Atlantic Salmon (Salmo salar) GILL Primary Cell Culture Oxidative Stress and Cellular Damage Response Challenged with Oxytetracycline Antibiotic
Abstract
1. Introduction
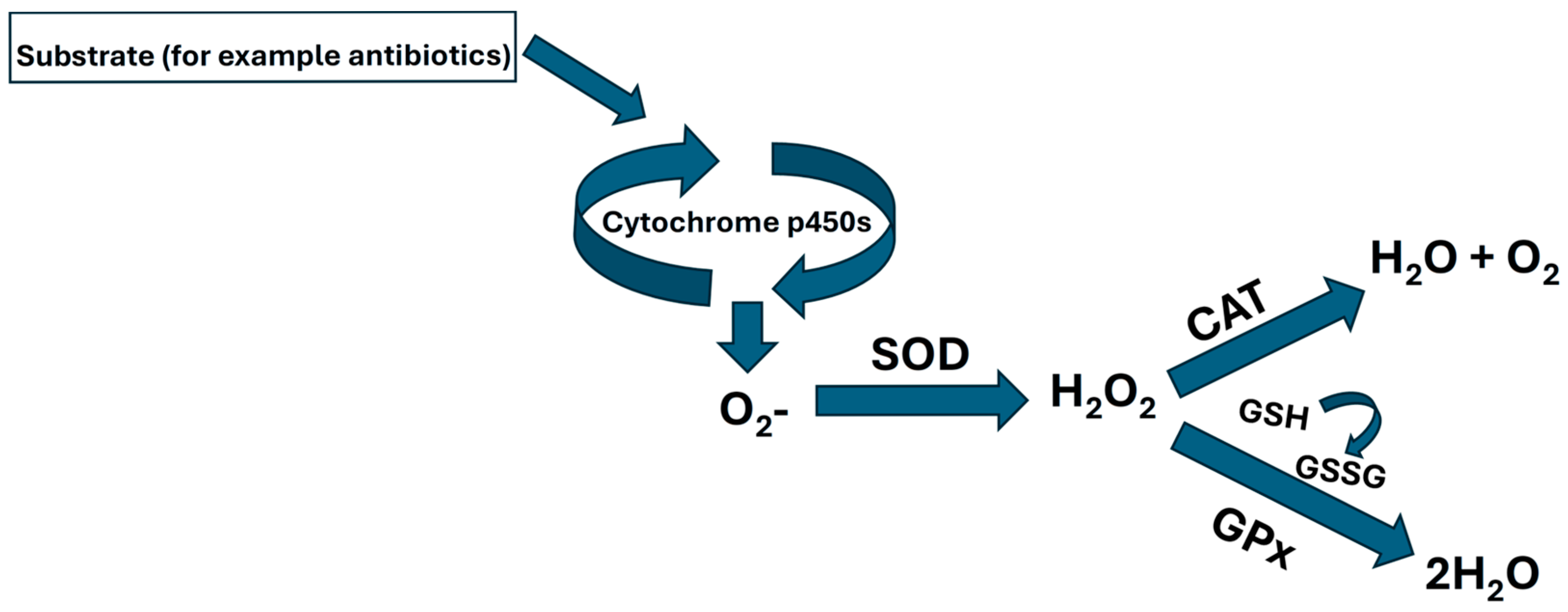
- Phase I or oxidative-phase enzymes. Cytochrome p450 is the largest enzyme complex involved in this phase of metabolism. In general terms, we can say that Phase I is a set of oxidation reactions that prepare toxicants for transformation by Phase II reactions.
2. Materials and Methods
2.1. The Ethics Statement
2.2. Sampling Procedure
2.3. Primary Culture
2.4. In Vitro Experimental Treatment
2.5. Extraction of Total RNA from Gill Cells
2.6. qRT-PCR Analysis
2.7. Antioxidant Enzyme Activity
2.7.1. Homogenization
2.7.2. Specific Enzymatic Conditions
2.7.3. Protein Quantification
2.7.4. Statistical Analyses
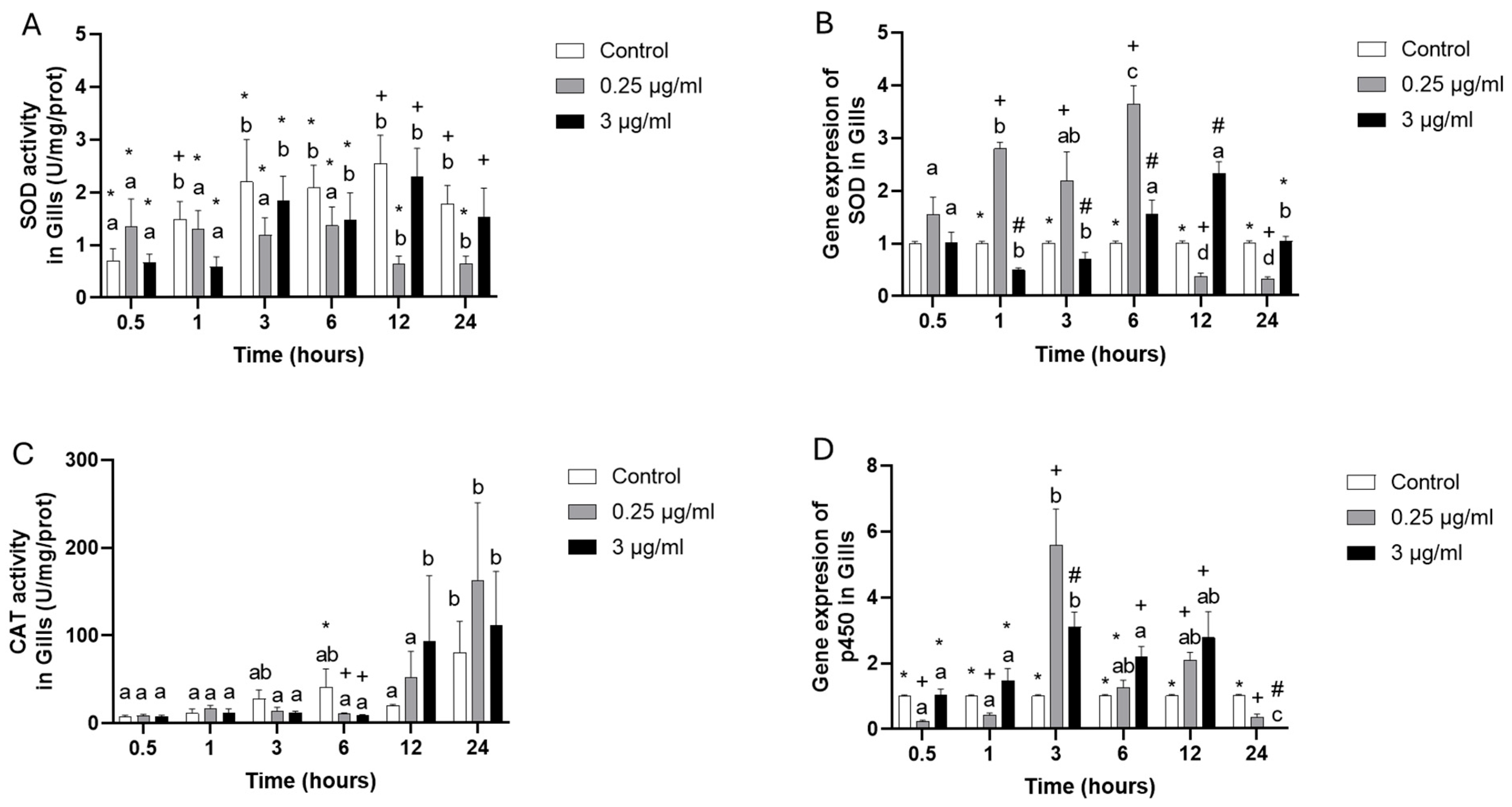
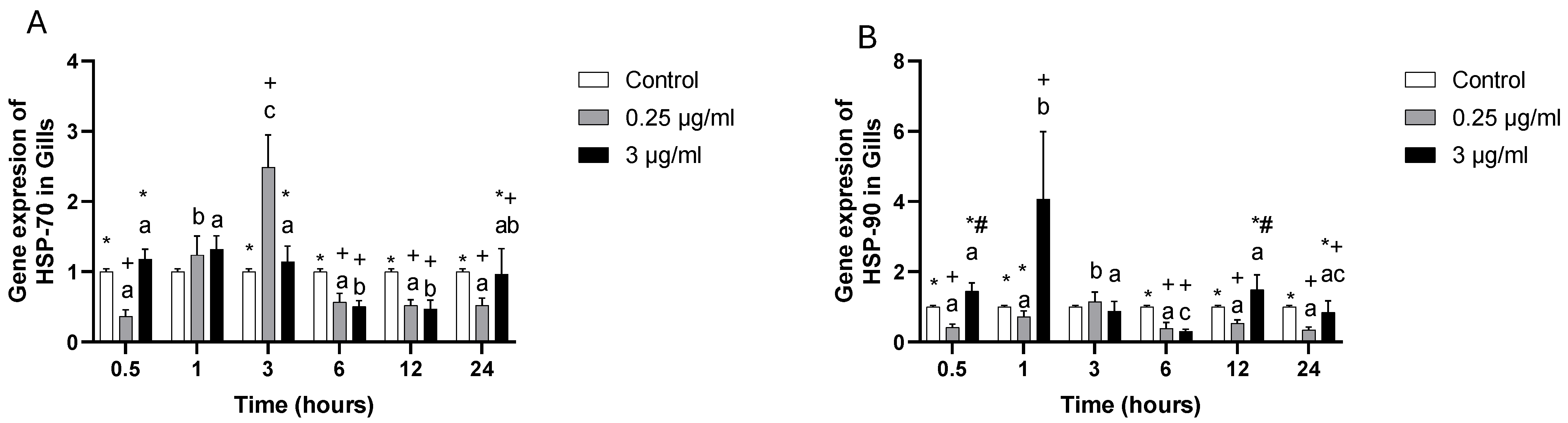
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormick, S.D. Smolt physiology and endocrinology. In Euryhaline Fishes; McCormick, S.D., Farrell, A.P., Brauner, C., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 199–251. [Google Scholar]
- Fry, F.E. Effects of the Environment on Animal Activity Biological Series; Ontario Fisheries Research Laboratory Publication: Nipissing, Canada, 1947; Volume 55. [Google Scholar]
- Saravia, J.; Nualart, D.; Paschke, K.; Pontigo, J.P.; Navarro, J.M.; Vargas-Chacoff, L. Temperature and immune challenges modulate the transcription of genes of the ubiquitin and apoptosis pathways in two high-latitude Notothenioid fish across the Antarctic Polar Front. Fish Physiol. Biochem. 2024, 50, 1429–1443. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Moneva, F.; Oyarzún, R.; Martínez, D.; Munoz, J.L.P.; Bertran, C.; Mancera, J.M. Environmental salinity-modified osmoregulatory response in the sub-Antarctic notothenioid fish Eleginops maclovinus. Polar Biol. 2014, 37, 1235–1245. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Arjona, F.J.; Ruiz-Jarabo, I.; García-López, A.; Flik, G.; Mancera, J.M. Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J. Therm. Biol. 2020, 88, 102526. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Bjornsson, B.T.; McCormick, S.D. Effects of long-term cortisol treatment on growth and osmoregulation of Atlantic salmon and brook trout. Gen. Comp. Endocrinol. 2021, 308, 113769. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Martínez, D.; Oyarzún, R.; Paschke, K.; Navarro, J.M. The osmotic response capacity of the Antarctic fish Harpagifer antarcticus is insufficient to cope with projected temperature and salinity under climate change. J. Therm. Biol. 2021, 96, 102835. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Dann, F.; Oyarzún-Salazar, R.; Nualart, D.; Muñoz, J.L.P. Oxidative Stress Response of Liver Cell Culture in Atlantic Salmon Challenged Under Two Antibiotics: Oxytetracycline and Florfenicol. Toxics 2025, 13, 361. [Google Scholar] [CrossRef]
- Nualart, D.; Paschke, K.; Guerreiro, P.M.; McCormick, S.D.; González-Wevar, C.; Cheng, C.C.-H.; Vargas Chacoff, L. Combined effects of PVC microplastics and thermal rise alter the oxidative stress response in Antarctic fish Harpagifer antarcticus and Sub-Antarctic Harpagifer bispinis. Mar. Pollut. Bull. 2025, 220, 118438. [Google Scholar] [CrossRef]
- Rand, G.M. (Ed.) Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995; p. 1148. [Google Scholar] [CrossRef]
- Van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Borković, S.S.; Šaponjić, J.S.; Pavlović, S.Z.; Blagojević, D.P.; Milošević, S.M.; Kovačević, T.B.; Radojičić, R.M.; Spasić, M.B.; Žikić, R.V.; Saičić, Z.S. The activity of antioxidant defences enzymes in the mussel Mytilus galloprovincialis from the Adriatic Sea. Comp. Biochem. Physiol. C 2005, 141, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Anastasi, V.; Martin Dörr, A.J. Hepatic antioxidant enzymes and total glutathione of Cyprinus carpio exposed to three disinfectants, chlorine dioxide, sodium hypochlorite and peracetic acid, for superficial water potabilization. Chemosphere 2006, 64, 1633–1641. [Google Scholar] [CrossRef]
- Winston, G.W.; Di Giulio, R.T. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat. Toxicol. 1991, 19, 137–161. [Google Scholar] [CrossRef]
- Soldatov, A.A.; Gostyukhina, O.L.; Golovina, I.V. Antioxidant enzymes compplex of tissues of the bivalve Mytilus galloprovincialis Lam. Under normal and oxidative-stress conditions: A review. Appl. Biochem. Microbiol. 2007, 43, 556–562. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2024. Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2024. [Google Scholar]
- SERNAPESCA. Report with Health Background of Freshwater and Sea Year 1st Semester; Department of Animal Health Sub-Directorate of Aquaculture National Fisheries and Aquaculture Service: Valparaiso, Chile, 2023; p. 55. [Google Scholar]
- Christensen, A.M.; Ingerslev, F.; Baun, A. Ecotoxicity of mixtures of antibiotics used in aquacultures. Environ. Toxicol. Chem. 2006, 25, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.; Mancilla, M.; Miranda, C.D. Use of antimicrobials in Chilean Salmon farming: Facts, myths and perspectives. Rev. Aquac. 2023, 15, 89–111. [Google Scholar] [CrossRef]
- Elema, M.O.; Hoff, K.A.; Kristensen, H.G. Bioavailability of oxytetracycline from medicated feed administered to Atlantic salmon (Salmo salar L.) in seawater. Aquaculture 1996, 143, 7–14. [Google Scholar] [CrossRef]
- Nualart, D.; Muñoz, J.L.P.; Vargas-Chacoff, L. Intestinal Immune System Expression of Coho Salmon Challenged with Oxytetracycline: In Vivo and In Vitro Approach. Int. J. Mol. Sci. 2025, 26, 6330. [Google Scholar] [CrossRef]
- Muñoz, J.L.P.; Martínez, D.; Nualart, D.P.; Mardones, O.; Delmoral, I.; Morera, F.; Vargas-Chacoff, L. Antibiotic oxytetracycline is affecting the dynamics of serotonergic response in brain of coho salmon. Aquaculture 2025, 603, 742376. [Google Scholar] [CrossRef]
- Schnell, S.; Kawano, A.; Porte, C.; Lee, L.E.J.; Bols, N.C. Effects of Ibuprofen on the Viability and Proliferation of Rainbow Trout Liver Cell Lines and Potential Problems and Interactions in Effects Assessment. Environ. Toxicol. Int. J. 2008, 24, 157–165. [Google Scholar] [CrossRef]
- Pontigo, J.P.; Vargas-Chacoff, L. Growth hormone (GH) and growth hormone release factor (GRF) modulate the immune response in the SHK-1 cell line and leukocyte cultures of head kidney in Atlantic salmon. Gen. Comp. Endocrinol. 2021, 300, 113631. [Google Scholar] [CrossRef]
- Nualart, D.P.; Dann, F.; Oyarzun-Salazar, R.; Morera, F.J.; Vargas-Chacoff, L. Immune Transcriptional Response in Head Kidney Primary Cell Cultures Isolated from the Three Most Important Species in Chilean Salmonids Aquaculture. Biology 2023, 12, 924. [Google Scholar] [CrossRef]
- Tafalla, C.; Novoa, B.; Alvarez, J.M.; Figueras, A. In vivo and in vitro effect of oxytetracycline treatment on the immune response of turbot, Scophthalmus maximus (L.). J. Fish Dis. 1999, 22, 271–276. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rasmussen, R. Quantification on the Light Cycler. In Rapid Cycle Real-Time PCR, Methods and Applications; Meuer, S., Wittwer, C., Nakagawara, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 21–34. [Google Scholar]
- Pedro, A.V.F.; Martínez, D.; Pontigo, J.P.; Vargas-Lagos, C.; Hawes, C.; Wadsworth, S.; Morera, F.J.; Vargas-Chacoff, L.; Yáñez, A.J. Transcriptional activation of genes involved in oxidative stress in Salmo salar challenged with Piscirickettsia salmonis. Comp. Biochem. Physiol. B 2019, 229, 18–25. [Google Scholar] [CrossRef]
- Martínez, D.; Vargas-Lagos, C.; Oyarzún, R.; Loncoman, C.A.; Pontigo, J.P.; Yáñez, A.J.; Vargas-Chacoff, L. Temperature modulates the immunological response of the sub-antarctic notothenioid fish Eleginops maclovinus injected with Piscirickettsia salmonis. Fish Shellfish. Immunol. 2018, 82, 492–503. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Muñoz, J.L.P.; Saravia, J.; Oyarzún, R.; Pontigo, J.P.; González, M.P.; Mardones, O.; Hawes, C.; Pino, J.; Wadsworth, S.; et al. Neuroendocrine stress response in Atlantic salmon (Salmo salar) and Coho salmon (Oncorynchus kisutch) during sea lice infestation. Aquaculture 2019, 507, 329–340. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Lopez-Galindo, C.; Vargas-Chacoff, L.; Nebot, E.; Casanueva, J.F.; Rubio, D.; Sole, M.; Mancera, J.M. Sublethal effects of the organic antifoulant Mexel (R) 432 on osmoregulation and xenobiotic detoxification in the flatfish Solea senegalensis. Chemosphere 2010, 79, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 113, pp. 484–490. [Google Scholar]
- Flohe, L.; Gunzler, G.A. Assays of glutathione peroxidase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 114–121. [Google Scholar]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 8, 715–748. [Google Scholar] [CrossRef]
- Gheorghe, S.; Stan, M.S.; Mitroi, D.N.; Staicu, A.C.; Cicirma, M.; Lucaciu, I.E.; Nita-Lazar, M.; Dinischiotu, A. Oxidative Stress and Histopathological Changes in Gills and Kidneys of Cyprinus carpio following Exposure to Benzethonium Chloride, a Cationic Surfactant. Toxics 2022, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Gjessing, M.; Sandvik, M.; Eriksen, G.S. The gill epithelial cell lines RTgill-W1, from Rainbow trout and ASG-10, from Atlantic salmon, exert different toxicity profiles towards rotenone. Cytotechnology 2023, 75, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Sujitha, M.; Anila, P.A.; Ren, Z.; Poopal, R.K. Responses of Cirrhinus mrigala to second-generation fluoroquinolone (ciprofloxacin) toxicity: Assessment of antioxidants, tissue morphology, and inorganic ions. Environ. Toxicol. 2021, 36, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, L.; Ou, R.; Nie, X.; Yang, Y.; Wang, F.; Li, K. Effects of norfloxacin on hepatic genes expression of P450 isoforms (CYP1A and CYP3A), GST and P-glycoprotein (P-gp) in Swordtail fish (Xiphophorus Helleri). Ecotoxicology 2015, 24, 1566–1573. [Google Scholar] [CrossRef]
- Iftikhar, N.; Zafar, R.; Hashmi, I. Multi-biomarkers approach to determine the toxicological impacts of sulfamethoxazole antibiotic on freshwater fish Cyprinus carpio. Ecotoxicol. Environ. Saf. 2022, 233, 113331. [Google Scholar] [CrossRef]
- Bainy, A.C.D.; Saito, E.; Carvalho, P.S.M.; Junqueira, V.B.C. Oxidative stress in gill, erythrocytes, liver and kidney of Nile tilapia (Oreochromis niloticus) from a polluted site. Aquat. Toxicol. 1996, 34, 151–162. [Google Scholar] [CrossRef]
- Antão-Barboza, L.G.; Russo Vieira, L.; Branco, V.; Carvalho, C.; Guilhermino, L. Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Sci. Rep. 2018, 8, 15655. [Google Scholar] [CrossRef]
- Regoli, F.; Winston, G.W.; Gorbi, S.; Frenzilli, G.; Nigro, M.; Corsi, I.; Focardi, S. Integrating enzymatic responses to organic chemical exposure with total oxyradical absorbing capacity and DNA damage in the European eel Anguilla anguilla. Environ. Toxicol. Chem. 2003, 22, 2120e2129. [Google Scholar] [CrossRef]
- Regoli, F.; Nigro, M.; Benedetti, M.; Gorbi, S.; Pretti, C.; Gervasi, P.G.; Fattorini, D. Interactions between metabolism of trace metals and xenobiotic agonists of the aryl hydrocarbon receptor in the Antarctic fish Trematomus bernacchii: Environmental perspectives. Environ. Toxicol. Chem. 2005, 24, 1475e1482. [Google Scholar] [CrossRef]
- Benedetti, M.; Martuccio, G.; Fattorini, D.; Canapa, A.; Barucca, M.; Nigro, M.; Regoli, F. Oxidative and modulatory effects of trace metals on metabolism of polycyclic aromatic hydrocarbons in the Antarctic fish Trematomus bernacchii. Aquat. Toxicol. 2007, 85, 167e175. [Google Scholar] [CrossRef]
- Benedetti, M.; Fattorini, D.; Martuccio, G.; Nigro, M.; Regoli, F. Interactions between trace metals (Cu, Hg, Ni, Pb) and 2,3,7,8-tetrachlorodibenzo-p-dioxin in the Antarctic fish Trematomus bernacchii: Oxidative effects on biotransformation pathway. Environ. Toxicol. Chem. 2009, 28, 818e825. [Google Scholar] [CrossRef] [PubMed]
- Vakharia, D.D.; Liu, N.; Pause, R.; Fasco, M.; Bessette, E.; Zhang, Q.-Y.; Kaminsky, L.S. Effect of metals on polycyclic aromatic hydrocarbon induction of CYP1A1 and CYP1A2 in human hepatocyte cultures. Toxicol. Appl. Pharmacol. 2001, 170, 93e103. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lazado, C.C.; Brinchmann, M.F.; Berg, I.; Kiron, V. In vivo modulation of immune response and antioxidant defense in Atlantic cod, Gadus morhua following oral administration of oxolinic acid and florfenicol. Comp. Biochem. Physiol. Part. C 2009, 150, 459–464. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 30, 620–650. [Google Scholar] [CrossRef]
- García-Pimentel, M.M.; Mezzelani, M.; Valdés, N.J.; Giuliani, M.E.; Gorbi, S.; Regoli, F.; León, V.M.; Campillo, J.A. Integrative oxidative stress biomarkers in gills and digestive gland of the combined exposure to citalopram and bezafibrate with polyethylene microplastics on mussels Mytilus galloprovincialis. Environ. Pollut. 2025, 366, 125508. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Correia, A.T.; Nunes, B. Rainbow trout (Oncorhynchus mykiss) pro-oxidant and genotoxic responses following acute and chronic exposure to the antibiotic oxytetracycline. Ecotoxicology 2017, 26, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Atessahin, A.; Sahna, E.; Karahan, I.; Ozer, S. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology 2006, 218, 164–171. [Google Scholar] [CrossRef]
- Gibson, B.W. The human mitochondrial proteome: Oxidative stress protein modifications and oxidative phosphorylation. Int. J. Biochem. Cell Biol. 2005, 37, 927e34. [Google Scholar] [CrossRef] [PubMed]
- Gnanasoundari, M.; Pari, L. Impact of naringenin on oxytetracycline-mediated oxidative damage in kidney of rats. Ren. Fail. 2006, 28, 599–605. [Google Scholar] [CrossRef]
- Álvarez-Muñoz, D.; Gómez-Parra, A.; Blasco, J.; Sarasquete, C.; González-Mazo, E. Oxidative stress and histopathology damage related to the metabolism of dodecylbenzene sulfonate in Senegalese sole. Chemosphere 2009, 74, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Li, Z.B.; Guo, X.Q.; Guo, J.P. Effects of trichlorfon and sodium dodecyl sulphate on antioxidant defense system and acetylchloniesterase of Tilapia nilotica in vitro. Pest. Biochem. Phys. 2008, 92, 107–113. [Google Scholar] [CrossRef]
- Kładna, A.; Michalska, T.; Berczýnski, P.; Kruk, I.; Aboul-Enein, H.Y. Evaluation of the antioxidant activity of tetracycline antibiotics in vitro. Luminescence 2012, 27, 249–255. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Astola, A.; Arjona, F.J.; Martín del Río, M.P.; García-Cózar, F.; Mancera, J.M.; Martínez-Rodríguez, G. Pituitary gene and protein expression under experimental variation on salinity and temperature in gilthead sea bream Sparus aurata. Comp. Biochem. Physiol. B 2009, 154, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Iwama, G.; Afonso, L.; Todgham, A.; Ackerman, P.; Nakano, K. Are HSPs suitable for indicating stressed states in fish? J. Exp. Biol. 2004, 204, 15–19. [Google Scholar] [CrossRef]
- Fowler, S.; Hamilton, D.; Currie, S. A comparison of the heat shock response in juvenile and adult rainbow trout (Oncorhynchus mykiss)—Implications for increased thermal sensitivity with age. Can. J. Fish. Aquat. Sci. 2009, 66, 91–100. [Google Scholar] [CrossRef]
- Hori, T.S.; Gamperl, A.K.; Afonso, L.O.B.; Johnson, S.C.; Hubert, S.; Kimball, J.; Bowman, S.; Rise, M.L. Heat-shock responsive genes identified and validated in Atlantic cod (Gadus morhua) liver, head kidney and skeletal muscle using genomic techniques. BMC Genom. 2010, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.; Höglund, E.; Gilmour, K.M.; Currie, S. Hormonal modulation of the heat shock response: Insights from fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R184–R192. [Google Scholar] [CrossRef]
- LeBlanc, S.; Middleton, S.; Gilmour, K.M.; Currie, S. Chronic social stress impairs thermal tolerance in the rainbow trout (Oncorhynchus mykiss). J. Exp Biol. 2011, 214, 1721–1731. [Google Scholar] [CrossRef]
- Niforou, K.; Cheimonidou, C.; Trougakos, I.P. Molecular chaperones and proteostasis regulation during redox imbalance. Redox Biol. 2014, 1, 323–332. [Google Scholar] [CrossRef]
- Martínez, D.; Vargas-Lagos, C.; Saravia, J.; Oyarzún, R.; Loncoman, C.; Pontigo, J.P.; Vargas-Chacoff, L. Cellular stress responses of Eleginops maclovinus fish injected with Piscirickettsia salmonis and submitted to thermal stress. Cell Stress Chaperones 2020, 25, 93–104. [Google Scholar] [CrossRef]

| Primer | Nucleotide Sequences (5′→3′) | Efficiency (%) | GenBank No/Reference |
|---|---|---|---|
| p450Fw | TCGTTCCTTGTCCGAAAGCAGA | 100.4 | Pedro et al., 2019 [30] |
| p450 Rv | TGTCGGTACCAGCACCAAACAT | ||
| GR Fw | AAAGTGCCAGTACCAAGCCC | 101.7 | Martinez et al., 2018 [31] |
| GR Rv | CATGCTGATGAGCTACTGTTGTT | ||
| SOD Fw | GGGCAATGCCAATAACTCCACA | 104.5 | Pedro et al., 2019 [30] |
| SOD Rv | AGGACCATGGTGATCCATGAGAAG | ||
| GPx Fw | GAACTGCAGCAATGGTGAGA | 100.3 | Pedro et al., 2019 [30] |
| GPx Rv | CATGAGAGAGATGGGGTCGT | ||
| HSP70-Fw | AGGGAACGCAACGTCCTGATTT | 102 | Vargas-Chacoff et al., 2019 [32] |
| HSP70-Rv | ACTAACCAGGCGGTTGTCAAAGTC | ||
| HSP90-Fw | CATTCGTGGAACGCCTTCGAAA | 101 | Vargas-Chacoff et al., 2019 [32] |
| HSP90-Rv | AGAGACAAGGGTCTTGCCGTCATA | ||
| 18S Fw | GTCCGGGAAACCAAAGTC | 103.5 | Pedro et al., 2019 [30] |
| 18S Fw | TTGAGTCAAATTAAGCCGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Chacoff, L.; Ramírez-Mora, J.; Nualart, D.; Dann, F.; Muñoz, J.L.P. Atlantic Salmon (Salmo salar) GILL Primary Cell Culture Oxidative Stress and Cellular Damage Response Challenged with Oxytetracycline Antibiotic. Toxics 2025, 13, 914. https://doi.org/10.3390/toxics13110914
Vargas-Chacoff L, Ramírez-Mora J, Nualart D, Dann F, Muñoz JLP. Atlantic Salmon (Salmo salar) GILL Primary Cell Culture Oxidative Stress and Cellular Damage Response Challenged with Oxytetracycline Antibiotic. Toxics. 2025; 13(11):914. https://doi.org/10.3390/toxics13110914
Chicago/Turabian StyleVargas-Chacoff, Luis, José Ramírez-Mora, Daniela Nualart, Francisco Dann, and José Luis P. Muñoz. 2025. "Atlantic Salmon (Salmo salar) GILL Primary Cell Culture Oxidative Stress and Cellular Damage Response Challenged with Oxytetracycline Antibiotic" Toxics 13, no. 11: 914. https://doi.org/10.3390/toxics13110914
APA StyleVargas-Chacoff, L., Ramírez-Mora, J., Nualart, D., Dann, F., & Muñoz, J. L. P. (2025). Atlantic Salmon (Salmo salar) GILL Primary Cell Culture Oxidative Stress and Cellular Damage Response Challenged with Oxytetracycline Antibiotic. Toxics, 13(11), 914. https://doi.org/10.3390/toxics13110914








