus, soil and earthworms by ultra-performance liquid chromatography with time-of-flight mass spectrometry. Chemosphere 2019, 226, 782–790. [Google Scholar] [CrossRef]
- Malhat, F.; Badawy, H.; Barakat, D.; Saber, A. Determination of etoxazole residues in fruits and vegetables by SPE clean-up and HPLC-DAD. J. Environ. Sci. Health B 2013, 48, 331–335. [Google Scholar] [CrossRef]
- Jang, J.; Rahman, M.; El-Aty, A.M.A.; Ko, A.; Park, J.; Choi, J.; Park, K.H.; Yang, A.; Seo, Y.M.; Shim, J. Analysis of etoxazole in red pepper after major modification of QuEChERS for gas chromatography-nitrogen phosphorus detection. Biomed. Chromatogr. 2014, 28, 767–773. [Google Scholar] [CrossRef]
- Yao, Z.; Li, Z.; Zhuang, S.; Li, X.; Xu, M.; Lin, M.; Wang, Q.; Zhang, H. Enantioselective determination of acaricide etoxazole in orange pulp, peel, and whole orange by chiral liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2015, 38, 599–604. [Google Scholar] [CrossRef]
- Sun, D.; Pang, J.; Fang, Q.; Zhou, Z.; Jiao, B. Stereoselective toxicity of etoxazole ato MCF-7 cells and its dissipation behavior in citrus and soil. Environ. Sci. Pollut. Res. Int. 2016, 23, 24731–24738. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Rencuzogullari, E.; Canli, M. Investigations on the effects of etoxazole in the liver and kidney of Wistar rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 19635–19639. [Google Scholar] [CrossRef]
- Park, W.; Lim, W.; Park, S.; Whang, K.Y.; Song, G. Exposure to etoxazole induces mitochondria-mediated apoptosis in porcine trophectoderm and uterine luminal epithelial cells. Environ. Pollut. 2020, 257, 113480. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.Y.; Park, S.; Song, G.; Lim, W. Developmental toxicity and angiogenic defects of etoxazole exposed zebrafish (Danio rerio) larvae. Aquat. Toxicol. 2019, 217, 105324. [Google Scholar] [CrossRef] [PubMed]
- Blow, N. Metabolomics: Biochemistry’s new look. Nature 2008, 455, 697–700. [Google Scholar] [CrossRef]
- Van Ravenzwaay, B.; Cunha, G.C.; Leibold, E.; Looser, R.; Mellert, W.; Prokoudine, A.; Walk, T.B.; Wiemer, J.C. The use of metabolomics for the discovery of new biomarkers of effect. Toxicol. Lett. 2007, 172, 21–28. [Google Scholar] [CrossRef]
- Labine, L.M.; Simpson, M.J. The use of nuclear magnetic resonance (NMR) and mass spectrometry (MS)-based metabolomics in environmental exposure assessment. Curr. Opin. Environ. Sci. Health 2020, 15, 7–15. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Hoyt, D.W.; Nicora, C.D.; Kinmonth-Schultz, H.A.; Ward, J.K.; Bingol, K. Structure elucidation of unknown metabolites in metabolomics by combined NMR and MS/MS prediction. Metabolites 2018, 8, 8. [Google Scholar] [CrossRef]
- Wishart, D.S. Advances in metabolite identification. Bioanalysis 2011, 3, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Zhou, Z.; Zhu, W. Subacute oral toxicity assessment of benalaxyl in mice based on metabolomics methods. Chemosphere 2018, 191, 373–380. [Google Scholar] [CrossRef]
- McKelvie, J.R.; Yuk, J.; Xu, Y.; Simpson, A.J.; Simpson, M.J. 1H NMR and GC/MS metabolomics of earthworm responses to sub-lethal DDT and endosulfan exposure. Metabolomics 2009, 5, 84–94. [Google Scholar] [CrossRef]
- Yuk, J.; Simpson, M.J.; Simpson, A.J. 1-D and 2-D NMR-based metabolomics of earthworms exposed to endosulfan and endosulfan sulfate in soil. Environ. Pollut. 2013, 175, 35–44. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Earthworm, Acute Toxicity Tests; Test No. 207; OECD: Paris, France, 1984. [Google Scholar]
- International Union of Pure and Applied Chemistry (IUPAC). Etoxazole (Ref: S 1283). Available online: https://sitem.herts.ac.uk/aeru/iupac/Reports/284.htm (accessed on 10 October 2025).
- Hemmer, S.; Manier, S.K.; Wagmann, L.; Meyer, M.R. Impact of four different extraction methods and three different reconstitution solvents on the untargeted metabolomics analysis of human and rat urine samples. J. Chromatogr. A 2024, 1725, 464930. [Google Scholar] [CrossRef]
- Schippers, P.; Rasheed, S.; Park, Y.M.; Risch, T.; Wagmann, L.; Hemmer, S.; Manier, S.K.; Müller, R.; Herrmann, J.; Meyer, M.R. Evaluation of extraction methods for untargeted metabolomic studies for future applications in zebrafish larvae infection models. Sci. Rep. 2023, 13, 7489. [Google Scholar] [CrossRef]
- Bao, X.; Xu, W.; Cui, J.; Yan, Z.; Wang, J.; Chen, X.; Meng, Z. NMR-based metabolomics approach to assess the ecotoxicity of prothioconazole on the earthworm (Eisenia fetida) in soil. Pestic. Biochem. Physiol. 2023, 190, 105320. [Google Scholar] [CrossRef]
- Hecklau, C.; Pering, S.; Seibel, R.; Schnellbaecher, A.; Wehsling, M.; Eichhorn, T.; von Hagen, J.; Zimmer, A. S-Sulfocysteine simplifies fed-batch processes and increases the CHO specific productivity via antioxidant activity. J. Biotechnol. 2016, 218, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Sandberg, M.; Weber, S.G. Development of a liquid chromatographic method for picomole determination of S-sulfocysteine in trifluoroacetic acid extracts of neonatal rat brain. J. Pharm. Biomed. Anal. 1999, 19, 261–268. [Google Scholar] [CrossRef]
- Katz, J.; Wals, P.; Lee, W.N.P. Isotopomer studies of gluconeogenesis and the Krebs cycle with 13C-labeled lactate. J. Biol. Chem. 1993, 268, 25509–25521. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Toyomizu, M.; Kikusato, M. Possible role of corticosterone in proteolysis, glycolytic, and amino acid metabolism in primary cultured avian myotubes incubated at high-temperature conditions. Domest. Anim. Endocrinol. 2021, 76, 106608. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.G.; Good, R.A. Quantitative effects of nutritional essential amino acid deficiency upon immune responses to tumors in mice. J. Exp. Med. 1973, 137, 19. [Google Scholar] [CrossRef]
- Yin, Y.; Zhao, X.; Yang, L.; Wang, K.; Sun, Y.; Ye, J. Dietary High Glycinin Reduces Growth Performance and Impairs Liver and Intestinal Health Status of Orange-Spotted Grouper (Epinephelus coioides). Animals 2023, 13, 2605. [Google Scholar] [CrossRef]
- Mi, M.; Chang, M.; Huang, Y.; Zhao, J.; Pan, L.; Bao, N.; Qin, G.; Zhao, Y. Fructo-oligosaccharides Ameliorate Intestinal Mechanical Barrier Injury in Piglets Induced by Soybean Antigen in vitro and in vivo. Curr. Protein. Pept. Sci. 2023, 24, 267–276. [Google Scholar] [CrossRef]
- Cuaron, J.A.; Chapple, R.P.; Easter, R.A. Effect of lysine and threonine supplementation of sorghum gestation diets on nitrogen balance and plasma constituents in first-litter gilts. J. Anim. Sci. 1984, 58, 631–637. [Google Scholar] [CrossRef]
- Batatinha, H.A.P.; Biondo, L.A.; Lira, F.S.; Castell, L.M.; Rosa-Neto, J.C. Nutrients, immune system, and exercise: Where will it take us? Nutrition 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Hiramoto, K.; Muramatsu, T.; Okumara, J. Effect of methionine and lysine deficiencies on protein synthesis in the liver and oviduct and in the whole body of laying hens. Poult. Sci. 1990, 69, 84–89. [Google Scholar] [CrossRef]
- Lehnert, H.; Wurtman, R.J. Amino acid control of neurotransmitter synthesis and release: Physiological and clinical implications. Psychother. Psychosom. 1993, 60, 18–32. [Google Scholar] [CrossRef]
- Kaneko, J.; Enya, A.; Enomoto, K.; Ding, Q.; Hisatsune, T. Anserine (beta-alanyl-3-methyl-L-histidine) improves neurovascular-unit dysfunction and spatial memory in aged AβPPswe/PSEN1dE9 Alzheimer’s-model mice. Sci. Rep. 2017, 7, 12571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, J.; Liu, X.; Yang, Q.; Dong, Y.; Jia, L. The antioxidant activities of alkalic-extractable polysaccharides from Coprinus comatus on alcohol-induced liver injury in mice. Sci. Rep. 2018, 8, 11695. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, L.; Hao, S.; Yang, X.; Huang, H.; Cen, J.; Wang, Y. Fatty-acid Profiles and Fingerprints of Seven Types of Fish Roes as Determined by Chemometric Methods. J. Oleo Sci. 2020, 69, 1199–1208. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jin, H.; Chu, X.; Dai, W.; Tang, W.; Zhu, J.; Wang, F.; Yang, X.; Li, W.; Liu, G.; et al. The Host CYP1A1-Microbiota Metabolic Axis Promotes Gut Barrier Disruption in Methicillin-Resistant Staphylococcus aureus-Induced Abdominal Sepsis. Front. Microbiol. 2022, 13, 802409. [Google Scholar] [CrossRef]
- Akhova, A.; Nesterova, L.; Shumkov, M.; Tkachenko, A. Cadaverine biosynthesis contributes to decreased Escherichia coli susceptibility to antibiotics. Res. Microbiol. 2021, 172, 103881. [Google Scholar] [CrossRef]
- Mikó, E.; Kovács, T.; Sebő, É.; Tóth, J.; Csonka, T.; Ujlaki, G.; Sipos, A.; Szabó, J.; Méhes, G.; Bai, P. Microbiome-Microbial Metabolome-Cancer Cell Interactions in Breast Cancer-Familiar, but Unexplored. Cells 2019, 8, 293. [Google Scholar] [CrossRef]
- Olin-Sandoval, V.; Yu, J.S.L.; Miller-Fleming, L.; Alam, M.T.; Kamrad, S.; Correia-Melo, C.; Haas, R.; Segal, J.; Peña Navarro, D.A.; Herrera-Dominguez, L.; et al. Lysine harvesting is an antioxidant strategy and triggers underground polyamine metabolism. Nature 2019, 572, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.P.; Jochem, A.; Johnson, S.E.; Reddy, T.R.; Russell, J.D.; Coon, J.J.; Pagliarini, D.J. Defining intermediates and redundancies in coenzyme Q precursor biosynthesis. J. Biol. Chem. 2021, 296, 100643. [Google Scholar] [CrossRef]
- Meleti, M.; Quartieri, E.; Antonelli, R.; Pezzi, M.E.; Ghezzi, B.; Viani, M.V.; Setti, G.; Casali, E.; Ferrari, E.; Ciociola, T.; et al. Metabolic Profiles of Whole, Parotid and Submandibular/Sublingual Saliva. Metabolites 2020, 10, 318. [Google Scholar] [CrossRef]
- Niemann, B.; Haufs-Brusberg, S.; Puetz, L.; Feickert, M.; Jaeckstein, M.Y.; Hoffmann, A.; Zurkovic, J.; Heine, M.; Trautmann, E.M.; Müller, C.E.; et al. Apoptotic brown adipocytes enhance energy expenditure via extracellular inosine. Nature 2022, 609, 361–368. [Google Scholar] [CrossRef]
- Shaikh, F.Y.; Sears, C.L. Messengers from the microbiota. Science 2020, 369, 1427–1428. [Google Scholar] [CrossRef]
- Hui, R.; Xu, J.; Zhou, M.; Xie, B.; Zhou, M.; Zhang, L.; Cong, B.; Ma, C.; Wen, D. Betaine improves METH-induced depressive-like behavior and cognitive impairment by alleviating neuroinflammation via NLRP3 inflammasome inhibition. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 135, 111093. [Google Scholar] [CrossRef]
- Caradonna, E.; Abate, F.; Schiano, E.; Paparella, F.; Ferrara, F.; Vanoli, E.; Difruscolo, R.; Goffredo, V.M.; Amato, B.; Setacci, C.; et al. Trimethylamine-N-Oxide (TMAO) as a Rising-Star Metabolite: Implications for Human Health. Metabolites 2025, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Aspinall, J.V.; Lv, W.; Zheng, X.; Zhang, H.; Li, S.; Zhang, J.; Bai, N.; Zhang, Y.; Wang, X. Differences in kinetic metabolomics in Eisenia fetida under single and dual exposure of imidacloprid and dinotefuran at environmentally relevant concentrations. J. Hazard. Mater. 2021, 417, 126001. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, Y.; Sang, W. Integration of transcriptomic and metabolomic reveals metabolic pathway alteration in earthworms (Eisenia fetida) under copper exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 260, 109400. [Google Scholar] [CrossRef] [PubMed]
- Gillis, J.D.; Price, G.W.; Prasher, S.O.; Asgari Lajayer, B. Metabolomics reveals increased toxicity in earthworms (Eisenia fetida) of the transformation product methyl-triclosan. Ecotoxicol. Environ. Saf. 2025, 294, 118128. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, C.; Zhang, Q.; Wang, Z.; Chen, Z.; He, Y.; He, J.; Sun, D. Etoxazole Exposure Triggers Sublethal Metabolic Responses in Earthworms (Eisenia fetida): An NMR-Based Metabolomics Study. Toxics 2025, 13, 923. https://doi.org/10.3390/toxics13110923
Liao C, Zhang Q, Wang Z, Chen Z, He Y, He J, Sun D. Etoxazole Exposure Triggers Sublethal Metabolic Responses in Earthworms (Eisenia fetida): An NMR-Based Metabolomics Study. Toxics. 2025; 13(11):923. https://doi.org/10.3390/toxics13110923
Chicago/Turabian StyleLiao, Chaoxuan, Qinghai Zhang, Zelan Wang, Zuyong Chen, Yu He, Ji He, and Dali Sun. 2025. "Etoxazole Exposure Triggers Sublethal Metabolic Responses in Earthworms (Eisenia fetida): An NMR-Based Metabolomics Study" Toxics 13, no. 11: 923. https://doi.org/10.3390/toxics13110923
APA StyleLiao, C., Zhang, Q., Wang, Z., Chen, Z., He, Y., He, J., & Sun, D. (2025). Etoxazole Exposure Triggers Sublethal Metabolic Responses in Earthworms (Eisenia fetida): An NMR-Based Metabolomics Study. Toxics, 13(11), 923. https://doi.org/10.3390/toxics13110923






