The Effects of Urbanization on Chronic Kidney Disease and Renal Function Decline: Findings from a Nation-Wide Longitudinal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Urbanization Level Assessment
2.3. Health Outcomes
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Descriptive Analyses
3.2. Regression Analyses
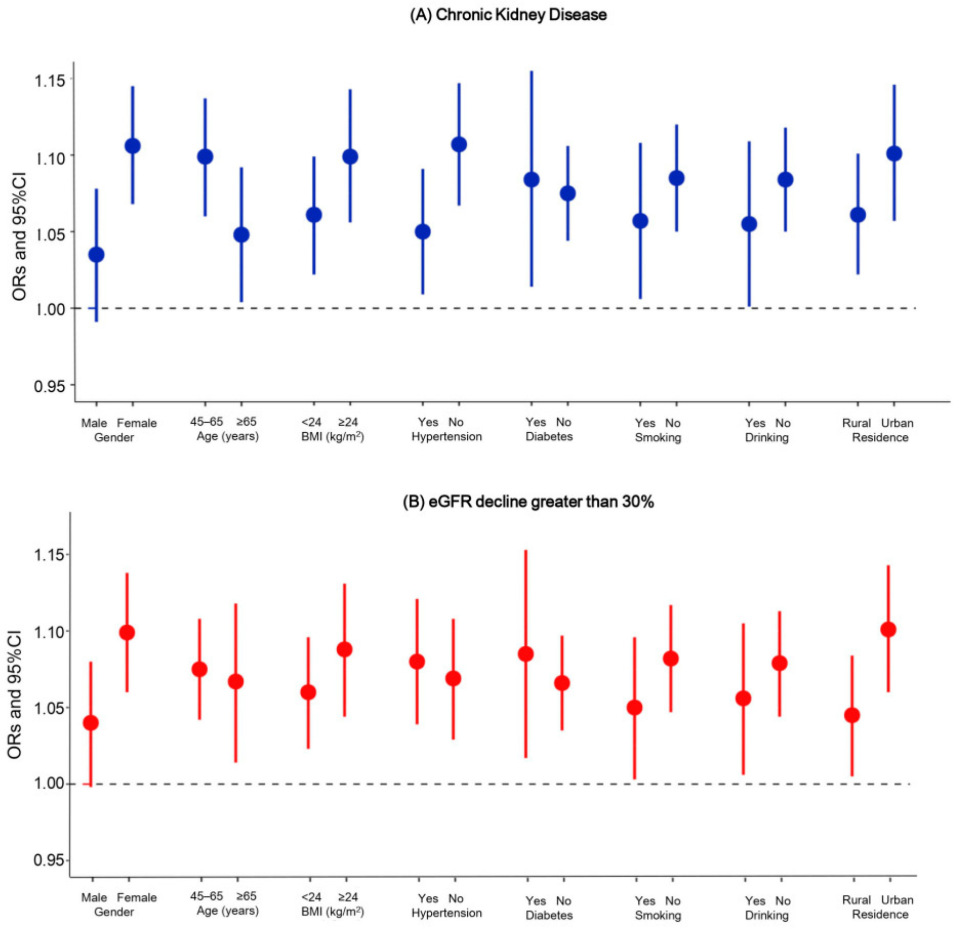
3.3. Stratification Analyses
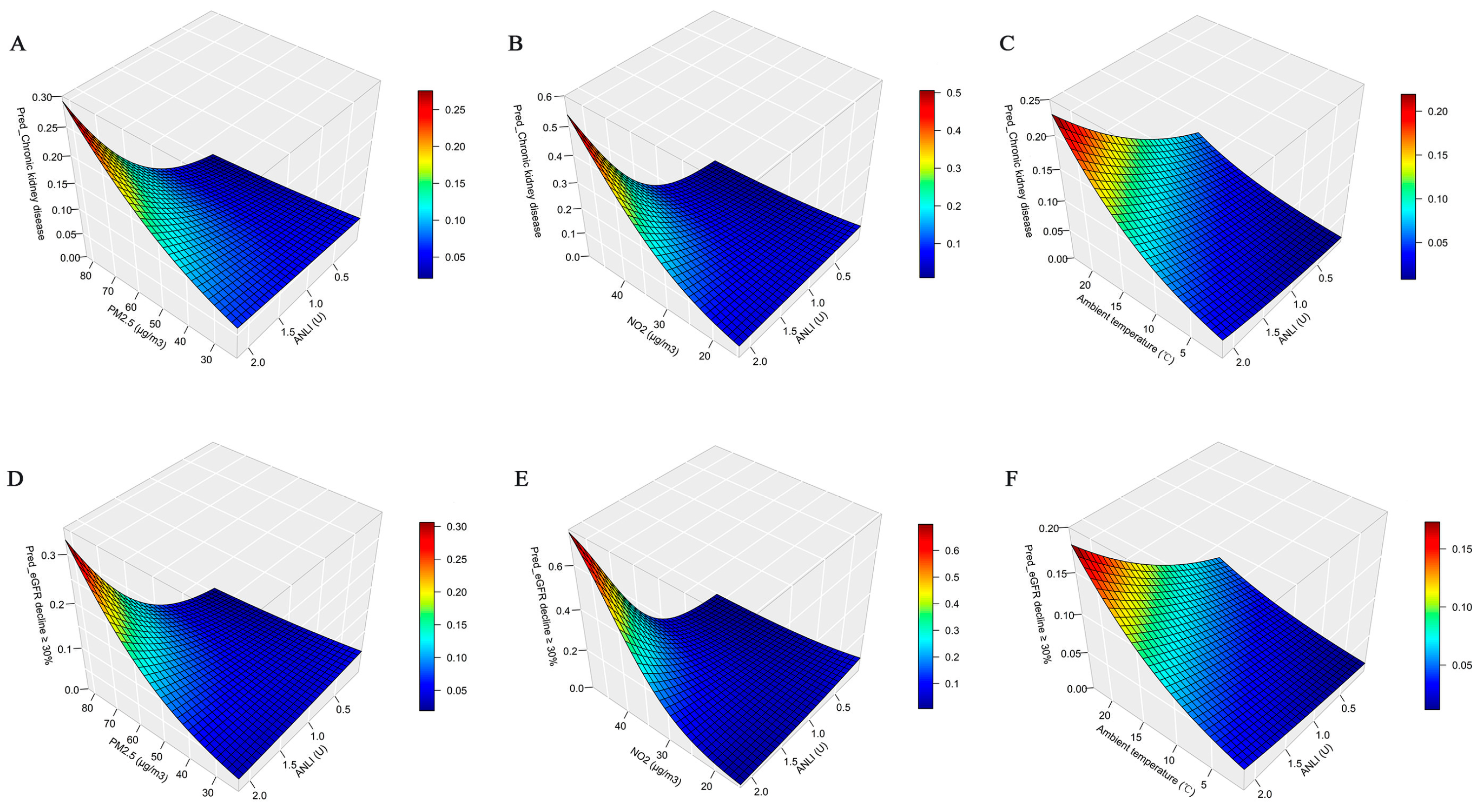
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM2.5 | fine particulate matter |
| NO2 | nitrogen dioxide |
| NCDs | non-communicable disease |
| CKD | chronic kidney disease |
| CHARLS | China Health and Retirement Longitudinal Study |
| ANLI | average nighttime light index |
| NLI | nighttime light index |
| IDW | inverse distance weighted |
| BMI | body mass index |
| eGFR | estimated glomerular filtration rate |
| OR | odds ratio |
| CI | confidence interval |
References
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Vassalotti, J.A.; Centor, R.; Turner, B.J.; Greer, R.C.; Choi, M.; Sequist, T.D. Practical Approach to Detection and Management of Chronic Kidney Disease for the Primary Care Clinician. Am. J. Med. 2016, 129, 153–162.e7. [Google Scholar] [CrossRef]
- GBD. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [PubMed]
- Zhang, L.; Wang, F.; Wang, L.; Wang, W.; Liu, B.; Liu, J.; Chen, M.; He, Q.; Liao, Y.; Yu, X.; et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet 2012, 379, 815–822. [Google Scholar] [CrossRef]
- Vanholder, R.; Annemans, L.; Bello, A.K.; Bikbov, B.; Gallego, D.; Gansevoort, R.T.; Lameire, N.; Luyckx, V.A.; Noruisiene, E.; Oostrom, T.; et al. Fighting the unbearable lightness of neglecting kidney health: The decade of the kidney. Clin. Kidney J. 2021, 14, 1719–1730. [Google Scholar] [CrossRef]
- Jagannathan, R.; Patzer, R.E. Urbanization and kidney function decline in low and middle income countries. BMC Nephrol. 2017, 18, 276. [Google Scholar] [CrossRef]
- Inoue, Y.; Howard, A.G.; Thompson, A.L.; Mendez, M.A.; Herring, A.H.; Gordon-Larsen, P. The association between urbanization and reduced renal function: Findings from the China Health and Nutrition Survey. BMC Nephrol. 2017, 18, 160. [Google Scholar] [CrossRef]
- Allender, S.; Wickramasinghe, K.; Goldacre, M.; Matthews, D.; Katulanda, P. Quantifying Urbanization as a Risk Factor for Noncommunicable Disease. J. Urban Health Bull. N. Y. Acad. Med. 2011, 88, 906–918. [Google Scholar] [CrossRef]
- Oyebode, O.; Pape, U.J.; Laverty, A.A.; Lee, J.T.; Bhan, N.; Millett, C. Rural, urban and migrant differences in non-communicable disease risk-factors in middle income countries: A cross-sectional study of WHO-SAGE data. PLoS ONE 2015, 10, e0122747. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Lin, T.; Dixon, J.; Zhang, G.; Ye, H. Urbanization and health in China, thinking at the national, local and individual levels. Environ. Health 2016, 15 (Suppl. 1), 32. [Google Scholar] [CrossRef]
- Soderland, P.; Lovekar, S.; Weiner, D.E.; Brooks, D.R.; Kaufmann, J.S. Chronic Kidney Disease Associated With Environmental Toxins and Exposures. Adv. Chronic Kidney Dis. 2010, 17, 254–264. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Ding, H.Y.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- McKinley, J.M.; Mueller, U.; Atkinson, P.M.; Ofterdinger, U.; Cox, S.F.; Doherty, R.; Fogarty, D.; Egozcue, J.J.; Pawlowsky-Glahn, V. Chronic kidney disease of unknown origin is associated with environmental urbanisation in Belfast, UK. Environ. Geochem. Health 2021, 43, 2597–2614. [Google Scholar] [CrossRef]
- Bello, A.K.; Peters, J.; Rigby, J.; Rahman, A.A.; El Nahas, M. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin. J. Am. Soc. Nephrol. 2008, 3, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Ustaoglu, E.; Bovkir, R.; Aydinoglu, A.C. Spatial distribution of GDP based on integrated NPS-VIIRS nighttime light and MODIS EVI data: A case study of Turkey. Environ. Dev. Sustain. 2021, 23, 10309–10343. [Google Scholar] [CrossRef]
- Propastin, P.; Kappas, M. Assessing Satellite-Observed Nighttime Lights for Monitoring Socioeconomic Parameters in the Republic of Kazakhstan. Giscience Remote Sens. 2012, 49, 538–557. [Google Scholar] [CrossRef]
- Forbes, D.J. Multi-scale analysis of the relationship between economic statistics and DMSP-OLS night light images. Giscience Remote Sens. 2013, 50, 483–499. [Google Scholar] [CrossRef]
- Fu, D.; Xia, X.; Duan, M.; Zhang, X.; Li, X.; Wang, J.; Liu, J. Mapping nighttime PM2.5 from VIIRS DNB using a linear mixed-effect model. Atmos. Environ. 2018, 178, 214–222. [Google Scholar] [CrossRef]
- Xu, Z.; Xia, X.P.; Liu, X.N.; Qian, Z.G. Combining DMSP/OLS Nighttime Light with Echo State Network for Prediction of Daily PM2.5 Average Concentrations in Shanghai, China. Atmosphere 2015, 6, 1507–1520. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, W.Z.; Wang, Y.Y.; Ma, L.; Liang, C.Y.; Li, P.F.; Yang, C.; Wei, F.L.; Li, S.C.; Zhang, L.X. Urbanization, ambient air pollution, and prevalence of chronic kidney disease: A nationwide cross-sectional study. Environ. Int. 2021, 156, 106752. [Google Scholar] [CrossRef]
- Lai, K.Y.; Sarkar, C.; Ni, M.Y.; Gallacher, J.; Webster, C. Exposure to light at night (LAN) and risk of obesity: A systematic review and meta-analysis of observational studies. Environ. Res. 2020, 187, 109637. [Google Scholar] [CrossRef]
- Sun, S.; Cao, W.; Ge, Y.; Ran, J.; Sun, F.; Zeng, Q.; Guo, M.; Huang, J.; Lee, R.S.; Tian, L.; et al. Outdoor light at night and risk of coronary heart disease among older adults: A prospective cohort study. Eur. Heart J. 2021, 42, 822–830. [Google Scholar] [CrossRef]
- Wu, Y.; Gui, S.Y.; Fang, Y.; Zhang, M.; Hu, C.Y. Exposure to outdoor light at night and risk of breast cancer: A systematic review and meta-analysis of observational studies. Environ. Pollut. 2021, 269, 116114. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhu, G.; Zhao, Z.X.; Sabel, C.E.; Ma, Z.W.; Jiao, Z.H.; Zhao, J.; Wang, H.K. Population aging might have delayed the alleviation of China’s PM2.5 health burden. Atmos. Environ. 2022, 270, 118895. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Y.; Smith, J.P.; Strauss, J.; Yang, G. Cohort profile: The China Health and Retirement Longitudinal Study (CHARLS). Int. J. Epidemiol. 2014, 43, 61–68. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Yu, B.L.; Yang, C.S.; Zhou, Y.Y.; Yao, S.J.; Qian, X.J.; Wang, C.X.; Wu, B.; Wu, J.P. An extended time series (2000–2018) of global NPP-VIIRS-like nighttime light data from a cross-sensor calibration. Earth Syst. Sci. Data 2021, 13, 889–906. [Google Scholar] [CrossRef]
- Xavier, A.C.; King, C.W.; Scanlon, B.R. Daily gridded meteorological variables in Brazil (1980–2013). Int. J. Climatol. 2016, 36, 2644–2659. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.; Knibbs, L.D.; Hamm, N.A.S.; Cao, W.; Li, T.; Guo, J.; Ren, H.; Abramson, M.J.; Guo, Y. A machine learning method to estimate PM2.5 concentrations across China with remote sensing, meteorological and land use information. Sci. Total Environ. 2018, 636, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Dong, B.; Li, S.S.; Chen, G.B.; Yang, Z.G.; Dong, Y.H.; Wang, Z.H.; Ma, J.; Guo, Y.M. Exposure to ambient particulate matter air pollution, blood pressure and hypertension in children and adolescents: A national cross-sectional study in China. Environ. Int. 2019, 128, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Turin, T.C.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Appel, L.J.; Arima, H.; Chadban, S.J.; Cirillo, M.; Djurdjev, O.; et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014, 311, 2518–2531. [Google Scholar] [CrossRef] [PubMed]
- GB/T 2260–2007; Codes for the Administrative Divisions of the People’s Republic of China. Standards Press of China: Beijing, China, 2007.
- Masimango, M.I.; Sumaili, E.K.; Wallemacq, P.; Malembaka, E.B.; Hermans, M.P.; Fillee, C.; D’Hoore, W.; Winkler, C.A.; Limou, S.; Jadoul, M. Prevalence and Risk Factors of CKD in South Kivu, Democratic Republic of Congo: A Large-Scale Population Study. Kidney Int. Rep. 2020, 5, 1251–1260. [Google Scholar] [CrossRef]
- Bello, A.K.; Ronksley, P.E.; Tangri, N.; Kurzawa, J.; Osman, M.A.; Singer, A.; Grill, A.; Nitsch, D.; Queenan, J.A.; Wick, J.; et al. Prevalence and Demographics of CKD in Canadian Primary Care Practices: A Cross-sectional Study. Kidney Int. Rep. 2019, 4, 561–570. [Google Scholar] [CrossRef]
- Gan, T.; Liang, W.; Yang, H.C.; Liao, X.C. The effect of Economic Development on haze pollution (PM2.5) based on a spatial perspective: Urbanization as a mediating variable. J. Clean. Prod. 2020, 266, 121880. [Google Scholar] [CrossRef]
- Chen, L.; Frauenfeld, O.W. Impacts of urbanization on future climate in China. Clim. Dyn. 2016, 47, 345–357. [Google Scholar] [CrossRef]
- Patil, R.R. Urbanization as a Determinant of Health: A Socioepidemiological Perspective. Soc. Work. Public Health 2014, 29, 335–341. [Google Scholar] [CrossRef]
- Gassasse, Z.; Smith, D.; Finer, S.; Gallo, V. Association between urbanisation and type 2 diabetes: An ecological study. BMJ Glob. Health 2017, 2, e000473. [Google Scholar] [CrossRef]
- Du, W.W.; Wang, H.J.; Su, C.; Jia, X.F.; Zhang, B. Thirty-Year Urbanization Trajectories and Obesity in Modernizing China. Int. J. Environ. Res. Public Health 2022, 19, 1943. [Google Scholar] [CrossRef] [PubMed]
- Navara, K.J.; Nelson, R.J. The dark side of light at night: Physiological, epidemiological, and ecological consequences. J. Pineal Res. 2007, 43, 215–224. [Google Scholar] [CrossRef]
- Luo, X.M.; Sun, K.; Li, L.; Wu, S.; Yan, D.; Fu, X.S.; Luo, H. Impacts of urbanization process on PM2.5 pollution in “2 + 26” cities. J. Clean. Prod. 2021, 284, 124761. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Li, T.T.; Yan, Y.; Xian, H.; Al-Aly, Z. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: A cohort study. Lancet Planet. Health 2017, 1, E267–E276. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Li, T.T.; Yan, Y.; Xian, H.; Al-Aly, Z. Particulate Matter Air Pollution and the Risk of Incident CKD and Progression to ESRD. J. Am. Soc. Nephrol. 2018, 29, 218–230. [Google Scholar] [CrossRef]
- Liu, X.X.; Zhao, X.L.; Ye, L.; Hu, C.F.; Xie, Z.H.; Ma, J.N.; Wang, X.; Liang, W. The TyG Index Mediates Air-Pollution-Associated Chronic Kidney Disease Incidence in HIV/AIDS Patients: A 20-Year Cohort Study. Toxics 2025, 13, 669. [Google Scholar]
- Ma, H.F.; Liang, W.; Han, A.J.; Zhang, Q.; Gong, S.; Bai, Y.; Gao, D.M.; Xiang, H.; Wang, X. Ambient particulate matter and renal function decline in people with HIV/AIDS. AIDS 2024, 38, 713–721. [Google Scholar] [PubMed]
- Hansson, E.; Glaser, J.; Jakobsson, K.; Weiss, I.; Wesseling, C.; Lucas, R.A.I.; Wei, J.L.K.; Ekstrom, U.; Wijkstrom, J.; Bodin, T.; et al. Pathophysiological Mechanisms by which Heat Stress Potentially Induces Kidney Inflammation and Chronic Kidney Disease in Sugarcane Workers. Nutrients 2020, 12, 1639. [Google Scholar] [CrossRef]
- Ho, J.Y.; Zijlema, W.L.; Triguero-Mas, M.; Donaire-Gonzalez, D.; Valentin, A.; Ballester, J.; Chan, E.Y.Y.; Goggins, W.B.; Mo, P.K.H.; Kruize, H.; et al. Does surrounding greenness moderate the relationship between apparent temperature and physical activity? Findings from the PHENOTYPE project. Environ. Res. 2021, 197, 110992. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, R.; Xu, Z.; Jin, J.; Wang, J.; Yang, T.; Wei, J.; Huang, J.; Li, G. Long-term exposure to ozone and diabetes incidence: A longitudinal cohort study in China. Sci. Total Environ. 2022, 816, 151634. [Google Scholar] [CrossRef]
- Elliot, S.J.; Berho, M.; Korach, K.; Doublier, S.; Lupia, E.; Striker, G.E.; Karl, M. Gender-specific effects of endogenous testosterone: Female alpha-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int. 2007, 72, 464–472. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, X.; Guo, Y.; Wang, Y.; Liu, Y.; Zhang, S.; Xie, H.Q.; Xiang, T.; Li, Z.; Nie, T.; et al. Effect-directed analysis of estrogenic chemicals in sediments from an electronic-waste recycling area. Environ. Pollut. 2022, 306, 119369. [Google Scholar] [CrossRef]
- Gong, P.; Liang, S.; Carlton, E.J.; Jiang, Q.; Wu, J.; Wang, L.; Remais, J.V. Urbanisation and health in China. Lancet 2012, 379, 843–852. [Google Scholar] [CrossRef]
- Tripathy, J.P.; Thakur, J.S.; Jeet, G.; Chawla, S.; Jain, S.; Prasad, R. Urban rural differences in diet, physical activity and obesity in India: Are we witnessing the great Indian equalisation? Results from a cross-sectional STEPS survey. BMC Public Health 2016, 16, 816. [Google Scholar] [CrossRef]
- Andrassy, K.M. Comments on ‘KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef]
- Li, G.X.; Huang, J.; Wang, J.W.; Zhao, M.H.; Liu, Y.; Guo, X.B.; Wu, S.W.; Zhang, L.X. Long-Term Exposure to Ambient PM2.5 and Increased Risk of CKD Prevalence in China. J. Am. Soc. Nephrol. 2021, 32, 448–458. [Google Scholar] [CrossRef]
- Chan, T.C.; Zhang, Z.L.; Lin, B.C.; Lin, C.Q.; Deng, H.B.; Chuang, Y.C.; Chan, J.W.M.; Jiang, W.K.; Tam, T.; Chang, L.Y.; et al. Long-Term Exposure to Ambient Fine Particulate Matter and Chronic Kidney Disease: A Cohort Study. Environ. Health Perspect. 2018, 126, 107002. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chu, D.C.; Lee, J.H.; Yang, Y.R.; Chan, C.C. Traffic-related air pollution associated with chronic kidney disease among elderly residents in Taipei City. Environ. Pollut. 2018, 234, 838–845. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD or N (%) | |
|---|---|---|
| NO. | 5298 | |
| Age (years) | 58.6 ± 8.5 | |
| Gender (n, %) | ||
| Male | 2395 (45.2) | |
| Female | 2903 (54.8) | |
| BMI (kg/m3) | 23.6 ± 3.8 | |
| Education level (n, %) | ||
| Illiteracy | 4850 (91.5) | |
| Elementary school or above | 448 (8.5) | |
| Marital status (n, %) | ||
| Married | 4705 (88.8) | |
| Separated/Divorced/Widowed | 593 (11.2) | |
| Smoking status (n, %) | ||
| Smoker | 2008 (37.9) | |
| Non-smoker | 3290 (62.1) | |
| Drinking status (n, %) | ||
| Drinker | 1727 (32.6) | |
| Non-drinker | 3571 (67.4) | |
| Current residence (n, %) | ||
| Rural | 3649 (68.9) | |
| Urban | 1649 (31.1) | |
| Hypertension (n, %) | 2080 (39.3) | |
| Diabetes (n, %) | 702 (13.3) | |
| Cardiovascular disease (n, %) | 585 (11.0) | |
| Stroke (n, %) | 97 (1.8) | |
| Baseline eGFR (mL/min/1.73 m2) | 96.2 ± 14.6 | |
| Temperature (°C) | 15.4 ± 4.3 | |
| PM2.5 (μg/m3) | 53.3 ± 13.6 | |
| NO2 (μg/m3) | 28.4 ± 8.4 | |
| CKD | eGFR Decline Greater than 30% | ||||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | ||
| Continuous variables | |||||
| Crude model | 1.080 (1.055, 1.105) | <0.001 | 1.067 (1.041, 1.092) | <0.001 | |
| Model I | 1.077 (1.050, 1.103) | <0.001 | 1.067 (1.040, 1.093) | <0.001 | |
| Model II | 1.080 (1.053, 1.107) | <0.001 | 1.068 (1.041, 1.095) | <0.001 | |
| Model III | 1.073 (1.045, 1.101) | <0.001 | 1.07 (1.042, 1.097) | <0.001 | |
| Categorical variables | |||||
| Q1 (0.017~0.107) | ref. | ref. | |||
| Q2 (0.107~0.265) | 1.082 (0.663, 1.773) | 0.754 | 1.421 (0.893, 2.276) | 0.140 | |
| Q3 (0.265~0.610) | 2.622 (1.610, 4.324) | <0.001 | 2.402 (1.480, 3.935) | <0.001 | |
| Q4 (0.610~2.176) | 2.521 (1.573, 4.092) | <0.001 | 2.528 (1.596, 4.046) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, W.; Hou, D.; Li, X.; Qiu, J.; Wang, M.; Zhao, X.; Peng, S.; Lu, G. The Effects of Urbanization on Chronic Kidney Disease and Renal Function Decline: Findings from a Nation-Wide Longitudinal Study. Toxics 2025, 13, 907. https://doi.org/10.3390/toxics13110907
Liang W, Hou D, Li X, Qiu J, Wang M, Zhao X, Peng S, Lu G. The Effects of Urbanization on Chronic Kidney Disease and Renal Function Decline: Findings from a Nation-Wide Longitudinal Study. Toxics. 2025; 13(11):907. https://doi.org/10.3390/toxics13110907
Chicago/Turabian StyleLiang, Wei, Dong Hou, Xiaoyu Li, Jiayi Qiu, Mei Wang, Xiuli Zhao, Shouxin Peng, and Guangyu Lu. 2025. "The Effects of Urbanization on Chronic Kidney Disease and Renal Function Decline: Findings from a Nation-Wide Longitudinal Study" Toxics 13, no. 11: 907. https://doi.org/10.3390/toxics13110907
APA StyleLiang, W., Hou, D., Li, X., Qiu, J., Wang, M., Zhao, X., Peng, S., & Lu, G. (2025). The Effects of Urbanization on Chronic Kidney Disease and Renal Function Decline: Findings from a Nation-Wide Longitudinal Study. Toxics, 13(11), 907. https://doi.org/10.3390/toxics13110907